Abstract
Combined deficiency of factor V and factor VIII (F5F8D) is a bleeding disorder caused by mutations in either LMAN1 or MCFD2. LMAN1 (ERGIC-53) and MCFD2 form a Ca2+-dependent cargo receptor that cycles between the endoplasmic reticulum (ER) and the ER-Golgi intermediate compartment for efficient transport of FV/FVIII from the ER to the Golgi. Here we show that the C-terminal EF-hand domains are both necessary and sufficient for MCFD2 to interact with LMAN1. MCFD2 with a deletion of the entire N-terminal non-EF hand region still retains the LMAN1-binding function. Deletions that disrupt core structure of the EF-hand domains abolish LMAN1 binding. Circular dichroism spectroscopy studies on missense mutations localized to different structural elements of the EF-hand domains suggest that Ca2+-induced folding is important for LMAN1 interaction. The EF-hand domains also mediate the interaction with FV and FVIII. However, mutations in MCFD2 that disrupt the tertiary structure and abolish LMAN1 binding still retain the FV/FVIII binding activities, suggesting that this interaction is independent of Ca2+-induced folding of the protein. Our results suggest that the EF-hand domains of MCFD2 contain separate binding sites for LMAN1 and FV/FVIII that are essential for cargo receptor formation and cargo loading in the ER.
Introduction
Combined deficiency of factor V and factor VIII (F5F8D) is an autosomal recessive disorder. Patients with F5F8D show mild to moderate bleeding symptoms. Levels of FV and FVIII in plasma generally fall to between 5% and 30% of the normal.1–3 Genetic studies identified mutations in LMAN1 (lectin, mannose binding 1) or MCFD2 (multiple coagulation factor deficiency protein 2) as the cause of F5F8D.4,5 LMAN1 (also known as ERGIC-53) is a type 1 transmembrane protein with homology to leguminous lectins6,7 that cycles between the endoplasmic reticulum (ER) and the ER-Golgi intermediate compartment (ERGIC).8–10 Previous studies show that LMAN1 exists as hexamers in cells,11 and it forms a protein complex with MCFD2 with a 1:1 stoichiometry.12 Missense mutations of MCFD2 found in F5F8D patients disrupt its interaction with LMAN1,5,13 suggesting that LMAN1-MCFD2 complex formation is important for efficient FV and FVIII secretion.
The ER luminal side of LMAN1 contains a mannose-binding carbohydrate recognition domain (CRD) and a series of 4 α-helices that are predicted to form coiled-coil domains. The crystal structure of the CRD is similar to Ca2+-dependent leguminous lectins and the ER chaperone calnexin, with 2 Ca2+-binding sites.14,15 FVIII can interact with the LMAN1-MCFD2 complex in a Ca2+-dependent manner,12 suggesting that the LMAN1-MCFD2 complex is a cargo receptor, which directly binds and recruits FV and FVIII in the ER for packaging into ER exit vesicles. The cytoplasmic tail of LMAN1 contains a diphenylalanine ER exit motif,16 which binds to the Sec24 subunit of COPII coat proteins that are responsible for generating cargo-containing transport vesicles that bud from the ER.17,18 FV- and FVIII-containing vesicles are then moved to the ERGIC and eventually to the cis-Golgi. The cytoplasmic tail of LMAN1 also has a dilysine ER retrieval signal that directs the protein into the COPI-coated retrograde vesicles that can bring the LMAN1-MCFD2 complex back to the ER, presumably on releasing FV/FVIII cargo in the ERGIC.8
MCFD2 is a 16-kDa soluble protein with an N-terminal sequence of unknown structure and 2 calmodulin-like EF-hand domains at the C terminus.5 MCFD2 lacks sorting signals and is localized to the ERGIC via its interaction with LMAN1.5 MCFD2 can also bind FVIII independent of LMAN1 and the glycosylation state of FVIII.12 A recently solved NMR structure revealed that in the absence of Ca2+ or with EF-hand domain mutations, MCFD2 largely exists in a disordered apo state.19 In the presence of Ca2+, the EF-hand domains form a structure similar to that of calmodulin. In contrast, the N-terminal sequence of the molecule remains disordered even in the Ca2+-bound state. The linker region between the 2 EF-hand domains is also largely unstructured. Despite our knowledge on the individual structures of LMAN1 and MCFD2, it is not clear how LMAN1 and MCFD2 bind to each other to form a complex, and how the complex recognizes FV/FVIII.
In mammals, the LMAN1-MCFD2 complex is so far the only known example of a specific receptor for soluble cargo proteins. The requirement of both a transmembrane component (LMAN1) and a soluble cofactor (MCFD2) is a unique feature of this secretory pathway. Understanding how the receptor complex is organized and how it recognizes the FV/FVIII cargo proteins is important in understanding the mechanism of this receptor-mediated secretory pathway. In this study, we present evidence that the EF hands of MCFD2 contain separate binding sites required for LMAN1-MCFD2 receptor complex formation and for the interaction between MCFD2 and its client cargo proteins, FV and FVIII.
Methods
Plasmid constructs
MCFD2 mutant constructs for mammalian cell expression are schematically shown in Figure 1A. The wild-type MCFD2 was previously cloned into the pcDNA3.1-myc-His expression vector.5 N-terminal deletion mutants (Δ35 (E27-N61), Δ40 (E27-E66), Δ45 (E27-E71), Δ46 (E27-L7-21), Δ47 (E27-Q73), Δ48 (E27-H74), Δ49 (E27-Y75), and Δ50 (E27-N76)) were made by polymerase chain reaction-based methods. We took advantage of a unique KasI restriction site located near the end of the signal sequence. MCFD2 DNA fragments were amplified by polymerase chain reaction and then ligated into the pcDNA3.1-MCFD2-myc-His vector digested with KasI and HindIII to produce the resulting constructs. Missense mutants and deletion mutants ΔL1, ΔL2, ΔEF2-N, ΔEF1 (P69-Q102), and ΔEF2 (M112-Q146) were introduced into MCFD2 using the Stratagene mutagenesis II XL kit (Stratagene) according to manufacturer's instruction. All mutation constructs were confirmed by DNA sequencing. Mutagenesis primer sequences are available on request. A human ceruloplasmin expression construct (in pcDNA3 vector) is a gift from P. Fox (Cleveland Clinic Foundation).
