Abstract
Results from biochemical and structural studies of the RSC chromatin-remodeling complex prompt a proposal for the remodeling mechanism: RSC binding to the nucleosome releases the DNA from the histone surface and initiates DNA translocation (through one or a small number of DNA base pairs); ATP binding completes translocation, and ATP hydrolysis resets the system. Binding energy thus plays a central role in the remodeling process. RSC may disrupt histone-DNA contacts by affecting histone octamer conformation and through extensive interaction with the DNA. Bulging of the DNA from the octamer surface is possible, and twisting is unavoidable, but neither is the basis of remodeling.
Keywords: histones, nucleosomes, RSC, DNase, exonuclease III
Nucleosomes inhibit transcription, DNA repair, and other chromosome transactions. SWI/SNF and RSC chromatin-remodeling complexes relieve this inhibition by sliding or disassembling nucleosomes (1–4). In the case of sliding, the structure of the nucleosome is unaltered at the end of the reaction. Studies to date have revealed an important principle of the remodeling process, DNA translocation driven by ATP hydrolysis, but the underlying mechanism remains obscure. Translocation slides the DNA around the histone octamer (5–7). Sliding exposes DNA at one end of an isolated nucleosome particle and in the linker regions between nucleosomes in an array. Exposure of the DNA may be detected by an increase in susceptibility to attack by nucleases.
Translocation is effected by the Sth1 subunit of RSC, a member of the DEAD/H-box family of helicase/translocases. These enzymes contact DNA through two domains and step along one strand by a scissors-like motion between them (8). A gap in one strand stops this stepping and blocks translocation. It could be inferred from the effect of gaps in nucleosomal DNA that Sth1 contacts one strand about two turns from the dyad of the nucleosome (6). A favored notion for remodeling is that the translocase draws DNA into the nucleosome from one side, creating a bulge, which is expelled on the other side. The alternative of DNA twisting strain rather than a bulge traversing the nucleosome has been excluded on the basis of studies with nicked or gapped nucleosomal DNA (6, 9).
An outstanding question for remodeling by DNA translocation is how the remodeler effects DNA sliding in the face of histone–DNA interaction. Translocation by Sth1 entails DNA sliding from the end of the nucleosome to a point near the dyad, requiring the disruption of all histone–DNA contacts along the way. Neither the mechanism nor the energetics of this process has been described.
Here we report on the nuclease digestion of RSC–nucleosome complexes, alone and in the presence of nucleotides. The results are supportive of structural studies showing a perturbation of nucleosome structure in the presence of RSC alone (10). These and other findings lead to a proposal for the mechanism of chromatin remodeling by RSC.
Results
DNase I Digestion of a RSC-Nucleosome Complex.
DNase I digestion of the nucleosome has been most informative about histone–DNA interaction in the past. Digestion of end-labeled nucleosomal DNA and gel electrophoresis produces a pattern of bands with the periodicity of the DNA double helix, due to exposure of the DNA to solution alternating with protection by the histone surface (Fig. 1A, lane 3). As previously reported (11), the band pattern obtained from a RSC–nucleosome complex is also modulated with the periodicity of the double helix (Fig. 1A, lane 4). It has been noted, however, that RSC has a general protective effect (6, 12), consistent with the proposal from structural studies for binding of the nucleosome in a central cavity. This general protection is evidenced by a 3.2-fold greater amount of uncut nucleosomal DNA in the presence of RSC at the level of digestion used here (Fig. 1A, lanes 3 and 4). RSC-binding also results in a hypersensitive site two turns from the dyad [Fig. 1A (arrow), and C], attributed to interaction with Sth1 (6).
Fig. 1.
