Abstract
Cartilage is the first skeletal tissue to be formed during embryogenesis leading to the creation of all mature cartilages and bones, with the exception of the flat bones in the skull. Therefore, errors occurring during the process of chondrogenesis, the formation of cartilage, often lead to severe skeletal malformations such as dysplasias. There are hundreds of skeletal dysplasias, and the molecular genetic etiology of some remains more elusive than of others. Many efforts have aimed at understanding the morphogenetic event of chondrogenesis in normal individuals, of which the main morphogenetic and regulatory mechanisms will be reviewed here. For instance, many signaling molecules that guide chondrogenesis—for example, transforming growth factor-β, bone morphogenetic proteins, fibroblast growth factors, and Wnts, as well as transcriptional regulators such as the Sox family—have already been identified. Moreover, extracellular matrix components also play an important role in this developmental event, as evidenced by the promotion of the chondrogenic potential of chondroprogenitor cells caused by collagen II and proteoglycans like versican. The growing evidence of the elements that control chondrogenesis and the increasing number of different sources of progenitor cells will, hopefully, help to create tissue engineering platforms that could overcome many developmental or degenerative diseases associated with cartilage defects.
Introduction
Mature cartilage develops from the mesodermal lineage and has multiple functions in adult organisms such as articulation capacity in joints or elastic and loading capacity in intervertebral discs. During embryogenesis, chondrogenic precursors play a key role in the formation of the two mature skeletal tissues in long bones: bone and adult cartilage.1 Long bone formation is achieved through a sequence of events called endochondral ossification initiated from mesenchymal condensations. Briefly, this developmental progression involves the formation of cartilage tissue by mesenchymal cell (MC) differentiation and the subsequent replacement of the cartilage tissue by bone2 and is tightly controlled by regulation of gene expression and cell–cell and cell–extracellular matrix (ECM) interactions.3
These regulatory events conduct a set of morphogenetic and phenotypic changes to the MC within the embryonic mesoderm, giving rise to a preskeletal tissue composed mainly of chondroblasts. Here, chondrogenesis is defined as the process through which the MCs differentiate into chondroblasts that subsequently either develop into adult chondrocytes or undergo hypertrophy and apoptosis.4 In endochondral ossification, a part of this embryonic cartilage subsequently turns into bone.5
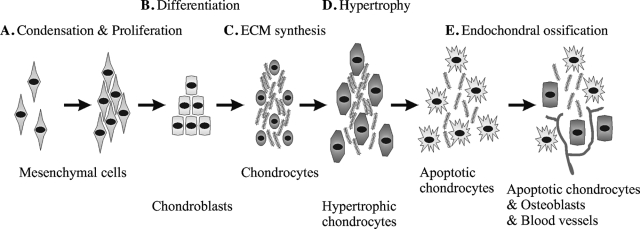
Endochondral ossification can be divided into five main stages (Fig. 1). These stages are clearly defined by specific events:
FIG. 1.
Schematic representation of the chondrogenesis and endochondral ossification. (A) First MCs condense to form a dense cell mass. (B) MCs proliferate and differentiate into chondroblasts. (C) These cells start secreting cartilage ECM and become mature chondrocytes. (D) Eventually, chondrocytes grow to become hypertrophic, and if the tissue undergoes endochondral ossification, (E) cartilage is vascularized, ECM is degraded, hypertrophic chondrocytes become apoptotic, and osteoblasts invade the free space within the tissue.
(A) Commitment of MCs to become cartilage caused by paracrine factors, such as fibroblast growth factor (FGF) and Hedgehog pathways.
(B) Differentiation of the condensed MCs into chondrocytes in the process of which the transcription factor Sox9 plays an essential role in controlling the expression of downstream genes specific for cartilage tissue development.
(C) Rapid division of chondrocytes and production of the cartilage-specific ECM.
(D) Halted proliferation of chondrocytes and a several fold increase in size entering hypertrophy. The composition of the ECM (mainly collagen type X and fibronectin) is also changed, and chondrocytes start to mineralize the environment with calcium salts.
(E) Invasion of blood vessels takes place, and the hypertrophic chondrocytes die by apoptosis. At this stage, osteoblast precursors invade the remodeling tissue and start forming bone using the cartilaginous matrix as template and replacing it by mineralized matrix.
In this review, we will focus on the morphogenetic changes, the regulatory mechanisms, and the interactions that take place during developmental chondrogenesis. In this context, a precise knowledge on the extrinsic factors (environmental) and intrinsic factors (genetic regulation) controlling chondrogenesis can be easily translated into tissue engineering research to obtain more reliable therapeutic products. Therefore, we will place special emphasis on the importance of the ECM during the development of cartilage and its biomechanical function to help recreate similar processes during the development of regenerative platforms. Moreover, we will also summarize recent descriptions of chondroblastic progenitors that are expected to mimic natural chondrogenesis and might play a key role in biomedical research in the near future. Finally, we will discuss some of the novel biomaterials and its applications currently used for cartilage and, eventually, bone repair.
Morphogenetic Changes Associated with Chondrogenesis
Natural chondrogenesis of the different skeletal structures begins at different time points during embryonic development, but all chondrogenic events share the same cellular origin, the MCs. These MCs may arise from three different sources: (1) neural crest cells of the neural ectoderm that eventually form the craniofacial bones; (2) the sclerotome of the paraxial mesoderm, which gives rise to the axial skeleton; and (3) the somatopleure of the lateral plate mesoderm, which yields the skeleton of the long bones.6 Under the proper signaling, these MCs begin a physical compaction called mesenchymal condensation, which begins with the MC increasing their cellular interaction established both with the ECM and with the surrounding cells (Fig. 1A). Specifically, N-cadherin and neural cell adhesion molecule (N-CAM)–mediated cell–cell interactions are of outermost importance during mesenchymal condensation.7
When the cells aggregate, MCs begin to produce collagen I, fibronectin, and proteoglycans.8 The result of the strong interactions that cells establish with their environment is the formation of a dense mass of MCs that immediately begins to differentiate into chondroblasts (Fig. 1B). Condensed MCs start expressing mainly the transcription factor Sox9 that controls downstream genes involved in chondrogenesis, promoting these progenitor cells to secrete cartilage-specific ECM molecules (Fig. 1C).9–12
The tissue then undergoes a series of morphological and physical changes during maturation, including an increase in rigidity to acquire its typical cartilage stiffness due mainly to ECM components. Moreover, if cartilage turns into bone, chondrocytes increase in size turning into hypertrophic chondrocytes and enriching the ECM mainly with collagen X (Fig. 1D).10 Afterward, ECM is progressively degraded, and hypertrophic chondrocytes undergo apoptosis generating spaces used by blood vessels to invade the cartilage (Fig. 1E). Simultaneously, osteoblast progenitors use the blood stream to enter this vascularized tissue. The progressive death of chondrocytes and degradation of cartilage collagens and proteoglycans is synchronized with the synthesis of bone ECM and the proliferation of the osteoblast population within the tissue. In addition to this endochondral ossification that requires a cartilaginous intermediate, there is a second type of bone formation called intramembranous ossification that does not require the transitional step of chondrogenesis. This direct osteogenic process consists of the conversion of MCs into bone tissue (reviewed in Franz-Odendaal et al.13). Intramembranous ossification is the characteristic way in which flat bones of the skull develop. During this process, neural crest–derived MCs (NCMCs) proliferate and condense forming aggregates where some of the cells form capillaries and other differentiate into osteoblasts that secrete a collagen–proteoglycan matrix that is able to promote calcium salt deposition.14
Finally, it has been noted above that expression of proteoglycans is not exclusive of the synthesis of ECM in mature cartilage formation, but is also important during mesenchymal condensation. For instance, versican, a chondroitin sulfate proteoglycan, has recently been shown to be crucial in mesenchymal condensation to the extent that the absence of the active form of this proteoglycan disrupts subsequent chondrogenesis.15
Onset of Chondrogenesis: Transcription Factor Sox9 and Genes Related to Its Regulation
Cartilage formation is the first step in the development of almost all skeletal elements in mammal organisms and presents two independent ontogenetic origins. As stated above, craniofacial cartilage proceeds mainly from NCMCs, while other cartilages come from MCs of mesodermal origin.16,17 Although these origins are significantly different from each other, transition of the MC to mature cartilage or bone is very similar in both cases.
