Abstract
There has long been a described relationship between mesenchymal stem cells (MSCs) and blood vessels in aspects of bone and other skeletal tissues with regard to their embryonic formation and their adult repair and regeneration dynamics. The use of exogenously added MSCs to supplement the naturally available progenitor cell stock has been a standard practice in several orthopedic surgeries by adding bone marrow to the repair constructs. This, coupled with the well-established need for vasculature to orient and drive bone formation, firmly established the functional relationship between MSCs, osteoprogenitors, and blood vessels. It is now apparent that MSCs are pericytes (cells that surround blood vessels) throughout the body. In addition, MSCs can function to secrete bioactive factors that are immunomodulatory, thus allowing allogeneic MSCs to be infused into patients requiring clinically relevant treatments. Such infused MSCs trophically establish microenvironments that support the regeneration of the injured tissue. These new functions usher in a new era of cell-based therapies.
Introduction
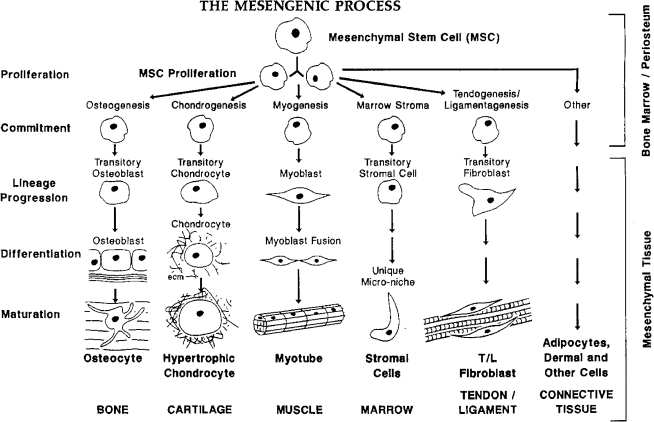
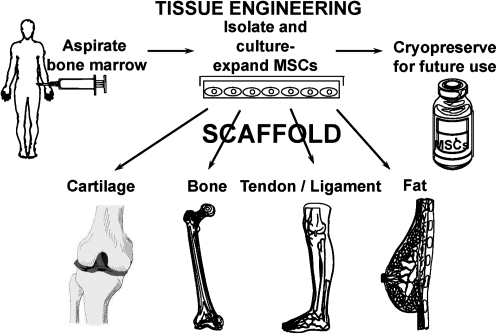
There are several truisms regarding the management of reparative therapies in orthopedic medicine. First, the presence of vasculature, bone marrow, or periosteum is a key feature in the reformation of structural bone at break sites. Indeed, marrow-rich autograft is often taken from the iliac crest to give a boost to fracture repair or spinal fusion surgeries. Second, we long ago identified blood vessels and their positional context as the orientors and drivers of bone formation in the long bones of chick, mouse, and human embryos.1–3 Age-related decrease of vascular density or localized ischemia has long been identified as a cause for poor bone repair and maintenance as well as for nonunions. Third, we identified unique cells in adult bone marrow, referred to as mesenchymal stem cells (MSCs), that readily form bone at both ectopic and orthotopic sties.4–9 As seen in Figure 1, these cells are capable of differentiating down a number of mesenchymal tissue lineage pathways, including the formation of bone, cartilage, muscle, fat, tendon/ligament, and other connective tissues. Together with site-specific scaffolds, MSCs have been used by our lab and others in distinctive tissue engineering repair schemes for skeletal tissues (Fig. 2). Further, the use of microfracture of the subchondral bone plate to cause a bleed has short-term positive healing benefit to cartilage defects.10,11
FIG. 1.
The mesengenic process. Adult mesenchymal stem cells (MSCs) are able to differentiate into bone, cartilage, muscle, marrow stroma, tendon/ligament, fat, and other connective tissues in a sequence of lineage transitions. The figure was first drawn to mirror the sequence of events observed in hematopoietic differentiation. The state of knowledge in the late 1980s and early 1990s provided the most information for the lineages on the left and the least information for the pathways on the right. This information is reviewed in Refs.4–9
FIG. 2.
