Abstract
Osteoarthritis (OA) is a degenerative joint disease that can result in joint pain, loss of joint function, and deleterious effects on activity levels and lifestyle habits. Current therapies for OA are largely aimed at symptomatic relief and may have limited effects on the underlying cascade of joint degradation. Local drug delivery strategies may provide for the development of more successful OA treatment outcomes that have potential to reduce local joint inflammation, reduce joint destruction, offer pain relief, and restore patient activity levels and joint function. As increasing interest turns toward intra-articular drug delivery routes, parallel interest has emerged in evaluating drug biodistribution, safety, and efficacy in preclinical models. Rodent models provide major advantages for the development of drug delivery strategies, chiefly because of lower cost, successful replication of human OA-like characteristics, rapid disease development, and small joint volumes that enable use of lower total drug amounts during protocol development. These models, however, also offer the potential to investigate the therapeutic effects of local drug therapy on animal behavior, including pain sensitivity thresholds and locomotion characteristics. Herein, we describe a translational paradigm for the evaluation of an intra-articular drug delivery strategy in a rat OA model. This model, a rat interleukin-1β overexpression model, offers the ability to evaluate anti-interleukin-1 therapeutics for drug biodistribution, activity, and safety as well as the therapeutic relief of disease symptoms. Once the action against interleukin-1 is confirmed in vivo, the newly developed anti-inflammatory drug can be evaluated for evidence of disease-modifying effects in more complex preclinical models.
Introduction
Osteoarthritis (OA) is a degenerative joint disease that is classically described as the breakdown and eventual loss of joint cartilage.1 OA is predicted to affect more than 25% of the adult U.S. population by 2030.2,3 Patients afflicted with OA may experience pain and loss of joint function with associated deleterious effects on patient activity level and lifestyle habits. Current OA treatments are largely targeted at symptomatic relief of joint pain and may only have limited effects on the underlying cascade of joint degeneration. There is great interest in the development of more successful OA treatments that would reduce joint inflammation and cartilage destruction, offer relief from disease symptoms, and restore patient activity levels and joint functions. While numerous disease-modifying drugs have been developed for inflammatory arthropathies such as rheumatoid arthritis, the widely used route of systemic administration may not achieve therapeutic effects in the joint space because of relatively slow and nonspecific transport of molecules to the joint tissues. As OA is a disease often localized to a single joint, local delivery of a therapeutic may be preferred.4–6 Intra-articular injection is widely used for delivery of corticosteroids and hyaluronans,4,5,7 although their disease-modifying actions are nonexistent, not well understood, or not widely accepted. There is increasing interest in the delivery of disease-modifying agents directly to the joint space for the treatment of OA, to achieve maximal drug activity and residence time in the joint tissues.6 In recent years, we have seen clinical studies that describe intra-articular delivery of several powerful anti-inflammatory agents, including the tumor necrosis factor (TNF) antagonist—infliximab—for the treatment of rheumatoid arthritis,8 the naturally occurring interleukin-1 receptor antagonist (IL1Ra)—anakinra—for the treatment of OA,9–12 and solutions enriched in IL1Ra for the treatment of OA.13 These studies differ in their dosing administration and delivery vehicles, in their focus on different measurement outcomes, and in their disease-modifying effects, but share a similar interest in understanding the effects of intra-articular drug delivery on patient safety and structural or other surrogate markers of disease-modifying effects.
As increasing interest turns toward the intra-articular drug delivery route, parallel interest has emerged in appropriate means to assess drug biodistribution and safety, dosing, and efficacy against OA in the single affected joint. The focus of this review is to describe a translational paradigm for the evaluation of intra-articular protein and small-molecule drug delivery using a rat model, based on our experience with the delivery of a vehicle for sustained IL1Ra release. Rodent models, in general, provide major advantages for development of drug delivery strategies, chiefly because of lower cost, successful replication of many human OA-like characteristics, rapid disease development, and small joint volumes that enable use of lower total drug amounts during protocol development. We begin with a brief overview of common practices for the treatment of OA, followed by a model of how to determine drug distribution to the joint space and other compartments in the rat, and end with the means to determine OA drug efficacy through joint function and behavioral assessments in small animals.
Disease-Modifying Agents in the Treatment of OA
It is now widely accepted that OA results from a combination of changes occurring throughout the joint tissues, including cartilage, synovium, ligament, and bone.14 Amongst the earliest histopathological signs for OA are collagen and aggrecan depletion in hyaline cartilage.15 Although not a classical inflammatory arthropathy, inflammation in OA is believed to be a key player in the progression of cartilage destruction and joint disease.16–20 Elevated levels of proinflammatory cytokines are regularly present in the synovial fluid and tissues of OA-affected joints,18,21 and chondrocytes from OA cartilage express higher mRNA levels for inflammation products including nitric-oxide synthase, cyclo-oxygenase-2, stromelysin, and IL6 and IL8.16,22–24 IL1β, in particular, directly contributes to reduced anabolic and enhanced catabolic activities in OA-affected joints through regulation of protease expression that acts to degrade cartilage.18,25,26 Hence, a chronic cycle of joint destruction ensues with inflammation, increasing proteolytic activity that changes local tissue mechanics and generates extracellular matrix fragment release, factors that further promote inflammation. Therapeutic agents aiming to interrupt or modify this local proinflammatory, catabolic joint environment are of great interest for the potential to alter the progression of joint destruction and reduce the functional and symptomatic consequences of OA.
Numerous disease-modifying OA drugs have been proposed that largely focus on antagonizing the production or activity of inflammatory mediators, such as IL1 and TNF. The goal of these compounds is to block or modify the local proinflammatory, catabolic environment found in the OA-affected joint. As for corticosteroids and viscosupplements, therapeutic efficacy depends in part upon the joint-residence time of the drug, which may only be on the period of hours.6 Intra-articular drug delivery is compromised by the presence of a highly efficient lymphatic system that rapidly eliminates molecules from the synovial cavity,27 requiring frequent administration of the therapeutic drug (e.g., weekly for 3–6 weeks). This, in turn, may be costly and result in adverse side effects and high levels of patient discomfort. A common approach to partly overcome the challenges of rapid drug clearance from the joint space has been delivery of the drug as an insoluble suspension, promoting delayed drug clearance from the intrasynovial space as shown for some corticosteroids28 (see Refs.5,6). Molecular modification to increase the compound's molecular weight has also been widely used, such as PEGylation, as we have seen for the TNF antagonist etanercept,29,30 or through molecular crosslinking, as we have seen for hyaluronan.31 Numerous polymeric or lipid-based drug carrier systems have been developed for application to the joint space, borrowing in large part from local drug delivery advances for other pathologies including cancer, bone healing, and ocular disorders.32 Liposomes are a common vehicle of interest for encapsulation of inflammation-modifying drugs such as methotrexate or corticosteroids.33–35 Microencapsulation of drugs has also been studied using nano- or microparticles made of polymers or biocompatible materials such as poly(lactic-co-glycolic acid),36–38 albumin,39 chitosan,40 and silk.41 Still other modifications have been made directly to protein drugs to increase longevity, modify molecular weight, or include an environmentally responsive component such as thermally or pH-triggered drug release.42–44
Although numerous advances have been made in the area of arthritis drug development, only a very small fraction of these have been investigated for local drug delivery applications in the treatment of arthritis. A key requirement for drug delivery is demonstration of an ability to provide for increased intra-articular drug concentrations and residence time following administration to the joint space, as well as decreased systemic exposure to the drug. Many factors influence the efficacy of a drug delivery vehicle for promoting sustained intra-articular residence time, including vehicle safety, composition, hydrophobicity, particle size, and charge. Several review articles presented excellent discussion of the factors necessary for a successful drug delivery vehicle.4–6,45 Below we describe our experience with a rat model, wherein we evaluated the biodistribution of a specific thermogelling drug delivery system for the sustained release of an IL1 antagonist for application to OA.