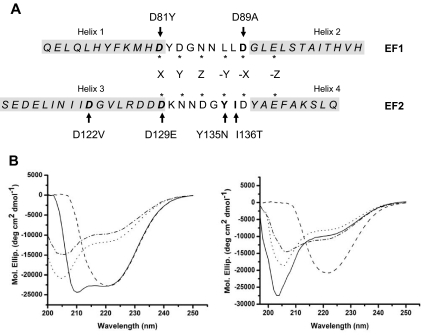
Figure 1.
CD spectra of MCFD2 proteins. (A) Sequences of the 2 EF-hand domains of MCFD2. Sequences are aligned to match the locations of 6 Ca2+ coordination residues in the Ca2+-binding loops, which are indicated with asterisks. Residues that are mutated in F5F8D patients are shown in boldface, and mutations are indicated by arrows. Amino acid residues in the 4 α-helical regions are shown in italics and are boxed in light gray shade. (B) CD spectra of the wild-type MCFD2, and the D89A, the D122V, and the I136T mutants. The x-axis represents the wavelengths scanned. The y-axis shows molar ellipticity (Mol. Ellip.) values expressed as degrees cm2/dmol.
Antibodies
We purchased monoclonal anti-myc from Santa Cruz Biotechnology, monoclonal anti-Flag from Sigma-Aldrich, and monoclonal anti–human FV from Haematologic Technologies. Monoclonal anti-LMAN1 was a gift from H.-P. Hauri (University of Basel). Monoclonal anti–human FVIII was a gift from D. Pittman (Bayer). Rabbit anti–human MCFD2 was described previously.5 Anti-ceruloplasmin was a gift from P. Fox (Cleveland Clinic Foundation).
Cell culture, transfection, and metabolic labeling
COS-1 cells were grown in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum, 100 IU/mL penicillin, and 100 IU/mL streptomycin. Cells were transfected using FuGENE 6 (Roche Diagnostics) according to the manufacturer's instructions. Twenty hours after transfection, cells were metabolically labeled with [35S] methionine/cysteine (250 μCi/mL in methionine/cysteine-free Dulbecco modified Eagle medium) (MP Biomedicals) followed by a 30-minute incubation in complete medium.
Crosslinking and immunoprecipitation
Crosslinking using dithio-bis(succinimidyl propionate) (DSP; Thermo Electron) and immunoprecipitation were described previously.12 Briefly, cells were washed 3 times in ice-cold phosphate-buffered saline, pH 7.4, containing 0.9mM CaCl2 and 0.5mM MgCl2 and then incubated with 1mM DSP in crosslinking buffer (phosphate-buffered saline, pH 7.4, with 1mM CaCl2 and 100mM glucose) on ice for 1 hour with gentle shaking. The crosslinking reaction was quenched in 20mM Tris-HCl, pH 7.5, for 5 minutes. Cells were lysed in Nonidet P-40 buffer (50mM Tris-HCl, pH 7.5, 150mM NaCl, 1% Nonidet P-40, 0.05% sodium dodecyl sulfate, 2mM CaCl2) plus protease inhibitors and subjected to immunoprecipitation using various antibodies. Immunoprecipitates were washed 3 times using NP-40 buffer with 0.5M NaCl. Proteins were separated on 4% to 12% Criterion Bis-Tris gels (Bio-Rad) using MES running buffer and visualized by exposing to a Kodak Bio-Max film using a Kodak Biomax Transcreen LE. Each experiment was performed at least twice.
Immunofluorescence
Immunofluorescence staining of HeLa cells transfected with different MCFD2 and LMAN1 expression vectors was previously described.5 Images were viewed on a Leica DMRXE confocal microscope (Wetzlar) using a 40× oil-immersion objective with a 1.25 numeric aperture. Confocal images were acquired using the Leica Confocal Software, Version 2.61.
Purification of MCFD2 proteins
Cloning of MCFD2 into pET15b was described previously.5 The D89A and D122V point mutations were introduced into the DNA construct using the Stratagene mutagenesis II XL kit. MCFD2 constructs were expressed in Escherichia coli Rosetta (DE3)pLysS strain (Novagen). The MCFD2 proteins were purified from cell lysates by Ni affinity chromatography using HisTrap HP (GE Healthcare) followed by buffer exchange on a PD-10 desalting column. The His tag of the fusion protein was removed by incubating with thrombin overnight followed by reverse Ni affinity chromatography. The proteins were further purified using a Superdex 75 gel-filtration column (GE Healthcare) in 5mM Bis-Tris, pH 7.0.
Circular dichroism spectroscopy
CD spectra were determined on a Jasco J-815 CD spectropolarimeter using a cuvette of 2-mm path length. Before measurement, all protein concentrations were adjusted to 0.1 mg/mL in 5mM Bis-Tris buffer, pH 7.0, with 10mM CaCl2. To remove Ca2+ from MCFD2, protein was incubated with 5mM Bis-Tris buffer, pH 7.0, with 10mM ethylenediaminetetraacetic acid for 2 hours and dialyzed against 5mM Bis-Tris buffer, pH 7.0, at 4°C overnight. The spectra were measured from 200 to 250 nm at room temperature. Each sample was scanned 11 times. The scans were averaged after correction for the buffer spectrum. Secondary structure proportions were calculated using programs SELCON3, CDSSTR, and CONTINLL (CDPro package; Colorado State University).
