Abstract
Despite improvements in the understanding of rotator cuff pathology and advances in surgical treatment options, repairs of chronic rotator cuff tears often re-tear or fail to heal after surgery. Hence, there is a critical need for new regenerative repair strategies that provide effective mechanical reinforcement of rotator cuff repair as well as stimulate and enhance the patient's intrinsic healing potential. This article will discuss and identify appropriate models for translating regenerative medicine therapies for rotator cuff repair. Animal models are an essential part of the research and development pathway; however, no one animal model reproduces all of the features of the human injury condition. The rat shoulder is considered the most appropriate model to investigate the initial safety, mechanism, and efficacy of biologic treatments aimed to enhance tendon-to-bone repair. Whereas large animal models are considered more appropriate to investigate the surgical methods, safety and efficacy of the mechanical—or combination biologic/mechanical—strategies are ultimately needed for treating human patients. The human cadaver shoulder model, performed using standard-of-care repair techniques, is considered the best for establishing the surgical techniques and mechanical efficacy of various repair strategies at time zero. While preclinical models provide a critical aspect of the translational pathway for engineered tissues, controlled clinical trials and postmarketing surveillance are also needed to define the efficacy, proper indications, and the method of application for each new regenerative medicine strategy.
Introduction
Unmet clinical needs
Rotator cuff tendon tears are a common cause of debilitating pain, reduced shoulder function, and weakness in the adult human population. It has been estimated that as much as 30% of the patient population seen by subspecialty shoulder surgeons may be related to rotator cuff pathology. In the absence of surgical repair, full-thickness cuff tears result in a persistent tendon defect, which may have detrimental effects on cells and tissues in both the extra- and intraarticular joint space. Chronic, full-thickness rotator cuff tendon tears demonstrate evidence of degeneration and edema that becomes more pronounced as the tear size increases.1 Further, large tears have markedly less reparative vascular and cellular components than small tears, which compromised their ability to heal.1 In many cases, particularly with large tears, the unloaded muscle progresses to severe and irreversible atrophy, fatty infiltration, and fibrosis,2 and the affected muscle–tendon unit stiffens and becomes clinically difficult to mobilize and repair.3
Surgical repair of chronic tears is indicated when conservative treatment fails to improve the patients' symptoms.4 Despite improvements in the understanding of rotator cuff pathology and advances in surgical treatment options, repairs of large, chronic cuff tears fail to heal in 20–95% of cases.5–7 Several factors have been suggested to be responsible for the high failure rate of chronic tears. These include patient age,8,9 smoking,10 size of tear,8,9,11 time from injury to repair,12 tendon quality,13 muscle quality,14 biologic healing response,15 and surgical technique.16,17 Further, many recurrent and chronic rotator cuff tears are considered not repairable at all. Treatment of symptomatic irreparable tears is extremely challenging and limited to nonsurgical management, debridement with partial repair,18–21 or major reconstructive procedures such as muscle transfers22 or shoulder arthroplasty.23
The high failure rate and morbidity associated with chronic tears form the basis for recommending early surgical repair for acute, full-thickness rotator cuff tears. Yet, there remains a critical need for new tissue engineering and regenerative repair strategies that target the clinically challenging, large, and chronic injury condition. These strategies should provide both effective mechanical reinforcement of a rotator cuff repair as well as stimulate and enhance the patient's intrinsic healing potential.24,25 Discriminating preclinical models are also needed to evaluate these new approaches, to predict their safety and efficacy in the human population. It is the objective of this article to discuss and identify appropriate models for translating new regenerative medicine therapies for rotator cuff repair
Animal Models
Animal models are the primary translational pathway for investigating the efficacy, safety, and mechanisms of action of engineered tissues and regenerative medicine therapies. Over the past two decades, animal shoulder models have been widely used to investigate rotator cuff repair strategies. Each model has advantages and disadvantages that must be considered in the context of the specific research questions being asked. Features of the human injury condition that would be ideal to achieve in an animal shoulder model include (1) similar soft tissue and bony anatomy as human, (2) similar shoulder function as human, (3) an intrasynovial injury environment, (4) a chronic injury condition, (5) a tendon size that allows for standard-of-care repair techniques used in humans, (6) the incidence of tendon re-tear in a percentage of subjects, (7) an absence of spontaneous tendon healing or scar formation without treatment, (8) the ability to control postoperative mechanical loading on the repair, and (9) the ability to evaluate clinically relevant outcome measures such as functional assessment, pain, and imaging; if investigation of the associated muscle pathology is the objective, then the animal model should also demonstrate (10) muscle atrophy, stiffening, and fatty infiltration after the creation of a tendon tear that is irreversible without successful surgical repair.
Rat Model
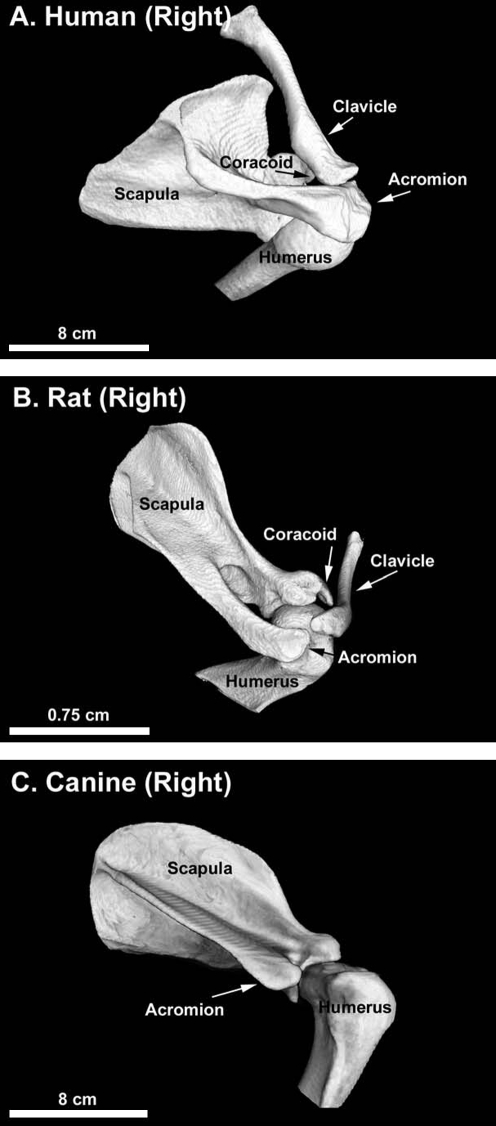
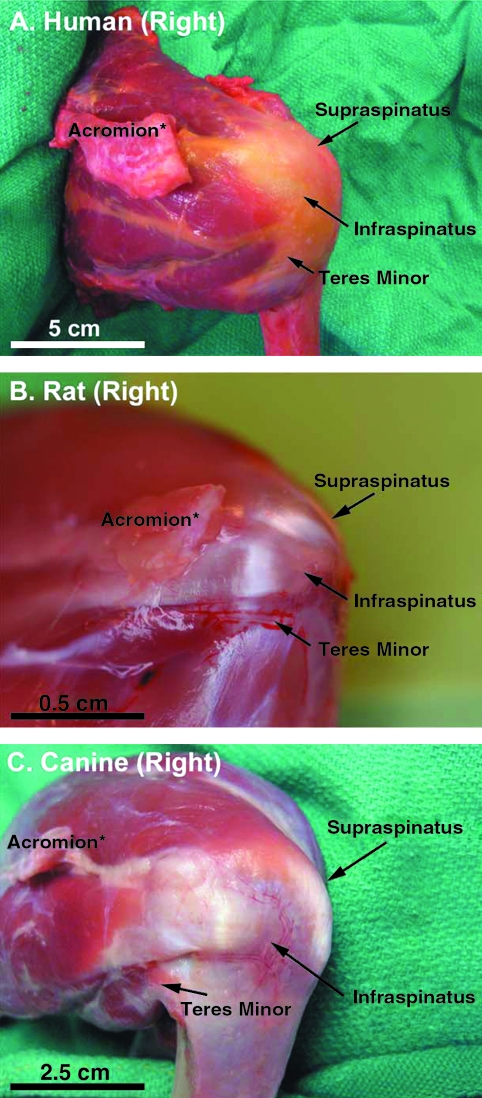
The rat model developed by Soslowsky et al. is considered to have the greatest similarity to human with respect to bony anatomy and activity (overhead reaching).26 Similar to the human shoulder (Fig. 1A), the rat acromion projects anteriorly over the humeral head to the clavicle, creating an enclosed arch over the supraspinatus tendon (Fig. 1B). However, the rat rotator cuff tendons are dissimilar from human (Fig. 2A) in that they are aligned and not interdigitated (Fig. 2B). They appear to be confluent with the underlying joint capsule only at their insertions. In general, the supraspinatus tendon has been the target of most investigations in the rat model. When the rat locomotes, burrows, and reaches overhead (such as for food), excursion of the supraspinatus tendon occurs immediately below the acromial arch, similar to the human shoulder.26 Hence, the rat model has been particularly useful to study the mechanisms of supraspinatus tendon injury involved in the pathogenesis of rotator cuff disease, especially those processes related to extrinsic tendon damage caused by repetitive motion injuries (treadmill running) or impingement.26–28
FIG. 1.