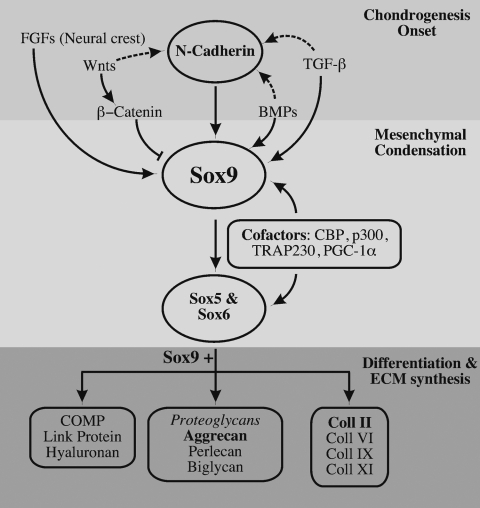
As we mentioned above, one of the first steps in chondrogenesis is the mesenchymal condensation first mediated by paracrine factors and subsequently by Sox9, a transcription factor that belongs to the SRY family and contains the high mobility group box (HMG-box) DNA binding domain.18 The expression of Sox9 is regulated by members of the FGF, transforming growth factor-β (TGF-β), bone morphogenetic protein (BMP), and Wnt families (Fig. 2).8,19 The key role of the transcription factor Sox9 in skeletal development is evidenced by its tight regulation and by the fact that abnormal expression of Sox9 leads to severe skeletal disorders like campomelic dysplasia. Sox9 is responsible for the expression of some of the key genes in chondrogenesis: Sox5, Sox6, and collagen II α1 (Col2a1) (Fig. 2). Moreover, Sox9 regulates expression of collagen XI α2 (Col11a2) and CD-RAP (cartilage-derived retinoic acid-sensitive protein) before matrix deposition in the cartilage anlagen.20–22 L-Sox5 and Sox6 in turn are required for the expression of collagen IX α1 (Col9a1) and aggrecan, which is the most abundant proteoglycan in cartilage (Fig. 2).23 Moreover, Akiyama et al. have proven that all osteo-chondroprogenitors stem from Sox9-expressing cells, confirming the principal role of Sox9 in chondrogenesis.24
FIG. 2.
Upstream and downstream regulation of Sox9. Chondrogenesis begins with the upregulation of N-cadherin and Sox9 by paracrine factors like TGF-β, FGFs, or BMPs, while Wnts downregulate the expression of Sox9. Expression of N-cadherins and Sox9 is accompanied by the condensation and proliferation of MCs, a process called mesenchymal condensation. Sox9 activates the expression of Sox5 and Sox6, and with them and the help of cofactors such as CBP, induces the MC to differentiate into chondroblasts after the condensation. Chondroblasts produce cartilage-specific ECM, including collagen II, IV, IX, and XI; proteoglycans such as aggrecan; and also link proteins and COMP.
In the interdigital region of developing limbs, it is TGF-β that induces the expression of Sox9. This induction is a quick process as the upregulation of Sox9 has been proven to happen only 30 min after exposure of chicken limb bud cells to TGF-β.19 Apart from regulating the expression of Sox9, TGF-β ultimately initiates the expression of cartilage oligomeric matrix protein (COMP), aggrecan, N-cadherin, N-CAM, Coll II, Coll XI, fibronectin, and tenascin.25 The strong chondrogenic effect of TGF-β is commonly used to induce chondrogenic commitment of mesenchymal stem cells (MSCs) in vitro, which will be discussed below.26,27
BMPs are another family of the TGF-β superfamily of growth factors that mediate important events within skeletogenesis and body patterning. BMPs induce and maintain the expression of the Hox genes Msx-1 and Msx-2, which are key regulators in antero-posterior limb signaling.28 Further, BMP-4 and BMP-7 are responsible for specification of neural plate cells to neural crest–derived cells.29,30 In addition, the expression of Sox9 is dependent on BMP signaling via the BMP receptors 1RA and 1RB (Fig. 2).31 Apart from these early patterning functions, BMPs are also related to the proliferation and maturation of chondrocytes.32 Gene knockout experiments with mice have demonstrated that BMP-4 promotes the expression of the cartilage-specific (α2) collagen XI, and that BMP receptors promote the synthesis of collagen I, a type of collagen present in the ECM of MSCs throughout most of the chondrogenesis.33
There is considerable crosstalk between the TGF and BMP signaling pathways, which is evidenced by a synergistic effect of TGF-β and BMP-2 on collagen X α1 (Col10a1) and an additive effect on Col2a1 mRNA expression.34 Activation of both pathways simultaneously seems to promote chondrogenic maturation. In summary, the members of the TGF-β superfamily (TGF-β and BMPs) promote very early events in chondrogenesis and, directly or indirectly, maintain their regulation during the differentiation and maturation of chondrocytes.
During the development of the craniofacial skeletal structure, maturation of the neural crest cells is also initiated with the expression of Sox9, as happens in all other skeletal tissues laid down through endochondral ossification. However, in craniofacial bone development, expression of Sox9 can be activated by TGF-β–related growth factors and FGFs. The TGF-β superfamily is more commonly responsible for activation of Sox9, but in certain areas like the posterior facial suture, Sox9 is under the control of FGF-2, FGF-3, FGF-8, and its receptors.35 Further, the FGF family of growth factors is important for the proliferation and survival of cranial neural crest–derived cells.35 Apart from being involved in the craniofacial skeletogenesis, FGFs are very important patterning growth factors in limb development and regeneration. FGF-8, FGF-4, and FGF-10 have been identified as important factors for the formation and maintenance of the apical epidermal ridge, a structure that directs proximo-distal development of limbs.36,37
Finally, the Wnt family of signaling molecules is also involved in chondrogenesis (Fig. 2). Wnt proteins are distinguished by their capability to activate β-catenin into canonical Wnts (i.e., Wnt3a) and noncanonical Wnts (i.e., Wnt5a). The influence of Wnts on chondrogenesis is twofold and time dependent: at low levels Wnts promote chondroprogenitor differentiation, specifically through modulating Sox9 expression, and later at high levels they promote chondrocyte hypertrophy and subsequent endochondral ossification.38,39 CatnB controls the chondrogenic commitment of chondroprogenitors by negatively regulating Sox9 expression in particular through competing with Sox9 for the CatnB cofactor TCF, (T-cell factor) and upregulation of Wnt4.40,41 Because CatnB is a mutual antagonist of Sox9,19 noncanonical Wnts, such as Wnt5a, promote early chondrogenesis in vitro while inhibiting terminal differentiation in vivo.42 Specifically, Wnt5a misexpression delays the maturation of chondrocytes, while Wnt4 misexpression accelerates this process.41 Further, Wnts also regulate the expression of N-cadherin, an important cell–cell adhesion protein that is directly involved in mesenchymal condensation.43
Fine regulation of Sox9—more specifically, the balance between Sox9 and the osteogenic transcription factor Runx2/Cbfa1—is what drives osteo-chondroprogenitors to chondroblasts or osteoblasts. For instance, BMP-induced Smad1 and interactions between Smad1 and Runx2 regulate the transition of chondrocytes into hypertrophy44–46 as evidenced in Runx2-deficient mice, in which the late stages of chondrocyte hypertrophy are blocked.47,48 The expression patterns of TGF-β–related proteins, FGFs, and Wnts are responsible for regulating the balance between Sox9 and Runx2 and, therefore, the patterning of the skeletal tissue.
Events Happening After the Expression of Sox9
The expression of Sox9 is the first step toward chondrogenesis, for it induces MCs to become osteo-chondroprogenitors during mesenchymal condensation. After these events, two other Sox transcription factors, Sox5 and Sox6, are upregulated. Sox9 is required for the expression of Sox5 and Sox6, and these three Sox transcription factors (the Sox trio) are then expressed until chondrocytes enter hypertrophy.18 The Sox trio regulates many important events throughout chondrogenesis, thanks to the different spatiotemporal expression of the transcriptional cofactors (CBP [CREB binding protein], p300, TRAP230, PGC-1α, and TCF) that bind to the Sox proteins (Fig. 2). Other members of the Sox genes, like Sox10 in skull development, can also be involved in chondrogenesis, but they are less frequent.29
Members of the Sox family of transcription factors directly or indirectly rule the complexity and richness of the cartilaginous ECM. ECM molecules like collagens, COMP, or proteoglycans are only synthesized after the expression of Sox transcription factors, but few of the regulatory mechanisms are understood to date. One of them is the control of the expression of Col2a1, the gene encoding collagen II, which is regulated by Sox9, Sox5, and Sox10 in avian neural crest cells.49 Other studies also show that Sox9 stimulates the synthesis of collagen II,19,50,51 collagen IX,52 and collagen XI proteins.53 Finally, the synthesis of COMP is also regulated by the Sox trio.54
After the ECM of cartilage is synthesized, eventually chondrocytes enter hypertrophy to render either into a calcified cartilage tissue or into bone, via endochondral ossification as described above. Hypertrophy is enhanced by the thyroid hormone triiodothyronine,55 but its regulation relies on Indian Hedgehog/parathyroid hormone–related protein (Ihh/PTHrP) signaling. These two proteins create negative feedback gradients that control the fate and proliferation of prehypertrophic and hypertrophic chondrocytes within the cartilage.56 On the other hand, Sox9 inhibits chondrocytic hypertrophy, explaining why Sox9 and PTHrP regulate each other.57,58
ECM Influence on Mesenchymal Condensation
The tightly regulated transition of MCs to chondrocytes involves not only the time and space–dependent expression of transcription factors, but also cell–cell and cell–matrix interactions. Cells interact with the ECM or with other cells using membrane receptors or different types of adhesion molecules, and these recognitions translate into intracellular signals affecting gene expression, cell proliferation, and migration.
