Abstract
Animals anticipating a meal initiate a series of responses enabling them to better cope with the meal’s metabolic impact. These responses, such as cephalic insulin, occur prior to the onset of ingestion and are especially evident in animals maintained on a meal-feeding schedule with limited but predictable access to food each day. We tested the hypothesis that meal-fed rats secrete the incretin hormone glucagon-like peptide-1 (GLP-1) cephalically when anticipating a large meal. Male Long-Evans rats were fed ad libitum (controls) or adapted to a schedule on which food was available for the same 4-h period each day (meal fed animals). Plasma GLP-1 increased in meal-fed rats over an interval from 75 to 60 min prior to feeding time, from a baseline of 10 to around 40 pm, and then returned to baseline prior to food presentation. Controls had steady plasma GLP-1 levels (10–15 pm) over the same span. Meal-fed rats also secreted cephalic insulin starting around 15 min prior to food presentation. Administration of the selective GLP-1 receptor antagonist exendin-4[desHis-1,Glu-9] prior to the premeal spike of GLP-1 caused meal-fed rats to eat significantly less food than normal, whereas administration of the antagonist after the GLP-1 spike but prior to food presentation resulted in a significant increase in food consumption. These findings document for the first time a cephalic increase of plasma GLP-1 and suggest that it functions to facilitate consumption of a large meal.
Plasma GLP-1 increases sharply and then returns to baseline an hour before a scheduled meal in rats, implying a neurally mediated anticipatory control mechanism.
Consumption of large meals is a useful adaptive strategy to optimize survival under certain challenging environments. However, intake of large nutrient loads represents a substantial threat to homeostasis in that excessive substrate fluxes challenge the normal physiological processes of disposal (1). To facilitate postprandial homeostasis, animals routinely make premeal anticipatory responses to enhance digestion and metabolism of a meal. The term cephalic is used to describe these anticipatory responses because they are initiated by central nervous system responses to cues in the environment that reliably predict the opportunity to eat and digest nutrients. Moreover, in rats habituated to receive their entire daily food ration over a fixed, shortened interval each day, preprandial anticipatory responses are augmented (2). This finding suggests that animals adapt to the metabolic requirements of experimentally induced large meals, i.e. they adapt and are said to be meal fed.
Because meal-fed animals must balance the conflicting necessities of daily caloric requirements and metabolic homeostasis, they are an excellent model for studying preprandial cephalic responses. We previously documented increased preprandial secretion of insulin and ghrelin in meal-fed rats (2,3), and other groups used similar approaches to show increased secretion of digestive enzymes, reduced metabolic rate, increased body temperature, and preprandial insulin secretion (see reviews in Refs. 1 and 4,5,6,7,8,9,10,11). These responses would be expected to enhance meal digestion, compensate for prandial changes in energy expenditure, and promote clearance of glucose from the circulation. Indeed, cephalic control of meal-induced hyperglycemia appears to be an important adaptation in that there is evidence that the secretion of hormones that influence blood glucose, such as cholecystokinin (12,13,14,15,16) and glucagon (17,18,19,20), is also conditioned to meal time.
The incretin glucagon-like peptide (GLP)-1 is secreted from the intestine during meals and augments insulin secretion and promotes glucose disposal (21). In addition, GLP-1 contributes to satiation (22,23,24) and regulates gastric function (25,26). GLP-1 is essential for normal glucose tolerance and compounds based on GLP-1 receptor activation have been developed as therapeutics for diabetes (27,28). We therefore reasoned that GLP-1 might be secreted before anticipated meals to augment pre- and postprandial nutrient homeostasis. The purpose of these experiments therefore was to assess anticipatory GLP-1 in meal-fed rats.
Materials and Methods
Animals
Male Long-Evans rats (Harlan, Indianapolis, IN) weighing between 250 and 300 g at the onset of the experiments were housed in individual plastic shoebox cages with ad libitum access to pelleted rat chow (Teklad; Harlan, Madison, WI) and water in a temperature-controlled vivarium (22 ± 2 C) on a 12-h light, 12-h dark schedule, with lights on at 0700 h. The research was approved by the University of Cincinnati Institutional Animal Care and Use Committee and carried out in Association for Assessment and Accreditation of Laboratory Animal Care-approved facilities conforming to National Institutes of Health and U.S. Department of Agriculture regulations.