Effects of RSC- and ATPγS binding upon DNase I digestion of nucleosomal DNA. (A) DNase I digestion patterns of naked DNA and nucleosomes, under the conditions indicated above the lanes. Digestion was with 0.33 units of DNase I (ProMega) for 15 sec (lanes 1 and 2) or with 0.6 units for 40 sec (lanes 3–8). Positions of size markers produced by digestion of naked DNA with either Msp I or Alu I are indicated by DNA length on the right. (B) Profile of radioactivity in lane 4 of the region indicated by the bracket on the right of (A). Arrows identify cutting sites exposed by RSC–nucleosome interaction. (C) DNase I digestion pattern in the vicinity of the hypersensitive site indicated by the arrow in (A), under the conditions indicated above the lanes. In this case, the nucleosomal DNA was labeled at the end opposite to that in (A). Amounts of enzyme and times of digestion were the same as in (A).
Close examination reveals further differences between the DNase I digestion patterns of a RSC–nucleosome complex and the nucleosome alone: RSC-binding results in increased exposure of sites in periodically protected regions. Indeed, RSC exposes all major cutting sites in naked DNA within protected regions up to 100 bp from the labeled end of the nucleosome [Fig. 1A and B (arrows)]. A similar result was obtained upon mapping from the opposite end of the nucleosome: RSC binding exposed all major DNA cutting sites in protected regions up to 90 bp from the labeled end in the nucleosome. Thus RSC binding even in the absence of ATP diminishes the interaction of the histones with almost the entirety of the nucleosomal DNA.
RSC was shown previously to bind naked DNA as tightly as a nucleosome (13). We now find, by gel shift analysis, that the affinity of RSC for DNA is approximately twofold greater for 160 bp DNA than for 64 bp. RSC can evidently accommodate the full length of nucleosomal DNA.
DNase I Digestion of a RSC-Nucleosome Complex in the Presence of Nucleotides.
As previously noted (11), a RSC–nucleosome complex in the presence of ATP gave a DNase I digestion pattern closely similar to that of naked DNA (Fig. 1A, lane 5). The hypersensitive site was abolished. Prior destruction of the ATP by treatment with hexokinase and glucose produced a digestion pattern similar to that of the RSC–nucleosome complex in the absence of ATP (Fig. 1A, lane 6). Once attributed to an alteration of nucleosome structure by RSC and ATP, this result can now be understood in terms of sliding of the DNA and its exposure in truly naked form beyond the ends of the nucleosome.
Addition of the “nonhydrolyzable” ATP analog ATPγS to a RSC–nucleosome complex enhanced DNase I cutting in protected regions and, like ATP, abolished the hypersensitive site (Fig. 1A, lane 7). As little as 15 sec exposure to ATPγS was sufficient to obtain this result. Pretreatment with a vast excess of hexokinase and glucose led to the reappearance of the hypersensitive site (Fig. 1A, lane 8), indicative of the destruction of ATPγS, due to a low activity of kinase on the analog, as has been observed with other ATPases and phosphotransferases.
It may be asked whether the effects of ATPγS on the RSC–nucleosome digestion pattern are attributable to a low level of RSC activity on the analog as well. To investigate this possibility, we measured rates of restriction endonuclease cutting of nucleosomal DNA. Xmn I, which cleaves a site 20 bp from one end of the nucleosome, cut about 50% of the DNA in the presence of ATP in 1 h, but showed no detectable cutting in the presence of ATPγS or in the absence of any nucleotide (Fig. S1). Msp I, which cleaves a site 39 bp from the opposite end of the nucleosome, cut about 70% of the DNA in the presence of ATP in 10 min, but cut only 10% in 1 h in the presence of ATPγS or in the absence of any nucleotide (Fig. S1). ATPγS evidently supports little if any RSC action on the nucleosome.
Exonuclease III Digestion of a RSC-Nucleosome Complex.