During the mesenchymal condensation and differentiation, cell–matrix and cell–cell contacts have special significance. For instance, many approaches have shown that N-cadherin–mediated cell–cell interactions play a very important role in mesenchymal condensation.7 Activation of N-cadherin is important for the expression of many chondrogenic markers like Sox9, Sox6, Sox5, aggrecan, and collagen II.59 Nevertheless, chondrogenesis is also possible in N-cadherin–deficient limbs, thanks to other cadherins, for example, cadherin-11.60 In any case, cadherins and cell–cell contact are necessary for the condensation of MCs. Therefore, the expression of N-cadherin is regulated by similar mechanisms to those controlling Sox9: TGF-β, BMP-2, and different Wnts influence in the overall N-cadherin expression.43,61
Cell–matrix interactions play an important role in establishing the mechanical strength necessary to pull the MC together in a condensed cell mass. Cells condense and subsequently migrate out from the condensation areas, which requires substantial ECM remodeling. Thus, it is not surprising that the ECM is tightly regulated throughout chondrogenesis to meet the specific mechanical needs in each step of this morphogenetic process. Before the onset of chondrogenesis, MCs are surrounded by an ECM rich in hyaluronan and collagen I.8,62 First of all, during mesenchymal condensation, the ECM is partially degraded to enhance cell mobility and cell–cell interactions. The concentration of hyaluronan in the ECM drops at this initial step.62 Moreover, matrix metalloproteinases (MMPs) are upregulated to reduce the protein content of the ECM.63 Although collagen I is detected along all the mesenchymal condensation,11 MMPs reduce the presence of structural proteins like collagen I. These changes in the protein and polysaccharide content soften the ECM and facilitate a more intimate cell–cell contact that is critical for the mesenchymal condensation to occur. The different MMPs seem to have different roles in chondrogenic development that are dependent on the developmental stage of the chondrocyte. In particular, MMP1 and MMP2 have the capacity to degrade cartilage matrix, and they are characterized as the MMPs that are involved in earlier chondrogenesis. Specifically, blockage of MMP2 function supports precartilage condensation and chondrogenesis,64 and MMP1 knockout mice show decreased chondrocyte proliferation in the proliferative zone of the growth plates of long bones.65 In contrast, MMP13 appears to be one of the most important MMPs in late-stage chondrogenesis in terms of cartilage remodeling and mineralization because it is characterized by a substrate specificity for collagen II, the cartilage-specific collagen.66 Specifically, MMP13 seems to make the space for cell enlargement that goes hand in hand with hypertrophy by degradation of the cartilage matrix.
Finally, mesenchymal condensation is also affected by small proteoglycans such as versican, perlecan, or syndecan. Versican is a chondroitin sulfate proteoglycan that enhances mesenchymal condensation,15 but the mechanism by which versican is able to trigger cellular aggregation is not fully understood. Versican can bind many structures that are present in the ECM of precartilage MCs such as fibronectin, collagen I, hyaluronan, or tenascin, and it has been shown that versican is necessary for chondrogenic gene expression.15 Similarly, perlecan is present in very early stages of chondrogenesis (day 12.5 of gestation) during mouse embryo development, and is also capable of inducing cell aggregation, condensation, and chondrogenic differentiation of C3H10T1/2 fibroblasts in vitro.67 Perlecan is a heparan sulfate proteoglycan that is known to bind other ECM molecules and basement membrane proteins, such as laminin and collagen IV, as well as growth factors, such as FGFs, especially FGF-2 or FGF-9,68 and BMPs like BMP-2, -4, and -6.69–71 All the interactions established by perlecan explain the importance of this proteoglycan within chondrogenesis, as shown in perlecan-deficient mouse experiments.72 Especially strong expression of perlecan is observed in the prehypertrophic and hypertrophic zones,72 but the exact mechanism by which perlecan regulates mesenchymal condensation and chondrogenesis remains elusive.
Finally, syndecans are transmembrane heparan sulfate proteoglycans that have different isoforms and are reported to be involved in cancers, wound healing, angiogenesis, and chondrogenesis.73 Syndecan-3 is present in tissues undergoing chondrogenesis and shares with perlecan many binding affinities, such as BMP-2.74
In summary, ECM greatly affects mesenchymal condensation in two different ways: it establishes the adequate mechanical environment and moreover modulates the concentration or activity of important growth factors like BMPs or FGFs. This complex and important role of the ECM during mesenchymal condensation still remains unclear and needs further investigation.
Evolution of the ECM During Cartilage Maturation
Mature cartilage is a tissue with an ECM composed of collagenous and noncollagenous elements. The exact composition of the matrix depends on the type of cartilage, but they all share that collagen II is the main collagenous element (about 90% of the collagenous fraction) within the cartilage.75 Collagens I, VI, IX, X, and XI are also present in the collagen fibrils with different percentages depending on the particular cartilage.8 The noncollagenous fraction of the cartilage is basically composed of proteoglycans, hyaluronan, link protein, and interfibrillar proteins like COMP or decorin. Proteoglycans are important components of the noncollagenous cartilage matrix because they are responsible for the mechanical properties of cartilage. Aggrecan accounts for about 90% of the proteoglycan content, and the rest contains decorin, fibromodulin, lumican, biglycan, and perlecan. All the collagenous and noncollagenous components together form an interconnected elastic network surrounding chondrocytes in mature cartilaginous tissues76 accounting for its unique rigidity and flexibility.
The complexity of the ECM of cartilage arises after the mesenchymal condensation, when cells activate all the chondrogenic machinery and gradually transform the ECM into that of a mature cartilage. Collagen I gradually disappears and is substituted by collagen II, VI, IX, and XI. Collagen II is, by far, the most abundant, and its synthesis is regulated by three members of the Sox family of transcription factors, Sox9, Sox6, and Sox5.77 More interesting is that collagens I and II can induce chondrogenesis in bone marrow–derived MSCs, which suggests that ECM molecules also regulate gene expression.78 Collagen X is expressed later on, when chondrocytes acquire the hypertrophic phenotype, and either form calcified cartilage or enter endochondral ossification to become bone tissue.
The short proteoglycans that are present in the mesenchymal condensation (versican, perlecan, or syndecan-3) are gradually substituted by larger and more complex proteoglycans, mainly aggrecan.15 Link protein, COMP, and all other components of the cartilage ECM are also synthesized to obtain mature cartilage. The regulatory mechanisms that control the synthesis of the noncollagenous elements of the cartilage remain unknown, but are probably dependent on the expression of Sox9.
Finally, if mature cartilage is to become bone or calcified cartilage, chondrocytes acquire the hypertrophic phenotype and express vascular endothelium growth factor.79 When ossification occurs, the cartilaginous ECM is gradually degraded and invaded by blood vessels, while chondrocytes become apoptotic. Osteoblasts coming from the blood stream invade the tissue, and cartilage gradually transforms into bone. Then, collagen II is replaced by collagen I, the predominant collagen in bone.80
Sources of Chondroprogenitor Cells
Cartilage has two main ontogenetic origins: the mesoderm and the neural crest. In both cases, MCs are generated from less differentiated Sox9-expressing cells that start skeletogenesis with the mesenchymal condensation.24 The NCMCs that give rise to the craniofacial skeletal structure are formed from ectodermal cells, which suggests that ectodermal and mesodermal cells are embryonic origins for chondrocytes. Because two out of the three germ layers produce chondroprogenitor cells, it is quite probable that many cells of mesodermal or ectodermal origin could turn into chondrocytes with the proper signaling mechanisms.
In vitro chondrogenesis has been described with adult multipotent cells from bone marrow,26,81,82 muscle,83,84 synovial or periosteal cartilage,84 and adipose tissue.26,82,84 To date, the results show that the best chondroprogenitors of adult MSC origin are those of the synovial or periosteal cartilage, followed by muscular MSCs and bone marrow stromal cells, which have better chondrogenic commitment than progenitor cells from adipose tissue.84 MSCs possess a multipotent capacity that is characterized by their ability to differentiate into chondrocytes, osteoblasts, and adipocytes. Further, in their function as stem cells they are able to self-renew, however, only for a limited number of passages in vitro.85 The best way to isolate MSCs remains elusive. Originally, isolation protocols have been described that rely on the fact that MSCs readily adhere to plastic.86 Intermittently, the multilineage potential of MSCs has been characterized after isolation by FACS (Fluorescence-activated cell sorting) using a monoclonal antibody against STRO-187,88 and the low-affinity nerve growth factor receptor, also named CD271.89 Only recently, Pittenger et al.90 published a landmark paper describing surface marker expression characteristic of MSCs as being CD29+, CD44+, CD71+, CD90+, CD106+, CD120+, and CD124+.