Inferior vena cava catheterization
Rats were anesthetized with 1.0 ml/kg ketamine/xylazine (10:6.5 ratio), and a small skin incision was made on the dorsum of the skull. The rat was then turned over and the abdomen opened applying sterile technique. The small intestine and the cecum were lifted to expose the inferior vena cava. After clearing the retroperitoneal fat from the vein, a 21-gauge needle was used to puncture the vein just superior to the right renal vein and a SILASTIC brand cannula (Dow Corning, Midland, MI) was inserted. The catheter was secured with 4-0 silk suture at the entrance site and 1 cm proximally and thereafter was passed sc to the incision over the skull in which it was anchored with acrylic cement. This procedure is routine in our laboratory, and we are able to maintain patency for several weeks by filling the lines with heparinized glycerol (500 U heparin/ml) between samplings.
Experiment 1
Rats with indwelling iv catheters had access to chow plus water from 1200 to 1600 h daily and water alone for the remainder of the day. Food intake and body weight were recorded each day. Beginning 14 d after the start of this meal-feeding schedule, blood sampling was initiated. In past studies we found that handling meal-fed rats and taking one or more blood samples at any point within 2 or 3 h preceding a scheduled meal can cause less-than-normal food intake during the subsequent meal (our unpublished observations). We therefore harvested only a single sample on a given day from any animal, leaving it undisturbed on most days. In this manner we were able to accumulate a relatively undisturbed premeal profile of plasma GLP-1 from eight to 12 rats at each of the specified time points over the course of several weeks. In other words, each rat was sampled once at a specified time during the premeal period (the time on any particular sampling day was randomly determined for each rat but never at the same time that it had been previously sampled). On subsequent days spaced at least 3 d apart, each rat was sampled at a different time. All samples were assayed for GLP-1 or insulin in the same assay.
A group of weight-matched control rats with ad libitum food and water but lacking iv catheters had food intake and body weight assessed daily. Cohorts of these controls were killed by decapitation at 1030 and 1100 h after 20 d into the experiment and trunk blood collected. Blood samples were collected in 0.5 m EDTA, 500 kIU/ml aprotinin, stored on ice until centrifugation, and the plasma frozen at −20 C until assayed.
Experiment 2
A group of comparably meal-fed rats, lacking iv catheters, was used in an alternative protocol for assessing premeal GLP-1. After 17 d on the meal-feeding schedule, cohorts were killed by decapitation at 1100, 1130, and 1145 h (i.e. 60, 30, and 15 min before meal feeding time). Trunk blood was collected and processed as in experiment 1.
Experiment 3
After 3 wk on the same feeding schedule (chow available from 1200 to 1600 h), half of a novel cohort of meal-fed rats received the selective GLP-1 receptor antagonist exendin-4[desHis-1,Glu-9] (dH-Ex-4; American Peptides, Sunnyvale, CA; 20 μg iv; see Ref. 29), and half received the vehicle, physiological saline, iv, at 1000 h. Food was returned at 1200 h and intake assessed after 15 and 30 min and 1 and 4 h. One week later the groups were reversed, such that each rat received both exendin and vehicle, in random order. Several weeks later the same rats underwent the same two procedures except that the saline or exendin was administered at 1145 h. The meal-feeding schedule was maintained throughout.
Plasma analyses
Glucose was measured using a glucose oxidase method. Insulin concentrations were determined by RIA using a guinea pig antiinsulin serum, iodinated insulin, and a double-antibody separation method (30). Total GLP-1 immunoreactivity was measured by RIA using antiserum 89390 (kindly provided by Dr. Jens Holst, Panum Institute, Copenhagen, Denmark) from ethanol extracts of plasma, as previously described (31,32). This antibody recognizes both the intact hormone GLP-1-(7-36)-NH2 and the metabolite GLP-1-(9-36)-NH2 and is a reliable index of secreted hormone. The intraassay variability is less than 7% and the interassay variability is less than 11%.
Statistical analyses
Between-subjects ANOVAs were used where appropriate to determine differences between meal-fed and ad libitum groups. One-way repeated-measures ANOVA was used to analyze meal-fed food intake and body weight in experiment 3. All pair-wise comparisons of mean differences were conducted using Tukey honestly significant differences post hoc comparisons. Differences between group means were considered statistically significant if P < 0.05.