Exonuclease III (exo III), which degrades duplex DNA from the 3′ end, is inhibited by histone–DNA interaction, and can be used to define the boundaries of the core particle of the nucleosome (14). On more extensive digestion, exo III invades a nucleosome and produces a pattern of bands differing by multiples of 10 bases (15) (Fig. 2, lane 1). This pattern, attributed to the dissociation of DNA–histone contacts occurring every turn of the helix, and to the advance of exo III upon dissociation, provided evidence of sequential unraveling of DNA from the ends of the nucleosome. Exo III digestion reaches a distance 40–50 bp from the ends before stalling (Fig. 2, lane 1), showing that the DNA associated with H2A-H2B dimers is more accessible than that associated with the central H3-H4 tetramer. In the presence of RSC, digestion proceeds even further, to about half the length of the starting DNA [Fig. 2, lane 2 (asterisk); digestion further than seen in ref. 13, due to the use of a three- to fivefold greater excess of RSC]. Exo III digestion cannot proceed beyond this length because the enzyme is specific for duplex DNA, and upon digestion from both 3′ ends to the midpoint, no duplex remains. In the presence of ATP, digestion proceeds even more rapidly to the midpoint, as expected from sliding of the nucleosome and exposure of the DNA (Fig. 2, lane 3). Digestion in the presence of ATPγS was indistinguishable from that with RSC alone (Fig. 2, lane 4).
Fig. 2.

Effect of RSC-binding upon exo III digestion of nucleosomal DNA. Digestion of nucleosomes (2.5 ng DNA) was performed with 40 u exo III (New England Biolabs) for 5 min under the conditions indicated above the lanes (0.3 μg RSC, 0.5 mM ATP or ATPγS). Position of size marker produced by digestion of naked DNA with Msp I is indicated by DNA length on the right. Product of limit exo III digestion, approximately half the length of the starting nucleosomal DNA, is indicated by an asterisk.
Reversible Dissociation of H2A-H2B Dimers.
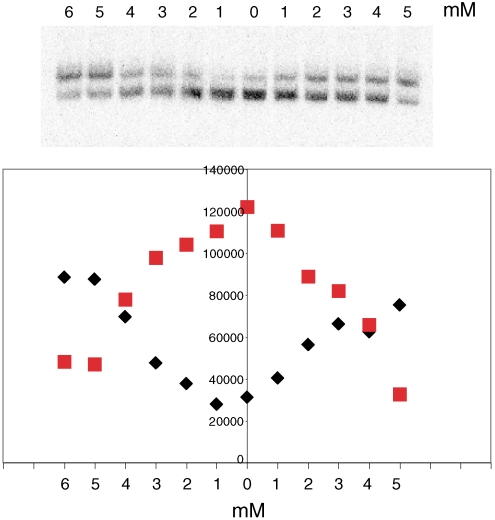
As mentioned above, one H2A-H2B dimer is undetectable in the structure of a RSC–nucleosome complex (10). It has long been known that histones are lost from chromatin in solutions of very low ionic strength in the presence of naked DNA (16). We observed the loss of histones from an isolated nucleosome in a solution of 15 mM Hepes, 3 mM MgCl2, in the presence of a 1,000-fold excess of nonspecific DNA, as shown by an electrophoretic mobility shift of the nucleosome (Fig. 3 Center). The addition of as little as 5 mM potassium acetate prevented this loss of histones (left half of Fig. 3). The process required nonspecific DNA as a histone acceptor and was entirely reversible: Addition of potassium acetate following histone loss restored the particle to the original mobility (right half of Fig. 3). The histone-depleted particle was indistinguishable in mobility from a complex of the H3-H4 tetramer and DNA. We conclude that H2A-H2B dimers are in association equilibrium with the tetramer particle of the nucleosome.
Fig. 3.
Dissociation of H2A-H2B dimers from nucleosomes and reversal in the presence of acetate. Nucleosomes (1 ng DNA) were incubated with naked DNA (1 μg pUC19) for 5 min and analyzed by gel electrophoresis. For lanes to the left of the center line, incubation was in the presence of potassium acetate at the concentrations indicated; for lanes to the right of the center line, incubation was with naked DNA alone, followed by the addition of potassium acetate at the concentrations indicated. The bands in the gel correspond to the nucleosome and tetramer particle. The relative intensities are plotted as black diamonds and red squares below.
We investigated the specificity of the potassium acetate effect. Substitution of sodium or ammonium for potassium made no difference, showing the cation was not responsible for the effect. The nature of the anion was important, however, as the midpoint of the tetramer particle—nucleosome transition shifted from 3 mM for acetate to 5–10 mM for formate, glutamate, and phosphate, whereas chloride failed to protect the nucleosome against histone loss at any concentration tested.