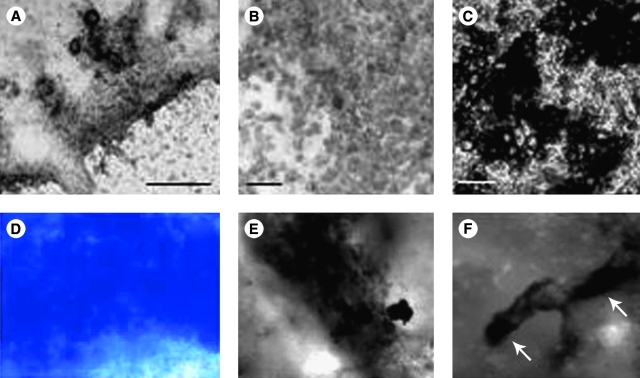
Chondrogenesis from embryonic stem cells (ESCs) has also been reported.91,92 In contrast to MSCs, ESCs have to go through additional developmental stages to reach the fully differentiated chondrocyte state because they represent a pluripotent population. This is achieved through induction with BMP-2 or BMP-4 in murine and human ESCs.91,93 Similar to the in vivo embryonic chondrogenic events, additional induction with TGFs can augment chondrogenesis,92 underlining the importance of resembling stem cell differentiation systems according to in vivo embryonic development (Fig. 3A, B). Interestingly, these chondrocytic cultures can undergo hypertrophy, mineralize, and express bone-specific markers such as collagen I when challenged with osteogenic inducers like vitamin D3.92 It could therefore be hypothesized that this particular culture model represents endochondral bone formation (Fig. 3C, F, and Fig. 4). Further, overexpression of human Sox9 in murine ESCs (mESCs) leads to upregulated expression of the cartilage markers collagen IIA, aggrecan, and pax1 even in undifferentiated ESCs.94 Upon induced differentiation, the adult form of collagen II (the isoform collagen IIB) was expressed by the Sox9 overexpressing ESCs, again emphasizing the importance of this transcriptional regulator.
FIG. 3.
In vitro models for chondrogenesis and osteogenesis with embryonic primitive cells (mESCs and mouse embryonic fibroblasts [MEFs]). (A, B) Chondrocytes were derived from mESCs with BMP-2 and TGF-β1 as described.92 After 4 weeks of in vitro culture, cartilage nodules appear that contain small round cells (B) that secrete a grayish matrix that differs from mature mineralized osteogenic cultures shown in (C). (D) Toluidine blue staining of MEFs cultured in RAD16-I with Dulbecco's modified Eagle's medium (DMEM) supplemented with fetal bovine serum (FBS), L-Glu, and antibiotics reveals that these cells spontaneously undergo chondrogenesis and produce proteoglycans.102 (E) Von Kossa staining of MEFs after 21 days cultured in RAD16-I with DMEM supplemented with FBS, L-Glu, β-glycerophosphate, dexamethasone, sodium ascorbate, and antibiotics. MEFs can also promote calcium deposition and become osteoblast-like cells.99 (F) mESCs after osteogenic induction in RAD16-I hydrogels produce osteoblasts-like cells that induce calcium salts deposition, indicated by white arrows.99 Scale bars: 100 μm. Color images available online at www.liebertonline.com/ten.
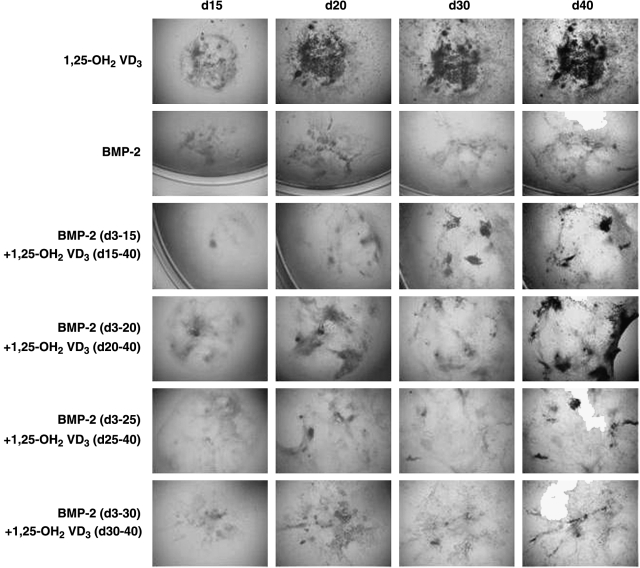
FIG. 4.
Endochondral bone formation from mESCs in vitro. mESCs were differentiated with 1,25-OH2 vitamin D3 toward osteoblasts, which appear black in phase contrast microscopy. Chondrogenic cultures were treated with BMP-2 from day 3 of differentiation onward. Interestingly, chondrogenic cultures can undergo mineralization when additionally triggered with the osteogenic inducer 1,25-OH2 vitamin D3. However, a window of opportunity seems to exist that falls between day 15 and day 20 of differentiation.92
Further evidence for how critical it is to modulate stem cell differentiation according to developmental events found in vivo comes from two papers by Hwang et al., who have utilized the supportive effects of ECM molecules to enhance chondrogenic differentiation from ESCs in vitro. In the presence of glucosamine, a component of glycosaminoglycans such as heparin sulfate and hyaluronan, or encapsulated in RGD-modified (arginine-glycine-aspartic acid integrim binding motif ) hydrogels, neocartilage formation from ESCs could be detected accompanied by an increase in cartilage-specific gene expression.95,96
Usage of both ESCs and MSCs in tissue engineering and clinical application is characterized by certain caveats. Although being the stem cells with the highest differentiation potential, ESCs represent a population of cells encircled by moral and ethical dilemma. MSCs, in turn, can be harvested from the patient, however, not without invasive surgery. The search for alternative cell sources is therefore still ongoing. Other possible sources of chondroprogenitor cells appear from the natural process of the limb regeneration in amphibians. There, the first step in limb regeneration is the formation of a cell mass called blastema containing dedifferentiated chondroblasts, myoblasts, and fibroblasts.97 Although there is evidence that myotubes can enter cellularization in mammals,98 no chondrogenesis has been achieved with adult myotubes. On the other hand, fibroblasts have extensively shown multipotential capacity99–101 and specifically chondrogenic capacity.67,69 Moreover, fibroblasts can undergo spontaneous chondrogenesis in simple three-dimensional culture conditions (Fig. 3D) showing a high synthesis of proteoglycans.102 The same chondrogenic cultures can be induced to differentiate into bone-like tissue (Fig. 3E) by inducing the system with osteogenic media.99 This makes fibroblasts (where embryonic or adult) good and reliable candidates in future therapeutic approaches for cartilage and bone repair.
Mimicking Cell–ECM Interactions with Biomaterials
The broad incidence of the diseases related to cartilage and bone degeneration explains the emergence of a great amount of engineered tissues for cartilage repair. All of them are formed with biomaterials, cells, or a combination of both. The cells to be used vary from mature chondrocytes to any type of the previously described chondroprogenitor cells.
Polylactic acid (PLA), polyglycolic acid (PGA), and polylactic-coglycolic acid (PLGA) are U.S. Food and Drug Administration–approved materials for medical use that are extensively studied for cartilage repair applications.103,104 PLA and PLGA have also been tested in vivo in combination with progenitor cells from the bone marrow105 and also mixed with articular chondrocytes.106 The good performance of PLA and PLGA in vivo makes of them one of the reference tools for the medical reconstruction of cartilage and bone defects such as Bankart's reconstruction, which is the reattachment of the capsule and glenoid labrum to the glenoid lip.107,108
Apart from PLA and PGA, other polymers to regenerate cartilage or bone are currently being studied, including polycaprolactones,109,110 polyfumarates,111 polyethyleneglycol,112 polyvinyl alcohol,113,114 and polyethylene oxide.115 All of them are nontoxic, nonimmunogenic, and bioresorbable and have proven to be effective in allowing chondrocyte growth.
Apart from polymers, peptide-based matrices are the other important family of scaffolds used in regenerative medicine. Many natural ECM, such as collagen ECM or the basement membrane, have a high-protein content.116,117 Consequently, many bioinspired engineered tissues have peptides or large proteins within their structure. A whole family of self-assembling peptides has been isolated or engineered over the last 15 years, and many of them have been tested promising for tissue engineering applications.26,118–120 The self-assembly of peptides is driven by noncovalent interactions, such as hydrogen bonds, and polar and hydrophobic interactions giving rise to interweaving nanofibers.121
An example of a peptide matrix is RAD16-I, a 16–amino acid peptide that self-assembles to form a scaffold with a distribution of pore sizes between 50 and 100 nm.122 This soft scaffold has proved to be permissive for the growth of different cell types and nontoxic to rats.119,123,124 Recently, RAD16-I was used to recreate mesenchymal condensation in vitro. We have developed an assay with which embryonic fibroblasts, encapsulated in RAD16-I, proliferate and condense to form a dense cell mass with bilateral symmetry.102 The volume reduction can be as high as 50%, but, more interestingly, bilaterality is accompanied by spontaneous chondrogenesis of the fibroblasts. This phenomenon has many similarities compared to mesenchymal condensation and limb bud regeneration. It is interesting that the condensation of fibroblasts only happens when the peptide that forms the nanofiber matrix is diluted enough to render a soft three-dimensional environment to the cells. When fibroblasts are cultured in slightly higher concentrations of the peptide, which give rise to stiffer hydrogels, they fail to condense although they form cellular networks. This work supports the previously discussed fact that cell–cell contact and a soft ECM are necessary in mesenchymal condensation.