Postero-lateral-superior view of the right shoulder bony anatomy in (A) human, (B) rat, and (C) canine. Similar to the human shoulder (A), the rat acromion projects anteriorly over the humeral head to the clavicle, creating an enclosed arch over the supraspinatus tendon (B). The bony anatomy of large animals such as the canine (C) diverges from human in that the acromion, clavicle, and the coracoid process are generally minimal or nonexistent and do not cover the rotator cuff.
FIG. 2.
Posterior-lateral view of the right rotator cuff in (A) human, (B) rat, and (C) canine. The rotator cuff tendons in the rat shoulder (B) are dissimilar from human (A) in that they are aligned and not interdigitated. They appear to be confluent with the underlying joint capsule only at their insertions. In general, the supraspinatus tendon has been the target of most investigations in the rat model. The rotator cuff tendons of large animals such as the canine (C) are also dissimilar from human (A) in that they are extraarticular, highly aligned, and not interdigitated. The infraspinatus tendon has been the target of most investigations in large animal models, and this tendon is not integrated with the underlying joint capsule. *The acromion has been partially resected in the photographs of human and rat rotator cuff to fully observe the supraspinatus tendon.
The rat model has also been used to study the mechanisms,29–31 healing,32–35 and regenerative strategies36–38 for acute tendon-to-bone repair. It is an appealing model for molecular, histologic, and immunohistochemical investigations because of the large number of specimens that can be readily obtained and its utility as an extensively bred and genetically defined tool for these types of assays.31 Further, re-tear of rotator cuff repairs performed with a Mason-Allon–like stitch has not been observed postoperatively in the rat model.39 Hence, the rat model lends itself particularly well to studying regenerative strategies for tendon-to-bone repair that are biologically based as the effectiveness of these types of approaches, for example, growth factor therapy, depend to a large extent on maintaining an intact tendon–bone repair interface. Recently, the rat has also been used to study the use of scaffold devices for rotator cuff repair augmentation40 or interposition grafting across a large rotator cuff defect,41–43 which is a regenerative strategy that fundamentally targets joint closure and not anatomic (mechanical) repair.
Further, the rat allows for measures to control postoperative loading on the tendon-to-bone repair. Hence, the rat has been successfully used to study the effect of postoperative activity levels (casting, muscle paralysis, free cage activity, and exercise) on the acute tendon-to-bone healing.34,35,39 The rat tolerates bilateral shoulder surgery (e.g., Refs.31,40,43), which offers the experimental advantage of having a paired control. As well, the rat model has been used to investigate the pervasive clinical problems of chronic rotator cuff repair44–49 and two tendon tears.50,51 Because chronic tendon tears in the rat are reparable through at least 16 weeks,48 the rat allows for studies of tendon-to-bone repair in the context of a clinically relevant chronic tendon injury, although in the absence of persistent degenerative muscle changes.47 Finally, the rat model has the advantages of low cost, ease of management, allowance for large sample size, and availability of biologic agents.
Limitations of the rat model include the absence of irreversible muscle fat accumulation with a chronic tear,47 making the rat less suited for studying the mechanism and treatment of associated muscle pathology. Further, the absence of postoperative re-tears in the rat is a significant departure from the human condition and makes the rat a less suitable model for evaluating repair strategies that are engineered to target the critical need for mechanical efficacy in human rotator cuff repair. As well, the small size of the rat shoulder tendons makes the study of standard-of-care repair techniques utilized in human rotator cuff repair impossible. Like other animals, the rat is quadrupedal and its forelimb is weight bearing and used for gait (as well as overhead reaching). Finally, also like other animal models, the rat undergoes scar tissue formation and healing of the rotator cuff injury in the absence of treatment,42,43 which limits the ability to discriminate nonefficacious treatments for the human condition, where spontaneous healing does not occur.
Large Animal Models
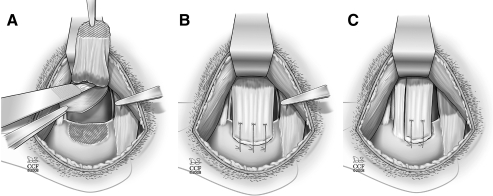
Rotator cuff injury and repair has been investigated in large animal models, including rabbit,52–60 goat,61,62 sheep,63–68 and dog.69–76 (Although the rabbit may be considered by some to be a small animal, it is grouped with the large animals because it is in many ways more similar to the large animals than to the rat with respect to the points of this discussion.) The bony anatomy of these larger animals diverges from human (Fig. 1A) in that the acromion, clavicle, and the coracoid process are generally minimal or nonexistent and do not cover the rotator cuff77,78 (Fig. 1C). (In the rabbit, the bony anatomy differs from the goat, sheep, and dog in that the acromion is directed inferiorly and partially covers the infraspinatus and teres minor tendons, and the subscapularis tendon travels through a bony tunnel on the anterior aspect of the scapula.79) The soft tissue anatomy of the large animal shoulder is also significantly different from human (Fig. 2A) in that the rotator cuff tendons in large animals are extraarticular, highly aligned, and not interdigitated (Fig. 2C). The infraspinatus tendon has been the target of most investigations in large animal models because these animals become lame if the supraspinatus tendon is injured, though recently the subscapularis tendon has been investigated in the rabbit model.79 The infraspinatus tendon in large animals is not integrated with the underlying joint capsule.77 Hence, a portion of the joint capsule must be deliberately resected in the infraspinatus injury model, to model the intraarticular nature of the injury that occurs in the human condition76 (Fig. 3A).