Discussion
RSC-Binding Reduces DNA–Histone Interaction.
Remarkably, RSC binding alone, without ATP, exposes nucleosomal DNA to attack by nucleases. In the presence of RSC, DNase I cuts the underside of the double helix, normally protected by interaction with the histone octamer of the nucleosome; and exo III invades DNA normally protected by interaction with the central H3-H4 tetramer, digesting all the way to the dyad of the nucleosome.
These results are consistent with cryoelectron microscopy (cryoEM) and 3D reconstruction of the RSC–nucleosome complex (10). Whereas density for all the histones except one H2A-H2B dimer could be seen in the reconstruction, density for much of the DNA, including that near the ends (entry/exit to the nucleosome) and near the dyad, was conspicuously absent. The perturbation of histone–DNA interaction revealed by nuclease digestion may be manifest in the cryoEM reconstruction as a loss of DNA density due to motion or disorder.
The surprising disappearance of an H2A-H2B dimer from the cryoEM reconstruction also receives support from our work. We find that H2A-H2B dimers are reversibly dissociable from the nucleosome, in a manner that is critically dependent on ionic conditions. Perturbation of histone-DNA interaction by RSC-binding may promote the same dimer dissociation as occurs in the absence of RSC under appropriate ionic conditions. Binding in the RSC cavity may even create such ionic conditions.
Biochemical and structural studies thus converge on the consequences of RSC–nucleosome interaction. The energy of the interaction is sufficient to disrupt histone-DNA contacts and release the DNA from the histone octamer surface. The DNA is free to slide as required for translocation.
RSC-Binding Promotes DNA Exposure near the Dyad of the Nucleosome.
The most prominent feature of the DNase I digestion pattern of the RSC–nucleosome complex is a hypersensitive site about two turns from the dyad. Hypersensitivity is abolished by the addition of ATPγS. Two observations indicate this effect is not due to hydrolysis of ATPγS. First, the DNase I digestion pattern in the presence of ATPγS is otherwise the same as that obtained with RSC alone, in contrast with that in the presence of ATP. Second, sliding through many base pairs, evidenced by exposure of restriction endonuclease sites, and which requires ATP hydrolysis, does not occur in the presence of ATPγS. Hypersensitivity is most simply explained by strain on the DNA, perturbing the double helical structure or enhancing its displacement from the histone octamer surface. Strain may be caused when the translocase engages the nucleosomal DNA, initiating DNA translocation, and be relieved when ATP binding completes the translocation process.
Proposed Remodeling Mechanism.
The prevailing view of chromatin remodeling by RSC and the related SWI/SNF complex starts from the lack of effect of nicks or gaps on the process. Remodeling occurs up to the point where the translocase encounters a strand break (6, 7). On the basis of these findings, torsional strain, resulting from screw rotation of the DNA, has been ruled out as a driving force for the process (6, 9). In the absence of screw rotation, translocation of the DNA can only occur by sliding. As the structure of the nucleosome is conserved during remodeling, the double helix must remain in register with interaction sites on the histone surface, so sliding must occur through one or more turns of the double helix. Action of the translocase, drawing in a turn of the helix, would create a small bulge protruding from the histone surface, thought to diffuse to the opposite end of the nucleosome in the manner of a wave traversing the particle.
There are several limitations to this line of reasoning. First, the lack of effect of nicks and gaps does not exclude a mechanism involving screw rotation of the DNA. It only shows that torsional strain is not required for the process. Second, sliding through a turn of the double helix is unlikely, because translocases closely related to Sth1 move one base step at a time. Finally, a small bulge of a turn or two of the double helix is energetically disfavored by the low bending flexibility of DNA.