We consider that the use of new paradigms in tissue engineering and regenerative medicine is crucial to obtain in vitro constructs and in vivo therapies with the capacity of truly regenerate damage tissues and organs. Hence, future medical standards for tissue-engineered–derived products will require not only to achieve functionality but also to be safe (nongenotoxic and nontumorigenic), to present high degree of integration with the local tissue (nonrejection and noninflammatory), and to recover natural shape and volume (esthetics). This is way the material reviewed here aims to integrate new concepts and ideas learned by looking at the natural developmental process of chondrogenesis with the hope to create more efficient therapeutic modalities.
Conclusions and Future Directions
Cartilage development is a tightly regulated morphogenetic event where many balances define the fate of chondroprogenitor cells. Much has been studied on gene regulation by different types of signaling molecules (TGF-β, BMPs, FGFs, Wnt, Ihh, and others). Moreover, the onset of the key chondrogenesis transcription factors Sox5, Sox6, and specially Sox9 is well characterized, but less is known of their downstream regulations.
The structure and changes of the ECM during cartilage formation are very well described in the literature. It is also clear that chondrogenesis is affected by the ECM surrounding the MCs. But very little is known of this matter, and major efforts should be undertaken to achieve this goal. The control over cell fate by the ECM could be a very powerful tool for regenerative medicine. We can speculate that for a future therapeutic approach, freshly isolated progenitor cells could be implanted with an ECM analog capable of assisting differentiation and integration of these cells into the host tissue.
Finally, the last 20 years have been very prolific in obtaining mature chondrocytes out of other differentiated or multipotent cells and new biomaterials suitable for tissue regeneration. Briefly, cartilage-like tissues have been obtained from many cell types using different scaffolds. From all chondroprogenitors, fibroblasts have shown spontaneous and high chondrogenic commitment. The fact that fibroblasts can be easily extracted from the skin combined with their chondrogenic potential makes of them good candidates for cartilage repair, and eventually bone.
Acknowledgments
We gratefully acknowledge Alan Grodzinsky for his outstanding help and discussion during the writing of this manuscript. We thank the Catalan Government for the predoctoral fellowship 2005 FI 00864 to L.Q. and to Grant NIH 1-ROI-EB003805-01A1 and Translational Centre for Regenerative Medicine (Leipzig University) award TEMAT 1098SF to C.E.S. that supported this work.
Disclosure Statement
No competing financial interests exist.
References
- 1.Newman S.A. Frisch H.L. Dynamics of skeletal pattern formation in developing chick limb. Science. 1979;205:662. doi: 10.1126/science.462174. [DOI] [PubMed] [Google Scholar]
- 2.Horton W.A. Hood O.J. Machado M.A. Ahmed S. Griffey E.S. Abnormal ossification in thanatophoric dysplasia. Bone. 1988;9:53. doi: 10.1016/8756-3282(88)90027-0. [DOI] [PubMed] [Google Scholar]
- 3.Hall B.K. Miyake T. Divide, accumulate, differentiate: cell condensation in skeletal development revisited. Int J Dev Biol. 1995;39:881. [PubMed] [Google Scholar]
- 4.Hoffman L.M. Weston A.D. Underhill T.M. Molecular mechanisms regulating chondroblast differentiation. J Bone Joint Surg Am. 2003;85-A Suppl 2:124. doi: 10.2106/00004623-200300002-00017. [DOI] [PubMed] [Google Scholar]
- 5.Mackie E.J. Ahmed Y.A. Tatarczuch L. Chen K.S. Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol. 2008;40:46. doi: 10.1016/j.biocel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Olsen B.R. Reginato A.M. Wang W. Bone development. Annu Rev Cell Dev Biol. 2000;16:191. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- 7.DeLise A.M. Tuan R.S. Alterations in the spatiotemporal expression pattern and function of N-cadherin inhibit cellular condensation and chondrogenesis of limb mesenchymal cells in vitro. J Cell Biochem. 2002;87:342. doi: 10.1002/jcb.10308. [DOI] [PubMed] [Google Scholar]
- 8.DeLise A.M. Fischer L. Tuan R.S. Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage. 2000;8:309. doi: 10.1053/joca.1999.0306. [DOI] [PubMed] [Google Scholar]
- 9.Goldring M.B. Tsuchimochi K. Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97:33. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 10.Ichinose S. Tagami M. Muneta T. Sekiya I. Morphological examination during in vitro cartilage formation by human mesenchymal stem cells. Cell Tissue Res. 2005;322:217. doi: 10.1007/s00441-005-1140-6. [DOI] [PubMed] [Google Scholar]
- 11.Sahar D.E. Longaker M.T. Quarto N. Sox9 neural crest determinant gene controls patterning and closure of the posterior frontal cranial suture. Dev Biol. 2005;280:344. doi: 10.1016/j.ydbio.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Shibata S. Fukada K. Imai H. Abe T. Yamashita Y. In situ hybridization and immunohistochemistry of versican, aggrecan and link protein, and histochemistry of hyaluronan in the developing mouse limb bud cartilage. J Anat. 2003;203:425. doi: 10.1046/j.1469-7580.2003.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franz-Odendaal T.A. Hall B.K. Witten P.E. Buried alive: how osteoblasts become osteocytes. Dev Dyn. 2006;235:176. doi: 10.1002/dvdy.20603. [DOI] [PubMed] [Google Scholar]
- 14.Opperman L.A. Cranial sutures as intramembranous bone growth sites. Dev Dyn. 2000;219:472. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1073>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 15.Kamiya N. Watanabe H. Habuchi H. Takagi H. Shinomura T. Shimizu K. Kimata K. Versican/PG-M regulates chondrogenesis as an extracellular matrix molecule crucial for mesenchymal condensation. J Biol Chem. 2006;281:2390. doi: 10.1074/jbc.M509341200. [DOI] [PubMed] [Google Scholar]
- 16.Chai Y. Jiang X. Ito Y. Bringas P., Jr. Han J. Rowitch D.H. Soriano P. McMahon A.P. Sucov H.M. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- 17.Melnick M. Witcher D. Bringas P., Jr. Carlsson P. Jaskoll T. Meckel's cartilage differentiation is dependent on hedgehog signaling. Cells Tissues Organs. 2005;179:146. doi: 10.1159/000085950. [DOI] [PubMed] [Google Scholar]
- 18.Akiyama H. Chaboissier M.C. Martin J.F. Schedl A. de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawakami Y. Rodriguez-Leon J. Belmonte J.C. The role of TGFbetas and Sox9 during limb chondrogenesis. Curr Opin Cell Biol. 2006;18:723. doi: 10.1016/j.ceb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Eames B.F. de la Fuente L. Helms J.A. Molecular ontogeny of the skeleton. Birth Defects Res C Embryo Today. 2003;69:93. doi: 10.1002/bdrc.10016. [DOI] [PubMed] [Google Scholar]
- 21.Lefebvre V. Behringer R.R. de Crombrugghe B. L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. Osteoarthritis Cartilage. 2001;9 Suppl A:S69. doi: 10.1053/joca.2001.0447. [DOI] [PubMed] [Google Scholar]
- 22.Ng L.J. Wheatley S. Muscat G.E. Conway-Campbell J. Bowles J. Wright E. Bell D.M. Tam P.P. Cheah K.S. Koopman P. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev Biol. 1997;183:108. doi: 10.1006/dbio.1996.8487. [DOI] [PubMed] [Google Scholar]
- 23.Smits P. Li P. Mandel J. Zhang Z. Deng J.M. Behringer R.R. de Crombrugghe B. Lefebvre V. The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Dev Cell. 2001;1:277. doi: 10.1016/s1534-5807(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 24.Akiyama H. Kim J.E. Nakashima K. Balmes G. Iwai N. Deng J.M. Zhang Z. Martin J.F. Behringer R.R. Nakamura T. de Crombrugghe B. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc Natl Acad Sci USA. 2005;102:14665. doi: 10.1073/pnas.0504750102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Derfoul A. Perkins G.L. Hall D.J. Tuan R.S. Glucocorticoids promote chondrogenic differentiation of adult human mesenchymal stem cells by enhancing expression of cartilage extracellular matrix genes. Stem Cells. 2006;24:1487. doi: 10.1634/stemcells.2005-0415. [DOI] [PubMed] [Google Scholar]