Results
Experiment 1
Once the meal-feeding protocol was started, mean daily intake of meal-fed rats gradually increased and was stable by d 12. After 14 d on the schedule, the meal-fed rats consumed slightly less chow during the 4-h access period (18.5 ± 0.4 g; mean ± sem) than what they consumed over 24 h on the day before the start of meal feeding (21.0 ± 0.6 g; P < 0.05). Accordingly, they weighed slightly less than the controls (322 ± 4 g vs. 368 ± 2 g; P < 0.05). However, once they had acclimated to the meal-feeding regimen, rats gained weight at the same rate as ad libitum-fed controls (∼2.5 g/d for each group, P = n.s.).
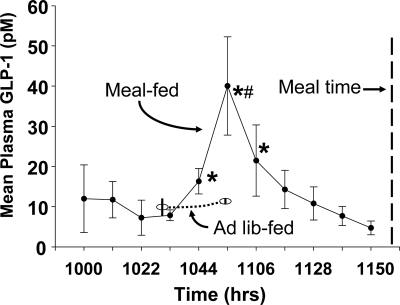
As depicted in Fig. 1, plasma GLP-1 increased sharply in the meal-fed rats approximately 75 min before the expected meal, from a baseline of near 10 pm to just over 40 pm and then returned to baseline by 40 min before food presentation. These data from a group of 12 meal-fed rats were generated over several weeks because each rat had only one sample, at one randomly determined time point, taken on any given day, to develop the temporal profile. The baseline fasting concentration of GLP-1 was computed as the mean of the four time points from 1000 to 1033 h. The mean levels of GLP-1 at the subsequent three time points (centered at 1044, 1055, and 1106 h) were each significantly elevated relative to the baseline (all P < 0.05). Subsequent values were not different from the baseline. Plasma GLP-1 levels in a group of ad libitum-fed controls are also depicted in Fig. 1. The peak GLP-1 (at 1055 h) in the meal-fed rats was significantly higher than the values at 1030 and 1100 h in the controls (all P < 0.05). Based on these findings, meal-fed rats have a previously undescribed anticipatory increase of GLP-1 before a scheduled meal.
Figure 1.
Mean plasma GLP-1 in meal-fed and control rats before the time of food presentation for the meal-fed rats. The plasma data for each meal-fed rat were collected from iv catheters on different days. They were grouped into 11-min blocks for statistical analysis and graphing; each point represents the mean value for eight to 12 rats. The plasma data for meal-fed controls were obtained at the time the animals were killed. All of the blood samples were analyzed in the same assay. *, Statistically different from baseline; #, statistically different from the controls at that time.
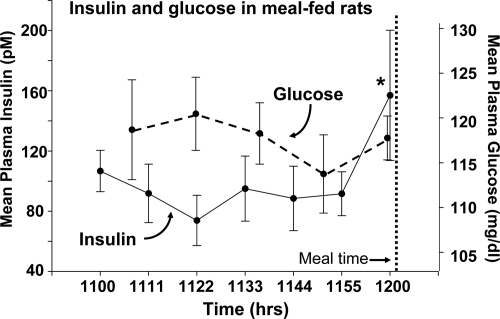
Plasma insulin and glucose levels in the meal-fed rats are depicted in Fig. 2. Plasma insulin levels were stable until the final sample taken just before food was presented, at which time it was significantly increased in the meal-fed rats. Plasma glucose levels were stable throughout the preprandial period.
Figure 2.
Mean plasma insulin and glucose values for the rats described in Fig. 1. *, Significantly different from the prior time point.
Experiment 2
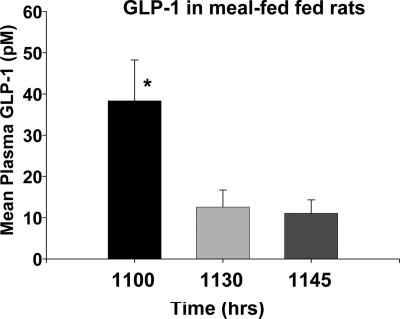
Meal-fed rats lacking iv catheters were comparable in mean food intake and body weight as the rats in experiment 1 after 14 d of meal feeding. On d 17 cohorts were killed and the plasma assessed for GLP-1 as depicted in Fig. 3. Rats in the −60 min cohort had plasma GLP-1 around 40 pm, and this was significantly higher than levels of GLP-1 at −30 and −15 min (both P < 0.05). Thus, the results from meal-fed animals studied using a second blood sampling paradigm also demonstrate a preprandial peak in GLP-1 levels.
Figure 3.
Mean plasma GLP-1 in cohorts of meal-fed rats in experiment 2 killed at 1100, 1130, and 1145 h, i.e. at 60, 30, and 15 min before the time of their scheduled food. *, Significantly different from the other two points.