The fundamental problem confronting any proposed mechanism of remodeling is the extensive histone–DNA interaction in the nucleosome. The histones bind through an intricate pattern of dipolar, nonpolar, hydrogen bonding, and electrostatic interactions to both strands of the DNA every turn of the helix (17). Simple rotation or sliding, requiring the simultaneous rupture of all contacts, is evidently impossible. Mechanisms involving torsional strain or DNA bulge formation solve the problem through a local perturbation of the double helix that diffuses along the nucleosomal DNA, disrupting only one or a small number of histone–DNA interactions at a time. Neither mechanism, however, addresses the central question of how the translocase draws in DNA, whether for strain or bulge formation, in the first place. The translocase, located two turns from the dyad of the nucleosome, must draw in DNA from the linker five turns away, requiring the disruption of dozens of histone–DNA contacts along the way. This problem arises at every step of the translocase on the DNA.
It may be asked whether the spontaneous unwrapping of nucleosomal DNA, well characterized by many procedures, might relieve the inhibitory effect of histone–DNA interaction upon translocase activity. The answer is that the rate of spontaneous unwrapping is far too slow. Unwrapping an end of the nucleosomal DNA occurs on the order of a second (18); the equilibrium constant for unwrapping to the middle of the nucleosome is at least 103-fold less than that for an end (19, 20), so the frequency of unwrapping to the middle is on the order of one per hour (on the reasonable assumption that rewrapping is not slower in the middle than at an end).
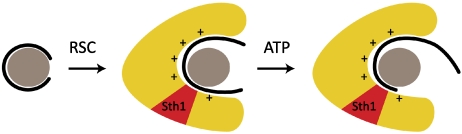
The solution of the problem is unwrapping upon RSC binding to the nucleosome (Fig. 4). Both cryoEM and nuclease digestion provide clear evidence of unwrapping all the way to the dyad of the nucleosome. The DNA is substantially free of the histones and available for translocation. A nuclease hypersensitive site appears at the location of Sth1 due to engagement of the active center, straining the DNA toward translocation. ATP-binding results in movement, relieving the strain. ATP hydrolysis resets the system.
Fig. 4.
Proposed mechanism of chromatin remodeling. Schematic of nucleosome at left depicts histone octamer as gray disk and DNA as solid curve. Schematic of RSC–nucleosome complex in center depicts RSC as yellow volume surrounding the nucleosome, with Sth1 region in red, with positively charged surface for interaction with the nucleosome, and with DNA largely dissociated from surface of the histone octamer. Schematic of RSC–nucleosome at right shows DNA translocated in the presence of ATP.
Movement of the DNA must occur by screw rotation, in view of the structural conservation of Sth1 with DEDxx helicases and duplex DNA translocases (8). X-ray studies of helicases make a compelling case for stepping along a single DNA strand, driven by ATP binding (8). The x-ray structure of Rad54 (21), a translocase closely related to Sth1, shows binding to one strand of duplex DNA nearly identical to that observed for helicases. Rotation of the DNA due to stepping along one strand imparts approximately one superhelical turn for every 10 bases. The resulting torsional strain need not diffuse past histone–DNA interaction sites nor will it accumulate and impede translocation because the DNA is largely free of the histone surface, allowing passage of the strain to the next linker region. There the strain will be relieved by the action of untwisting enzymes, abundant in eukaryotic cells, or it will be canceled by translocation in opposite directions by RSC associated with neighboring particles.
The key to the RSC remodeling mechanism is the role of binding energy in destabilizing the nucleosome and releasing the DNA. It may seem paradoxical that RSC protects nucleosomal DNA from nuclease digestion and at the same time exposes previously protected sites to nuclease attack. It is this paradox, however, that explains how RSC binding achieves a destabilizing effect. RSC contains a deep cavity for binding the nucleosome (10, 12, 22, 23). It envelops the particle, contacting both DNA and histones, thereby conferring general protection against nuclease digestion. The cavity is likely positively charged, as RSC binds naked DNA as tightly as a nucleosome. Moreover, as shown here, the cavity can accommodate the entire length of nucleosomal DNA. We may therefore suppose that entry in the cavity facilitates unwrapping of the nucleosomal DNA, enhancing the separation of the nucleic acid and protein components of the nucleosome, while maintaining them in close proximity. The rate of unwrapping to the middle of the nucleosome would be most affected because the rate presumably decays exponentially from the end of the DNA toward the middle. Although positively charged, the RSC cavity is unlikely to bind DNA so tightly or in a manner that prevents rotation and consequent relaxation of torsional strain. A single molecule study of RSC–DNA interaction is supportive of this view (24). RSC imparts negative superhelicity, and so must bind to naked DNA at two sites, one undoubtedly the translocase active center, and the second presumably the interior of the RSC cavity. The number of negative supercoils introduced is, however, far less than one per turn of the helix translocated, indicative of rotation in the RSC cavity.