- 26.Kisiday J.D. Kopesky P.W. Evans C.H. Grodzinsky A.J. McIlwraith C.W. Frisbie D.D. Evaluation of adult equine bone marrow- and adipose-derived progenitor cell chondrogenesis in hydrogel cultures. J Orthop Res. 2008;26:322. doi: 10.1002/jor.20508. [DOI] [PubMed] [Google Scholar]
- 27.Terraciano V. Hwang N. Moroni L. Park H.B. Zhang Z. Mizrahi J. Seliktar D. Elisseeff J. Differential response of adult and embryonic mesenchymal progenitor cells to mechanical compression in hydrogels. Stem Cells. 2007;25:2730. doi: 10.1634/stemcells.2007-0228. [DOI] [PubMed] [Google Scholar]
- 28.Lallemand Y. Nicola M.A. Ramos C. Bach A. Cloment C.S. Robert B. Analysis of Msx1; Msx2 double mutants reveals multiple roles for Msx genes in limb development. Development. 2005;132:3003. doi: 10.1242/dev.01877. [DOI] [PubMed] [Google Scholar]
- 29.Basch M.L. Bronner-Fraser M. Garcia-Castro M.I. Specification of the neural crest occurs during gastrulation and requires Pax7. Nature. 2006;441:218. doi: 10.1038/nature04684. [DOI] [PubMed] [Google Scholar]
- 30.Liem K.F., Jr. Tremml G. Roelink H. Jessell T.M. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell. 1995;82:969. doi: 10.1016/0092-8674(95)90276-7. [DOI] [PubMed] [Google Scholar]
- 31.Yoon B.S. Ovchinnikov D.A. Yoshii I. Mishina Y. Behringer R.R. Lyons K.M. Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proc Natl Acad Sci USA. 2005;102:5062. doi: 10.1073/pnas.0500031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minina E. Wenzel H.M. Kreschel C. Karp S. Gaffield W. McMahon A.P. Vortkamp A. BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development. 2001;128:4523. doi: 10.1242/dev.128.22.4523. [DOI] [PubMed] [Google Scholar]
- 33.Tsumaki N. Yoshikawa H. The role of bone morphogenetic proteins in endochondral bone formation. Cytokine Growth Factor Rev. 2005;16:279. doi: 10.1016/j.cytogfr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Mehlhorn A.T. Niemeyer P. Kaschte K. Muller L. Finkenzeller G. Hartl D. Sudkamp N.P. Schmal H. Differential effects of BMP-2 and TGF-beta1 on chondrogenic differentiation of adipose derived stem cells. Cell Prolif. 2007;40:809. doi: 10.1111/j.1365-2184.2007.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall C. Flores M.V. Murison G. Crosier K. Crosier P. An essential role for zebrafish Fgfrl1 during gill cartilage development. Mech Dev. 2006;123:925. doi: 10.1016/j.mod.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Kawakami A. Fukazawa T. Takeda H. Early fin primordia of zebrafish larvae regenerate by a similar growth control mechanism with adult regeneration. Dev Dyn. 2004;231:693. doi: 10.1002/dvdy.20181. [DOI] [PubMed] [Google Scholar]
- 37.Nye H.L. Cameron J.A. Chernoff E.A. Stocum D.L. Regeneration of the urodele limb: a review. Dev Dyn. 2003;226:280. doi: 10.1002/dvdy.10236. [DOI] [PubMed] [Google Scholar]
- 38.Day T.F. Guo X. Garrett-Beal L. Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Tamamura Y. Otani T. Kanatani N. Koyama E. Kitagaki J. Komori T. Yamada Y. Costantini F. Wakisaka S. Pacifici M. Iwamoto M. Enomoto-Iwamoto M. Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J Biol Chem. 2005;280:19185. doi: 10.1074/jbc.M414275200. [DOI] [PubMed] [Google Scholar]
- 40.Akiyama H. Lyons J.P. Mori-Akiyama Y. Yang X. Zhang R. Zhang Z. Deng J.M. Taketo M.M. Nakamura T. Behringer R.R. McCrea P.D. de Crombrugghe B. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18:1072. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartmann C. Tabin C.J. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development. 2000;127:3141. doi: 10.1242/dev.127.14.3141. [DOI] [PubMed] [Google Scholar]
- 42.Church V. Nohno T. Linker C. Marcelle C. Francis-West P. Wnt regulation of chondrocyte differentiation. J Cell Sci. 2002;115:4809. doi: 10.1242/jcs.00152. [DOI] [PubMed] [Google Scholar]
- 43.Tuli R. Tuli S. Nandi S. Huang X. Manner P.A. Hozack W.J. Danielson K.G. Hall D.J. Tuan R.S. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem. 2003;278:41227. doi: 10.1074/jbc.M305312200. [DOI] [PubMed] [Google Scholar]
- 44.Enomoto H. Enomoto-Iwamoto M. Iwamoto M. Nomura S. Himeno M. Kitamura Y. Kishimoto T. Komori T. Cbfa1 is a positive regulatory factor in chondrocyte maturation. J Biol Chem. 2000;275:8695. doi: 10.1074/jbc.275.12.8695. [DOI] [PubMed] [Google Scholar]
- 45.Leboy P. Grasso-Knight G. D'Angelo M. Volk S.W. Lian J.V. Drissi H. Stein G.S. Adams S.L. Smad-Runx interactions during chondrocyte maturation. J Bone Joint Surg Am. 2001;83-A Suppl 1:S15. [PubMed] [Google Scholar]
- 46.Zheng Q. Zhou G. Morello R. Chen Y. Garcia-Rojas X. Lee B. Type X collagen gene regulation by Runx2 contributes directly to its hypertrophic chondrocyte-specific expression in vivo. J Cell Biol. 2003;162:833. doi: 10.1083/jcb.200211089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Komori T. Yagi H. Nomura S. Yamaguchi A. Sasaki K. Deguchi K. Shimizu Y. Bronson R.T. Gao Y.H. Inada M. Sato M. Okamoto R. Kitamura Y. Yoshiki S. Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 48.Otto F. Thornell A.P. Crompton T. Denzel A. Gilmour K.C. Rosewell I.R. Stamp G.W. Beddington R.S. Mundlos S. Olsen B.R. Selby P.B. Owen M.J. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki T. Sakai D. Osumi N. Wada H. Wakamatsu Y. Sox genes regulate type 2 collagen expression in avian neural crest cells. Dev Growth Differ. 2006;48:477. doi: 10.1111/j.1440-169X.2006.00886.x. [DOI] [PubMed] [Google Scholar]
- 50.Hattori T. Eberspaecher H. Lu J. Zhang R. Nishida T. Kahyo T. Yasuda H. de Crombrugghe B. Interactions between PIAS proteins and SOX9 result in an increase in the cellular concentrations of SOX9. J Biol Chem. 2006;281:14417. doi: 10.1074/jbc.M511330200. [DOI] [PubMed] [Google Scholar]
- 51.Huang W. Zhou X. Lefebvre V. de Crombrugghe B. Phosphorylation of SOX9 by cyclic AMP-dependent protein kinase A enhances SOX9's ability to transactivate a Col2a1 chondrocyte-specific enhancer. Mol Cell Biol. 2000;20:4149. doi: 10.1128/mcb.20.11.4149-4158.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Genzer M.A. Bridgewater L.C. A Col9a1 enhancer element activated by two interdependent SOX9 dimers. Nucleic Acids Res. 2007;35:1178. doi: 10.1093/nar/gkm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bridgewater L.C. Walker M.D. Miller G.C. Ellison T.A. Holsinger L.D. Potter J.L. Jackson T.L. Chen R.K. Winkel V.L. Zhang Z. McKinney S. de Crombrugghe B. Adjacent DNA sequences modulate Sox9 transcriptional activation at paired Sox sites in three chondrocyte-specific enhancer elements. Nucleic Acids Res. 2003;31:1541. doi: 10.1093/nar/gkg230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu C.J. Zhang Y. Xu K. Parsons D. Alfonso D. Di Cesare P.E. Transcriptional activation of cartilage oligomeric matrix protein by Sox9, Sox5, and Sox6 transcription factors and CBP/p300 coactivators. Front Biosci. 2007;12:3899. doi: 10.2741/2359. [DOI] [PubMed] [Google Scholar]
- 55.Mello M.A. Tuan R.S. Effects of TGF-beta1 and triiodothyronine on cartilage maturation: in vitro analysis using long-term high-density micromass cultures of chick embryonic limb mesenchymal cells. J Orthop Res. 2006;24:2095. doi: 10.1002/jor.20233. [DOI] [PubMed] [Google Scholar]
- 56.Shimizu H. Yokoyama S. Asahara H. Growth and differentiation of the developing limb bud from the perspective of chondrogenesis. Dev Growth Differ. 2007;49:449. doi: 10.1111/j.1440-169X.2007.00945.x. [DOI] [PubMed] [Google Scholar]
- 57.Guimont P. Grondin F. Dubois C.M. Sox9-dependent transcriptional regulation of the proprotein convertase furin. Am J Physiol Cell Physiol. 2007;293:C172. doi: 10.1152/ajpcell.00349.2006. [DOI] [PubMed] [Google Scholar]