Experiment 3
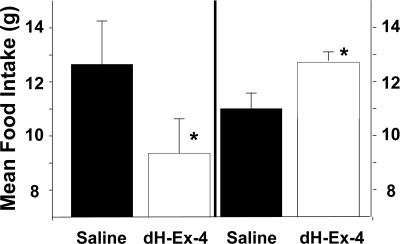
When the GLP-1 antagonist dH-Ex-4 was administered at 1000 h, timed to overlap the premeal GLP-1 spike, meal-fed rats consumed less food than when administered saline, a difference that was significant at each time point (15 and 30 min and 1 and 4 h, all P < 0.05). The left side of Fig. 4 depicts the 60-min data. These observations imply that blocking the action of the endogenous spike of GLP-1 before a scheduled meal reduces food intake in meal-fed rats. However, because it is possible that the infused exendin continued to exert some action after the spike of GLP-1, around 4 wk later the same animals were subsequently administered, the same doses of GLP-1 receptor antagonist and vehicle immediately before the meal, at 1145 h. As seen in the right side of Fig. 4, dH-Ex-4 administered at 1145 h had the opposite action, increasing food intake in meal-fed animals. Animals ate more at each time point and the differences were significant at 30 and 60 min.
Figure 4.
Mean 60-min food intake in meal-fed rats administered the GLP-1 antagonist dH-Ex-4 or saline at 1000 h (left side) or 1145 h (right side). *, Significantly different from saline administered at the same time in the same animals.
Discussion
We observed that rats on a meal-feeding schedule in which food is available during the same 4-h period each day secrete GLP-1 transiently starting around 75 min before feeding time and that plasma GLP-1 subsequently returns to baseline by the time food is presented. To our knowledge, this transient surge of GLP-1 has not previously been reported. Interestingly, although previous studies indicate that central or peripheral GLP-1 reduces food intake, our data suggest that preprandial GLP-1 secretion actually contributes to the increased food consumption that is characteristic of meal feeding. These findings are consistent with a role for GLP-1 release in the adaptation to meal feeding and suggest that this incretin plays a role in the anticipatory signals related to eating.
In approaching the question of cephalic GLP-1 secretion, we faced one major technical challenge. As discussed above, handling rats and taking one or more blood samples at any point within 2 or 3 h preceding a scheduled meal reduces food intake during that meal (our unpublished observations). We presume that even in rats habituated to these experiments, the procedure causes sufficient stress to interfere with the ongoing coordinated cephalic endocrine response that enables the individual to consume the large meal. Consequently, we adopted a sampling procedure to minimize the handling of rats in the 4 h before their habituated meal and limit the disturbance of anticipatory signals adapted for meal feeding. By sampling over the course of several weeks, we were able to generate complete preprandial GLP-1 profiles for the meal-fed animals in an unbiased manner. The success of this method is based on, and in fact demonstrates clearly, the degree of entrainment rats get as a result of meal feeding. We used an alternative protocol, killing meal-fed rats at key time points over the preprandial period in experiment 2 to confirm a premeal increment of GLP-1. This procedure was sufficient to support our initial finding but lacks the temporal resolution of random repeated blood sampling.
Most premeal anticipatory responses, such as cephalic insulin, occur much closer in time to the pending meal than what we observed with GLP-1 (7,8,10). Indeed, a premeal increase of insulin was not observed until the final sampling time in the present experiment (cf., Fig. 2). The increase of GLP-1 beginning more than an hour before the scheduled meal might therefore seem at odds with other cephalic responses. However, we presume that in highly meal-fed animals, there is an intricate and well-coordinated balance of numerous cephalic responses, with the timing of each having an important role for the overall process. Consistent with this, meal-fed rats have been observed to have an increased release of neuropeptide Y in the hypothalamus beginning around 2 h before the scheduled meal time (33,34). It may well be that changes in the brain trigger subsequent neuroendocrine responses to achieve the most efficient handling of the impending large meal, and in this light, the timing of the premeal GLP-1 surge does not seem inappropriate.