RSC–histone interaction may also contribute to destabilization of the nucleosome. The cryoEM studies (10) suggest RSC interaction with the histones, primarily H2A and H2B, on the flat faces of the nucleosome. RSC may perturb the structure of the histone octamer, for example, by extending the histone superhelix (increasing the pitch), causing a reduction in diameter and release of the DNA. For example, a 1 Å change in diameter, sufficient to perturb histone-DNA interaction, would correspond to a 1 bp change in contour length of the DNA. The displacement of an H2A-H2B dimer in the structure of a RSC–nucleosome complex (10) directly demonstrates a perturbation of octamer structure, though a small change in octamer diameter would not have been detectable at the resolution of the analysis.
Unwrapping of nucleosomal DNA and release of a dimer, which enable DNA translocation, are natural processes, intrinsic to the nucleosome. The binding of H2A-H2B dimers is delicately balanced against the bending strain imparted to nucleosomal DNA. The balance is critically dependent on counterion composition and concentration, and is tipped toward dissociation by the removal of carboxylate ions that contribute little to the overall ionic strength. RSC-binding similarly tips the balance, increasing the rates of unwrapping and of dimer dissociation, while conserving the structure of the nucleosome upon its release. It is the extended lifetime of the unwrapped, partially dissociated state that results in the apparent loss of DNA and of a dimer from the cryoEM structure, as well as in the greater accessibility to nuclease attack. The unwrapped DNA may remain associated with the dimer in the RSC cavity, because exo III digestion still pauses every 10 bases from the end of the nucleosome (Fig. 2).
The natural propensity for unwrapping may also explain why Sth1 alone is capable of chromatin remodeling (6), although an order of magnitude more slowly than the entire RSC complex. Sth1 alone may stimulate the rates of unwrapping and dimer dissociation, but to a lesser extent than RSC. Sth1 can then translocate nucleosomal DNA, but at a lower rate than RSC (6).
Other chromatin-remodeling complexes may also stimulate the natural processes of unwrapping and dimer dissociation. Many remodeling complexes contain members of the DEDxx helicase/translocase family and may be presumed to function by DNA translocation (6, 25), thus depending on nucleosomal DNA unwrapping. The Drosophila ACT complex has been reported to expose DNA near the entry and exit points of the nucleosome (26). The SWR1 complex (27), whose ATPase subunit is related to Sth1, catalyzes the exchange of H2A-H2B dimers for Htz1-H2B dimers, presumably by promoting the intrinsic process of dimer dissociation.
Materials and Methods
Nucleosomes were prepared with the use of 160 bp DNA 32P-labeled at one 5′ end (by PCR with end-labeled primer) and rat liver histones (9, 28). RSC was prepared as described (28). Except for DNase I digestion, all reactions were in 15 mM Hepes, pH 8.0, 3 mM MgCl2, 100 μg/mL BSA in a volume of 15 μL at 30 °C. Electrophoresis was in a 7% polyacrylamide—7 M urea gel in Tris/Borate/EDTA buffer for DNase I and exo III experiments, in a 7% polyacrylamide gel in Tris/Acetate/EDTA buffer for restriction endonuclease digestion experiments, and in a 3.2% polyacrylamide gel in 10 mM TrisCl, pH 7.5, 1 mM EDTA for nucleosome dissociation experiments. DNA was extracted with phenol–chloroform–isoamyl alcohol except for nucleosome dissociation experiments, in which reaction mixtures were applied directly to the gel. Data were obtained with the use of a PhosphorImager and analyzed with ImageQuant.