- 58.Huang W. Chung U.I. Kronenberg H.M. de Crombrugghe B. The chondrogenic transcription factor Sox9 is a target of signaling by the parathyroid hormone-related peptide in the growth plate of endochondral bones. Proc Natl Acad Sci USA. 2001;98:160. doi: 10.1073/pnas.011393998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woods A. Wang G. Dupuis H. Shao Z. Beier F. Rac1 signaling stimulates N-cadherin expression, mesenchymal condensation, and chondrogenesis. J Biol Chem. 2007;282:23500. doi: 10.1074/jbc.M700680200. [DOI] [PubMed] [Google Scholar]
- 60.Luo Y. Kostetskii I. Radice G.L. N-cadherin is not essential for limb mesenchymal chondrogenesis. Dev Dyn. 2005;232:336. doi: 10.1002/dvdy.20241. [DOI] [PubMed] [Google Scholar]
- 61.Modarresi R. Lafond T. Roman-Blas J.A. Danielson K.G. Tuan R.S. Seghatoleslami M.R. N-cadherin mediated distribution of beta-catenin alters MAP kinase and BMP-2 signaling on chondrogenesis-related gene expression. J Cell Biochem. 2005;95:53. doi: 10.1002/jcb.20396. [DOI] [PubMed] [Google Scholar]
- 62.Woitge H.W. Seibel M.J. Molecular markers of bone and cartilage metabolism. Curr Opin Rheumatol. 1999;11:218. doi: 10.1097/00002281-199905000-00011. [DOI] [PubMed] [Google Scholar]
- 63.Kondo S. Shukunami C. Morioka Y. Matsumoto N. Takahashi R. Oh J. Atsumi T. Umezawa A. Kudo A. Kitayama H. Hiraki Y. Noda M. Dual effects of the membrane-anchored MMP regulator RECK on chondrogenic differentiation of ATDC5 cells. J Cell Sci. 2007;120:849. doi: 10.1242/jcs.03388. [DOI] [PubMed] [Google Scholar]
- 64.Jin E.J. Choi Y.A. Kyun Park E. Bang O.S. Kang S.S. MMP-2 functions as a negative regulator of chondrogenic cell condensation via down-regulation of the FAK-integrin beta1 interaction. Dev Biol. 2007;308:474. doi: 10.1016/j.ydbio.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 65.Zhou Z. Apte S.S. Soininen R. Cao R. Baaklini G.Y. Rauser R.W. Wang J. Cao Y. Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci USA. 2000;97:4052. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ohkubo K. Shimokawa H. Ogawa T. Suzuki S. Fukada K. Ohya K. Ohyama K. Immunohistochemical localization of matrix metalloproteinase 13 (MMP-13) in mouse mandibular condylar cartilage. J Med Dent Sci. 2003;50:203. [PubMed] [Google Scholar]
- 67.French M.M. Smith S.E. Akanbi K. Sanford T. Hecht J. Farach-Carson M.C. Carson D.D. Expression of the heparan sulfate proteoglycan, perlecan, during mouse embryogenesis and perlecan chondrogenic activity in vitro. J Cell Biol. 1999;145:1103. doi: 10.1083/jcb.145.5.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knox S.M. Whitelock J.M. Perlecan: how does one molecule do so many things? Cell Mol Life Sci. 2006;63:2435. doi: 10.1007/s00018-006-6162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gomes R.R., Jr. Joshi S.S. Farach-Carson M.C. Carson D.D. Ribozyme-mediated perlecan knockdown impairs chondrogenic differentiation of C3H10T1/2 fibroblasts. Differentiation. 2006;74:53. doi: 10.1111/j.1432-0436.2005.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hopf M. Gohring W. Kohfeldt E. Yamada Y. Timpl R. Recombinant domain IV of perlecan binds to nidogens, laminin-nidogen complex, fibronectin, fibulin-2 and heparin. Eur J Biochem. 1999;259:917. doi: 10.1046/j.1432-1327.1999.00127.x. [DOI] [PubMed] [Google Scholar]
- 71.Takada T. Katagiri T. Ifuku M. Morimura N. Kobayashi M. Hasegawa K. Ogamo A. Kamijo R. Sulfated polysaccharides enhance the biological activities of bone morphogenetic proteins. J Biol Chem. 2003;278:43229. doi: 10.1074/jbc.M300937200. [DOI] [PubMed] [Google Scholar]
- 72.Arikawa-Hirasawa E. Watanabe H. Takami H. Hassell J.R. Yamada Y. Perlecan is essential for cartilage and cephalic development. Nat Genet. 1999;23:354. doi: 10.1038/15537. [DOI] [PubMed] [Google Scholar]
- 73.Fears C.Y. Woods A. The role of syndecans in disease and wound healing. Matrix Biol. 2006;25:443. doi: 10.1016/j.matbio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 74.Fisher M.C. Li Y. Seghatoleslami M.R. Dealy C.N. Kosher R.A. Heparan sulfate proteoglycans including syndecan-3 modulate BMP activity during limb cartilage differentiation. Matrix Biol. 2006;25:27. doi: 10.1016/j.matbio.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 75.Plainfosse M. Hatton P.V. Crawford A. Jin Z.M. Fisher J. Influence of the extracellular matrix on the frictional properties of tissue-engineered cartilage. Biochem Soc Trans. 2007;35:677. doi: 10.1042/BST0350677. [DOI] [PubMed] [Google Scholar]
- 76.Poole A.R. Kojima T. Yasuda T. Mwale F. Kobayashi M. Laverty S. Composition and structure of articular cartilage: a template for tissue repair. Clin Orthop Relat Res. 2001;39l Suppl:S26. doi: 10.1097/00003086-200110001-00004. [DOI] [PubMed] [Google Scholar]
- 77.Stokes D.G. Liu G. Dharmavaram R. Hawkins D. Piera-Velazquez S. Jimenez S.A. Regulation of type-II collagen gene expression during human chondrocyte de-differentiation and recovery of chondrocyte-specific phenotype in culture involves Sry-type high-mobility-group box (SOX) transcription factors. Biochem J. 2001;360:461. doi: 10.1042/0264-6021:3600461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bosnakovski D. Mizuno M. Kim G. Takagi S. Okumura M. Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng. 2006;93:1152. doi: 10.1002/bit.20828. [DOI] [PubMed] [Google Scholar]
- 79.Bluteau G. Julien M. Magne D. Mallein-Gerin F. Weiss P. Daculsi G. Guicheux J. VEGF and VEGF receptors are differentially expressed in chondrocytes. Bone. 2007;40:568. doi: 10.1016/j.bone.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 80.Termine J.D. Robey P.G. Bone matrix proteins and the mineralization process. In: Favus M.J., editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Philadelphia: Lippincott-Raven; 1996. pp. 24–28. [Google Scholar]
- 81.Evans J.F. Niu Q.T. Canas J.A. Shen C.L. Aloia J.F. Yeh J.K. ACTH enhances chondrogenesis in multipotential progenitor cells and matrix production in chondrocytes. Bone. 2004;35:96. doi: 10.1016/j.bone.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 82.Huang J.I. Kazmi N. Durbhakula M.M. Hering T.M. Yoo J.U. Johnstone B. Chondrogenic potential of progenitor cells derived from human bone marrow and adipose tissue: a patient-matched comparison. J Orthop Res. 2005;23:1383. doi: 10.1016/j.orthres.2005.03.008.1100230621. [DOI] [PubMed] [Google Scholar]
- 83.Stringa E. Love J.M. McBride S.C. Suyama E. Tuan R.S. In vitro characterization of chondrogenic cells isolated from chick embryonic muscle using peanut agglutinin affinity chromatography. Exp Cell Res. 1997;232:287. doi: 10.1006/excr.1997.3532. [DOI] [PubMed] [Google Scholar]
- 84.Yoshimura H. Muneta T. Nimura A. Yokoyama A. Koga H. Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- 85.Stenderup K. Justesen J. Clausen C. Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 86.Friedenstein A.J. Gorskaja J.F. Kulagina N.N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267. [PubMed] [Google Scholar]
- 87.Gronthos S. Graves S.E. Ohta S. Simmons P.J. The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood. 1994;84:4164. [PubMed] [Google Scholar]
- 88.Simmons P.J. Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55. [PubMed] [Google Scholar]
- 89.Quirici N. Soligo D. Bossolasco P. Servida F. Lumini C. Deliliers G.L. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30:783. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- 90.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 91.Kramer J. Hegert C. Guan K. Wobus A.M. Muller P.K. Rohwedel J. Embryonic stem cell-derived chondrogenic differentiation in vitro: activation by BMP-2 and BMP-4. Mech Dev. 2000;92:193. doi: 10.1016/s0925-4773(99)00339-1. [DOI] [PubMed] [Google Scholar]
- 92.zur Nieden N.I. Kempka G. Rancourt D.E. Ahr H.J. Induction of chondro-, osteo- and adipogenesis in embryonic stem cells by bone morphogenetic protein-2: effect of cofactors on differentiating lineages. BMC Dev Biol. 2005;5:1. doi: 10.1186/1471-213X-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Toh W.S. Yang Z. Liu H. Heng B.C. Lee E.H. Cao T. Effects of culture conditions and bone morphogenetic protein 2 on extent of chondrogenesis from human embryonic stem cells. Stem Cells. 2007;25:950. doi: 10.1634/stemcells.2006-0326. [DOI] [PubMed] [Google Scholar]