Results
MCFD2 missense mutations differentially affect the protein structure
To date, 6 MCFD2 missense mutations have been identified in F5F8D patients,3 all of which are localized to the EF-hand domains. The EF-hand domains are characteristic structures consisting of a Ca2+-binding loop flanked by 2 α-helices. The 4 α-helices are hereby designated helix 1 through 4 (Figure 1A). Most mutations (D81Y, D89A, D129E, Y135N, and I136T) are localized to the Ca2+-binding loops of both EF-hand domains (Figure 1A). D122V, the only mutation located outside the Ca2+ binding loops, replaces a charged amino acid with a hydrophobic one on helix 3 in the second EF-hand domain (Figure 1A; supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
To investigate the consequences of different missense mutations on MCFD2 secondary structure, we performed CD spectroscopy measurements on the wild-type MCFD2, as well as MCFD2 with the D89A, D122V, or I136T mutations in the presence of Ca2+ (Figure 1B). The spectrum of the wild-type MCFD2 shows minima at approximately 208 nm and 222 nm. The spectra of D89A and I136T mutants show a shallow 222-nm band and a minimum shifted from 208 nm to approximately 205 nm, consistent with previous reports.19 Interestingly, MCFD2 with the D122V mutation displays a completely different CD spectrum (Figure 1B). The 208-nm band is much smaller than the 222-nm band, so that it largely overlaps with the 222-nm band to form a single broad minimum at 222 nm. We calculated portions of secondary structures in MCFD2 proteins using 3 different computer programs SELCON3, CDSSTR, and CONTINLL.20 All 3 programs predict that the D122V mutant contains a smaller portion of α-helix than the wild-type MCFD2, whereas the D89A and I136T mutations have no significant effect on the amount of α-helical content (data not shown). In the absence of Ca2+, CD spectrum of the wild-type MCFD2 changed to a pattern similar to those of D89A and I136T mutants (Figure 1B), consistent with the previous report.19 On the other hand, spectra of all 3 mutant proteins showed only slight changes in the absence of Ca2+ (Figure 1B), indicating that the folding status of these mutants is not significantly affected by Ca2+ in the buffer. Taken together, our results suggest that, although all 3 mutations likely abolish calcium binding, there are independent and separable effects of mutations in calcium binding loops (D89A, D129E, and I136T) and mutations in helical regions (D122V) on MCFD2 conformation.
The EF-hand domains of MCFD2 are sufficient and necessary for LMAN1 binding
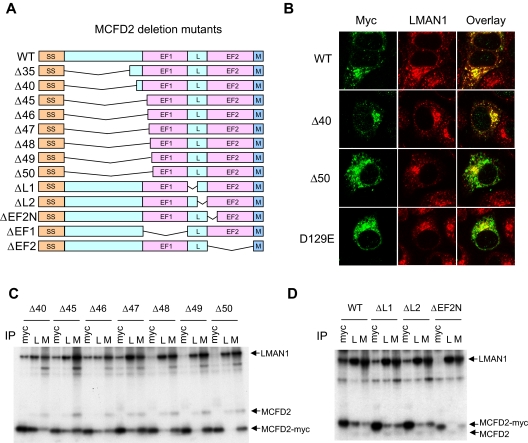
The N-terminal sequence of mature MCFD2 contains 42 amino acid residues that are conserved among vertebrates.19 To investigate whether this N-terminal domain is required for LMAN1 binding, we constructed a series of deletion mutants with a myc tag at the C terminus (Figure 2A). COS-1 cells were transfected with these constructs and metabolically labeled with 35S-methionine/cysteine. Lysates prepared from labeled cells were subjected to immunoprecipitation with anti-myc, anti-MCFD2, and anti-LMAN1 antibodies. Anti-myc detects the myc-tagged mutant MCFD2, whereas anti-MCFD2 detects both the endogenous and myc-tagged MCFD2. MCFD2 with deletion of the entire N-terminal sequence plus 3 amino acids in the first EF-hand domain (Δ45) can still efficiently coimmunoprecipitate with LMAN1 (Figure 2C). Immunofluoresecence staining of cells transfected with the Δ40 deletion mutant demonstrated colocalization with endogenous LMAN1, similar to the wild-type MCFD2 (Figure 2B), indicating that MCFD2 mutants are retained in the ERGIC via their interactions with LMAN1. These results suggest that the N-terminal sequence of MCFD2 is not essential for LMAN1 binding and that the EF-hand domains are sufficient for LMAN1 binding.
Figure 2.
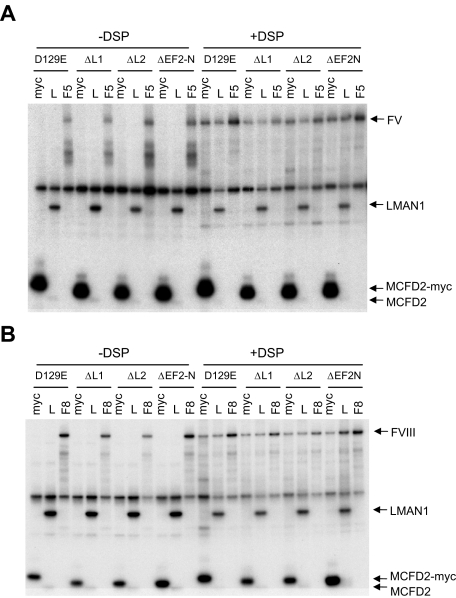
Effects of MCFD2 deletion mutations on LMAN1 binding. (A) Diagram of MCFD2 deletion constructs used in the study. SS indicates signal sequence; L, linker; and M, myc tag. The N-terminal domain is defined as the first 42 amino acids after the signal sequence. (B) Immunofluorescence staining of the wild-type (WT), Δ40, and the Δ50 N-terminal deletion mutants, as well as the D129E missense mutant MCFD2. (C) co-IP of various N-terminal deletion mutants with LMAN1. (D) co-IP of ΔL1, ΔL2, and ΔEF2-N mutants with LMAN1. Cell lysates were immunopreciptated with anti-myc (myc), anti-LMAN1 (L), and anti-MCFD2 (M).