Drug Biodistribution Following Intra-Articular Drug Delivery
The diarthrodial joint is surrounded by a highly vascularized synovial membrane that efficiently filters most solutes and drugs in the intrasynovial joint space, with an intra-articular concentration that is generally proportional to plasma concentrations.6 The synovium also secretes large amounts of hyaluronan and low-molecular-weight solutes and may secrete proinflammatory cytokines and catabolic proteases in the case of synovitis or OA. And, diffusion from intrasynovial spaces into the capillary bed and extrasynovial interstitial spaces is also known to occur, although lymphatic drainage may be the principal route for eliminating many molecules from the joint space.6 We know from the work of Levick27 that low-molecular-weight solutes (<500 Da) may persist for less than 1 h after injection in the joint space, whereas those greater than 1 kDa have residence times in the order of hours. The large fat pads in the joint space may retain larger molecules, including a majority of microparticles, leading to sustained presence and delayed (or enhanced) lymphatic clearance depending on molecular characteristics. Gene delivery has been developed to overcome some of the limitations of rapid drug clearance, investigating sustained expression of compounds such as an IL1 antagonist and TNF receptor.46–48
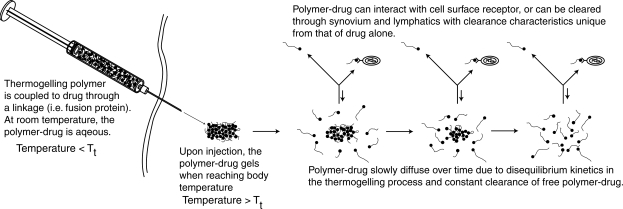
We have experience with a drug delivery strategy originally developed for solid tumor delivery that involves conjugation of a thermally responsive polymer to a protein drug to promote spontaneous gelation or nanoparticle formation at body temperature.42 The thermally responsive polymer is a repeating pentapeptide sequence present in native elastin (V-P-G-X-G, where X is any amino acid except proline). These elastin-like polypeptides (ELPs) are soluble in aqueous solution and exhibit an inverse temperature phase transition above a given transition temperature (Tt), such that formation of insoluble micron- to submicron-sized particles occurs above Tt, and hence, an intra-articular “drug depot” can be formed (Fig. 1). Taking advantage of disequilibrium kinetics, the concept is for the intra-articular drug depot to slowly resolubilize, thereby releasing an active molecule that is bioavailable to bind to its target or be cleared through the synovium and lymphatic system. ELPs have some unique advantages for biomedical applications in that they are biocompatible, are nonimmunogenic, and can be designed at the gene level for precise control of molecular weight and chemistry.
FIG. 1.
Formation of an intra-articular drug depot using a thermally responsive polymer. Elastin-like polypeptides (ELPs) are composed of a repeating pentapeptide sequence present in native elastin (V-P-G-X-G, where X is any amino acid except proline). ELPs can be designed such that they are soluble in aqueous solution at room temperature and form a gel at temperatures above transition temperature (Tt); hence, ELPs can be designed to form intra-articular “drug depots” upon injection. These depots allow active therapeutics to be stored in the drug depot and slowly released over time. Soluble molecules not in the drug depot are available to bind to its target or be cleared through the synovium and lymphatic systems.
Intra-articular drug biodistribution has been evaluated first for the ELP thermally responsive carrier alone, as a means to assess safety and to test the broader application and concept of the ELP drug depot.49 Additional studies would be required to evaluate intra-articular drug biodistribution for any specific drug or ELP fusion protein, as the biodistribution characteristics will depend on charge, hydrophobicity, and other features of the chosen drug. Nevertheless, the approach to track radiolabeled molecule distribution as described here follows well-documented procedures (see Ref.6) and is sufficiently general to be extended to a particular drug. In this work, two ELPs were designed from their corresponding gene and expressed in Escherichia coli; one was selected to be thermally responsive and depot forming (Tt = 32°C, molecular weight = 47 kDa), whereas the other remained soluble and of higher molecular weight than the depot-forming molecule (Tt = 50°C, molecular weight = 61 kDa). The purified proteins were 14C labeled to evaluate protein distribution following intra-articular injection into a rat knee joint. In brief, the right knee joints of female Wistar rats received one intra-articular injection of 30 μL of either 14C-ELP at a concentration of approximately 650 μM (maximum dose of 1 μCi/injection), with numerous tissues and fluids collected at multiple time points. Tissues included right and left knee synovial fluid, meniscus, cartilage, synovium, blood, heart, lung, liver, kidney, and bladder. Radioactivity was measured in body fluids and tissue digests. Values for joint tissues and fluids were summed into a total “joint” value (synovial fluid, meniscus, joint cartilages, synovium) to test for comparisons between left (uninjected control) and right (injected) joints. All radioactive readings were reported as total counts per weight of the harvested tissue and normalized by the amount specifically distributed to the “joint” at 10 min (i.e., the injected dose [ID]). In this manner, the percent of ID per gram (% ID/g) of tissue relative to the recovered amount at early times (i.e., 10 min) was determined for all tissues and fluids. This approach closely follows that used for biodistribution studies in other organ systems, although the normalization here for total “joint” values appears to be a novelty compared with prior studies reporting values for fluid only.
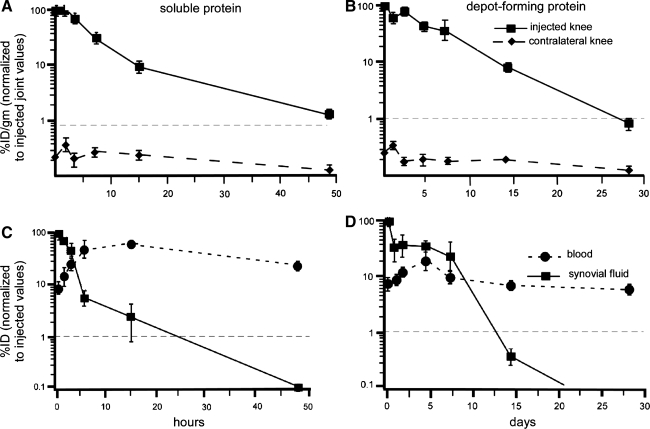
For the soluble, non–depot-forming ELP, the amount of radiolabeled protein in the injected knee decreased with time, reaching background levels (<1% ID/g) within the first 48 h after injection (Fig. 2A). In contrast, the depot-forming ELP was detected at high levels in the right knee for an extended period of time, with levels as high as 80% ID/g at 48 h after injection to less than 10% ID/g at 14 days (Fig. 2B). The kinetics of the 14C-ELP decay was fit to an exponential model to determine an intra-articular half-life for both soluble and depot-forming proteins. The results suggested a joint space half-life of 3.4 h for the soluble protein and 3.7 days for the depot-forming protein. Neither ELP was found to preferentially accumulate in any of the organs studied, with lower than background levels everywhere but blood and kidney (Fig. 2C). As expected, the peak amount of soluble ELP measured in blood occurred at 15 h after injection (4.3% of ID/g or 60% of ID for estimated total blood volume) and at 48 h for the depot-forming ELP (<10% ID for total blood volume). These findings suggest that a thermally triggered drug-depot has the potential to increase protein longevity in the joint space and simultaneously decrease peak serum exposure, which has advantages for costly drugs and for protection from adverse effects.
FIG. 2.
Biodistribution of a thermogelling drug delivery vehicles composed of 14C-labeled ELPs after intra-articular injection in a rat knee. Data for a soluble ELP that does not thermogel (A, C) is compared with that of a thermogelling ELP that spontaneously forms submicron-sized particles at 37°C (B, D). (A) and (B) show the amount of 14C recovered from the injected and contralateral knees as a percentage of values measured at time 0 (10 min postinjection, considered the injected dose [ID]). (C) and (D) show the amount of 14C recovered from blood volume and synovial fluid also as a percentage of values measured at time 0. The 14C-dose per joint compartment is normalized by the weight of all tissues and reported as a normalized % ID/g; 14C-values per fluid is given as % ID. The results illustrate that the thermally responsive ELP (B, D) contribute to longer residence time in the joint space in the order of days, rather than hours as for the soluble ELP. In addition, the results illustrate that the thermally responsive ELP (B, D) is associated with lower peak serum levels when compared with the soluble ELP. This figure was modified from Betre et al.49
This study, which evaluates depot biodistribution in the rat knee joint space, largely parallels other studies tracking molecular distribution and clearance from the intra-articular space.6 Further studies are also needed to incorporate histological assessments or high-resolution imaging to determine if proteins are localized to meniscus, cartilage, synovium, or fat tissues within the joint space, and to determine if cellular uptake (i.e., phagocytosis) is involved in the clearance from the diarthrodial joint.