- 94.Kim J.H. Do H.J. Yang H.M. Oh J.H. Choi S.J. Kim D.K. Cha K.Y. Chung H.M. Overexpression of SOX9 in mouse embryonic stem cells directs the immediate chondrogenic commitment. Exp Mol Med. 2005;37:261. doi: 10.1038/emm.2005.35. [DOI] [PubMed] [Google Scholar]
- 95.Hwang N.S. Varghese S. Theprungsirikul P. Canver A. Elisseeff J. Enhanced chondrogenic differentiation of murine embryonic stem cells in hydrogels with glucosamine. Biomaterials. 2006;27:6015. doi: 10.1016/j.biomaterials.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 96.Hwang N.S. Varghese S. Zhang Z. Elisseeff J. Chondrogenic differentiation of human embryonic stem cell-derived cells in arginine-glycine-aspartate-modified hydrogels. Tissue Eng. 2006;12:2695. doi: 10.1089/ten.2006.12.2695. [DOI] [PubMed] [Google Scholar]
- 97.Brockes J.P. Kumar A. Plasticity and reprogramming of differentiated cells in amphibian regeneration. Nat Rev Mol Cell Biol. 2002;3:566. doi: 10.1038/nrm881. [DOI] [PubMed] [Google Scholar]
- 98.Duckmanton A. Kumar A. Chang Y.T. Brockes J.P. A single-cell analysis of myogenic dedifferentiation induced by small molecules. Chem Biol. 2005;12:1117. doi: 10.1016/j.chembiol.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 99.Garreta E. Genove E. Borros S. Semino C.E. Osteogenic differentiation of mouse embryonic stem cells and mouse embryonic fibroblasts in a three-dimensional self-assembling peptide scaffold. Tissue Eng. 2006;12:2215. doi: 10.1089/ten.2006.12.2215. [DOI] [PubMed] [Google Scholar]
- 100.Wang J. Li A. Wang Z. Feng X. Olson E.N. Schwartz R.J. Myocardin sumoylation transactivates cardiogenic genes in pluripotent 10T1/2 fibroblasts. Mol Cell Biol. 2007;27:622. doi: 10.1128/MCB.01160-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang L.H. Chen T.M. Yu S.T. Chen Y.H. Olanzapine induces SREBP-1-related adipogenesis in 3T3-L1 cells. Pharmacol Res. 2007;56:202. doi: 10.1016/j.phrs.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 102.Quintana L. Fernández T. Genové E. Olmos M.M. Borrós S. Semino C.E. Early tissue patterning is recreated by mouse embryonic fibroblasts in a three-dimensional environment. Tissue Eng. 2009 doi: 10.1089/ten.tea.2007.0296. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sherwood J.K. Riley S.L. Palazzolo R. Brown S.C. Monkhouse D.C. Coates M. Griffith L.G. Landeen L.K. Ratcliffe A. A three-dimensional osteochondral composite scaffold for articular cartilage repair. Biomaterials. 2002;23:4739. doi: 10.1016/s0142-9612(02)00223-5. [DOI] [PubMed] [Google Scholar]
- 104.Zwingmann J. Mehlhorn A.T. Sudkamp N. Stark B. Dauner M. Schmal H. Chondrogenic differentiation of human articular chondrocytes differs in biodegradable PGA/PLA scaffolds. Tissue Eng. 2007;13:2335. doi: 10.1089/ten.2006.0393. [DOI] [PubMed] [Google Scholar]
- 105.Zhou G. Liu W. Cui L. Wang X. Liu T. Cao Y. Repair of porcine articular osteochondral defects in non-weightbearing areas with autologous bone marrow stromal cells. Tissue Eng. 2006;12:3209. doi: 10.1089/ten.2006.12.3209. [DOI] [PubMed] [Google Scholar]
- 106.Sedrakyan S. Zhou Z.Y. Perin L. Leach K. Mooney D. Kim T.H. Tissue engineering of a small hand phalanx with a porously casted polylactic acid-polyglycolic acid copolymer. Tissue Eng. 2006;12:2675. doi: 10.1089/ten.2006.12.2675. [DOI] [PubMed] [Google Scholar]
- 107.Magnusson L. Ejerhed L. Rostgard-Christensen L. Sernert N. Eriksson R. Karlsson J. Kartus J.T. A prospective, randomized, clinical and radiographic study after arthroscopic Bankart reconstruction using 2 different types of absorbable tacks. Arthroscopy. 2006;22:143. doi: 10.1016/j.arthro.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 108.Martinez Martin A.A. Herrera Rodriguez A. Panisello Sebastian J.J. Tabuenca Sanchez A. Domingo Cebollada J. Perez Garcia J.M. Use of the suture anchor in modified open Bankart reconstruction. Int Orthop. 1998;22:312. doi: 10.1007/s002640050267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eyrich D. Wiese H. Maier G. Skodacek D. Appel B. Sarhan H. Tessmar J. Staudenmaier R. Wenzel M.M. Goepferich A. Blunk T. In vitro and in vivo cartilage engineering using a combination of chondrocyte-seeded long-term stable fibrin gels and polycaprolactone-based polyurethane scaffolds. Tissue Eng. 2007;13:2207. doi: 10.1089/ten.2006.0358. [DOI] [PubMed] [Google Scholar]
- 110.Xie J. Han Z. Kim S.H. Kim Y.H. Matsuda T. Mechanical loading-dependence of mRNA expressions of extracellular matrices of chondrocytes inoculated into elastomeric microporous poly(L-lactide-co-epsilon-caprolactone) scaffold. Tissue Eng. 2007;13:29. doi: 10.1089/ten.2006.0060. [DOI] [PubMed] [Google Scholar]
- 111.Park H. Temenoff J.S. Tabata Y. Caplan A.I. Mikos A.G. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials. 2007;28:3217. doi: 10.1016/j.biomaterials.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee S.H. Shin H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv Drug Deliv Rev. 2007;59:339. doi: 10.1016/j.addr.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 113.Bonzani I.C. George J.H. Stevens M.M. Novel materials for bone and cartilage regeneration. Curr Opin Chem Biol. 2006;10:568. doi: 10.1016/j.cbpa.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 114.Mohan N. Nair P.D. Polyvinyl alcohol-poly(caprolactone) semi IPN scaffold with implication for cartilage tissue engineering. J Biomed Mater Res B Appl Biomater. 2008;84:584. doi: 10.1002/jbm.b.30906. [DOI] [PubMed] [Google Scholar]
- 115.Sharma B. Williams C.G. Khan M. Manson P. Elisseeff J.H. In vivo chondrogenesis of mesenchymal stem cells in a photopolymerized hydrogel. Plast Reconstr Surg. 2007;119:112. doi: 10.1097/01.prs.0000236896.22479.52. [DOI] [PubMed] [Google Scholar]
- 116.Engel J. Laminins and other strange proteins. Biochemistry. 1992;31:10643. doi: 10.1021/bi00159a001. [DOI] [PubMed] [Google Scholar]
- 117.Yurchenco P.D. O'Rear J.J. Basal lamina assembly. Curr Opin Cell Biol. 1994;6:674. doi: 10.1016/0955-0674(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 118.Dhadwar S.S. Bemman T. Anderson W.A. Chen P. Yeast cell adhesion on oligopeptide modified surfaces. Biotechnol Adv. 2003;21:395. doi: 10.1016/s0734-9750(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 119.Holmes T.C. de Lacalle S. Su X. Liu G. Rich A. Zhang S. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc Natl Acad Sci USA. 2000;97:6728. doi: 10.1073/pnas.97.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nowak A.P. Breedveld V. Pakstis L. Ozbas B. Pine D.J. Pochan D. Deming T.J. Rapidly recovering hydrogel scaffolds from self-assembling diblock copolypeptide amphiphiles. Nature. 2002;417:424. doi: 10.1038/417424a. [DOI] [PubMed] [Google Scholar]
- 121.Kisiday J. Jin M. Kurz B. Hung H. Semino C. Zhang S. Grodzinsky A.J. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci USA. 2002;99:9996. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang S. Holmes T.C. DiPersio C.M. Hynes R.O. Su X. Rich A. Self-complementary oligopeptide matrices support mammalian cell attachment. Biomaterials. 1995;16:1385. doi: 10.1016/0142-9612(95)96874-y. [DOI] [PubMed] [Google Scholar]
- 123.Semino C.E. Kasahara J. Hayashi Y. Zhang S. Entrapment of migrating hippocampal neural cells in three-dimensional peptide nanofiber scaffold. Tissue Eng. 2004;10:643. doi: 10.1089/107632704323061997. [DOI] [PubMed] [Google Scholar]
- 124.Semino C.E. Merok J.R. Crane G.G. Panagiotakos G. Zhang S. Functional differentiation of hepatocyte-like spheroid structures from putative liver progenitor cells in three-dimensional peptide scaffolds. Differentiation. 2003;71:262. doi: 10.1046/j.1432-0436.2003.7104503.x. [DOI] [PubMed] [Google Scholar]