MCFD2 with deletions of up to 47 amino acids, or 5 amino acids into helix 1 can still significantly coimmunoprecipitate with LMAN1 (Figure 2C). Further deletions into this helix markedly decreased the coimmunoprecipitation (co-IP). Consequently, immunofluorescence staining of the Δ50 mutant (Figure 2B) showed a diffused pattern that resembles that of the D129E missense mutant, which is unable to bind LMAN1.12 Similarly, deletion of the first 5 amino acids of helix 3 (ΔEF2-N) abolishes the LMAN1 interaction, indicating that the structure of this helix is important for LMAN1 binding. Consistent with our findings, a previous report showed that a patient mutation that truncates the last 3 amino acids of helix 4 markedly decreased MCFD2 binding to LMAN1.21 This mutation significantly altered MCFD2 3-dimensional structure.21 Similarly, our partial helix 1 and helix 3 deletion mutants probably also alter the structure of the EF-hand domains. Taken together, these results suggest that the integrity of the core structures of both EF-hand domains of MCFD2 is necessary for LMAN1 interaction.
We next asked whether the linker region between the 2 EF-hand domains is critical for the interaction with LMAN1. The linker region of MCFD2 is defined as the sequence between the 2 EF-hand domains and is also well conserved in vertebrate species.19 In the NMR structure, this region is 10 amino acids long and largely unstructured.19 We deleted 5 amino acids at a time and assessed the LMAN1 binding by co-IP. Results showed that partial deletions of the linker region had minimal effect on the interaction with LMAN1 (Figure 2D), suggesting that the length or the amino acid sequence of the linker region is not critical for LMAN1 binding.
MCFD2 missense mutants found in F5F8D patients can interact with FV and FVIII
The interaction between the LMAN1-MCFD2 complex with FV/FVIII is detected with a crosslinking/co-IP assay. In this assay, cells are treated with a membrane-permeable, thiol-cleavable chemical crosslinker, DSP, before lysis and immunoprecipitation. We previously demonstrated specific crosslinking of FVIII to the LMAN1-MCFD2 complex.12 We now further demonstrate the specificity of this interaction by cotransfecting MCFD2 with ceruloplasmin (Cp), a protein with homology to the FVIII A domains. Cells were treated with DSP and analyzed by immunoprecipitation. The co-IP of LMAN1 and MCFD2 with FVIII was readily detected with anti-myc and anti-LMAN1 antibodies. In contrast, no co-IP was detected between LMAN1 or MCFD2 and Cp with anti-myc, anti-LMAN1, or anti-Cp antibodies (supplemental Figure 2).
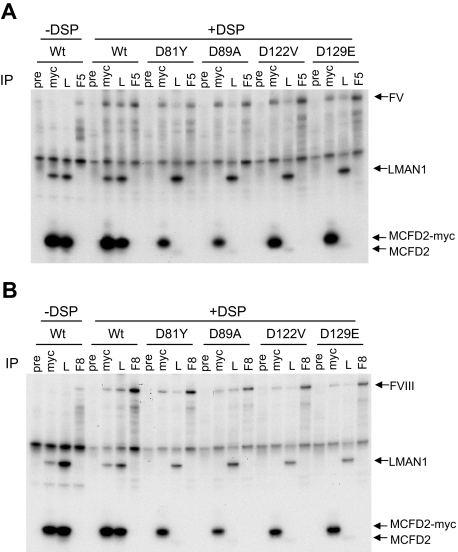
Our CD spectroscopy experiments suggest that different MCFD2 missense mutations exert different effects on the MCFD2 structure, although all previously reported missense mutations abolish LMAN1 binding.12,13 The D129E mutant has previously been shown to maintain interaction with FVIII, despite loss of LMAN1 binding.12 Using the crosslinking/co-IP assay, we investigated whether the other missense mutations affect the interactions of MCFD2 with FV/FVIII. We cotransfected FV or FVIII with wild-type or myc-tagged missense MCFD2 (D81Y, D89A, D122V, and D129E) in COS1 cells and monitored the interactions of mutant MCFD2 with FV and FVIII (Figure 3). As expected, all mutant MCFD2 failed to coimmunoprecipitate with LMAN1. In the presence of DSP, anti-myc antibody pulled down both FV and FVIII in cells transfected with wild-type MCFD2, as well as in cells transfected with all missense mutant MCFD2 (Figure 3). As a positive control, anti-LMAN1 antibody also pulled down FV and FVIII in all co-IP experiments because of the presence of endogenous LMAN1-MCFD2 complex. Thus, all MCFD2 missense mutants identified to date retain the FV/FVIII binding activity, despite separate effects of tertiary structure disruptions induced by these mutations (Figure 1).
Figure 3.
MCFD2 missense mutations identified in F5F8D patients do not abolish crosslinking to FV and FVIII. COS-1 cells were cotransfected with the wild-type or indicated MCFD2 missense mutants along with either FV (A) or FVIII (B). Cells were incubated with or without DSP before lysis. Cell lysates were immunoprecipitated with preimmune serum (pre), anti-myc (myc), anti-LMAN1 (L), and either anti-FV (F5 in panel A) or anti-FVIII (F8 in panel B) antibodies.