FIG. 3.
Rotator cuff tendon injury and repair in the large animal (canine) model. (A) To model an intraarticular injury of infraspinatus tendon injury and repair, a portion of the joint capsule must be deliberately resected. (B) A full-width infraspinatus tendon injury and repair model is demonstrated. (C) A partial-width infraspinatus tendon injury and repair model is demonstrated; in this instance, the superior two-thirds of the infraspinatus tendon was released and repaired.
Large animal shoulder models facilitate accuracy and reproducibility of injury and repair manipulations.80 Because their size allows for many standard-of-care surgical techniques to be reproduced, large animal models have been used to study various surgical repair techniques at time zero81–86 and after healing.87,88 It should be appreciated, however, that animal tendons in these models are generally acutely severed and healthy, in contrast to chronically injured and perhaps diseased human rotator cuff tendons. On the other hand, the highly aligned structure of the animal tendons makes them less effective at retaining sutures than healthy, human cuff tissue. To some extent, both of these issues in large animals confound our conclusion on the mechanical effectiveness of various repair strategies for the human condition. As well, large animals have been used to study regenerative strategies for acute tendon-to-bone healing using growth factors,25,89 scaffold interposition,42,59,75,90–92 and scaffold augmentation.65,76,93
Large animal models have been used to a lesser extent to study chronic rotator cuff repair54,63,64,67,68 as the tendons become irreparable to their anatomic footprint after approximately 6 weeks, resulting from excessive tendon retraction and muscle atrophy and stiffening.68 When tendons are chronically released in large animal models, robust scar tissue forms in the gap between the tendon edge and the bone.77 To identify the released tendon edge for subsequent, chronic repair procedures, a nonresorbable membrane such as Gore-Tex must be used to cover the tendon stump at the time it is released.68 As a consequence of chronic tendon release, significant muscle atrophy and fatty infiltration develop and persist in large animal models,54,64,66,67,94,95 making them well suited to study the mechanism and treatment of associated rotator cuff muscle pathology.
Several studies have reported that rotator cuff repairs in large animals undergo high re-tear rates postoperatively. Using tantalum bead markers in the canine model, it has been reported that acute, full-width tendon repairs (Fig. 3B) re-tear within the first days after surgery, regardless of suture type, suture configuration, or modulation of postoperative activity using slinging or low-ceiling housing.77 These observations are supported by similar re-tear rates for full-width primary repairs in the sheep model,25,63,96 even when postoperative activity is limited using small pens or a softball affixed to the operatively treated leg. Clearly, the activity and postoperative management of large animals is challenging to control, and likely plays a role in the high re-tear rates observed. However, it should be noted that the canine shoulder in particular allows for clinically relevant rehabilitation modalities, including slinging, hobbles, casting, swimming, walking through obstacles, jumping down from graduated heights, exercise bands, and treadmill walking or running (in air or underwater).97 Researchers using the canine shoulder model might consider and adopt aspects of the well-established veterinary expertise related to canine shoulder rehabilitation.98
A partial width tendon injury and repair model has recently been used in the canine (Fig. 3C), based on the reasoning that a partial width injury might moderate the incidence of repair failures and mimic the mechanical environment of many single tendon tears in the human injury condition.76 However, a 100% incidence of re-tear was also reported with this partial-width model, though tendon retraction distance was somewhat reduced compared to full-width injury and repairs. We conclude that either a full- or partial-width injury and repair model in a large animal will provide a rigorous test of the extent to which a new repair strategy or postoperative protocol can maintain the structural integrity of a repair in a high-load environment. However, large animal models may be a more rigorous test for a mechanical repair strategy than the human condition, given the highly aligned structure of the animal tendons (less effective suture retention than human cuff tissue) and the relative difficulty in controlling the postoperative load environment of the animals.
Limitations of the large animal models include quadrupedal gait with limited overhead reaching. Further, like the rat, large animal models undergo robust scar tissue formation between the released tendon stump and bone in the absence of treatment. This gap scar tissue can be visually, mechanically, and histologically misconstrued as tendon.63,77 Further, the high incidence of tendon re-tear makes large animal models less suited to study the mechanism or efficacy of biologic treatments aimed at tendon-to-bone healing because of the difficulty keeping the tendon and bone in proximity after repair. When studied in large animal models where re-tear occurs, biologic treatments become difficult to maintain at the repair site and at best serve to influence scar tissue formation in the gap between the retracted tendon stump and the bone. As well there is an increased cost and complexity of management associated with large animals, which reduces the practical sample size for experimental studies. Finally, the activity of large animals can be challenging to control, though to varying degrees they do accommodate casting,99,100 slinging,101 and treadmill running.102–104
Human Cadaveric Models
As previously discussed, the mechanical effectiveness of various repair strategies for the human condition may be difficult to fully appreciate in a large animal model. Human cadaver models have been used historically to investigate the mechanical strength of various suture repair techniques.105–113 Human cadaver models offer the advantage of testing repair strategies in human rotator cuff tissue, where the suture retention properties and mechanical load environment of the human condition can be reasonably well reproduced. Recently, a human cadaver model was used to study scaffold strategies aimed at improving the mechanical properties of a rotator cuff repair at time zero (i.e., at the time of surgery).114 While this model did not reproduce all of the elements of a standard-of-care repair technique for augmentation with scaffolds, it provides the basis for future refinements of the human cadaver model for evaluating the appropriate surgical methods and mechanical efficacy of scaffold-based strategies. Further, it will be important to evaluate scaffold repair techniques under cyclic—not just load to failure—conditions, as cyclic loading models the physiologic environment of the rotator cuff repair during the postoperative period.105,111–113 Human cadaver models are limited by high variability in bone and tendon properties among donors, requiring paired studies to be performed.113 They are also limited by only providing information on the mechanical performance of a repair strategy at time zero. Nonetheless, together with large animal models, human cadaver models performed using standard-of-care repair techniques provide an important part of the translational pathway for evaluating the mechanical effectiveness of regenerative strategies for rotator cuff repair.
Preclinical Models: Which One to Choose?
One can appreciate that various animal models offer distinct advantages and disadvantages for studying rotator cuff tissue engineering and regenerative repair strategies. While a nonhuman primate shoulder may offer more anatomic, biomechanical, and immunologic similarity to humans than other animals, cost and management issues make use of this model impractical. A comparison of the animal models discussed in this article is summarized in Table 1. It is readily apparent that no one animal model reproduces all of the features of the human injury condition. All animals differ from human in terms of the biomechanical use of their shoulder. As well, because no animal is immunologically identical to the human, a possible adverse immunologic response to a regenerative medicine therapy in human patients may not be predicted from animal studies.115,116 Finally, all animals exhibit some degree of scar tissue formation in the absence of repair or treatment, which reduces the ability to discriminate nonefficacious treatments for the human condition using any animal model.
Table 1.