Tissue engineering using MSCs. A bone marrow aspirate provides the source for MSCs that can be expanded in culture. These MSCs can be stored frozen and thawed without loss of potency. Using tissue-specific scaffolds, cartilage, bone, tendon, and fat can be tissue engineered to fill defects and contribute to the functional regeneration of these tissues.
Because differentiated cells are the biosynthetic units that fabricate specific tissues, the management of reparative progenitor cells is the key to repair/regeneration strategies in orthopedics in adults.8 This fact brings us into the new era of cell-based therapies.
Autologous Chondrocyte Implantation
As a current clinical example of this new era, surgeons repairing massive articular cartilage defects have used autologous chondrocyte implantation (ACI) for more than 20 years. First introduced by Dr. Lars Pedersen and his student/colleague Dr. Mats Brittberg, this technology involves obtaining a small biopsy of non-weight-bearing cartilage from which autologous chondrocytes are obtained, culture-expanded, and subsequently introduced into a chondral lesion and covered with a periosteal flap.12,13 This procedure has been made broadly available by Genzyme Corporation with over 20,000 patients receiving the ACI procedure with generally good outcomes, mainly seen as years of pain-free activity. Several variations on this cell-based approach have been developed and used clinically. For example, Fidia Advanced Biopolymers of Italy12 and TiGenix of Belgium have conducted clinical trials using autologous,15,16 culture-expanded chondrocytes with Fidia Advanced Biopolymers recently reporting a 5-year follow-up. Key aspects of these cell-based therapies are the quality of the autologous chondrocytes and the properties of the engineered delivery vehicle.
MSCs
We have published studies using autologous MSCs in bone,17 cartilage,18 muscle,19 and tendon20 tissue engineering strategies for repairing (regenerating) skeletal tissues. The logics for these studies focused on driving cells down specific lineage pathways by using unique inductive agents or scaffolds. In addition, one must manage the vascular availability that must be plentiful for bone and absent for cartilage. Thus, there is an intimate relationship between success and failure of tissue-engineered skeletal tissues and the availability of both vasculature and progenitor cells. As hypothesized below, this natural relationship of MSCs to blood vessels is functionally quite specific.
It is important to stress that the definition of an MSC is a culture-adherent, multipotent progenitor capable of differentiating into, at the least, bone, cartilage, and fat. Additionally, these mesenchymal progenitors have a distinctive set of cell surface markers as recently reviewed.21 We long ago identified SH2 and SH3,422 (endoglin [CD105] and ecto-5′-nucleotidase [CD73]) as markers for MSCs.23,24 These progenitor populations exist in every tissue in the body and have been isolated from bone marrow, fat, muscle, synovium, dermis, brain, liver, tendon, and so on. Although these progenitors have been isolated from all of these tissues and they can develop into bone, cartilage, or fat, these progenitors are distinctive and reflect aspects of their tissue of origin. For example, if pellets of adult human marrow MSCs in chemically defined medium are exposed to TGF-β, they become chondrocytes and make cartilage extracellular matrix.25,26 The mesenchymal progenitors from adult fat require both TGF-β and BMP-6 to make cartilage.27
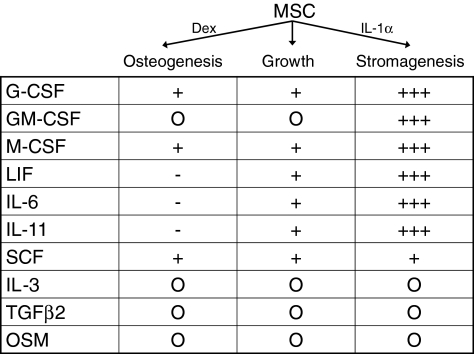
Lastly and now extremely important, we reported that human MSCs secrete substantial quantities of bioactive factors whether continuing in their mitotic expansion, in the osteogenic pathway or in the marrow stromal (hematopoietic support) pathway.28 Indeed, the quantity and identity of these secreted bioactive factors is a signature of the MSCs in mitosis or their presence in a lineage pathway (Fig. 3) as we pointed out previously.28 This last observation can now be understood in the context of the new era of MSC therapies.