Interactions with FV and FVIII are mediated through the EF-hand domains of MCFD2
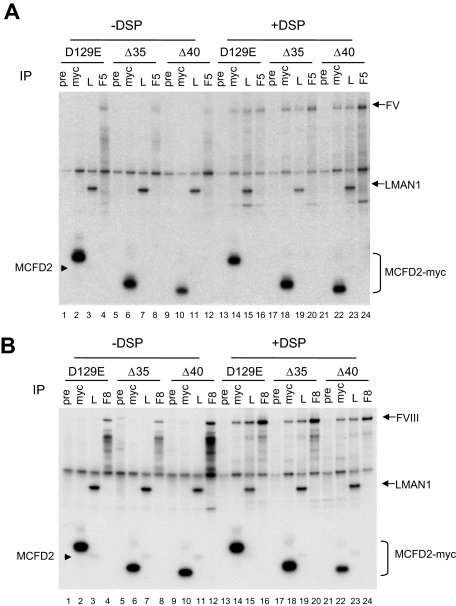
To study the structural requirements for the interactions between MCFD2 and FV/FVIII, we introduced a missense mutation, D129E, into 2 N-terminal MCFD2 deletion mutant constructs (Δ35 and Δ40) depicted in Figure 2A. The presence of the D129E mutation abolishes LMAN1 binding (Figure 3).5 Thus, the observed co-IP between these MCFD2 mutants and FV/FVIII indicate direct binding, not an indirect interaction mediated through LMAN1. We next asked the question whether the N-terminus of MCFD2 is involved in FV/FVIII binding by monitoring co-IP of the 2 N-terminal deletion mutants of MCFD2 with FV and FVIII. As expected, introducing the D129E mutation into either MCFD2 truncation mutant abolished the co-IP with LMAN1 (Figure 4). Little FV (Figure 4A) or FVIII (Figure 4B) was pulled down by anti-myc (to detect transfected MCFD2) or anti-LMAN1 (to detect endogenous LMAN1) in the absence of DSP (lanes 2, 3, 6, 7, 10, and 11). However, DSP treatment significantly increased the amount of FV/FVIII that was pulled down by both antibodies (lanes 14, 15, 18, 19, 22, and 23). The co-IP of LMAN1 with FV/FVIII was the result of the presence of endogenous MCFD2. The amount of FV or FVIII pulled down by the anti-myc antibody was indistinguishable from the wild-type MCFD2 for both N-terminal deletion mutants (lanes 14, 18, and 22), suggesting that the N-terminal domain of MCFD2 is not critical for binding FV and FVIII. As a negative control, no co-IP of FV/FVIII was detected using preimmune serum in the presence or absence of DSP.
Figure 4.
N-terminal domain of MCFD2 is not required for binding to FV and FVIII. COS-1 cells were cotransfected with the D129E missense mutant MCFD2 or N-terminal deletion mutants (Δ35 and Δ40) and FV (A) or FVIII (B). Both deletion mutants also contain a D129E missense mutation. Cells were metabolically labeled 20 hours after transfection, incubated with or without DSP before lysis. Cell lysates were immunoprecipitated with preimmune serum (pre), anti-myc (myc), anti-LMAN1 (L), and either anti-FV (F5 in panel A) or anti-FVIII (F8 in panel B) antibodies. Arrowheads indicate the endogenous MCFD2 bands.
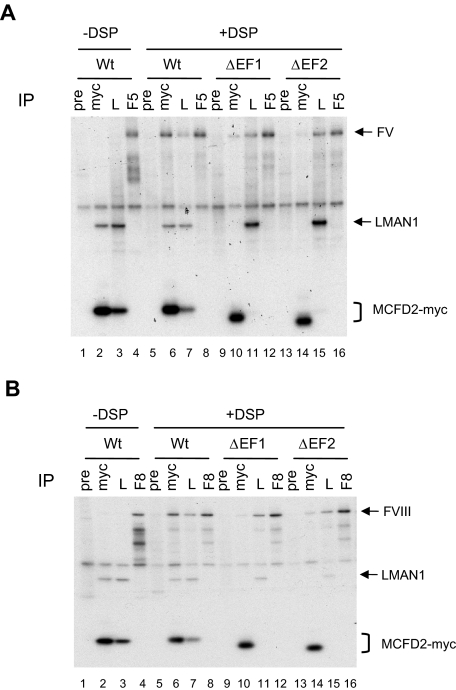
Next we investigated the effect of linker and second EF hand deletions on FV/FVIII binding, by introducing the D129E point mutation into the 2 linker-deletion mutants (ΔL1 and ΔL2) and the N-terminal helix 3 deletion mutant (ΔEF2-N) depicted in Figure 2A. As expected, introducing the D129E mutation into MCFD2 truncation mutants abolished co-IP with LMAN1 (Figure 5). However, both linker deletion mutants crosslinked to FV (Figure 5A) and FVIII (Figure 5B) with similar efficiency as the wild-type protein, suggesting that the linker region is not critical for FV/FVIII binding. The ΔEF-2N mutant, which removed 5 amino acids from the left helix of the second EF hand, could also be effectively cross-linked to FV/FVIII (Figure 5), in contrast to the inability of this mutation to bind LMAN1 (Figure 2D). These results, taken together with the observation that all MCFD2 missense mutants can still bind FV and FVIII (Figure 3), suggest that the interaction of MCFD2 with FV/FVIII is less dependent on the 3-dimensional structure of MCFD2 than its interaction with LMAN1. To investigate whether both EF-hand domains must be present for FV/FVIII binding, we deleted the entire first or second EF-hand domain (ΔEF1 and ΔEF2; Figure 6). Both deletions markedly decreased crosslinking to FV (Figure 6A) and FVIII (Figure 6B), as indicated by the amount of FV and FVIII pulled down by anti-myc (Figure 6A-B, compare lane 6 with lanes 10 and 14).