Comparison of Animal Models for Rotator Cuff Tissue Engineering and Regenerative Repair
| Feature | Rat shoulder model | Large animal shoulder models (rabbit, dog, goat, and sheep) |
|---|---|---|
| Bony anatomy1 | Human-like (Fig. 1) | Diverges from human (Fig. 1) |
| Rotator cuff (soft tissue) anatomy1 | Diverges from human (Fig. 2) | Diverges from human (Fig. 2) |
| Shoulder function2 | Quadruped, but some overhead range of motion such that excursion of the supraspinatus tendon occurs immediately below the acromial arch, similar to human | Quadruped, limited range of motion |
| Postoperative environment2 | Low absolute loads | Modest to high absolute loads |
| Intrasynovial injury environment3 | Readily achieved with (supraspinatus) tendon release | Deliberate joint capsule resection required with (infraspinatus) tendon release |
| Chronic tendon/joint capsule injury4 | Partially persists | Partially persists |
| Full-width chronic tendon injuries4 | Reparable through at least 16 weeks | Irreparable after approximately 6 weeks |
| Chronic muscle changes10 | Do not persist | Persist |
| Tendon size5 | Limits standard-of-care repair techniques | Permits standard-of-care repair techniques |
| Re-tear incidence6 | None/low | High |
| Healing response without treatment7 | Spontaneous, robust scar tissue | Spontaneous, robust scar tissue |
| Control of activity8 | Casting, muscle paralysis, treadmill | Casting, muscle paralysis, slinging, treadmill |
| Clinical outcome measures9 | Gait and pain analysis | Gait and pain analysis; MRI, ultrasound to assess tendon healing |
| Cost | Inexpensive | Expensive |
| Management | Easy | Challenging |
| Sample size | Large | Small |
| Antibodies | Readily available | Limited availability |
Superscript numbers refer to the features of the human injury condition that are listed in the Animal Models section.
MRI, magnetic resonance imaging.
Based on its anatomy, the rat is most appropriate to study the mechanism, pathogenesis, and/or management of rotator cuff disease. Further, because the rat rotator cuff can be repaired in a mechanically stable manner such that re-tears do not occur, the rat lends itself to studying healing mechanisms or the effect of biologic treatments at the tendon–bone interface. However, studies investigating mechanical repair strategies aimed to reduce re-tears and enhance gap tissue formation are better studied in large animal models where standard-of-care surgical techniques can be reproduced and the mechanical loads are demanding. A human cadaver model using standard-of-care repair techniques and cyclic loading may be the most appropriate way to test the mechanical effectiveness of a particular repair strategy at time zero.
Scaffolds for tendon interposition and augmentation are a common regenerative approach being investigated currently. Many important research questions can be studied in either the rat or large animal models, except to the extent the research question is related to mechanical efficacy. In these cases the reproducibility of a standard-of-care surgical technique and robust mechanical load environment in the large animal model would be preferable. As previously mentioned, however, the effectiveness of a mechanical repair strategy for human patients may be underappreciated in the large animal model where re-tears of even acute repairs currently do not appear to be preventable. Again, a human cadaver model may be the most appropriate way to test the efficacy of mechanically based scaffold strategies at time zero.
Finally, while a chronic tendon injury can be created in both the rat and the large animals, the time-frame at which the tendon becomes irreparable in the large animal is limited. As well, if the incidence of re-tear is high for acute repairs in large animals, one can assume that it is as high or higher for chronic tears. Hence, the rat is the preferable model to study chronic tendon-to-bone healing if maintenance of repair integrity is essential to the research question. However, the persistence of chronic muscle changes (atrophy and fatty infiltration) makes the large animal model preferable for studies of the mechanism and treatment of muscle pathology and the mechanical environment associated with chronic rotator cuff injury.
While no one model may be appropriate for assessing every rotator cuff regenerative medicine strategy, it is clear that our interpretation and comparison of various approaches would be greatly aided by the adoption of some commonalities in our animal studies with respect to study design, outcome measures, and spectrum of controls. We suggest that using a paired study design and including time zero and normal controls will facilitate interpretation of the efficacy of an approach within and across studies. Further, use of a common species, injury, and repair technique for large animal studies would aid in comparison of various approaches. Forums that foster critical discussion among the orthopedic research community should be directed at both identifying and defining best model systems for rotator cuff regenerative medicine strategies.
Summary
Animal models are a critical part of the preclinical pathway for identifying engineered tissues and regenerative medicine therapies that will successfully lead to improved outcomes and quality of life for patients suffering with chronic, debilitating rotator cuff injuries. The rat shoulder is arguably the most appropriate and cost-effective model to investigate the initial safety, mechanism, and efficacy of biologic treatments aimed to enhance acute or chronic tendon-to-bone repair. Due to high re-tear rates in the human population, however, an effective biologic treatment may be rendered useless for human use if it cannot be delivered and maintained at the rotator cuff repair site via a mechanically robust vehicle. Hence, regenerative medicine therapies for human rotator cuff repair must ultimately include a mechanical—and perhaps a combination biologic/mechanical—approach, the safety and efficacy of which are better investigated in human cadaver and large animal models using standard-of-care surgical techniques. While animal models may allow us to assess the extent to which a particular regenerative strategy induces an unfavorable host response, limits re-tear or gap formation during the postoperative period, or improves the biomechanical properties of a healed repair, we must recognize that no animal model entirely reproduces the biologic or mechanical environment of the human injury condition. Ultimately, the onus lies on the orthopedic profession, the regulatory bodies, and industry to perform controlled clinical trials and postmarketing surveillance to define the efficacy, proper indications, and the method of application for each new regenerative medicine strategy.
Disclosure Statement
Kathleen A. Derwin, Ph.D., previously received a grant from the NIH to develop a large animal canine model (R21AR052765). No other competing financial interests exist.
Andrew Ryan Baker, M.S., has no competing financial interests.
Joseph Iannotti, M.D., Ph.D., is a paid consultant for DePuy Johnson and Johnson and Tornier. He sits on the scientific advisory board for Wyeth Pharmaceuticals and United Healthcare, and receives royalties from Lippincott Wolters Kluwer Publisher.
Jesse A. McCarron, M.D., is a paid consultant for Wyeth Pharmaceuticals.