FIG. 3.
Bioactive factor secretion by MSCs. Culture-expanded MSCs were put into osteogenic, growth, or stromagenic (i.e., hematopoietic support conditions comparable to Dexter) conditions, and the 24 h media were assayed by ELISA for specific bioactive molecules as listed. MSCs from six donors were separately analyzed. The relative (to growth conditions) quantities are represented by “+” or “−” signs (+, increases; −, decreases); the “O” means none detected. Although the absolute quantities varied greatly from donor to donor, the percent change in osteogenic or stromagenic conditions relatively tightly clustered for all donors.28
New MSC Treatments
Recent clinical trials have been conducted by independent investigators29 and by Osiris Therapeutics (see website www.osiris.com) of Maryland (see Disclosure). These clinical trials and the reports of preclinical models using allogeneic or xenogenic MSCs28–32 usher us into a new, exciting era of cell-based therapies. Space does not permit a full exposition of all the properties and capacities of MSCs in this context. The two key functions of culture-expanded human MSCs are that they are strongly immunomodulatory and immunosuppressive especially toward T-cells (recognition, antigen presentation, and expansion), and, second, the MSCs are trophic33 (Fig. 3). This trophic activity involves (1) anti-apoptosis, especially in ischemic zones, that limits the field of cell death and injury, (2) anti-scarring/anti-fibrosis, (3) angiogenesis, and (4) mitotic stimulation of tissue-intrinsic progenitors.
The immunomodulatory capacity of MSCs33–37 is quite potent as documented by the results from completed phase I and II clinical trials (as well as pediatric compassionate use) for Graft versus Host Disease and Crohn's disease, which are quite striking. The results of these clinical trials involve completely inhibiting the graft versus host disease such that most of the treated patients are alive at 1 year posttreatment or for Crohn's disease most have vastly improved clinical scores. These immunomodulatory effects allow the use of allogeneic MSCs with intravenous delivery, the preferred route of administration for clinical uses. Some evidence exists to indicate that MSCs function at the tissue target sites, but these observations also indicate that very few of the MSCs are in the target tissues; nonetheless, substantial clinical efficacy is observed in the limited number of patients thus far infused. The oversimplification that MSCs home to sites of tissue injury may be correct, but relatively few cells are observed to dock in these afflicted tissues.
It is of interest to review the historic basis for using MSCs as immunomodulatory agents because this pathway does not appear in Figure 1 nor is it intuitively obvious why marrow MSCs might so function. Originally, we reasoned that marrow MSCs must be capable of differentiating into bone marrow stroma that is able to support in vitro and in vivo hematopoiesis. Indeed, there are clear experimental data to support this assertion: simply, MSCs maintained in culture under Dexter conditions with hydrocortisone and horse serum form a fibroblastic connective tissue layer that supports hematopoietic expansion as if it were bone marrow stroma.38 Likewise, the first clinical use of culture-expanded MSCs was as an additive to bone marrow transplantation35; this assumed that MSCs homed to bone marrow, engrafted, and re-fabricated the bone marrow stroma. Because allogeneic bone marrow transplantation has proven useful to cure certain genetic diseases involving mesenchymal tissues or their functions, it was a natural extension of such procedures to consider the delivery of allogeneic MSCs alone.39–42 To ensure that such delivery of allo-MSCs would not cause an immunologic event, MSCs were tested in the standard mixed lymphocyte reactions and found to be immunosuppressive. A number of studies have been conducted to delineate the mechanism of this immunomodulation, and this is far from being completed and is quite multi-factorial and complex.43,44 The bioactive molecules secreted by MSCs clearly act to decrease TNF-α and IFN-γ levels with a prostaglandin-mediated increase in IL-10. In addition to suppressing T-cell replication, there is also an inhibition of antigen presentation ability and possibly a B-cell component that is affected. The cumulative result is that any T-cell or immunosurveillance cell that comes into the range of MSCs will be suppressed. As discussed below, this has a profound effect in vivo for intrinsic MSCs.