Prior to DNase I digestion, DNA (1.2 ng), either naked or in nucleosomes, was incubated for 20 min at 30 °C in 20 mM Hepes, pH 8.0, 5 mM MgCl2, 7 mM potassium acetate, 125 μg/mL BSA, in a volume of 20 μL, containing 0.8 μg 18mer oligonucleotide (with Gcn4-binding sequence, but only important as carrier DNA for digestion), and where indicated, 0.5 mM ATP, 0.5 mM ATPγS, and 0.3 μg RSC. Also where indicated, hexokinase (100 μg) and glucose were added and incubated 20 min at 30 °C (for destruction of ATP or ATPγS) before the addition of RSC and subsequent incubation for another 20 min. Finally DNase I was added in 5 μL of 25 mM Hepes, pH 7.5, 5 mM MgCl2, 3 mM CaCl2, 50 mM potassium chloride, 200 μg/mL BSA. Digestion was stopped by the addition of 2.5 μL of 100 mM EDTA.
Supplementary Material
Acknowledgments.
We thank Brad Cairns and Michael Levitt for discussion. This research was supported by National Institutes of Health Grant GM36659.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/1000398107/DCSupplemental.
References
- 1.Lorch Y, Zhang M, Kornberg RD. Histone octamer transfer by a chromatin-remodeling complex. Cell. 1999;96(3):389–392. doi: 10.1016/s0092-8674(00)80551-6. [DOI] [PubMed] [Google Scholar]
- 2.Whitehouse I, et al. Nucleosome mobilization catalysed by the yeast SWI/SNF complex. Nature. 1999;400(6746):784–787. doi: 10.1038/23506. [DOI] [PubMed] [Google Scholar]
- 3.Saha A, Wittmeyer J, Cairns BR. Chromatin remodelling: The industrial revolution of DNA around histones. Nat Rev Mol Cell Biol. 2006;7(6):437–447. doi: 10.1038/nrm1945. [DOI] [PubMed] [Google Scholar]
- 4.Lorch Y, Maier-Davis B, Kornberg RD. Chromatin remodeling by nucleosome disassembly in vitro. Proc Natl Acad Sci USA. 2006;103(9):3090–3093. doi: 10.1073/pnas.0511050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saha A, Wittmeyer J, Cairns BR. Chromatin remodeling by RSC involves ATP-dependent DNA translocation. Genes Dev. 2002;16(16):2120–2134. doi: 10.1101/gad.995002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saha A, Wittmeyer J, Cairns BR. Chromatin remodeling through directional DNA translocation from an internal nucleosomal site. Nat Struct Mol Biol. 2005;12(9):747–755. doi: 10.1038/nsmb973. [DOI] [PubMed] [Google Scholar]
- 7.Zofall M, Persinger J, Kassabov SR, Bartholomew B. Chromatin remodeling by ISW2 and SWI/SNF requires DNA translocation inside the nucleosome. Nat Struct Mol Biol. 2006;13(4):339–346. doi: 10.1038/nsmb1071. [DOI] [PubMed] [Google Scholar]
- 8.Singleton MR, Dillingham MS, Wigley DB. Structure and mechanism of helicases and nucleic acid translocases. Annu Rev Biochem. 2007;76:23–50. doi: 10.1146/annurev.biochem.76.052305.115300. [DOI] [PubMed] [Google Scholar]
- 9.Lorch Y, Davis B, Kornberg RD. Chromatin remodeling by DNA bending, not twisting. Proc Natl Acad Sci USA. 2005;102(5):1329–1332. doi: 10.1073/pnas.0409413102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaban Y, et al. Structure of a RSC-nucleosome complex and insights into chromatin remodeling. Nat Struct Mol Biol. 2008;15(12):1272–1277. doi: 10.1038/nsmb.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cairns BR, et al. RSC, an essential, abundant chromatin-remodeling complex. Cell. 1996;87(7):1249–1260. doi: 10.1016/s0092-8674(00)81820-6. [DOI] [PubMed] [Google Scholar]