Figure 5.
Linker region and the adjacent EF2 sequence of MCFD2 are not required for binding to FV and FVIII. (A) COS-1 cells were cotransfected with the D129E missense mutant or the indicated MCFD2 deletion mutants along with either FV (A) or FVIII (B). All deletion mutants also contain the D129E missense mutation. Cells were incubated with or without DSP before lysis. Cell lysates were immunoprecipitated with anti-myc (myc), anti-LMAN1 (L), and either anti-FV (F5 in panel A) or anti-FVIII (F8 in panel B) antibodies.
Figure 6.
Deletion of either EF-hand domain of MCFD2 markedly decreases its interaction with FV and FVIII. COS-1 cells were cotransfected with the wild-type or the EF hand deletion mutants along with either FV (A) or FVIII (B). Cells were incubated with or without DSP before lysis. Cell lysates were immunoprecipitated with anti-myc (myc), anti-LMAN1 (L), and anti-MCFD2 (M).
Discussion
The LMAN1-MCFD2 cargo receptor is unique in that both a transmembrane component (LMAN1) and a soluble cofactor (MCFD2) are absolutely required for its function.3 Mutations in either LMAN1 or MCFD2 result in nearly identical manifestations.2,3,22 However, a systematic study of all available cases of F5F8D with known mutations uncovered a subtle difference between patients with LMAN1 mutations and patients with MCFD2 mutations.13 The mean FV and FVIII levels in the MCFD2 group are significantly lower than those of the LMAN1 group, suggesting that MCFD2 plays a more direct role in the cargo receptor function. In the current study, we demonstrated a central role for the EF-hand domains of MCFD2 in the formation of the LMAN1-MCFD2 complex, as well as in interactions with both FV and FVIII. These 2 interactions are distinct and probably involve separate binding sites of the protein.
The core structure of MCFD2 EF-hand domains consists of 2 helix-loop-helix units connected by a flexible linker.19 The 2 Ca2+-binding loops are localized in close proximity (supplemental Figure 1). Although all missense mutations previously reported in F5F8D patients abolish LMAN1 binding,12,13 their effects on MCFD2 structure vary. Most of these mutations directly change the Ca2+ chelating amino acids and are expected to abolish Ca2+ binding of the mutated EF hand. The I136T mutation is localized to the second EF-hand domain, but it does not directly change a Ca2+ chelating residue (Figure 1). Rather, side chains of Y135-I136 are thought to form a very short antiparallel β-sheet with the first Ca2+ binding loop.23 Because the CD spectra of D89A, D129E, and I136T are very similar (Figure 1),19,21 we speculate that mutations in either Ca2+-binding loops lead to the same structural consequence of disrupting interaction of the 2 loops. The conformational changes to the D89A, D129E, and I136T mutants are probably caused by their inability to bind Ca2+ because of the central role of Ca2+ in maintaining the structure of EF hand proteins.23 Although removal of Ca2+ significantly altered the CD spectrum of the wild-type MCFD2, it had little impact on the spectra of all 3 mutant proteins (Figure 1B), supporting the notion that all 3 mutations abolish the Ca2+ binding. The unique CD spectrum of the D122V mutant suggests different effect of this mutation on the protein structure. It probably primarily exhibits disruption of α-helices with secondary effects on the Ca2+-binding loops. The precise impact of missense mutations on MCFD2 structure requires further NMR studies of the mutant proteins.
We have shown that the EF-hand domains are sufficient for LMAN1 binding. Deletion of the entire N-terminal non-EF hand sequence has minimal effect on co-IP and colocalization of MCFD2 with LMAN1 (Figure 2). Several lines of evidence indicate that the LMAN1-MCFD2 interaction requires integrity of the core structure of the EF-hand domains of MCFD2. Deletions of 5 amino acids into the left helix of either EF hand (helix 1 or helix 3) abolished LMAN1 binding (Figure 2). Similarly, a nonsense mutation that deletes the last 3 amino acids of MCFD2 results in a protein that cannot bind LMAN1.21 Finally, all MCFD2 missense mutations identified to date in F5F8D patients abolish the interaction with LMAN1.12,13 It is worth pointing out that LMAN1-MCFD2 interaction tolerates some modifications of the EF-hand domains. Deletions of up to 4 amino acids into helix 1 still retain detectable amount of co-IP with LMAN1 (Figure 2). These results suggest that the interaction with LMAN1 may require the 3-dimensional coordination of α-helices from both EF-hand domains of MCFD2. One such candidate interaction site is a hydrophobic surface patch identified in the NMR structure that mainly consists of residues from helix 2 and helix 3.19 The linker region between 2 EF hands is important in maintaining the relative positions of helices in some EF-hand domain proteins.23 In MCFD2, this linker sequence is unusually long.19 Partial deletions of the MCFD2 linker region have no obvious effect on LMAN1 binding, suggesting that the linker length or the amino acid sequence is not a constraining factor. Additional sequence tags at the C-terminus of the EF-hand domains apparently do not interfere with function, as evident from our recombinant MCFD2 constructs (Figure 2A), along with other published reports.24
The interaction of MCFD2 with FV/FVIII is clearly distinct from the interaction of MCFD2 with LMAN1, although both interactions involve the EF-hand domains. The missense mutations and the ΔEF2-N mutation that fail to bind LMAN1 can still interact with FV and FVIII (Figures 3,5). As discussed in the second paragraph above, mutations in the Ca2+-binding loop probably disrupt the 3-dimensional structure of EF hands without significantly affecting the α-helical secondary structure. Thus, Ca2+ binding-induced 3-dimensional conformation of MCFD2 is not essential for this interaction. We speculate that secondary structure elements are sufficient to form the binding site for FV and FVIII. The D122V and ΔEF-2N mutations that damage helix 3 can also interact with FV/FVIII, suggesting that this helix is not directly involved in this interaction. We propose that MCFD2 is the protein that initiates the cargo capture in the ER and brings FV/FVIII to the proximity of LMAN1 for additional interactions. The less rigid structural requirement for FV/FVIII binding is consistent with this function.