References
- 1.Matthews T.J. Hand G.C. Rees J.L. Athanasou N.A. Carr A.J. Pathology of the torn rotator cuff tendon: reduction in potential for repair as tear size increases. J Bone Joint Surg Br. 2006;88:489. doi: 10.1302/0301-620X.88B4.16845. [DOI] [PubMed] [Google Scholar]
- 2.Meyer D.C. Pirkl C. Pfirrmann C.W. Zanetti M. Gerber C. Asymmetric atrophy of the supraspinatus muscle following tendon tear. J Orthop Res. 2005;23:254. doi: 10.1016/j.orthres.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Hersche O. Gerber C. Passive tension in the supraspinatus musculotendinous unit after long-standing rupture of its tendon: a preliminary report. J Shoulder Elbow Surg. 1998;7:393. doi: 10.1016/s1058-2746(98)90030-1. [DOI] [PubMed] [Google Scholar]
- 4.Mantone J.K. Burkhead W.Z., Jr. Noonan J., Jr. Nonoperative treatment of rotator cuff tears. Orthop Clin North Am. 2000;31:295. doi: 10.1016/s0030-5898(05)70149-8. [DOI] [PubMed] [Google Scholar]
- 5.Gazielly D.F. Gleyze P. Montagnon C. Functional and anatomical results after rotator cuff repair. Clin Orthop Relat Res. 1994;304:43. [PubMed] [Google Scholar]
- 6.Harryman D.T. Mack L.A. Wang K.Y. Jackins S.E. Richardson M.L. Matsen F.A., III Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73-A:982. [PubMed] [Google Scholar]
- 7.Galatz L.M. Ball C.M. Teefey S.A. Middleton W.D. Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A:219. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Romeo A.A. Hang D.W. Bach B.R., Jr. Shott S. Repair of full thickness rotator cuff tears. Gender, age, and other factors affecting outcome. Clin Orthop Relat Res. 1999;367:243. [PubMed] [Google Scholar]
- 9.Oh J.H. Kim S.H. Ji H.M. Jo K.H. Bin S.W. Gong H.S. Prognostic factors affecting anatomic outcome of rotator cuff repair and correlation with functional outcome. Arthroscopy. 2009;25:30. doi: 10.1016/j.arthro.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Mallon W.J. Misamore G. Snead D.S. Denton P. The impact of preoperative smoking habits on the results of rotator cuff repair. J Shoulder Elbow Surg. 2004;13:129. doi: 10.1016/j.jse.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Cofield R.H. Parvizi J. Hoffmeyer P.J. Lanzer W.L. Ilstrup D.M. Rowland C.M. Surgical repair of chronic rotator cuff tears. A prospective long-term study. J Bone Joint Surg Am. 2001;83-A:71. doi: 10.2106/00004623-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Bartolozzi A. Andreychik D. Ahmad S. Determinants of outcome in the treatment of rotator cuff disease. Clin Orthop Relat Res. 1994;308:90. [PubMed] [Google Scholar]
- 13.Riley G.P. Harrall R.L. Constant C.R. Chard M.D. Cawston T.E. Hazleman B.L. Tendon degeneration and chronic shoulder pain: changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann Rheum Dis. 1994;53:359. doi: 10.1136/ard.53.6.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goutallier D. Postel J.M. Gleyze P. Leguilloux P. Van Driessche S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg. 2003;12:550. doi: 10.1016/s1058-2746(03)00211-8. [DOI] [PubMed] [Google Scholar]
- 15.Hamada K. Tomonaga A. Gotoh M. Yamakawa H. Fukuda H. Intrinsic healing capacity and tearing process of torn supraspinatus tendons: in situ hybridization study of alpha 1 (I) procollagen mRNA. J Orthop Res. 1997;15:24. doi: 10.1002/jor.1100150105. [DOI] [PubMed] [Google Scholar]
- 16.Iannotti J.P. Full thickness rotator cuff tears: factors affecting surgical outcome. J Am Acad Orthop Surg. 1994;2:87. doi: 10.5435/00124635-199403000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Uhthoff H.K. Trudel G. Himori K. Relevance of pathology and basic research to the surgeon treating rotator cuff disease. J Orthop Sci. 2003;8:449. doi: 10.1007/s10776-002-0624-5. [DOI] [PubMed] [Google Scholar]
- 18.Melillo A.S. Savoie F.H., III Field LD. Massive rotator cuff tears: debridement versus repair. Orthop Clin North Am. 1997;28:117. doi: 10.1016/s0030-5898(05)70269-8. [DOI] [PubMed] [Google Scholar]
- 19.Rockwood C.A., Jr. Williams G.R., Jr. Burkhead W.Z., Jr. Debridement of degenerative, irreparable lesions of the rotator cuff. J Bone Joint Surg Am. 1995;77:857. doi: 10.2106/00004623-199506000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Hawkins R.H. Dunlop R. Nonoperative treatment of rotator cuff tears. Clin Orthop Relat Res. 1995;321:178. [PubMed] [Google Scholar]
- 21.Burkhart S.S. Partial repair of massive rotator cuff tears: the evolution of a concept. Orthop Clin North Am. 1997;28:125. doi: 10.1016/s0030-5898(05)70270-4. [DOI] [PubMed] [Google Scholar]
- 22.Dines D.M. Moynihan D.P. Dines J.S. McCann P. Irreparable rotator cuff tears: what to do and when to do it; the surgeon's dilemma. Instr Course Lect. 2007;56:13. [PubMed] [Google Scholar]
- 23.Feeley B.T. Gallo R.A. Craig E.V. Cuff tear arthropathy: current trends in diagnosis and surgical management. J Shoulder Elbow Surg. 2009;18:484. doi: 10.1016/j.jse.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Accousti K.J. Flatow E.L. Technical pearls on how to maximize healing of the rotator cuff. Instr Course Lect. 2007;56:3. [PubMed] [Google Scholar]
- 25.Rodeo S.A. Potter H.G. Kawamura S. Turner A.S. Kim H.J. Atkinson B.L. Biologic augmentation of rotator cuff tendon-healing with use of a mixture of osteoinductive growth factors. J Bone Joint Surg Am. 2007;89:2485. doi: 10.2106/JBJS.C.01627. [DOI] [PubMed] [Google Scholar]
- 26.Soslowsky L.J. Carpenter J.E. DeBano C.M. Banerji I. Moalli M.R. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5:383. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- 27.Soslowsky L.J. Thomopoulos S. Tun S. Flanagan C.L. Keefer C.C. Mastaw J. Carpenter J.E. Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9:79. [PubMed] [Google Scholar]
- 28.Soslowsky L.J. Thomopoulos S. Esmail A. Flanagan C.L. Iannotti J.P. Williamson J.D., III Carpenter J.E. Rotator cuff tendinosis in an animal model: role of extrinsic and overuse factors. Ann Biomed Eng. 2002;30:1057. doi: 10.1114/1.1509765. [DOI] [PubMed] [Google Scholar]
- 29.Wurgler-Hauri C.C. Dourte L.M. Baradet T.C. Williams G.R. Soslowsky L.J. Temporal expression of 8 growth factors in tendon-to-bone healing in a rat supraspinatus model. J Shoulder Elbow Surg. 2007;16:198S. doi: 10.1016/j.jse.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galatz L.M. Sandell L.J. Rothermich S.Y. Das R. Mastny A. Havlioglu N. Silva M.J. Thomopoulos S. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J Orthop Res. 2006;24:541. doi: 10.1002/jor.20067. [DOI] [PubMed] [Google Scholar]