Outcomes of Intra-Articular Drug Delivery
If the mechanism of action is known for a drug candidate, then an animal model can be developed to specifically evaluate the relevant mechanism in vivo. This is generally a recommended strategy to confirm drug bioactivity in vivo, as it reduces confounding factors arising from interfering molecular pathways. We discuss here our first steps toward evaluating the bioactivity of intra-articular drug delivery for a novel candidate—an ELP fused to an IL1Ra. For this work, we selected a rat model of IL1β overexpression, as developed by Gouze et al.50 This model provides a first test of dosing requirements for the ELP-IL1Ra fusion protein and is advantageous in the evaluation of anti-IL1 therapeutics because the pathology is initiated and driven by IL1β overexpression. It should be noted, however, that antigen-induced arthritis models are amongst the most popular for evaluating intra-articular anticytokine therapy,51–55 particularly as mouse models in this category are well established and have served to evaluate a broad category of drug targets including corticosteroids, hyaluronan, broad-acting anti-inflammators, and anticytokines. IL1 is known to be a cytokine of key importance in the mouse antigen-induced models of arthritis, and it would serve as a useful model for evaluating the bioactivity of ELP-IL1Ra in vivo. We selected the rat model of IL1β overexpression, however, as this model is also driven by IL1 and has direct relation to our ELP biodistribution work.
It has been more than 15 years since protein delivery of IL1Ra to the joint space was shown to slow the progression of cartilage lesions in a joint instability model of OA.9 Since that time, IL1Ra has gained use for the treatment of rheumatoid arthritis and has been developed for gene delivery to the joint space.47,48,56–58 Nevertheless, protein delivery to the joint space in human OA subjects has not revealed therapeutic benefits, likely because of the rapid clearance of the protein drug.11 For this reason, we created an IL1Ra-ELP fusion protein with the goal to increase drug longevity in the joint compartment through a thermally responsive drug-depot (see Fig. 1). The fusion protein was synthesized by subcloning the gene for human IL1Ra with a gene for a thermally responsive ELP and expressing it in E. coli.43 The resulting 31-kDa protein has been previously shown to retain a thermally triggered depot formation at 34° C, while retaining some of the IL1Ra bioactivity against IL1-induced lymphocyte proliferation in vitro. It is noteworthy that conjugation of the ELP peptide to the C or N-terminus of a bioactive drug has been associated with some reduced activity for the drug, for both IL1Ra and a soluble TNF.59 Nevertheless, the benefits of sustained drug release in the local, affected joint compartment may compensate for this loss of activity.
A retrovirally transduced rat skin fibroblast cell line carrying cDNA for the human IL1β gene (MFG-hIL1β) was delivered to the joint space of rat knees (right joints) via intra-articular injection, producing an inflammatory arthritis characterized by synovial hypertrophy and variable stages of cartilage destruction and subchondral bone remodeling. Prior studies showed that the temporal progression of arthritis and cartilage destruction was dependent on the total number of cells injected and could develop in as little as 2 days postinjection. In our work, we chose to deliver a modest number of rat skin fibroblasts (1.25 × 104 in 30 μL phosphate-buffered saline) to study moderate cartilage destruction. Twenty-four hours after the injection of IL1β overexpressing cells, rats received a second knee joint injection of either commercially available IL1Ra (0.24 μg; R&D Systems, Minneapolis, MN) or ELP-IL1Ra fusion protein (120 μg). The fusion protein dose was adjusted to correct for a majority of drug in depot form and the lower affinity of the soluble ELP-IL1Ra fusion drug, such that both treatment groups received nearly equivalent amounts of bioactive drug within the first 24 h. Evidence of joint inflammation was assessed by measuring coronal and sagittal knee joint diameters over time, as well as gross and histological grading upon sacrifice of the animals.
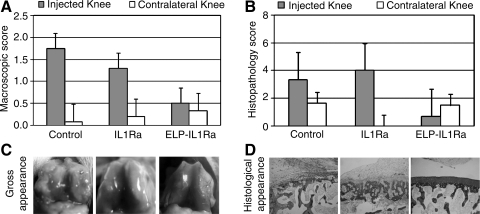
The injected (right) knee joints of all animals had evidence of greater knee joint diameters than uninjected contralateral control joints, starting at 3 days after delivery of IL1β overexpressing cells, suggesting that IL1β was driving the inflammatory events in the joint as expected. The injected knee joints from the ELP-IL1Ra fusion protein group had less evidence of gross pathology when compared with either control or commercially available IL1Ra groups (Fig. 3A, p < 0.001, Tukey's), with varying presentations of synovial erythema and hypertrophy in all joints of the no treatment controls, but not in treated groups. Uninjected left knee joints demonstrated little pathological changes at the time of sacrifice, with an average score that suggested no pathology. This observation suggests that the pathology was indeed localized to the joint receiving the IL1β overexpressing cells, and that there were few systemic effects of the injections. Histological sections were prepared from the patellofemoral grooves, femoral condyles, and tibial plateaus of all joints, although the greatest evidence of pathology induced by the IL1β overexpressing cells was observed in the tibial plateaus (Fig. 3B). Joints receiving ELP-IL1Ra injections demonstrated significantly less pathology than control joints (p < 0.0005, Tukey's) at the femoral condyle site and significantly less pathology than commercially available IL1Ra-treated joints at the tibial plateau (p < 0.007, Tukey's). Complete cartilage erosion was seen in all right knee joints, and subchondral bone remodeling was seen in a majority of knee joints obtained from animals in the control group. Again, histological evidence demonstrated that pathology was localized to the joint receiving IL1β overexpressing cells.
FIG. 3.
Gross pathology and histology scores for rat knee joints receiving an intra-articular injection of interleukin-1β (IL1β)-overexpressing cells. (A) Average morphological grades of degeneration for knee joints from animals receiving an intra-articular injection of IL1β-overexpressing fibroblasts, followed by delivery of a single dose of IL1 receptor antagonist (IL1Ra), a fusion protein of ELP and IL1Ra (ELP-IL1Ra), or no additional treatment (control). Data are shown for 1 week postinjection. Higher morphological grades were associated with greater evidence of synovial inflammation, as expected for the control group receiving no antiinflammatory proteins. Knee joints receiving an injection of ELP-IL1Ra fusion protein had significantly less evidence of gross pathology when compared with either control or IL1Ra treatment groups (p < 0.001, Tukey's). (B) Average histopathology scores for the tibial plateau regions of joints that received an intra-articular injection of IL1β-overexpressing fibroblasts, followed by delivery of a single dose of IL1Ra, a fusion protein of ELP and IL1Ra (ELP-IL1Ra), or no additional treatment (control). Data are shown for 1 week postinjection. Joints receiving ELP-IL1Ra injections demonstrated significantly less pathology than control and commercially available IL1Ra-treated joints at the tibial plateau (p < 0.007, Tukey's); femoral condyle and patellofemoral groove sites were also studied but not shown here. Complete cartilage erosion was seen in all six right knee joints, and subchondral bone remodeling in five of the six knee joints obtained from animals in the control group. In comparison, no evidence of either subchondral bone remodeling or cartilage erosion was observed for animals in the ELP-IL1Ra group, with only presentation of simple fissures (OARSI grade 3) in some knee joints. Higher histopathology scores were associated with cartilage erosion and subchondral bone remodeling, presumably mediated by the IL1. (C) Images of representative knee joints from animals in this study design. Evaluations of gross appearance are an important step in evaluating drug efficacy for an existing drug or newly developed drug delivery vehicle, as for the ELP-IL1Ra shown here. (D) Images of representative tibial plateau sections from animals in this study design. The figures were adapted from Adams et al.83
The results of this study are important for demonstrating that a fusion protein of ELP and IL1Ra retains some activity against IL1β-induced joint damage, thus confirming that the mechanism of action against IL1 is retained in vivo. Commercially available drug was similarly found to exhibit activity against IL1, although to a lesser extent than the ELP-IL1Ra that is likely related to the higher molar doses of ELP-IL1Ra used here. The observation that the ELP-IL1Ra compound was able to diminish both gross and histological evidences of inflammation is likely related, at least in part, to the sustained presence of the IL1Ra domain in the joint space after intra-articular delivery.