It is somewhat surprising that the N-terminus of MCFD2 appears to be dispensable for interactions with either LMAN1 or FV/FVIII. This sequence is conserved in vertebrate species. Although a defined structure cannot be assigned to the N-terminus of MCFD2, Guy et al pointed out that this sequence is not completely without order and it could still assume a folded state in a multisubunit protein complex.19 Kawasaki et al showed that MCFD2 can interact with the CRD of LMAN1 in vitro and that binding of MCFD2 enhanced the binding of CRD to the surface glycans of HeLa cells.25 Because our crosslinking assay was performed in living cells, it can potentially pick up weak and transient interactions. Therefore, we cannot rule out a role of the N-terminal sequence in further enhancing the interaction with LMAN1 or FV/FVIII. In addition to FV and FVIII, other potential LMAN1 cargo proteins have been identified.26–31 Some of these cargo proteins, such as CatC and CatZ, do not seem to require MCFD2.24 Thus, MCFD2 may be a specific cargo selection protein for the recruitment of FV and FVIII, but not other cargo proteins, such as CatC and CatZ. Detailed structure-function analysis of MCFD2 may lead to the development of a selective inhibitor of LMAN1-MCFD2 interaction as a new anticoagulant. Such an inhibitor would reduce FV/FVIII secretion without affecting other LMAN1-dependent cargo proteins.
Acknowledgments
The authors thank David Ginsburg for critical reading of the manuscript and Jun Yang for helpful discussions of the CD data.
This work was supported by the March of Dimes Foundation (Basil O'Connor Starter Scholar Research Award grant #5-FY08-06) and the National Institutes of Health (RO1 HL094505; B.Z.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.Z. and H.L. performed the experiments; and C.Z., H.L., J.Z., and B.Z. designed the research, analyzed the results, made the figures, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bin Zhang, Genomic Medicine Institute, Lerner Research Institute, Cleveland Clinic Foundation, 9500 Euclid Ave, Cleveland, OH 44195; e-mail: zhangb@ccf.org.
References
- 1.Spreafico M, Peyvandi F. Combined factor V and factor VIII deficiency. Semin Thromb Hemost. 2009;35(4):390–399. doi: 10.1055/s-0029-1225761. [DOI] [PubMed] [Google Scholar]
- 2.Zhang B, Ginsburg D. Familial multiple coagulation factor deficiencies: new biologic insight from rare genetic bleeding disorders. J Thromb Haemost. 2004;2(9):1564–1572. doi: 10.1111/j.1538-7836.2004.00857.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhang B. Recent developments in the understanding of the combined deficiency of FV and FVIII. Br J Haematol. 2009;145(1):15–23. doi: 10.1111/j.1365-2141.2008.07559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nichols WC, Seligsohn U, Zivelin A, et al. Mutations in the ER-Golgi intermediate compartment protein ERGIC-53 cause combined deficiency of coagulation factors V and VIII. Cell. 1998;93(1):61–70. doi: 10.1016/s0092-8674(00)81146-0. [DOI] [PubMed] [Google Scholar]
- 5.Zhang B, Cunningham MA, Nichols WC, et al. Bleeding due to disruption of a cargo-specific ER-to-Golgi transport complex. Nat Genet. 2003;34(2):220–225. doi: 10.1038/ng1153. [DOI] [PubMed] [Google Scholar]
- 6.Arar C, Carpentier V, Le Caer JP, Monsigny M, Legrand A, Roche AC. ERGIC-53, a membrane protein of the endoplasmic reticulum-Golgi intermediate compartment, is identical to MR60, an intracellular mannose-specific lectin of myelomonocytic cells. J Biol Chem. 1995;270(8):3551–3553. doi: 10.1074/jbc.270.8.3551. [DOI] [PubMed] [Google Scholar]
- 7.Itin C, Roche AC, Monsigny M, Hauri HP. ERGIC-53 is a functional mannose-selective and calcium-dependent human homologue of leguminous lectins. Mol Biol Cell. 1996;7(3):483–493. doi: 10.1091/mbc.7.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itin C, Kappeler F, Linstedt AD, Hauri HP. A novel endocytosis signal related to the KKXX ER-retrieval signal. EMBO J. 1995;14(10):2250–2256. doi: 10.1002/j.1460-2075.1995.tb07219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kappeler F, Klopfenstein DR, Foguet M, Paccaud JP, Hauri HP. The recycling of ERGIC-53 in the early secretory pathway: ERGIC-53 carries a cytosolic endoplasmic reticulum-exit determinant interacting with COPII. J Biol Chem. 1997;272(50):31801–31808. doi: 10.1074/jbc.272.50.31801. [DOI] [PubMed] [Google Scholar]
- 10.Nufer O, Kappeler F, Guldbrandsen S, Hauri HP. ER export of ERGIC-53 is controlled by cooperation of targeting determinants in all three of its domains. J Cell Sci. 2003;116(21):4429–4440. doi: 10.1242/jcs.00759. [DOI] [PubMed] [Google Scholar]
- 11.Neve EP, Lahtinen U, Pettersson RF. Oligomerization and intracellular localization of the glycoprotein receptor ERGIC-53 is independent of disulfide bonds. J Mol Biol. 2005;354(3):556–568. doi: 10.1016/j.jmb.2005.09.077. [DOI] [PubMed] [Google Scholar]