- 31.Thomopoulos S. Hattersley G. Rosen V. Mertens M. Galatz L. Williams G.R. Soslowsky L.J. The localized expression of extracellular matrix components in healing tendon insertion sites: an in situ hybridization study. J Orthop Res. 2002;20:454. doi: 10.1016/S0736-0266(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 32.Cohen D.B. Kawamura S. Ehteshami J.R. Rodeo S.A. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34:362. doi: 10.1177/0363546505280428. [DOI] [PubMed] [Google Scholar]
- 33.Galatz L.M. Silva M.J. Rothermich S.Y. Zaegel M.A. Havlioglu N. Thomopoulos S. Nicotine delays tendon-to-bone healing in a rat shoulder model. J Bone Joint Surg Am. 2006;88:2027. doi: 10.2106/JBJS.E.00899. [DOI] [PubMed] [Google Scholar]
- 34.Gimbel J.A. Van Kleunen J.P. Williams G.R. Thomopoulos S. Soslowsky L.J. Long durations of immobilization in the rat result in enhanced mechanical properties of the healing supraspinatus tendon insertion site. J Biomech Eng. 2007;129:400. doi: 10.1115/1.2721075. [DOI] [PubMed] [Google Scholar]
- 35.Thomopoulos S. Williams G.R. Soslowsky L.J. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng. 2003;125:106. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- 36.Murray D.H. Kubiak E.N. Jazrawi L.M. Araghi A. Kummer F. Loebenberg M.I. Zuckerman J.D. The effect of cartilage-derived morphogenetic protein 2 on initial healing of a rotator cuff defect in a rat model. J Shoulder Elbow Surg. 2007;16:251. doi: 10.1016/j.jse.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Dines J.S. Weber L. Razzano P. Prajapati R. Timmer M. Bowman S. Bonasser L. Dines D.M. Grande D.P. The effect of growth differentiation factor-5-coated sutures on tendon repair in a rat model. J Shoulder Elbow Surg. 2007;16:S215. doi: 10.1016/j.jse.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Thomopoulos S. Soslowsky L.J. Flanagan C.L. Tun S. Keefer C.C. Mastaw J. Carpenter J.E. The effect of fibrin clot on healing rat supraspinatus tendon defects. J Shoulder Elbow Surg. 2002;11:239. doi: 10.1067/mse.2002.122228. [DOI] [PubMed] [Google Scholar]
- 39.Galatz L.M. Charlton N. Das R. Kim H.M. Havlioglu N. Thomopoulos S. Complete removal of load is detrimental to rotator cuff healing. 2009;18:669. doi: 10.1016/j.jse.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 40.Cole B.J. Gomoll A.H. Yanke A. Pylawka T. Lewis P. MacGillivray J.D. Williams J.M. Biocompatibility of a polymer patch for rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2007;15:632. doi: 10.1007/s00167-006-0187-6. [DOI] [PubMed] [Google Scholar]
- 41.Perry S.M. Gupta R.R. Kleunen J.V. Ramsey M.L. Soslowsky L.J. Glaser D.L. Use of small intestine submucosa in a rat model of acute and chronic rotator cuff tear. J Shoulder Elbow Surg. 2007;15:179. doi: 10.1016/j.jse.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Zalavras C.G. Gardocki R. Huang E. Stevanovic M. Hedman T. Tibone J. Reconstruction of large rotator cuff tendon defects with porcine small intestinal submucosa in an animal model. J Shoulder Elbow Surg. 2006;15:224. doi: 10.1016/j.jse.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Ide J. Kikukawa K. Hirose J. Iyama K. Sakamoto H. Mizuta H. Reconstruction of large rotator-cuff tears with acellular dermal matrix grafts in rats. J Shoulder Elbow Surg. 2009;18:288. doi: 10.1016/j.jse.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Lin J.L. Carreira D. Ponnappan R. Volz B. Cole B.J. Use of bipolar radiofrequency energy in delayed repair of acute supraspinatus tears in rats. J Shoulder Elbow Surg. 2007;16:640. doi: 10.1016/j.jse.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Gimbel J.A. Mehta S. Van Kleunen J.P. Williams G.R. Soslowsky L.J. The tension required at repair to reappose the supraspinatus tendon to bone rapidly increases after injury. Clin Orthop Relat Res. 2004;426:258. doi: 10.1097/01.blo.0000136831.17696.80. [DOI] [PubMed] [Google Scholar]
- 46.Gimbel J.A. Van Kleunen J.P. Mehta S. Perry S.M. Williams G.R. Soslowsky L.J. Supraspinatus tendon organizational and mechanical properties in a chronic rotator cuff tear animal model. J Biomech. 2004;37:739. doi: 10.1016/j.jbiomech.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 47.Barton E.R. Gimbel J.A. Williams G.R. Soslowsky L.J. Rat supraspinatus muscle atrophy after tendon detachment. J Orthop Res. 2005;23:259. doi: 10.1016/j.orthres.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 48.Gimbel J.A. Van Kleunen J.P. Lake S.P. Williams G.R. Soslowsky L.J. The role of repair tension on tendon to bone healing in an animal model of chronic rotator cuff tears. J Biomech. 2007;40:561. doi: 10.1016/j.jbiomech.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 49.Galatz L.M. Rothermich S.Y. Zaegel M. Silva M.J. Havlioglu N. Thomopoulos S. Delayed repair of tendon to bone injuries leads to decreased biomechanical properties and bone loss. J Orthop Res. 2005;23:1441. doi: 10.1016/j.orthres.2005.05.005.1100230629. [DOI] [PubMed] [Google Scholar]
- 50.Perry S.M. Getz C.L. Soslowsky L.J. Alterations in function after rotator cuff tears in an animal model. J Shoulder Elbow Surg. 2009;18:296. doi: 10.1016/j.jse.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perry S.M. Getz C.L. Soslowsky L.J. After rotator cuff tears, the remaining (intact) tendons are mechanically altered. J Shoulder Elbow Surg. 2009;18:52. doi: 10.1016/j.jse.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fabis J. Danilewicz M. Omulecka A. Rabbit supraspinatus tendon detachment: effects of size and time after tenotomy on morphometric changes in the muscle. Acta Orthop Scand. 2001;72:282. doi: 10.1080/00016470152846637. [DOI] [PubMed] [Google Scholar]
- 53.Matsumoto F. Uhthoff H.K. Trudel G. Loehr J.F. Delayed tendon reattachment does not reverse atrophy and fat accumulation of the supraspinatus—an experimental study in rabbits. J Orthop Res. 2002;20:357. doi: 10.1016/S0736-0266(01)00093-6. [DOI] [PubMed] [Google Scholar]
- 54.Uhthoff H.K. Matsumoto F. Trudel G. Himori K. Early reattachment does not reverse atrophy and fat accumulation of the supraspinatus—an experimental study in rabbits. J Orthop Res. 2003;21:386. doi: 10.1016/S0736-0266(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 55.Koike Y. Trudel G. Uhthoff H.K. Formation of a new enthesis after attachment of the supraspinatus tendon: a quantitative histologic study in rabbits. J Orthop Res. 2005;23:1433. doi: 10.1016/j.orthres.2005.02.015.1100230628. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi M. Itoi E. Minagawa H. Miyakoshi N. Takahashi S. Tuoheti Y. Okada K. Shimada Y. Expression of growth factors in the early phase of supraspinatus tendon healing in rabbits. J Shoulder Elbow Surg. 2006;15:371. doi: 10.1016/j.jse.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 57.Funakoshi T. Majima T. Iwasaki N. Yamane S. Masuko T. Minami A. Harada K. Tamura H. Tokura S. Nishimura S. Novel chitosan-based hyaluronan hybrid polymer fibers as a scaffold in ligament tissue engineering. J Biomed Mater Res A. 2005;74:338. doi: 10.1002/jbm.a.30237. [DOI] [PubMed] [Google Scholar]