The trophic effects produced by the secretion of bioactive factors by MSCs have been seen in preclinical models of myocardial infarcts,45 stroke,30,31 spinal cord (cuts and contusions46), tendons,20 acute renal failure,47 and meniscal regeneration.48 A phase I study for acute myocardial infarct (AMI) has been completed by Osiris with rather substantial clinical success. The allogeneic MSCs were introduced intravenously within 48 h after the AMI with a fourfold improvement in arrhythmia and premature ventricular contractions and a prompt return of heart rate after exercise at all time points (Osiris website www.osiris.com). Importantly, a threefold improvement of lung function also suggested that the infused allogeneic MSCs positively affected damaged tissue in the lungs of some AMI patients.
Without reviewing all of the details of the clinical trials or preclinical models, I would propose that the MSCs protected cells that would have expired (apoptosis) as a result of ischemia or other tissue injuries and that this serves to limit the scope and extent of the field of injury.49 The MSCs also reduce or eliminate scarring either by inhibiting the entry of circulating or tissue-intrinsic cells capable of forming scar or inhibiting their scar-forming biosynthetic capacity. In all cases, there is an acceleration of angiogenesis and also a mitosis of tissue-intrinsic progenitors that results in the regeneration of cells and tissues that are damaged. I summarily refer to these multiple effects as “trophic” in keeping with the use of this term to describe the role of nerves on the formation of the regeneration blastema in amphibian limbs and the maintenance of innervated tissues and organs.49,50
The MSC Niche
Given this new information regarding the immunosuppressive and trophic activities of MSCs, the question arises how these capabilities reflect the in vivo functioning of MSCs and the unanswered question where MSCs reside in various tissues (i.e., the MSC niche). In addition, MSC-like cells have been isolated from almost every tissue of the body, including umbilical cord and placenta. Last, several investigators have suggested that isolated pericytes49,51–54 are capable of exhibiting some of the cell types pictured in Figure 1. Pericytes are mesenchymal cells that reside on blood vessels (large and small) on the tissue side of the vessel.51–53 Although studied by several groups, a definitive study has recently been completed that shows that human adult MSC markers are found on pericytes and vice versa; these observations were made for both in vitro and in situ situations.55 Given all of the above, I would propose that all MSCs are pericytes. (The reverse is not correct that all pericytes are MSCs.) When a focal injury occurs, blood vessels are disrupted and the pericytes are dislodged from their attachment domains on endothelial tubes. These dislodged pericytes divide and, in this mode, secrete massive amounts of bioactive factors and are, thus, identical to the MSCs that we culture as adherent cells on Petri dishes. The dividing and secretory MSC pericytes inhibit immunosurveillance of the damaged tissue and, thereby, prevent autoimmunity from developing.49 These MSCs also prevent scar-forming cells from entering the field of injury while secreting VEGF to bring in endothelial cells to reform vascular tubes. The MSCs reform pericytes as they physically and chemically stabilize the reforming vasculature.56 Some MSCs or their progeny may serve as a progenitor cell for the regeneration of the damaged tissue or might stimulate the mitosis of tissue-intrinsic progenitors that replace the damaged tissue.