Although this model and study were undertaken to specifically evaluate the concept of intra-articular delivery with the thermally responsive ELP depot, the induction of inflammation via overexpression of IL1 contributes to significant inflammatory and bony erosive changes in the joint that are not universal features of human OA. In addition, the presence of a constitutively active human IL1 gene mimics a chronic arthritis that again may not be the prominent feature of human OA. OA is a complex disease, however, with multiple mechanical, inflammatory, and metabolic changes that are not well represented by the IL1β overexpression model alone. Joint destruction-simulating OA can be initiated and promoted via several mechanisms in animal models, including spontaneous occurring (guinea pig), surgical ligament or meniscal resection, and modification of an animal's genotype (knockout, transgenic, and knockdown models). The next steps for application of this drug delivery strategy to treat OA, however, must explore intra-articular drug delivery in the more complex milieu of the unstable diarthrodial joint. Once the mechanism of action is confirmed in vivo, as shown here for the anti-IL1 effects of ELP-IL1Ra, the newly developed antiinflammatory drug can be evaluated for evidence of disease-modifying effects in the more complex, less repeatable, and involved pathology of knee joint instability-induced OA.60–62 Additional information is also needed on biodistribution of the fusion protein, dosing requirements for attenuation of disease progression, and evaluation of the drug safety profile; all of these would be well suited for study in a rat or mouse model. Nevertheless, the IL1 overexpression model had some utility in testing for a specific anti-IL1 activity for the ELP-IL1Ra fusion protein, and there remain many additional uses of the model that are a necessary part of any translational effort in drug delivery.
Disease Sequelae of OA in Small Animal Models
As illustrated above, the principal outcomes of interest in evaluating therapeutic efficacy in preclinical models of joint arthritis has historically relied upon anatomical evidence of cartilage and synovial pathology, as well as imaging appearance. From the clinical perspective, however, restoration of joint function and symptomatic pain relief are of primary importance, more so than anatomical or radiological evidence of pathology. Although measures of disease function and symptoms in preclinical models are less commonly utilized as outcomes, several pain and disability scales have come to wide-spread use for the study of musculoskeletal diseases in humans, and many items on these scales have analogs that may be translated to the preclinical model.63–65 The methods used to assess the functional and symptomatic consequences of pathology in the preclinical OA model primarily focus on measuring an animal's response thresholds to mechanical or thermal stimuli, analyzing the animal's characteristics of locomotion, and the animal's ability to perform a challenge or task. These data provide some quantifiable measures of the functional and symptomatic sequelae associated with disease.
Rodents are ideally suited for these studies because laboratory methods to collect functional and symptomatic data have been perfected over decades of behavioral testing in other disciplines and for other disease models.66 Many of these technologies, which have been prominently used in neurobiology and neuroscience, are easily translated to rodent musculoskeletal disease models. In addition, descriptors of animal gait and motion can be used to describe movements and behaviors associated with pathology in the musculoskeletal system. Challenges do exist, however, with scaling measurements down to the small sizes and rapid, sporadic movements of the rodents, and with correlating behavioral and functional assessments with more traditional biomarkers of OA pathology, such as radiological, serum, or joint fluid biomarkers. In our work, we have used multiple methods to describe the functional and symptomatic consequences of musculoskeletal pathology in rodents, including the rat IL1β overexpression model described earlier and a genetic knockout mouse susceptible to spontaneous joint degeneration. Here, we summarize the methods that we believe to have high utility for assessing animal function and sensitivity in preclinical rodent OA models.
Thermal sensitivity
Thermal sensitivity can be quantified by measuring an animal's latency to withdrawal from a noxious heat source and/or the period of time that pain-related behavior persists following withdrawal from the stimulus. These tests can be used to assess hyperalgesic states associated with pathology and, in the case of intra-articular drug delivery, can be indicative of a therapeutic, pain-relieving response. Tests for thermal hyperalgesia are amongst the most widely available, validated, and routinely used methods to assess pain-related behaviors in disease models.66 Briefly, an animal's latency to withdrawal from and/or the duration of pain related behaviors following application of a heat source (such as licking, flicking, and failing to bear weight) may be used to quantify animal sensitization. The heat source is generally applied through a hot plate or can be specifically applied to an area of interest via focused light; the latter may be preferred for unilateral injury models because differences between the affected and contralateral limbs can be acquired. Moreover, withdrawal of the tail from heat is often also acquired, because tail withdrawal tends to be associated with a spinal reflex, whereas paw withdrawal involves supraspinal processing.67–69
Although thermal withdrawal thresholds are widely used for arthritis models, it should be noted that many preclinical rodent models have an acute inflammation component associated with the induction of an arthritis-like pathology. Thus, heightened thermal sensitivity may be driven by inflammation associated with surgery or the injection of an adjuvant or antagonist. This pain response may not necessarily be indicative of OA, which is characterized by a chronic, low-grade inflammation. For example, we have shown that mice homozygous of Col9a1 gene inactivation prematurely develop OA-like lesions that progress in severity with age; when evaluating the thermal sensitivity of these animals at approximately 48 weeks of age, we did not observe any differences in thermal sensitivity in the hind limbs despite significant cartilage erosion in the mutant mice relative to wild-types. We did, however, observe other significant sensitizations and deficits.70 Thus, whenever possible, it is best to include multiple functional and symptomatic measures to characterize a preclinical model.
Mechanical sensitivity
Pain related data in a preclinical animal model can also be acquired from methods and instruments that assess mechanical sensitivity. Among the most common and accepted tests are methods using von Frey filaments to assess tactile allodynia (nonnoxius mechanical stimulus) and the Randall–Selitto instrument to assess mechanical hyperalgesia (noxius stimulus).71,72 Classically, the von Frey method uses a series of hand-held monofilaments with a known bending force. A series of von Frey monofilaments are applied to the plantar surface of the hind paws and the presence of a withdrawal event is recorded. Multiple trials may be used over a series of 4–9 monofilament applications to approximate the 50% paw withdrawal threshold in what is known as the “Chaplan up–down method.”73 The Randall–Selitto instrument assesses responsiveness to a noxious paw pressure with time to withdrawal, struggle, or vocalize recorded.
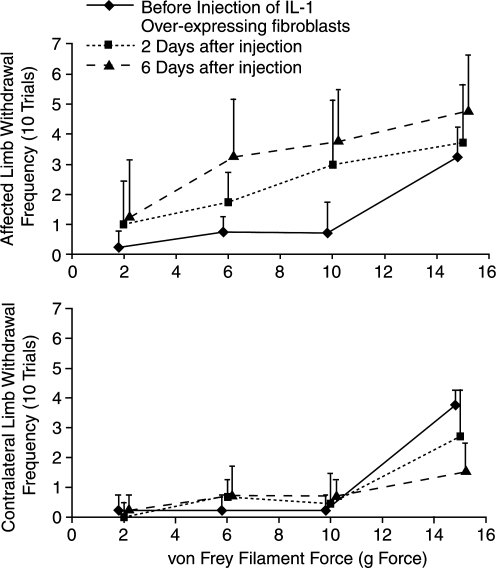
We have successfully used tests of mechanical sensitivity to characterize pain-related behaviors in both the IL1 overexpression model and a spontaneous mouse model of knee joint degeneration (type IX collagen knockout). Mice homozygous for Col9a1 inactivation had significantly higher sensitivity to von Frey filaments at 48 weeks of age, a result that coincided with marked cartilage erosion in the animals' knee joints.70 Following intra-articular knee injection of rat dermal fibroblasts genetically modified to overexpress human IL1β, we observed increased sensitivity to mechanical stimuli (decreased withdrawal threshold) in the injected limb as early as 2 days after injection, with sensitivity increasing in the affected limb up to 6 days relative to preinjection levels74 (Fig. 4). Changes in the contralateral limb were minimal with a tendency for the withdrawal threshold to increase relative to preinjection levels (not statistically significant), perhaps driven by an animal's unwillingness to shift weight from the unaffected limb to the affected limb. Although not statistically significant, this trend highlights the benefit of a preoperative control for sensitivity testing, as the contralateral limb is equally subject to postinjury changes and selected behaviors.
FIG. 4.
Sensitivity to mechanical stimuli increases in the affected limb following intra-articular injection of IL1β-overexpressing cells in the rat knee. An animal's sensitivity to mechanical stimuli was evaluated by measuring the frequency of paw withdrawal to the 2, 6, 10, and 15 g von Frey hairs in the affected and contralateral limb. Following intra-articular injection of IL1β-overexpressing cells, animal's withdrew their affected limb from the von Frey hairs more frequently than in preoperative measures, with sensitivity increasing to 6 days postinjection (p < 0.05, n = 4, Tukey's). Also, animals tended to be less likely to withdraw the contralateral limb from the 15 g hair following injection (nonsignificant).