- 58.Funakoshi T. Majima T. Suenaga N. Iwasaki N. Yamane S. Minami A. Rotator cuff regeneration using chitin fabric as an acellular matrix. J Shoulder Elbow Surg. 2006;15:112. doi: 10.1016/j.jse.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 59.Sano H. Kumagai J. Sawai T. Experimental fascial autografting for the supraspinatus tendon defect: remodeling process of the grafted fascia and the insertion into bone. J Shoulder Elbow Surg. 2002;11:166. doi: 10.1067/mse.2002.120808. [DOI] [PubMed] [Google Scholar]
- 60.Koike Y. Trudel G. Curran D. Uhthoff H.K. Delay of supraspinatus repair by up to 12 weeks does not impair enthesis formation: a quantitative histologic study in rabbits. J Orthop Res. 2006;24:202. doi: 10.1002/jor.20031. [DOI] [PubMed] [Google Scholar]
- 61.St Pierre P. Olson E.J. Elliott J.J. O'Hair K.C. McKinney L.A. Ryan J. Tendon-healing to cortical bone compared with healing to a cancellous trough. A biomechanical and histological evaluation in goats. J Bone Joint Surg Am. 1995;77:1858. doi: 10.2106/00004623-199512000-00010. [DOI] [PubMed] [Google Scholar]
- 62.Fealy S. Rodeo S.A. MacGillivray J.D. Nixon A.J. Adler R.S. Warren R.F. Biomechanical evaluation of the relation between number of suture anchors and strength of the bone-tendon interface in a goat rotator cuff model. Arthroscopy. 2006;22:595. doi: 10.1016/j.arthro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 63.Gerber C. Schneeberger A.G. Perren S.M. Nyffeler R.W. Experimental rotator cuff repair. A preliminary study. J Bone Joint Surg Am. 1999;81:1281. doi: 10.2106/00004623-199909000-00009. [DOI] [PubMed] [Google Scholar]
- 64.Gerber C. Meyer D.C. Schneeberger A.G. Hoppeler H. Von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004;86-A:1973. doi: 10.2106/00004623-200409000-00016. [DOI] [PubMed] [Google Scholar]
- 65.Schlegel T.F. Hawkins R.J. Lewis C.W. Motta T. Turner A.S. The effects of augmentation with Swine small intestine submucosa on tendon healing under tension: histologic and mechanical evaluations in sheep. Am J Sports Med. 2006;34:275. doi: 10.1177/0363546505279912. [DOI] [PubMed] [Google Scholar]
- 66.Meyer D.C. Lajtai G. von R.B. Pfirrmann C.W. Gerber C. Tendon retracts more than muscle in experimental chronic tears of the rotator cuff. J Bone Joint Surg Br. 2006;88:1533. doi: 10.1302/0301-620X.88B11.17791. [DOI] [PubMed] [Google Scholar]
- 67.Meyer D.C. Hoppeler H. Von Rechenberg B. Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22:1004. doi: 10.1016/j.orthres.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 68.Coleman S.H. Fealy S. Ehteshami J.R. MacGillivray J.D. Altchek D.W. Warren R.F. Turner A.S. Chronic rotator cuff injury and repair model in sheep. J Bone Joint Surg Am. 2003;85-A:2391. doi: 10.2106/00004623-200312000-00018. [DOI] [PubMed] [Google Scholar]
- 69.Oguma H. Murakami G. Takahashi-Iwanaga H. Aoki M. Ishii S. Early anchoring collagen fibers at the bone-tendon interface are conducted by woven bone formation: light microscope and scanning electron microscope observation using a canine model. J Orthop Res. 2001;19:873. doi: 10.1016/S0736-0266(01)00021-3. [DOI] [PubMed] [Google Scholar]
- 70.Kimura A. Aoki M. Fukushima S. Ishii S. Yamakoshi K. Reconstruction of a defect of the rotator cuff with polytetrafluoroethylene felt graft. Recovery of tensile strength and histocompatibility in an animal model. J Bone Joint Surg Br. 2003;85:282. doi: 10.1302/0301-620x.85b2.12823. [DOI] [PubMed] [Google Scholar]
- 71.Dejardin L.M. Arnoczky S.P. Ewers B.J. Haut R.C. Clarke R.B. Tissue-engineered rotator cuff tendon using porcine small intestine submucosa. Histologic and mechanical evaluation in dogs. Am J Sports Med. 2001;29:175. doi: 10.1177/03635465010290021001. [DOI] [PubMed] [Google Scholar]
- 72.Inoue N. Ikeda K. Aro H.T. Frassica F.J. Sim F.H. Chao E.Y. Biologic tendon fixation to metallic implant augmented with autogenous cancellous bone graft and bone marrow in a canine model. J Orthop Res. 2002;20:957. doi: 10.1016/S0736-0266(02)00037-2. [DOI] [PubMed] [Google Scholar]
- 73.Aoki M. Oguma H. Fukushima S. Ishii S. Ohtani S. Murakami G. Fibrous connection to bone after immediate repair of the canine infraspinatus: the most effective bony surface for tendon attachment. J Shoulder Elbow Surg. 2001;10:123. doi: 10.1067/mse.2001.111963. [DOI] [PubMed] [Google Scholar]
- 74.Aoki M. Miyamoto S. Okamura K. Yamashita T. Ikada Y. Matsuda S. Tensile properties and biological response of poly(L-lactic acid) felt graft: an experimental trial for rotator-cuff reconstruction. J Biomed Mater Res B Appl Biomater. 2004;71:252. doi: 10.1002/jbm.b.30084. [DOI] [PubMed] [Google Scholar]
- 75.Adams J.E. Zobitz M.E. Reach J.S., Jr. An K.N. Steinmann S.P. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22:700. doi: 10.1016/j.arthro.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 76.Derwin K.A. Codsi M.J. Milks R.A. Baker A.R. McCarron J.A. Iannotti J.P. Rotator cuff repair augmentation in a canine model using a woven PLLA device. 2009;91:1159. doi: 10.2106/JBJS.H.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Derwin K.A. Baker A.R. Codsi M.J. Iannotti J.P. Assessment of the canine model of rotator cuff injury and repair. J Shoulder Elbow Surg. 2007;16:S140. doi: 10.1016/j.jse.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turner A.S. Experiences with sheep as an animal model for shoulder surgery: strengths and shortcomings. J Shoulder Elbow Surg. 2007;16:S158. doi: 10.1016/j.jse.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 79.Grumet R.C. Hadley S. Diltz M.V. Lee T.Q. Gupta R. Development of a new model for rotator cuff pathology: the rabbit subscapularis muscle. Acta Orthop. 2009;80:97. doi: 10.1080/17453670902807425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carpenter J.E. Thomopoulos S. Soslowsky L.J. Animal models of tendon and ligament injuries for tissue engineering applications. Clin Orthop Relat Res. 1999;367 Suppl:S296. doi: 10.1097/00003086-199910001-00029. [DOI] [PubMed] [Google Scholar]