In the special case of bone marrow, these MSCs must be particularly vigilant in preventing T-cell-mediated immune events57,58 as a safeguard to the hematopoietic stem cell that could be damaged should such T-cell events take place in the marrow. Thus, my view is that the MSC niche is to sit as a functioning pericyte on vasculature in every tissue of the body (except avascular cartilage) to interact with vascular tissue to ensure homeostatic nutrient and cellular transport. In situations of tissue stress or damage, such as ischemia, the pericytes are detached, and they migrate to focused sites of tissue injury where they divide and serve to trophically31 establish a regenerative microenvironment while standing guard against self immunosurveillance. In cases of severe or extensive tissue damage in adults, the local MSCs are insufficient to handle these demands. In some cases, the MSCs call up sentinels from other deposits55 or we systemically supply additional contributors. Allogeneic MSCs can serve this purpose because they are immunomodulatory. Indeed, in some cases, it may be that allo-MSCs are more effective than autologous MSCs. In this regard, it maybe that deficiencies in autologous MSCs may account for some autoimmune diseases or immunodestructive conditions. Several assays can now be envisioned to determine if certain clinical conditions arise59 or are maintained because of defects in specific tissue MSCs/pericytes.
The Future
Increased use of both autologous and allogeneic MSCs for tissue engineering or their trophic capacities can be safely contemplated. To date, no adverse events have been reported in the over 1000 patients treated by physicians at our institution, independent investigators elsewhere, or by Osiris Therapeutics in their clinical trials. To date, defects in MSCs or MSC-mediated functions have not been reported. All of this will change as the use of MSCs increases for cell-based therapies; indeed, one of the biggest hurdles will be to establish potency assays that correlate MSC functions with clinical outcomes. Although we started working on MSCs in the late 1980s and started Osiris Therapeutics on December 23, 1992, as a bio-orthopedic company, the future is already here and we still have a long way to go to know all there is to know about this interesting family of perivascular stem cells and their use in cell-based therapies and regenerative medicine.
Acknowledgments
Supported in part by grants from NIH and funds from the David and Virginia Baldwin Fund. My thanks to my colleagues, who continue to inspire me by their clever and insightful experimentation. In particular, I dedicate this manuscript to Carl Brighten, who long ago saw the pericyte as a mesenchymal progenitor cell, and to Marshall Urist, for bringing orthopedics into the molecular arena; both individuals were talented orthopedic healthcare practitioners and inspirational scientists.
Disclosure Statement
I am a founder of Osiris, and I had stock in the company and, thus, could be considered to be conflicted although I have had no impact on or involvement with their current clinical trials.
References
- 1.Pechak D.G. Kujawa M.J. Caplan A.I. Morphological and histological events during first bone formation in embryonic chick limbs. Bone. 1986;7:441. doi: 10.1016/8756-3282(86)90004-9. [DOI] [PubMed] [Google Scholar]
- 2.Pechak D.G. Kujawa M.J. Caplan A.I. Morphology of bone development and bone remodeling in embryonic chick limbs. Bone. 1986;7:459. doi: 10.1016/8756-3282(86)90005-0. [DOI] [PubMed] [Google Scholar]
- 3.Caplan A.I. Pechak D.G. The cellular and molecular embryology of bone formation. In: Peck W.A., editor. Bone and Mineral Research. Vol. 5. New York: Elsevier; 1987. pp. 117–184. [Google Scholar]