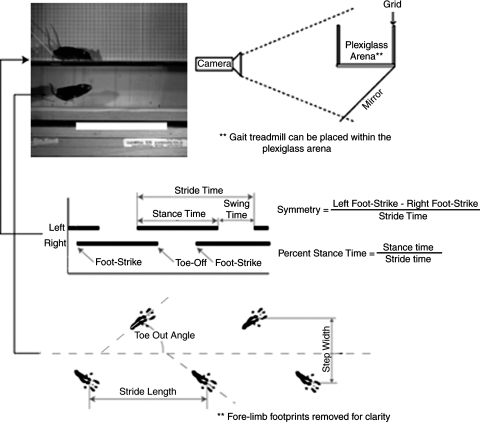
Footprinting
Characteristics of gait such as stride length, step width, and toe-out angle can reflect changes in joint function as a result of joint pathology (see Fig. 5). In footprinting, an animal's paws are inked; then, the animal is placed in a walkway lined with white paper and encouraged to walk. Footprints are collected and a number of geometric descriptions can be acquired. The major limitation in this method is that velocity, acceleration, and deceleration cannot be accurately determined during the trial. Stride length is highly correlated to the animal's selected velocity, and even step width and toe-out angles can change with an animal's speed. This affects this method's sensitivity to demonstrate differences, and it can also be difficult to discern whether changes are due to alteration of gait characteristics or selection of a different gait velocity with the resulting joint pathology. In our own studies of a type IX collagen knockout mouse, we were able to correlate stride lengths and step widths (from high-speed videography, see below) to selected speed, which then revealed that mutant mice used shorter stride lengths and different step widths than wild-types at a given velocity. These differences were not detectable with standard footprinting practices.
FIG. 5.
Temporal and geometric descriptors of rodent gait. Using high-speed videography, several key temporal and geometric descriptors of animal gait can be acquired and correlated to an animal's selected velocity. Stance, swing, and stride times can be determined by tracking the time of an animal's foot-strike and toe-off events. These data can be used to calculate an animal's percent stance time (the amount of time that a given limb can bear weight) and the gait symmetry of a limb pair (a variable that can indicate the presence of asymmetric limps). Moreover, geometric descriptors of gait similar to those acquired via footprinting can be measured, including stride lengths, toe-out angles, and step widths.
Finally, motion in footprinting trials is sometimes induced by brushing the animal's hind quarters, causing the animal to retreat from the stimulus. This induction of gait can reduce a method's sensitivity to detect abnormalities. We have previously shown that type IX collagen knockout gait deficiencies are greater when the animals were voluntarily exploring an open arena relative to when motion was prompted.70 Thus, although footprinting methods are capable of demonstrating differences between experimental and control animals, investigators must standardize the handling of the different animals and should be careful in the interpretation of both significant and nonsignificant results.
High-speed videography
High-speed video techniques have been developed to assess rodent gait in an open arena or on an illuminated walkway (AKA CatWalk, Noldus Information Technology, Wageningen, The Netherlands).75–77 Although velocity cannot be controlled in the open arena, these techniques do have the ability to accurately measure rodent velocity and the gait characteristics measured by footprinting. Post hoc analyses can then predict the effect of varying velocity on a given variable, and thereby, geometric descriptors of gait can be analyzed as deviations from an expected value for given velocity. In addition, high-speed videography can assess temporal descriptors of gait, such as stance times, swing times, and limb phases (Fig. 5). As such, limp-related variables can be quantified, such as symmetry and differences in percentage stance time. The open arena can also be used to assess the research animal in different types of motions, such as voluntary exploration and induced motion. Combined, these analyses may demonstrate gait differences at different stress levels and different velocities.
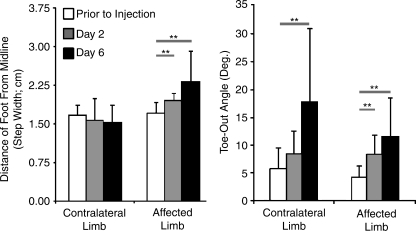
We have used these techniques to describe different models of degenerative disease, including the IL1 overexpression model described earlier. After IL1β overexpressing fibroblasts are injected in the rat knee, several detectable changes are observable and progress in significance as pathology increases. Most notably, percentage stance time decreases on the ipsilateral limb and increases on contralateral limb, and gait also becomes progressively asymmetric as animals spend more time shifting body weight from their contralateral limb to their ipsilateral limb than they do shifting from ipsilateral to contralateral. These shifts in temporal variables are quantifiable measures of an animal's limp, and both of these increase in severity from day 2 to 6 postoperation.74 Geometric changes were observed as well: toe-out angles increased in both limbs, and ipsilateral step width increased following injection of IL1β overexpressing cells (Fig. 6). These gait abnormalities are indicative of the animal's functional capabilities and joint pain, and thus, these data may be able to detect the efficacy of therapeutics targeted at joint disease.
FIG. 6.
Gait changes following intra-articular injection of IL1β-overexpressing cells in the rat knee. Following intra-articular injection of IL1β-overexpressing cells, animals changed the location and orientation of their hind paws while walking. Preoperatively, the left and right paws had similar distances from the animal's midline (step width); however, following intra-articular injection of IL1β-overexpressing cells, the animal placed the affected limb further away from their midline while walking (**p < 0.05, n = 4, Tukey's). Further, the animal increased the toe-out angle in both limbs during locomotion (**p < 0.05, n = 4, Tukey's).
Most preclinical animal models for joint disease describe a unilateral injury. It should be noted that for bilateral injuries, as seen in many genetic knockout models, the predicted gait changes are markedly different. Rather than a decrease on the injured limb, percentage stance times will likely increase when both limbs in a limb pair are injured. As an uninjured limb is unavailable to support weight, an increased percentage stance time in both injured limbs decreases the relative amount of time that a single limb must bear weight on its own. Further, in bilateral injury models, gait may not be asymmetric as we have observed in type IX collagen knockout mice,70 which exhibited a symmetric gait with higher hind-limb percentage stance times in both limbs, shorter stride lengths, and wider hind-limb step widths than wild-type controls.
Treadmill gait
As gait properties are highly correlated to velocity, a treadmill can provide the added advantage of assessing gait descriptors at various speeds. A standard treadmill does not allow for the full characterization of gait, as sight lines to the transverse plane are not available to assess many gait parameters. A few commercially available treadmills provide a clear belt as support, and thus accurate sight lines are obtained to assess geometric measures of gait. In addition to controlling velocity, a treadmill can be used to detect an animal's transition speed between walking and running gaits. This variable can also be a powerful tool as animals in pain may tend to transition to running gaits at slower velocities to reduce joint loading. It should also be noted, however, that a treadmill is a more stressful environment for the animal relative to exploring an open arena, and thus, gait abnormalities have the potential to present differently between these two environments.
We have designed a treadmill with clear supports to characterize rodent gait. Like our open arena design, this treadmill can sit upon a glass table with mirror underneath oriented at 45° (Fig. 5); this allows for recording of animal motion in both the sagittal and transverse planes. For the type IX collagen knockout mouse, we compared treadmill gait to open arena gait at similar velocities.78 On the treadmill, percentage stance times and stride lengths were shorter than in the open arena, revealing the significant effects of environment on gait. However, the relative differences between knockout and wild-type mice were similar in the two environments despite the shift in absolute values; thus, although the absolute values of the gait parameters may not be comparable between the two environments, a gait treadmill can be advantageous in examining the differences between animals at controlled velocities.
Weight-bearing, strength, and ground reaction forces
Weight-bearing and strength are other powerful descriptors of joint function and joint-associated pain. Several incapacitance meters are available commercially; these devices measure the percentage of weight on the left and right hind-limbs while an animal is rearing. This test is an efficient and reliable method to determine animal weight distribution during a stance phase, but does not offer information about limb strength or loading during motion. Similarly, grip strength meters are widely available; these instrumented bars record the maximum force achieved as an animal is withdrawn from the bar and can be performed for both the fore- and hind-limb pairs. Both of these meters are well-established technologies and have been used to characterize multiple musculoskeletal and neurobiology disease models. Dynamic assessment of ground reaction forces during locomotion is also possible in preclinical models. These data provide extensive information on both limb loading and strength. Although more commonly applied to larger animal models, such as the canine anterior cruciate ligament (ACL) transection model of OA,79,80 there exists the potential for similar characterizations in rodent models.81,82 Collection of this force data in rodents, however, requires highly-sensitive force plates, and similar to gait geometric and temporal descriptors, force data covary with the animal's body size and its selected velocity.