We hypothesized that a premeal increase of GLP-1 would contribute to the coordinated series of cephalic responses made by meal-fed rats and that a disruption of this signal would affect the ability of the animals to ingest the large meals characteristic of the meal-feeding response. We assessed the impact of the premeal surge of GLP-1 by giving the selective GLP-1 receptor antagonist, dH-Ex-4, 2 h before food presentation. The half-lives of exendin-9 and exendin-4, two congeners of dH-Ex-4, are 20–30 min in humans and rats (35,36,37) such that an effective dose should have been present throughout the duration of the GLP-1 peak, with lower levels and effects of the exendin at the time of the meal. Moreover, because GLP-1 given just before a meal decreases meal size, we reasoned that any lingering antagonism at the GLP-1 receptor would increase meal size. However, contrary to what would be predicted by the literature but consistent with our hypothesis that the early GLP-1 response is essential for other aspects of the cephalic response and ultimately for enabling the large meal to be eaten, food intake during the first hour was significantly decreased after dH-Ex-4. To rule out the possibility that the decrease of food intake was due to lingering effects of dH-Ex-4 rather than to its action to block GLP-1 activity an hour before the meal, several weeks later we ran a control experiment in which the same amount of exendin (or saline on an alternate day) was administered 15 min before meal time in the same animals. In this instance, meal size was actually increased during the first hour, consistent with the role of meal-related GLP-1 as a putative satiation signal (38). In these experiments, food intake on the control (saline) days was slightly lower when the timing of the injection occurred after rather than before the anticipatory increase of GLP-1, perhaps because of the long delay (around 1 month) between sessions. Nonetheless, there was not a significant difference between intakes on the two saline conditions, and for each assessment half the rats got saline and half got dH-Ex-4 on every test day. This procedure therefore precluded any bias in the findings based on differential conditions. The salient observation here is that blocking the action of the premeal GLP-1 surge reduced intake on the test day, the subsequent data suggesting that endogenous GLP-1 may actually suppress meal size slightly cannot be considered conclusive.
The question could be asked as to whether an infusion of exogenous GLP-1 that mimics the observed premeal pattern of GLP-1 observed in the plasma would increase food intake an hour or two later. The question cannot be easily addressed, however, because the levels of endogenous GLP-1 at key receptors that are responsible for anticipatory responses are not known, and raising plasma GLP-1 sufficiently in an attempt to raise tissue-fluid levels could create confounding effects at other GLP-1 receptor populations.
GLP-1 is a cleavage product of a prohormone, preproglucagon, that is known to be made in only three cell types: L cells in the mucosa of the small and large intestine, a small population of hindbrain neurons, and α-cells of the pancreatic islet (39). There is general consensus that under normal circumstances circulating GLP-1 originates almost entirely from the intestine (40,41). Plasma GLP-1 increases during meals, and its best-known actions are to augment insulin secretion as prandial glucose levels are increasing; hence, it is a potent incretin (42,43). Other effects of GLP-1 that are thought to contribute significantly to its glucose-lowering effect are inhibition of glucagon release and delayed gastric emptying (39). Interestingly, whereas the effect of GLP-1 to increase pyloric tone and reduce passage of gastric chyme into the intestine has received considerable attention, several studies demonstrated that GLP-1 also causes relaxation of the gastric fundus and increased capacitance of the stomach (25,26). In human subjects infusion of GLP-1 increased the amount of a test meal that could be consumed (25). These findings are all consistent with the results reported herein and suggest one plausible mechanism by which secretion of GLP-1 before eating could help prepare animals to better cope with a large meal.
Perhaps the best-known anticipatory endocrine response associated with feeding is cephalic insulin secretion. Whereas the typical 5- to 10-fold increase in postprandial insulin secretion requires elevated levels of blood glucose, and to a lesser degree other nutrient substrates, the incretin actions of GLP-1 and glucose-dependent insulinotropic polypeptide, and stimulation by the parasympathetic nervous system (44), make important contributions. This coordinated response to normal insulin secretion is exemplified by recent data suggesting that at least some of the incretin actions of GLP-1 use the neural input to the pancreas (45). Cephalic insulin describes insulin secreted before an anticipated meal before any increase in blood glucose from absorbed nutrients and is believed to be entirely dependent on neural stimuli to the islet. The consequence of the cephalic insulin response is improved glucose tolerance in response to a meal, as demonstrated by experiments in which prevention of cephalic insulin release caused a relative exaggeration in prandial blood glucose (46,47,48). Animals that cannot secrete insulin through the normal neural mechanism adopt an eating strategy in which they never eat large meals (49). Cephalic insulin can be elicited by any stimulus that reliably predicts the opportunity to eat; in other words, it can be brought under stimulus control (e.g. time of day, arbitrary odors, or sounds) through classical conditioning (3). If an individual can reliably anticipate food, the brain is able to respond by causing sufficient cephalic insulin secretion to allow adequate glucose tolerance. Thus, cephalic insulin (presumably in conjunction with other cephalic responses) enables an individual to take in larger amounts of food at one time and still be able to cope relatively well with the nutrient load. Consistent with what we and many others observed before, in the present experiment, the meal-fed rats had increased insulin at the final blood sampling before food presentation.