- 4.Caplan A.I. Stem cell delivery vehicle. Biomaterials. 1990;11:44. [PubMed] [Google Scholar]
- 5.Caplan A.I. Mesenchymal stem cells. J Orthop Res. 1991;9:641. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 6.Ohgushi H. Caplan A.I. Stem cell technology and bioceramics: from cell to gene. Eng J Biomed Mater Res. 1999;48:1. doi: 10.1002/(sici)1097-4636(1999)48:6<913::aid-jbm22>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 7.Dennis J.E. Konstantakos E.K. Arm D. Caplan A.I. In vivo osteogenesis assay: a rapid method for quantitative analysis. Biomaterials. 1998;19:1323. doi: 10.1016/s0142-9612(97)00170-1. [DOI] [PubMed] [Google Scholar]
- 8.Caplan A.I. Mesenchymal stem cell: cell-based reconstructive therapy in orthopaedics. Tissue Eng. 2005;11:1198. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 9.Bruder S.P. Caplan A.I. Bone regeneration through cellular engineering. In: Lanza R., editor; Langer R., editor; Vancanti J., editor. Principles in Tissue Engineering. New York: Springer; 2000. pp. 683–696. [Google Scholar]
- 10.Steadman J. Briggs K. Rodrigo J. Kocher M. Gill T. Rodkey W. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19:477. doi: 10.1053/jars.2003.50112. [DOI] [PubMed] [Google Scholar]
- 11.Mithoefer K. Williams R.J., III Warren R.F. Potter H.G. Spock C.R. Jones E.C. Wickiewicz T.L. Marx R.G. The microfracture technique for the treatment of articular cartilage lesions in the knee: a prospective cohort study. J Bone Joint Surg Am. 2005;87:1911. doi: 10.2106/JBJS.D.02846. [DOI] [PubMed] [Google Scholar]
- 12.Brittberg M. Lindahl A. Nilsson A. Ohlsson C. Isaksson O. Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 13.Peterson L. Minas T. Brittberg M. Nilsson A. Sjogren-Jansson E. Lindahl A. Two-to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop. 2000;374:212. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 14.Pavesio A. Abatangelo G. Borrione A. Brocchetta D. Hollander A.P. Kon E. Torasso F. Zanaasi S. Marcacci M. Hyaluronan-based scaffolds (Hyalograft® C) in the treatment of knee cartilage defects: preliminary clinical findings. In: Wiley, editor; Chichester, editor. Tissue Engineering of Cartilage and Bone. Novartis Foundation Symposium; 2003. pp. 203–217. [PubMed] [Google Scholar]
- 15.Dell'Accio F. De Bari C. Luyten F.P. Molecular markers predictive of the capacity of expanded human articular chondrocytes to form stable cartilage in vivo. Arthritis Rheum. 2001;44:1608. doi: 10.1002/1529-0131(200107)44:7<1608::AID-ART284>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 16.Dell'Accio F. Vanlauwe J. Bellemans J. Neys J. DeBari C. Luyten F.P. Expanded phenotypically stable chondrocytes persist in the repair tissue and contribute to cartilage matrix formation and structural integration in a goat model of autologous chondrocyte implantation. J Orthop Res. 2003;21:123. doi: 10.1016/S0736-0266(02)00090-6. [DOI] [PubMed] [Google Scholar]
- 17.Liebergall M. Young R.G. Ozawa N. Reese J.H. Davy D.T. Goldberg V.M. Caplan A.I. The effects of cellular manipulation and TGF-ß in a composite graft. In: Brighton C.T., editor; Friedlaender G.E., editor; Lane J.M., editor. Bone Formation and Repair. American Academy of Orthopaedic Surgeons Symposium; Tampa, Florida: 1994. pp. 367–378. [Google Scholar]
- 18.Wakitani S. Goto T. Pineda S.J. Young R.G. Mansour J.M. Goldberg V.M. Caplan A.I. Mesenchymal cell-based repair of large full-thickness defects of articular cartilage and underlying bone. J Bone Joint Surg. 1994;76:579. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Saito T. Dennis J.E. Lennon D.P. Young R.G. Caplan A.I. Myogenic expression of mesenchymal stem cells within myotubes of mdx mice in vitro and in vivo. Tissue Eng. 1996;1:327. doi: 10.1089/ten.1995.1.327. [DOI] [PubMed] [Google Scholar]