Conclusion
As new developments occur in intra-articular drug delivery for treatment of joint pathologies, so will interest grow in assessing drug safety, dosing, and efficacy in preclinical OA models. Using a translational paradigm for the evaluation of intra-articular drug delivery, we believe that there is great value in assessing drug biodistribution and efficacy against OA disease features in the affected joint using a rodent model. Several rodent models exist which simulate OA pathology with varying amounts of complexity. As such, therapeutic interventions can be evaluated for bioactivity and dosing requirements using a model driven by the relevant mechanisms prior to investigations in more complex pathology models. Rodent preclinical models provide major advantages for development of drug delivery strategies, including lower cost, successful replication of many human OA-like characteristics following rapid disease development, and small joint volumes that enable use of lower total drug amounts during protocol development. In addition, technologies exist, and continue to be developed, which can evaluate the ability of therapeutic interventions to restore joint function and alleviate joint-associated pain in rodents. Using this translational approach, critical information on a drug's applicability for intra-articular drug delivery strategies can be effectively obtained using preclinical OA models.
Acknowledgments
This work was supported with funds from the National Institutes of Health (R01EB002263, R21AR052745, P01AR050245, F32AR056190, and K99AR057426) and the North Carolina Biotechnology Center (CFG-8013). The contributions of Mr. Steve Johnson to animal studies and Dr. Elvire Gouze for the animal model are gratefully acknowledged.
Disclosure Statement
The author Lori A. Setton has received payment as a member of the Scientific Advisory Board of Phase Biosciences, Inc. (PhaseBio, Morrisville, NC). This organization did not provide funding for this study. The author, Lori A. Setton, is co-inventor of a patent application entitled “A direct drug delivery system based on thermally responsive biopolymers” that was filed by Duke University in July 2006 and licensed to Phase Biosciences, Inc. Phase Biosciences paid filing and licensing fees to Duke University.
All other authors have no competing financial interests to declare.
References
- 1.Moskowitz R.H.D. Goldberg V. Mankin H. 2nd. Cleveland, OH: W.B. Saunders Company; 1992. Osteoarthritis: Diagnosis and Medical/Surgical Management. [Google Scholar]
- 2.Buckwalter J.A. Stanish W.D. Rosier R.N. Schenck R.C., Jr. Dennis D.A. Coutts R.D. The increasing need for nonoperative treatment of patients with osteoarthritis. Clin Orthop Relat Res. 2001;385:36. doi: 10.1097/00003086-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Hootman J.M. Helmick C.G. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54:226. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 4.Gerwin N. Hops C. Lucke A. Intraarticular drug delivery in osteoarthritis. Adv Drug Deliv Rev. 2006;58:226. doi: 10.1016/j.addr.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Burt H.M. Tsallas A. Gilchrist S. Liang L.S. Intra-articular drug delivery systems: overcoming the shortcomings of joint disease therapy. Expert Opin Drug Deliv. 2009;6:17. doi: 10.1517/17425240802647259. [DOI] [PubMed] [Google Scholar]
- 6.Larsen C. Ostergaard J. Larsen S.W. Jensen H. Jacobsen S. Lindegaard C. Andersen P.H. Intra-articular depot formulation principles: role in the management of postoperative pain and arthritic disorders. J Pharm Sci. 2008;97:4622. doi: 10.1002/jps.21346. [DOI] [PubMed] [Google Scholar]
- 7.Brandt K.D. Smith G.N., Jr. Simon L.S. Intraarticular injection of hyaluronan as treatment for knee osteoarthritis: what is the evidence? Arthritis Rheum. 2000;43:1192. doi: 10.1002/1529-0131(200006)43:6<1192::AID-ANR2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 8.Conti F. Priori R. Chimenti M.S. Coari G. Annovazzi A. Valesini G. Signore A. Successful treatment with intraarticular infliximab for resistant knee monarthritis in a patient with spondylarthropathy: a role for scintigraphy with 99mTc-infliximab. Arthritis Rheum. 2005;52:1224. doi: 10.1002/art.20979. [DOI] [PubMed] [Google Scholar]
- 9.Caron J.P. Fernandes J.C. Martel-Pelletier J. Tardif G. Mineau F. Geng C. Pelletier J.P. Chondroprotective effect of intraarticular injections of interleukin-1 receptor antagonist in experimental osteoarthritis. Suppression of collagenase-1 expression. Arthritis Rheum. 1996;39:1535. doi: 10.1002/art.1780390914. [DOI] [PubMed] [Google Scholar]
- 10.Goupille P. Logeart I. Combe B. Naturalistic survey on nonsteroidal antiinflammatory treatment in patients with musculoskeletal pain. Joint Bone Spine. 2003;70:219. doi: 10.1016/s1297-319x(03)00047-2. [DOI] [PubMed] [Google Scholar]
- 11.Chevalier X. Giraudeau B. Conrozier T. Marliere J. Kiefer P. Goupille P. Safety study of intraarticular injection of interleukin 1 receptor antagonist in patients with painful knee osteoarthritis: a multicenter study. J Rheumatol. 2005;32:1317. [PubMed] [Google Scholar]
- 12.Goupille P. Mulleman D. Chevalier X. Is interleukin-1 a good target for therapeutic intervention in intervertebral disc degeneration: lessons from the osteoarthritic experience. Arthritis Res Ther. 2007;9:110. doi: 10.1186/ar2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wehling P. Moser C. Frisbie D. McIlwraith C.W. Kawcak C.E. Krauspe R. Reinecke J.A. Autologous conditioned serum in the treatment of orthopedic diseases: the orthokine therapy. BioDrugs. 2007;21:323. doi: 10.2165/00063030-200721050-00004. [DOI] [PubMed] [Google Scholar]
- 14.Buckwalter J.A. Lotz M. Stoltz J.F. Amsterdam. Washington, DC: IOS Press; 2007. Osteoarthritis, Inflammation, and Degradation: A Continuum. [Google Scholar]
- 15.Pritzker K.P. Gay S. Jimenez S.A. Ostergaard K. Pelletier J.P. Revell P.A. Salter D. van den Berg W.B. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Amin A.R. Attur M. Patel R.N. Thakker G.D. Marshall P.J. Rediske J. Stuchin S.A. Patel I.R. Abramson S.B. Superinduction of cyclooxygenase-2 activity in human osteoarthritis-affected cartilage. Influence of nitric oxide. J Clin Invest. 1997;99:1231. doi: 10.1172/JCI119280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers S.L. Brandt K.D. Ehlich J.W. Braunstein E.M. Shelbourne K.D. Heck D.A. Kalasinski L.A. Synovial inflammation in patients with early osteoarthritis of the knee. J Rheumatol. 1990;17:1662. [PubMed] [Google Scholar]
- 18.Pelletier J.P. Martel-Pelletier J. The pathophysiology of osteoarthritis and the implication of the use of hyaluronan and hylan as therapeutic agents in viscosupplementation. J Rheumatol Suppl. 1993;39:19. [PubMed] [Google Scholar]
- 19.Smith M.D. Triantafillou S. Parker A. Youssef P.P. Coleman M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol. 1997;24:365. [PubMed] [Google Scholar]
- 20.van de Loo A.A. Arntz O.J. Bakker A.C. van Lent P.L. Jacobs M.J. van den Berg W.B. Role of interleukin 1 in antigen-induced exacerbations of murine arthritis. Am J Pathol. 1995;146:239. [PMC free article] [PubMed] [Google Scholar]
- 21.Pickvance E.A. Oegema T.R., Jr. Thompson R.C., Jr. Immunolocalization of selected cytokines and proteases in canine articular cartilage after transarticular loading. J Orthop Res. 1993;11:313. doi: 10.1002/jor.1100110302. [DOI] [PubMed] [Google Scholar]