Because cephalic insulin secretion is enabled by a neural link from the brain to the pancreatic islets via the vagus nerves, the present results raise the likelihood that cephalic GLP-1 is also neurally mediated. There is experimental evidence that intestinal GLP-1 secretion after meals is mediated by vagal stimulation (50,51), and this mechanism has been used to explain the apparent paradox of a hormone made predominantly in the lower gut being secreted early in the course of meal ingestion. In our studies the preprandial peak of GLP-1 was independent of changes in blood glucose or insulin. Whereas we cannot rule out the possibility that the anticipatory GLP-1 response was mediated by another hormone, there is a very strong likelihood that a direct or indirect response was neurally initiated.
In summary, we used a meal-feeding model to demonstrate a preprandial pulse of GLP-1 secretion in rats. This entrained response has features in common with cephalic insulin secretion and suggests that neural signals initiate GLP-1 secretion in anticipation of eating as part of the coordinated response to meal feeding. This hypothesis is supported by the disparate effects of systemic blockade of the GLP-1 receptor before or during meal presentation. These findings suggest a novel role for GLP-1 and add to the understanding of the complex homeostatic requirements related to feeding.
Acknowledgments
We thank Kay Ellis for her careful measurement of plasma analytes.
Footnotes
This work was supported by National Institutes of Health Grants DK 067550, DK 054890, and DK 057900.
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 13, 2009
For editorial see page 445
Abbreviations: dH-Ex-4, Exendin-4[desHis-1,Glu-9]; GLP, glucagon-like peptide.
References
- Woods SC 1991 The eating paradox: how we tolerate food. Psychol Rev 98:488–505 [DOI] [PubMed] [Google Scholar]
- Drazen DL, Vahl TP, D'Alessio DA, Seeley RJ, Woods SC 2006 Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology 147:23–30 [DOI] [PubMed] [Google Scholar]
- Woods SC, Vasselli JR, Kaestner E, Szakmary GA, Milburn P, Vitiello MV 1977 Conditioned insulin secretion and meal feeding in rats. J Comp Physiol Psychol 91:128–133 [DOI] [PubMed] [Google Scholar]
- Katschinski M 2000 Nutritional implications of cephalic phase gastrointestinal responses. Appetite 34:189–196 [DOI] [PubMed] [Google Scholar]
- LeBlanc J 2000 Nutritional implications of cephalic phase thermogenic responses. Appetite 34:214–216 [DOI] [PubMed] [Google Scholar]
- Mattes RD 1997 Physiologic responses to sensory stimulation by food: nutritional implications. J Am Diet Assoc 97:406–413 [DOI] [PubMed] [Google Scholar]
- Powley TL 1977 The ventromedial hypothalamic syndrome, satiety, and a cephalic phase hypothesis. Psychol Rev 84:89–126 [PubMed] [Google Scholar]
- Powley TL 2000 Vagal circuitry mediating cephalic-phase responses to food. Appetite 34:184–188 [DOI] [PubMed] [Google Scholar]
- Strubbe JH, Woods SC 2004 The timing of meals. Psychol Rev 111:128–141 [DOI] [PubMed] [Google Scholar]
- Teff K 2000 Nutritional implications of the cephalic-phase reflexes: endocrine responses. Appetite 34:206–213 [DOI] [PubMed] [Google Scholar]
- Woods SC, Strubbe JH 1994 The psychobiology of meals. Psychon Bull Rev 1:141–155 [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Sipols AJ, Porte Jr D, Woods SC, Liddle RA 1989 IVT CCK inhibits food intake and gastric emptying in the baboon. Am J Physiol 256:R1313–R1317 [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Nadzan AM, Sipols AJ, Green PK, Liddle RA, Porte Jr D 1992 Intraventricular CCK-8 reduces single meal size in the baboon by interaction with type-A CCK receptors. Am J Physiol 263:R863–R867 [DOI] [PubMed] [Google Scholar]