- 20.Young R.G. Butler D.L. Weber W. Gordon S.L. Fink D.J. Caplan A.I. The use of mesenchymal stem cells in Achilles tendon repair. J Orthop Res. 1998;16:406. doi: 10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
- 21.da Silva Meirelles L. Caplan A.I. Nardi N.B. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 22.Haynesworth S.E. Baber M.A. Caplan A.I. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone. 1992;13:69. doi: 10.1016/8756-3282(92)90363-2. [DOI] [PubMed] [Google Scholar]
- 23.Barry F.P. Boynton R.E. Haynesworth S. Murphy J.M. Zaia J. The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope of endoglin (CD105) Biochem Biophys Res Commun. 1999;265:134. doi: 10.1006/bbrc.1999.1620. [DOI] [PubMed] [Google Scholar]
- 24.Barry F. Boynton R. Murphy M. Zaia J. The SH-3 and SH-4 antibodies recognize distinct epitopes on CD73 from human mesenchymal stem cells. Biochem Biophys Res Commun. 2001;28:519. doi: 10.1006/bbrc.2001.6013. [DOI] [PubMed] [Google Scholar]
- 25.Johnstone B. Hering T.M. Goldberg V.M. Yoo J.U. Caplan A.I. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 26.Yoo J.U. Barthel T.S. Nishimura K. Solchaga L.A. Caplan A.I. Goldberg V.M. Johnstone B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg. 1998;80:1745. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Estes B.T. Wu A.W. Guilak F. Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis Rheum. 2006;54:1222. doi: 10.1002/art.21779. [DOI] [PubMed] [Google Scholar]
- 28.Haynesworth S.E. Baber M.A. Caplan A.I. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1α. J Cell Physiol. 1996;166:585. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Le Blanc K. Frassoni F. Ball L. Locatelli F. Roelofs H. Lewis I. Lanino E. Sundberg G. Bernardo M.E. Remberger M. Dini G. Egeler R.M. Bacigalupo A. Fibbe W. Ringden O. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 30.Li Y. Chen J. Chen X.G. Wang L. Gautam S.C. Xu Y.X. Katakowski M. Zhang L.J. Lu M. Janakirman N. Chop M. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- 31.Mahmood A. Lu D. Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53:697. doi: 10.1227/01.neu.0000079333.61863.aa. [DOI] [PubMed] [Google Scholar]
- 32.Bai L. Lennon D.P. Eaton V. Maier K. Caplan A.I. Miller S.D. Miller R.H. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. GLIA. Epub ahead of print 2009. [DOI] [PMC free article] [PubMed]
- 33.Caplan A.I. Dennis J.E. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 34.Le Blanc K. Tammik C. Rosendahl K. Zetterberg E. Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 35.Bacigalupo A. Valle M. Podesta M. Pitto A. Zocchi E. DeFlora A. Possi S. Vassallo F. Lucchetti S. Figari O. Frassoni F. Piaggio G. The suppressive effect of bone marrow mesenchymal stem cells on T cell activation is deficient in patients with severe aplastic anemia. Blood. 2004;104:147A. [Google Scholar]
- 36.Beyth S. Borovsky Z. Mevorach D. Liebergall M. Gazit Z. Aslan H. Galun E. Rachmielwitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T cell unresponsiveness. Blood. 2005;105:2214. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 37.Le Blanc K. Rasmusson I. Gotherstrom C. Seidel C. Sundberg B. Sundin M. Rosendahl K. Tammik C. Ringden O. Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol. 2004;60:307. doi: 10.1111/j.0300-9475.2004.01483.x. [DOI] [PubMed] [Google Scholar]
- 38.Majumdar M.K. Thiede M.A. Mosca J.D. Moorman M. Gerson S.L. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998;176:186. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 39.Lazarus H.M. Haynesworth S.E. Gerson S.L. Rosenthal N. Caplan A.I. Ex-vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells) [MPCs]: implications for therapeutic use. Bone Marrow Transplant. 1995;16:557. [PubMed] [Google Scholar]