- 22.Mehraban F. Kasturi S. Gene transfer of type 1 interleukin-1 receptor extracellular-domain complementary DNA into rabbit synovial cell line HIG-82 results in cellular blockade of interleukin-1 signal transduction. Arthritis Rheum. 1998;41:515. doi: 10.1002/1529-0131(199803)41:3<515::AID-ART18>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 23.Pelletier J.P. Mineau F. Fernandes J.C. Duval N. Martel-Pelletier J. Diacerhein and rhein reduce the interleukin 1beta stimulated inducible nitric oxide synthesis level and activity while stimulating cyclooxygenase-2 synthesis in human osteoarthritic chondrocytes. J Rheumatol. 1998;25:2417. [PubMed] [Google Scholar]
- 24.van den Berg W.B. Lessons from animal models of osteoarthritis. Curr Rheumatol Rep. 2008;10:26. doi: 10.1007/s11926-008-0005-x. [DOI] [PubMed] [Google Scholar]
- 25.Fernandes J.C. Martel-Pelletier J. Pelletier J.P. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39:237. [PubMed] [Google Scholar]
- 26.Martel-Pelletier J. Pelletier J.P. Inflammatory factors involved in osteoarthritis. In: Buckwalter J.A., editor; Lotz M., editor; Stoltz J-F., editor. Osteoarthritis, Inflammation and Degradation: A Continuum. Amsterdam: IOS Press; 2007. pp. 3–13. [Google Scholar]
- 27.Levick J.R. Contributions of the lymphatic and microvascular systems to fluid absorption from the synovial cavity of the rabbit knee. J Physiol. 1980;306:445. doi: 10.1113/jphysiol.1980.sp013406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Derendorf H. Mollmann H. Gruner A. Haack D. Gyselby G. Pharmacokinetics and pharmacodynamics of glucocorticoid suspensions after intra-articular administration. Clin Pharmacol Ther. 1986;39:313. doi: 10.1038/clpt.1986.45. [DOI] [PubMed] [Google Scholar]
- 29.Roberts M.J. Bentley M.D. Harris J.M. Chemistry for peptide and protein PEGylation. Adv Drug Deliv Rev. 2002;54:459. doi: 10.1016/s0169-409x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- 30.Abuchowski A. McCoy J.R. Palczuk N.C. van Es T. Davis F.F. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem. 1977;252:3582. [PubMed] [Google Scholar]
- 31.Adams M.E. Atkinson M.H. Lussier A.J. Schulz J.I. Siminovitch K.A. Wade J.P. Zummer M. The role of viscosupplementation with hylan G-F 20 (Synvisc) in the treatment of osteoarthritis of the knee: a Canadian multicenter trial comparing hylan G-F 20 alone, hylan G-F 20 with non-steroidal anti-inflammatory drugs (NSAIDs) and NSAIDs alone. Osteoarthritis Cartilage. 1995;3:213. doi: 10.1016/s1063-4584(05)80013-5. [DOI] [PubMed] [Google Scholar]
- 32.Langer R. New methods of drug delivery. Science. 1990;249:1527. doi: 10.1126/science.2218494. [DOI] [PubMed] [Google Scholar]
- 33.Ceponis A. Waris E. Mönkkönen J. Laasonen L. Hyttinen M. Solovieva S.A. Hanemaaijer R. Bitsch A. Konttinen Y.T. Effects of low-dose, noncytotoxic, intraarticular liposomal clodronate on development of erosions and proteoglycan loss in established antigen-induced arthritis in rabbits. Arthritis Rheum. 2001;44:1908. doi: 10.1002/1529-0131(200108)44:8<1908::AID-ART329>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 34.Trif M. Guillen C. Vaughan D.M. Telfer J.M. Brewer J.M. Roseanu A. Brock J.H. Liposomes as possible carriers for lactoferrin in the local treatment of inflammatory diseases. Exp Biol Med. 2001;226:559. doi: 10.1177/153537020122600608. [DOI] [PubMed] [Google Scholar]
- 35.Hunziker E.B. Growth-factor induced healing of partial-thickness defects in adult articular cartilage. Osteoarthritis Cartilage. 2001;9:22. doi: 10.1053/joca.2000.0346. [DOI] [PubMed] [Google Scholar]
- 36.Horisawa E. Kubota K. Tuboi I. Sato K. Yamamoto H. Takeuchi H. Kawashima Y. Size-dependency of DL-lactide/glycolide copolymer particulates for intra-articular delivery system on phagocytosis in rat synovium. Pharm Res. 2002;19:132. doi: 10.1023/a:1014260513728. [DOI] [PubMed] [Google Scholar]
- 37.Lu L. Stamatas G.N. Mikos A.G. Controlled release of transforming growth factor. J Biomed Mater Res. 2000;50:440. doi: 10.1002/(sici)1097-4636(20000605)50:3<440::aid-jbm19>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 38.Hubbell J.A. Biomaterials science and high-throughput screening. Nat Biotechnol. 2004;22:828. doi: 10.1038/nbt0704-828. [DOI] [PubMed] [Google Scholar]
- 39.Ratcliffe J.H. Hunneyball I.M. Wilson C.G. Smith A. Davis S.S. Albumin microspheres for intraarticular drug delivery—investigation of their retention in normal and arthritic knee joints of rabbits. J Pharm Pharmacol. 1987;39:290. doi: 10.1111/j.2042-7158.1987.tb06268.x. [DOI] [PubMed] [Google Scholar]
- 40.Mattioli-Belmonte M. Gigante A. Muzzarelli R.A.A. Politano R. De Benedittis A. Specchia N. Buffa A. Biagini G. Greco F.N. N-dicarboxymethyl chitosan as delivery agent for bone morphogenetic protein in the repair of articular cartilage. Med Biol Eng Comput. 1999;37:130. doi: 10.1007/BF02513279. [DOI] [PubMed] [Google Scholar]
- 41.Wang X. Wenk E. Matsumoto A. Meinel L. Li C. Kaplan D.L. Silk microspheres for encapsulation and controlled release. J Control Release. 2007;117:360. doi: 10.1016/j.jconrel.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 42.Chilkoti A. Dreher M.R. Meyer D.E. Raucher D. Targeted drug delivery by thermally responsive polymers. Adv Drug Deliv Rev. 2002;54:613. doi: 10.1016/s0169-409x(02)00041-8. [DOI] [PubMed] [Google Scholar]
- 43.Shamji M.F. Betre H. Kraus V.B. Chen J. Chilkoti A. Pichika R. Masuda K. Setton L.A. Development and characterization of a fusion protein between thermally responsive elastin-like polypeptide and interleukin-1 receptor antagonist: sustained release of a local antiinflammatory therapeutic. Arthritis Rheum. 2007;56:3650. doi: 10.1002/art.22952. [DOI] [PubMed] [Google Scholar]
- 44.Shamji M.F. Whitlatch L. Friedman A.H. Richardson W.J. Chilkoti A. Setton L.A. An injectable and in situ-gelling biopolymer for sustained drug release following perineural administration. Spine. 2008;33:748. doi: 10.1097/BRS.0b013e3181695773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butoescu N. Jordan O. Doelker E. Intra-articular drug delivery systems for the treatment of rheumatic diseases: a review of the factors influencing their performance. Eur J Pharm Biopharm. 2009;73:205. doi: 10.1016/j.ejpb.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 46.Evans C.H. Gouze J.N. Gouze E. Robbins P.D. Ghivizzani S.C. Osteoarthritis gene therapy. Gene Ther. 2004;11:379. doi: 10.1038/sj.gt.3302196. [DOI] [PubMed] [Google Scholar]
- 47.Adriaansen J. Khoury M. de Cortie C.J. Fallaux F.J. Bigey P. Scherman D. Gould D.J. Chernajovsky Y. Apparailly F. Jorgensen C. Vervouordeldonk M.J. Tak P.P. Reduction of arthritis following intra-articular administration of an adeno-associated virus serotype 5 expressing a disease-inducible TNF-blocking agent. Ann Rheum Dis. 2007;66:1143. doi: 10.1136/ard.2006.064519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bloquel C. Denys A. Boissier M.C. Apparailly F. Bigey P. Scherman D. Bessis N. Intra-articular electrotransfer of plasmid encoding soluble TNF receptor variants in normal and arthritic mice. J Gene Med. 2007;9:986. doi: 10.1002/jgm.1088. [DOI] [PubMed] [Google Scholar]