- Forsyth PA, Weingarten HP, Collins SM 1985 Role of oropharyngeal stimulation in cholecystokinin-induced satiety in the sham feeding rat. Physiol Behav 35:539–543 [DOI] [PubMed] [Google Scholar]
- Katschinski M, Dahmen G, Reinshagen M, Beglinger C, Koop H, Nustede R, Adler G 1992 Cephalic stimulation of gastrointestinal secretory and motor responses in humans. Gastroenterology 103:383–391 [DOI] [PubMed] [Google Scholar]
- Konturek JW, Thor P, Maczka M, Stoll R, Domschke W, Konturek SJ 1994 Role of cholecystokinin in the control of gastric emptying and secretory response to a fatty meal in normal subjects and duodenal ulcer patients. Scand J Endocrinol 29:583–590 [DOI] [PubMed] [Google Scholar]
- de Jong A, Strubbe JH, Steffens AB 1977 Hypothalamic influence on insulin and glucagon release in the rat. Am J Physiol 233:E380–E388 [DOI] [PubMed] [Google Scholar]
- Fischer U, Hommel H, Gottschling HD, Nowak W 1976 The effect of meal feeding and of sham-feeding on insulin secretion in dogs. Eur J Clin Invest 30:465–471 [DOI] [PubMed] [Google Scholar]
- Secchi A, Caldara R, Caumo A, Monti LD, Bonfatti D, Di Carlo V, Pozza G 1995 Cephalic-phase insulin and glucagon release in normal subjects and in patients receiving pancreas transplantation. Metabolism 44:1153–1158 [DOI] [PubMed] [Google Scholar]
- Teff KL, Mattes RD, Engelman K 1991 Cephalic phase insulin release in normal weight males: verification and reliability. Am J Physiol 261:E430–E436 [DOI] [PubMed] [Google Scholar]
- D'Alessio DA, Vahl TP 2004 Glucagon-like peptide 1: evolution of an incretin into a treatment for diabetes. Am J Physiol Endocrinol Metab 286:E882–E890 [DOI] [PubMed] [Google Scholar]
- Tang-Christensen M, Larsen PJ, Goke R, Fink-Jensen A, Jessop DS, Møller M, Sheikh SP 1996 Central administration of GLP-1-(7-36) amide inhibits food and water intake in rats. Am J Physiol 271:R848–R856 [DOI] [PubMed] [Google Scholar]
- Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR 1996 A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379:69–72 [DOI] [PubMed] [Google Scholar]
- van Dijk G, Thiele TE, Seeley RJ, Woods SC, Bernstein IL 1997 Glucagon-like peptide-1 and satiety? Nature 385:214 [DOI] [PubMed] [Google Scholar]
- Delgado-Aros S, Kim DY, Burton DD, Thomforde GM, Stephens D, Brinkmann BH, Vella A, Camilleri M 2002 Effect of GLP-1 on gastric volume, emptying, maximum volume ingested, and postprandial symptoms in humans. Am J Physiol 282:G424–G431 [DOI] [PubMed] [Google Scholar]
- Schirra J, Wank U, Arnold R, Göke B, Katschinski M 2002 Effects of glucagon-like peptide-1(7–36)amide on motility and sensation of the proximal stomach in humans. Gut 50:341–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amori RE, Lau J, Pittas AG 2007 Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 298:194–206 [DOI] [PubMed] [Google Scholar]
- D'Alessio DA, Vahl TP 2005 Utilizing the GLP-1 signaling system to treat diabetes: sorting through the pharmacologic approaches. Curr Diab Rep 5:346–352 [DOI] [PubMed] [Google Scholar]
- Seeley RJ, Blake K, Rushing PA, Benoit SC, Eng J, Woods SC, D'Alessio D 2000 The role of CNS GLP-1-(7-36) amide receptors in mediating the visceral illness effects of lithium chloride. J Neurosci 20:1616–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder DA, Prigeon RL, Wadwa RP, Dolan LM, D'Alessio DA 2006 β-Cell function, insulin sensitivity, and glucose tolerance in obese diabetic and nondiabetic adolescents and young adults. J Clin Endocrinol Metab 91:185–191 [DOI] [PubMed] [Google Scholar]
- Orskov C, Rabenhøj L, Wettergren A, Kofod H, Holst JJ 1994 Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes 43:535–539 [DOI] [PubMed] [Google Scholar]
- Vahl TP, Paty BW, Fuller BD, Prigeon RL, D'Alessio DA 2003 Effects of GLP-1-(7-36)NH2, GLP-1-(7-37), and GLP-1-(9-36)NH2 on intravenous glucose tolerance and glucose-induced insulin secretion in healthy humans. J Clin Endocrinol Metab 88:1772–1779 [DOI] [PubMed] [Google Scholar]