- 12.Asturias FJ, Chung WH, Kornberg RD, Lorch Y. Structural analysis of the RSC chromatin-remodeling complex. Proc Natl Acad Sci USA. 2002;99(21):13477–13480. doi: 10.1073/pnas.162504299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorch Y, Cairns BR, Zhang M, Kornberg RD. Activated RSC-nucleosome complex and persistently altered form of the nucleosome. Cell. 1998;94(1):29–34. doi: 10.1016/s0092-8674(00)81218-0. [DOI] [PubMed] [Google Scholar]
- 14.Prunell A, Kornberg RD. Variable center-to-center distance of nucleo-somes in chromatin. J Mol Biol. 1982;154:515–523. doi: 10.1016/s0022-2836(82)80010-7. [DOI] [PubMed] [Google Scholar]
- 15.Prunell A, Kornberg RD. Relation of nucleosomes to DNA sequences. Cold Spring Harbor Symp Quant Biol. 1978;42:103–108. doi: 10.1101/sqb.1978.042.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Ilyin Yv, Varshavsky AY, Mickelsaar Un, Georgiev GP. Studies on deoxyribonucleoprotein structure. Redistribution of proteins in mixtures of deoxyribonucleoproteins, DNA and RNA. Eur J Biochem. 1971;22:235–245. doi: 10.1111/j.1432-1033.1971.tb01537.x. [DOI] [PubMed] [Google Scholar]
- 17.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389(6648):251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 18.Li G, Levitus M, Bustamante C, Widom J. Rapid spontaneous accessibility of nucleosomal DNA. Nat Struct Mol Biol. 2005;12(1):46–53. doi: 10.1038/nsmb869. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JD, Widom J. Sequence and position-dependence of the equilibrium accessibility of nucleosomal DNA target sites. J Mol Biol. 2000;296(4):979–987. doi: 10.1006/jmbi.2000.3531. [DOI] [PubMed] [Google Scholar]
- 20.Polach KJ, Widom J. Mechanism of protein access to specific DNA sequences in chromatin: A dynamic equilibrium model for gene regulation. J Mol Biol. 1995;254:130–149. doi: 10.1006/jmbi.1995.0606. [DOI] [PubMed] [Google Scholar]
- 21.Durr H, Korner C, Muller M, Hickmann V, Hopfner KP. X-ray structures of the Sulfolobus solfataricus SWI2/SNF2 ATPase core and its complex with DNA. Cell. 2005;121(3):363–373. doi: 10.1016/j.cell.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 22.Leschziner AE, et al. Conformational flexibility in the chromatin remodeler RSC observed by electron microscopy and the orthogonal tilt reconstruction method. Proc Natl Acad Sci USA. 2007;104(12):4913–4918. doi: 10.1073/pnas.0700706104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skiniotis G, Moazed D, Walz T. Acetylated histone tail peptides induce structural rearrangements in the RSC chromatin remodeling complex. J Biol Chem. 2007;282(29):20804–20808. doi: 10.1074/jbc.C700081200. [DOI] [PubMed] [Google Scholar]
- 24.Lia G, et al. Direct observation of DNA distortion by the RSC complex. Mol Cell. 2006;21(3):417–425. doi: 10.1016/j.molcel.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cairns BR. Chromatin remodeling: Insights and intrigue from single-molecule studies. Nat Struct Mol Biol. 2007;14(11):989–996. doi: 10.1038/nsmb1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strohner R, et al. A ‘loop recapture’ mechanism for ACF-dependent nucleosome remodeling. Nat Struct Mol Biol. 2005;12(8):683–690. doi: 10.1038/nsmb966. [DOI] [PubMed] [Google Scholar]
- 27.Mizuguchi G, et al. ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science. 2004;303(5656):343–348. doi: 10.1126/science.1090701. [DOI] [PubMed] [Google Scholar]
- 28.Lorch Y, Kornberg RD. Isolation and assay of the RSC chromatin-remodeling complex from Saccharomyces cerevisiae. Method Enzymol. 2003;377:316–322. doi: 10.1016/S0076-6879(03)77019-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.