- 40.Koc O.N. Day J. Nieder M. Gerson S.L. Lazarus H.M. Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH) Bone Marrow Transplant. 2002;30:215. doi: 10.1038/sj.bmt.1703650. [DOI] [PubMed] [Google Scholar]
- 41.Koc O.N. Gerson S.L. Cooper B.W. Dyhouse S.M. Haynesworth S.E. Caplan A.I. Lazarus H.M. Rapid hematopoietic recovery after confusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 42.Horwitz E.M. Gordon P.L. Koo W.K.K. Marx J.C. Neel M.D. McNall R.Y. Muul L. Hoffman T. Isolated allogeneic bone marrow derived mesenchymal ells engraft and stimulate growth in children with osteogenesis imperfeta: implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pittenger M. Mackay A. Beck S. Jaiswal R. Douglas R. Mosca J. Moorman M. Simonetti D. Craig S. Marshak D. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 44.Aggarwal S. Pittenger M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 45.Pittenger M.F. Martin B.J. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 46.Hofstetter C.P. Schwarz E.J. Hess D. Widenfalk J. El Manira A. Prockop D.J. Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci USA. 2002;99:2199. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lange C. Togel F. Ittrich H. Clayton F. Nolte-Ernsting C. Zander A.R. Westenfelder C. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005;68:1613. doi: 10.1111/j.1523-1755.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- 48.Murphy J.M. Fink D.J. Hunziker E.B. Barry F.P. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 49.da Silva Meirelles L. Caplan A.I. Nardi N.B. The mesenchymal stem cell niche. Stem Cells. 2008;26:2287. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 50.Singer M. The trophic quality of the neuron: some theoretical considerations. In: Singer M., editor; Schade J.P., editor. Progress in Brain Research. Vol. 13. Amsterdam, The Netherlands: Elsevier; 1964. pp. 228–232. [DOI] [PubMed] [Google Scholar]
- 51.Sacchetti B. Funari A. Michienzi S. Di Cesare S. Piersanti S. Saggio I. Tagliafico E. Ferrari S. Robey P.G. Riminucci M. Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 52.Crisan M. Deasy B. Gavina M. Zheng B. Huard J. Lazzaari L. Peault B. Purification and long-term culture of multipotent progenitor cells affiliated with the walls of human blood vessels: myoendothelial cells and pericytes. Methods Cell Biol. 2008;86:295. doi: 10.1016/S0091-679X(08)00013-7. [DOI] [PubMed] [Google Scholar]
- 53.Hirschi K. D'Amore P.A. Pericytes in the microvasculature. Cardiovasc Res. 1969;32:687. [PubMed] [Google Scholar]
- 54.Brighton C.T. Lorich D.G. Kupcha R. Reilly T.M. Jones A.R. Woodbury R.A. The pericyte as a possible osteoblast progenitor cell. Clin Orthop Relat Res. 1992;275:287. [PubMed] [Google Scholar]
- 55.Crisan M. Yap S. Casteilla L. Chen C. Corselli M. Park T.S. Andriolo G. Sun B. Zheng B. Zhang L. Norotte C. Teng P.N. Traas J. Schugar R. Deasy B.M. Badylak S. Bühring H.J. Giacobino J.P. Lazzari L. Huard J. Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 56.Sorrell J.M. Baber M.A. Caplan A.I. Influence of adult mesenchymal stem cells on in vitro vascular formation. Tissue Eng Part A. 2009;15 doi: 10.1089/ten.tea.2008.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Le Blanc K. Tammit L.L. Sundberg B. Haynesworth S.E. Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 58.Maitra B. Szekely E. Gjini K. Laughlin M.J. Dennis J. Haynesworth S.E. Koc O.N. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33:597. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- 59.Penn. M.S.,Khalil M.K.Exploitation of stem cell homing for gene delivery Expert Opin Biol Ther 817.2008 [DOI] [PubMed] [Google Scholar]