- Yoshihara T, Honma S, Honma K 1996 Effects of restricted daily feeding on neuropeptide Y release in the rat paraventricular nucleus. Am J Physiol 270:E589–E595 [DOI] [PubMed] [Google Scholar]
- Yoshihara T, Honma S, Honma K 1996 Prefeeding release of paraventricular neuropeptide Y is mediated by ascending noradrenergic neurons in rats. Am J Physiol 270:E596–E600 [DOI] [PubMed] [Google Scholar]
- Edwards CM, Todd JF, Mahmoudi M, Wang Z, Wang RM, Ghatei MA, Bloom SR 1999 Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9–39. Diabetes 48:86–93 [DOI] [PubMed] [Google Scholar]
- Edwards CM, Stanley SA, Davis R, Brynes AE, Frost GS, Seal LJ, Ghatei MA, Bloom SR 2001 Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab 281:E155–E161 [DOI] [PubMed] [Google Scholar]
- Gedulin BR, Smith PA, Jodka CM, Chen K, Bhavsar S, Nielsen LL, Parkes DG, Young AA 2008 Pharmacokinetics and pharmacodynamics of exenatide following alternate routes of administration. Int J Pharm 356:231–238 [DOI] [PubMed] [Google Scholar]
- Williams DL, Baskin DG, Schwartz MW 2009 Evidence that intestinal GLP-1 plays a physiological role in satiety. Endocrinology 150:1680–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ 2006 The biology of incretin hormones. Cell Metab 3:153–165 [DOI] [PubMed] [Google Scholar]
- Brubaker PL 1991 Regulation of intestinal proglucagon-derived peptide secretion by intestinal regulatory peptides. Endocrinology 128:3175–3182 [DOI] [PubMed] [Google Scholar]
- Brubaker PL, Drucker DJ 2002 Structure-function of the glucagon receptor family of G protein-coupled receptors: the glucagon, GIP, GLP-1, and GLP-2 receptors. Receptors Channels 8:179–188 [PubMed] [Google Scholar]
- D'Alessio DA, Vogel R, Prigeon R, Laschansky E, Koerker D, Eng J, Ensinck JW 1996 Elimination of the action of glucagon-like peptide 1 causes an impairment of glucose tolerance after nutrient ingestion by healthy baboons. J Clin Invest 97:133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi M, Vahl TP, D'Alessio DA 2008 Regulation of islet hormone release and gastric emptying by endogenous glucagon-like peptide 1 after glucose ingestion. J Clin Endocrinol Metab 93:4909–4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessio DA, Kieffer TJ, Taborsky Jr GJ, Havel PJ 2001 Activation of the parasympathetic nervous system is necessary for normal meal-induced insulin secretion in rhesus macaques. J Clin Endocrinal Metab 86:1253–1259 [DOI] [PubMed] [Google Scholar]
- Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, Ellis KS, Woods SC, Seeley RJ, Herman JP, D'Alessio DA 2007 Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology 148:4965–4973 [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Bereiter DA, Trimble ER, Siegel EG, Jeanrenaud B 1981 Cephalic phase, reflex insulin secretion. Diabetologia 20(Suppl): 393–401 [PubMed] [Google Scholar]
- Louis-Sylvestre J 1978 Feeding and metabolic patterns in rats with truncular vagotomy or with transplanted β-cells. Am J Physiol 235:E119–E125 [DOI] [PubMed] [Google Scholar]
- Trimble ER, Berthoud HR, Siegel EG, Jeanrenaud B, Renold AE 1981 Importance of cholinergic innervation of the pancreas for glucose tolerance in the rat. Am J Physiol 241:E337–E341 [DOI] [PubMed] [Google Scholar]
- Inoue S, Bray GA, Mullen YS 1978 Transplantation of pancreatic B-cells prevents development of hypothalamic obesity in rats. Am J Physiol 235:E266–E271 [DOI] [PubMed] [Google Scholar]
- Brubaker PL, Anini Y 2003 Direct and indirect mechanisms regulating secretion of glucagon-like peptide-1 and glucagon-like peptide-2. Can J Physiol Pharmacol 81:1005–1012 [DOI] [PubMed] [Google Scholar]
- Dubé PE, Brubaker PL 2004 Nutrient, neural and endocrine control of glucagon-like peptide secretion. Horm Metab Res 36:755–760 [DOI] [PubMed] [Google Scholar]