- 12.Zhang B, Kaufman RJ, Ginsburg D. LMAN1 and MCFD2 form a cargo receptor complex and interact with coagulation factor VIII in the early secretory pathway. J Biol Chem. 2005;280(27):25881–25886. doi: 10.1074/jbc.M502160200. [DOI] [PubMed] [Google Scholar]
- 13.Zhang B, Spreafico M, Yang A, et al. Genotype-phenotype correlation in combined deficiency of factor V and factor VIII. Blood. 2008;111(12):5592–5600. doi: 10.1182/blood-2007-10-113951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Velloso LM, Svensson K, Schneider G, Pettersson RF, Lindqvist Y. Crystal structure of the carbohydrate recognition domain of p58/ERGIC-53, a protein involved in glycoprotein export from the endoplasmic reticulum. J Biol Chem. 2002:15979–15984. doi: 10.1074/jbc.M112098200. [DOI] [PubMed] [Google Scholar]
- 15.Velloso LM, Svensson K, Pettersson RF, Lindqvist Y. The crystal structure of the carbohydrate-recognition domain of the glycoprotein sorting receptor p58/ERGIC-53 reveals an unpredicted metal-binding site and conformational changes associated with calcium ion binding. J Mol Biol. 2003;334(5):845–851. doi: 10.1016/j.jmb.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 16.Hauri HP, Kappeler F, Andersson H, Appenzeller C. ERGIC-53 and traffic in the secretory pathway. J Cell Sci. 2000;113(4):587–596. doi: 10.1242/jcs.113.4.587. [DOI] [PubMed] [Google Scholar]
- 17.Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116(2):153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- 18.Lee MC, Miller EA. Molecular mechanisms of COPII vesicle formation. Semin Cell Dev Biol. 2007;18(4):424–434. doi: 10.1016/j.semcdb.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Guy JE, Wigren E, Svard M, Hard T, Lindqvist Y. New insights into multiple coagulation factor deficiency from the solution structure of human MCFD2. J Mol Biol. 2008;381(4):941–955. doi: 10.1016/j.jmb.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 20.Manavalan P, Johnson WC. Sensitivity of circular-dichroism to protein tertiary structure class. Nature. 1983;305(5937):831–832. [Google Scholar]
- 21.Nyfeler B, Kamiya Y, Boehlen F, et al. Deletion of three residues from the C-terminus of MCFD2 affects binding to ERGIC-53 and causes combined factor V and factor VIII deficiency. Blood. 2007;111(3):1299–1301. doi: 10.1182/blood-2007-09-112854. [DOI] [PubMed] [Google Scholar]
- 22.Spreafico M, Peyvandi F. Combined FV and FVIII deficiency. Haemophilia. 2008;14(6):1201–1208. doi: 10.1111/j.1365-2516.2008.01845.x. [DOI] [PubMed] [Google Scholar]
- 23.Grabarek Z. Structural basis for diversity of the EF-hand calcium-binding proteins. J Mol Biol. 2006;359(3):509–525. doi: 10.1016/j.jmb.2006.03.066. [DOI] [PubMed] [Google Scholar]
- 24.Nyfeler B, Zhang B, Ginsburg D, Kaufman RJ, Hauri HP. Cargo selectivity of the ERGIC-53/MCFD2 transport receptor complex. Traffic. 2006;7(11):1473–1481. doi: 10.1111/j.1600-0854.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- 25.Kawasaki N, Ichikawa Y, Matsuo I, et al. The sugar-binding ability of ERGIC-53 is enhanced by its interaction with MCFD2. Blood. 2008;111(4):1972–1979. doi: 10.1182/blood-2007-06-097022. [DOI] [PubMed] [Google Scholar]
- 26.Anelli T, Ceppi S, Bergamelli L, et al. Sequential steps and checkpoints in the early exocytic compartment during secretory IgM biogenesis. EMBO J. 2007;26(19):4177–4188. doi: 10.1038/sj.emboj.7601844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Appenzeller C, Andersson H, Kappeler F, Hauri HP. The lectin ERGIC-53 is a cargo transport receptor for glycoproteins. Nat Cell Biol. 1999;1(6):330–334. doi: 10.1038/14020. [DOI] [PubMed] [Google Scholar]
- 28.Mattioli L, Anelli T, Fagioli C, Tacchetti C, Sitia R, Valetti C. ER storage diseases: a role for ERGIC-53 in controlling the formation and shape of Russell bodies. J Cell Sci. 2006;119(Pt 12):2532–2541. doi: 10.1242/jcs.02977. [DOI] [PubMed] [Google Scholar]
- 29.Morais VA, Brito C, Pijak DS, et al. N-glycosylation of human nicastrin is required for interaction with the lectins from the secretory pathway calnexin and ERGIC-53. Biochim Biophys Acta. 2006;1762(9):802–810. doi: 10.1016/j.bbadis.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Nyfeler B, Reiterer V, Wendeler MW, et al. Identification of ERGIC-53 as an intracellular transport receptor of a1-antitrypsin. J Cell Biol. 2008;180(4):705–712. doi: 10.1083/jcb.200709100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vollenweider F, Kappeler F, Itin C, Hauri HP. Mistargeting of the lectin ERGIC-53 to the endoplasmic reticulum of HeLa cells impairs the secretion of a lysosomal enzyme. J Cell Biol. 1998;142(2):377–389. doi: 10.1083/jcb.142.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]