- 49.Betre H. Liu W. Zalutsky M.R. Chilkoti A. Kraus V.B. Setton L.A. A thermally responsive biopolymer for intra-articular drug delivery. J Control Release. 2006;115:175. doi: 10.1016/j.jconrel.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 50.Gouze E. Pawliuk R. Gouze J.N. Pilapil C. Fleet C. Palmer G.D. Evans C.H. Leboulch P. Ghivizzani S.C. Lentiviral-mediated gene delivery to synovium: potent intra-articular expression with amplification by inflammation. Mol Ther. 2003;7:460. doi: 10.1016/s1525-0016(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 51.Blom A.B. van der Kraan P.M. van den Berg W.B. Cytokine targeting in osteoarthritis. Curr Drug Targets. 2007;8:283. doi: 10.2174/138945007779940179. [DOI] [PubMed] [Google Scholar]
- 52.Goldring M.B. Anticytokine therapy for osteoarthritis. Expert Opin Biol Ther. 2001;1:817. doi: 10.1517/14712598.1.5.817. [DOI] [PubMed] [Google Scholar]
- 53.Joosten L.A. Helsen M.M. van den Berg W.B. Protective effect of rimexolone on cartilage damage in arthritic mice: a comparative study with triamcinolone hexacetonide. Agents Actions. 1990;31:135. doi: 10.1007/BF02003233. [DOI] [PubMed] [Google Scholar]
- 54.Robbins P.D. Evans C.H. Chernajovsky Y. Gene therapy for arthritis. Gene Ther. 2003;10:902. doi: 10.1038/sj.gt.3302040. [DOI] [PubMed] [Google Scholar]
- 55.van de Loo F.A. Joosten L.A. van Lent P.L. Arntz O.J. van den Berg W.B. Role of interleukin-1, tumor necrosis factor alpha, and interleukin-6 in cartilage proteoglycan metabolism and destruction. Effect of in situ blocking in murine antigen- and zymosan-induced arthritis. Arthritis Rheum. 1995;38:164. doi: 10.1002/art.1780380204. [DOI] [PubMed] [Google Scholar]
- 56.Evans C.H. Robbins P.D. Ghivizzani S.C. Wasko M.C. Tomaino M.M. Kang R. Muzzonigro T.A. Vogt M. Elder E.M. Whiteside T.L. Watkins S.C. Herndon J.H. Gene transfer to human joints: progress toward a gene therapy of arthritis. Proc Natl Acad Sci USA. 2005;102:8698. doi: 10.1073/pnas.0502854102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gouze J.N. Ghivizzani S.C. Gouze E. Palmer G.D. Betz O.B. Robbins P.D. Evans C.H. Herndon J.H. Gene therapy for rheumatoid arthritis. Hand Surg. 2001;6:211. doi: 10.1142/s0218810401000709. [DOI] [PubMed] [Google Scholar]
- 58.Adriaansen J. Fallaux F.J. de Cortie C.J. Vervoordeldonk M.J. Tak P.P. Local delivery of beta interferon using an adeno-associated virus type 5 effectively inhibits adjuvant arthritis in rats. J Gen Virol. 2007;88(Pt 6):1717. doi: 10.1099/vir.0.82603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shamji M.F. Chen J. Friedman A.H. Richardson W.J. Chilkoti A. Setton L.A. Synthesis and characterization of a thermally-responsive tumor necrosis factor antagonist. J Control Release. 2008;129:179. doi: 10.1016/j.jconrel.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ameye L.G. Young M.F. Animal models of osteoarthritis: lessons learned while seeking the “Holy Grail.”. Curr Opin Rheumatol. 2006;18:537. doi: 10.1097/01.bor.0000240369.39713.af. [DOI] [PubMed] [Google Scholar]
- 61.Bendele A.M. Animal models of osteoarthritis. J Musculoskelet Neuronal Interact. 2001;1:363. [PubMed] [Google Scholar]
- 62.Bendele A.M. Animal models of osteoarthritis in an era of molecular biology. J Musculoskelet Neuronal Interact. 2002;2:501. [PubMed] [Google Scholar]
- 63.Bellamy N. Methods of clinical assessment of anti-rheumatic drugs. Baillieres Clin Rheumatol. 1988;2:339. doi: 10.1016/s0950-3579(88)80018-9. [DOI] [PubMed] [Google Scholar]
- 64.Daltroy L.H. Cats-Baril W.L. Katz J.N. Fossel A.H. Liang M.H. The North American spine society lumbar spine outcome assessment instrument: reliability and validity tests. Spine. 1996;21:741. doi: 10.1097/00007632-199603150-00017. [DOI] [PubMed] [Google Scholar]
- 65.Ware J.E., Jr. Sherbourne C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473. [PubMed] [Google Scholar]
- 66.Crawley J.N. New York: Wiley-Liss; 2000. What's Wrong with My Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice. [Google Scholar]
- 67.Konig M. Zimmer A.M. Steiner H. Holmes P.V. Crawley J.N. Brownstein M.J. Zimmer A. Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature. 1996;383:535. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- 68.Zimmer A. Zimmer A.M. Baffi J. Usdin T. Reynolds K. Konig M. Palkovitz M. Mezey E. Hypoalgesia in mice with a targeted deletion of the tachykinin 1 gene. Proc Natl Acad Sci USA. 1998;95:2630. doi: 10.1073/pnas.95.5.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zimmer A. Zimmer A.M. Hohmann A.G. Herkenham M. Bonner T.I. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Allen K.D. Griffin T.M. Rodriguiz R.M. Wetsel W.C. Kraus V.B. Huebner J.L. Boyd L.M. Setton L.A. Decreased physical function and increased pain sensitivity in mice deficient for type IX collagen. Arthritis Rheum. 2009;60:2684. doi: 10.1002/art.24783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.D'Amour F. Smith D. A method for determining loss of pain sensitization. J Pharmacol Exp Ther. 1941;41:419. [Google Scholar]
- 72.Eddy N.B. Leimbach D. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther. 1953;107:385. [PubMed] [Google Scholar]
- 73.Chaplan S.R. Bach F.W. Pogrel J.W. Chung J.M. Yaksh T.L. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 74.Allen K.D. Adams S.B., Jr. Shamji M.F. Gouze E. Setton L.A. Interleukin-1beta induced knee joint destruction causes gait abnormalities and mechanical allodynia in rats. Presented at the annual meeting of the Biomedical Engineering Society, St. Louis, MO. 2008.
- 75.Hamers F.P. Koopmans G.C. Joosten E.A. CatWalk-assisted gait analysis in the assessment of spinal cord injury. J Neurotrauma. 2006;23:537. doi: 10.1089/neu.2006.23.537. [DOI] [PubMed] [Google Scholar]
- 76.Vrinten D.H. Hamers F.F. “CatWalk” automated quantitative gait analysis as a novel method to assess mechanical allodynia in the rat; a comparison with von Frey testing. Pain. 2003;102:203. doi: 10.1016/s0304-3959(02)00382-2. [DOI] [PubMed] [Google Scholar]
- 77.Hamers F.P. Lankhorst A.J. van Laar T.J. Veldhuis W.B. Gispen W.H. Automated quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries. J Neurotrauma. 2001;18:187. doi: 10.1089/08977150150502613. [DOI] [PubMed] [Google Scholar]
- 78.Allen K.D. Chen K. Setton L.A. Gait deficiencies in young adult mice homozygous for inactivated Col9a1 gene. Presented at the annual meeting of the Biomedical Engineering Society, Pittsburg, PA. 2009.
- 79.O'Connor B.L. Visco D.M. Rogers P.I. Mamlin L.A. Brandt K.D. Serial force plate analyses of dogs with unilateral knee instability, with or without interruption of the sensory input from the ipsilateral limb. Osteoarthritis Cartilage. 1999;7:567. doi: 10.1053/joca.1999.0261. [DOI] [PubMed] [Google Scholar]
- 80.van Valburg A.A. van Roermund P.M. Marijnissen A.C. Wenting M.J. Verbout A.J. Lafeber F.P. Bijlsma J.W. Joint distraction in treatment of osteoarthritis (II): effects on cartilage in a canine model. Osteoarthritis Cartilage. 2000;8:1. doi: 10.1053/joca.1999.0263. [DOI] [PubMed] [Google Scholar]
- 81.Clarke K.A. Heitmeyer S.A. Smith A.G. Taiwo Y.O. Gait analysis in a rat model of osteoarthrosis. Physiol Behav. 1997;62:951. doi: 10.1016/s0031-9384(97)00022-x. [DOI] [PubMed] [Google Scholar]
- 82.Schmitt D. Zumwalt A.C. Hamrick M.W. Bone mechanical properties and ground reaction forces in normal and hypermuscular mice. J Morphol. (in press). [DOI] [PMC free article] [PubMed]
- 83.Adams S.B., Jr. Shamji M.F. Nettles D.L. Paranjape S.M. Evans C.H. Gouze E. Setton L.A. A thermally responsive fusion protein of interleukin-1 receptor antagonist attenuates the pathologic response of knee joint cartilage to interleukin-1 in an in vivo model. Trans Ortho Res Soc. 2008;33:0109. [Google Scholar]