- 81.Sileo M.J. Ruotolo C.R. Nelson C.O. Serra-Hsu F. Panchal A.P. A biomechanical comparison of the modified Mason-Allen stitch and massive cuff stitch in vitro. Arthroscopy. 2007;23:235. doi: 10.1016/j.arthro.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 82.Gerber C. Schneeberger A.G. Beck M. Schlegel U. Mechanical strength of repairs of the rotator cuff. J Bone Joint Surg Br. 1994;76-B:371. [PubMed] [Google Scholar]
- 83.Nelson C.O. Sileo M.J. Grossman M.G. Serra-Hsu F. Single-row modified mason-allen versus double-row arthroscopic rotator cuff repair: a biomechanical and surface area comparison. Arthroscopy. 2008;24:941. doi: 10.1016/j.arthro.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 84.Baums M.H. Buchhorn G.H. Spahn G. Poppendieck B. Schultz W. Klinger H.M. Biomechanical characteristics of single-row repair in comparison to double-row repair with consideration of the suture configuration and suture material. Knee Surg Sports Traumatol Arthrosc. 2008;16:1052. doi: 10.1007/s00167-008-0590-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Klinger H.M. Steckel H. Spahn G. Buchhorn G.H. Baums M.H. Biomechanical comparison of double-loaded suture anchors using arthroscopic Mason-Allen stitches versus traditional transosseous suture technique and modified Mason-Allen stitches for rotator cuff repair. Clin Biomech. 2007;22:106. doi: 10.1016/j.clinbiomech.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 86.Cummins C.A. Appleyard R.C. Strickland S. Haen P.S. Chen S. Murrell G.A. Rotator cuff repair: an ex vivo analysis of suture anchor repair techniques on initial load to failure. Arthroscopy. 2005;21:1236. doi: 10.1016/j.arthro.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 87.Ozbaydar M. Elhassan B. Esenyel C. Atalar A. Bozdag E. Sunbuloglu E. Kopuz N. Demirhan M. A comparison of single-versus double-row suture anchor techniques in a simulated repair of the rotator cuff: an experimental study in rabbits. J Bone Joint Surg Br. 2008;90:1386. doi: 10.1302/0301-620X.90B10.20862. [DOI] [PubMed] [Google Scholar]
- 88.Klinger H.M. Buchhorn G.H. Heidrich G. Kahl E. Baums M.H. Biomechanical evaluation of rotator cuff repairs in a sheep model: suture anchors using arthroscopic Mason-Allen stitches compared with transosseous sutures using traditional modified Mason-Allen stitches. Clin Biomech. 2008;23:291. doi: 10.1016/j.clinbiomech.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 89.Seeherman H.J. Archambault J.M. Rodeo S.A. Turner A.S. Zekas L. D'Augusta D. Li X.J. Smith E. Wozney J.M. rhBMP-12 accelerates healing of rotator cuff repairs in a sheep model. J Bone Joint Surg Am. 2008;90:2206. doi: 10.2106/JBJS.G.00742. [DOI] [PubMed] [Google Scholar]
- 90.McAdams T.R. Knudsen K.R. Yalamanchi N. Chang J. Goodman S.B. Deltoid flap combined with fascia lata autograft for rotator cuff defects: a histologic study. Knee Surg Sports Traumatol Arthrosc. 2007;15:1144. doi: 10.1007/s00167-006-0281-9. [DOI] [PubMed] [Google Scholar]
- 91.Chen J.M. Willers C. Xu J. Wang A. Zheng M.H. Autologous tenocyte therapy using porcine-derived bioscaffolds for massive rotator cuff defect in rabbits. Tissue Eng. 2007;13:1479. doi: 10.1089/ten.2006.0266. [DOI] [PubMed] [Google Scholar]
- 92.Dejardin L.M. Arnoczky S.P. Clarke R.B. Use of small intestinal submucosal implants for regeneration of large fascial defects: an experimental study in dogs. J Biomed Mater Res. 1999;46:203. doi: 10.1002/(sici)1097-4636(199908)46:2<203::aid-jbm9>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 93.Nicholson G.P. Breur G.J. Van S.D. Yao J.Q. Kim J. Blanchard C.R. Evaluation of a cross-linked acellular porcine dermal patch for rotator cuff repair augmentation in an ovine model. J Shoulder Elbow Surg. 2007;16:S184. doi: 10.1016/j.jse.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 94.Safran O. Derwin K.A. Powell K. Iannotti J.P. Changes in rotator cuff muscle volume, fat content, and passive mechanics after chronic detachment in a canine model. J Bone Joint Surg Am. 2005;87:2662. doi: 10.2106/JBJS.D.02421. [DOI] [PubMed] [Google Scholar]
- 95.Gerber C. Meyer D.C. Frey E. von R.B. Hoppeler H. Frigg R. Jost B. Zumstein M.A. Neer Award 2007: reversion of structural muscle changes caused by chronic rotator cuff tears using continuous musculotendinous traction. An experimental study in sheep. J Shoulder Elbow Surg. 2009;18:163. doi: 10.1016/j.jse.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 96.Schlegel T.F. Hawkins R.J. Lewis C.W. Turner A.S. An in vivo comparison of the modified Mason-Allen suture technique versus an inclined horizontal mattress suture technique with regard to tendon-to-bone healing: a biomechanical and histologic study in sheep. J Shoulder Elbow Surg. 2007;16:115. doi: 10.1016/j.jse.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 97.Marcellin-Little D.J. Levine D. Canapp S.O., Jr. The canine shoulder: selected disorders and their management with physical therapy. Clin Tech Small Anim Pract. 2007;22:171. doi: 10.1053/j.ctsap.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 98.Millis D.L. Levine D. Taylor R.A. Canine Rehabilitation and Physical Therapy. St. Louis; MO: WB Saunders: 2004. [Google Scholar]
- 99.Seiler J.G. Autogenous flexor-tendon grafts. A biomechanical and morphological study in dogs. J Bone Joint Surg Am. 1993;75:1004. doi: 10.2106/00004623-199307000-00006. [DOI] [PubMed] [Google Scholar]
- 100.Derwin K. Androjna C. Spencer E. Safran O. Bauer T.W. Hunt T. Caplan A. Iannotti J. Porcine small intestine submucosa as a flexor tendon graft. Clin Orthop Relat Res. 2004;423:245. doi: 10.1097/01.blo.0000131235.91264.d7. [DOI] [PubMed] [Google Scholar]
- 101.Setton L.A. Mow V.C. Muller F.J. Pita J.C. Howell D.S. Mechanical behavior and biochemical composition of canine knee cartilage following periods of joint disuse and disuse with remobilization. Osteoarthritis Cartilage. 1997;5:1. doi: 10.1016/s1063-4584(97)80027-1. [DOI] [PubMed] [Google Scholar]
- 102.Anderst W.J. Les C. Tashman S. In vivo serial joint space measurements during dynamic loading in a canine model of osteoarthritis. Osteoarthritis Cartilage. 2005;13:808. doi: 10.1016/j.joca.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 103.West J.R. Juncosa N. Galloway M.T. Boivin G.P. Butler D.L. Characterization of in vivo Achilles tendon forces in rabbits during treadmill locomotion at varying speeds and inclinations. J Biomech. 2004;37:1647. doi: 10.1016/j.jbiomech.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 104.Tapper J.E. Fukushima S. Azuma H. Sutherland C. Marchuk L. Thornton G.M. Ronsky J.L. Zernicke R. Shrive N.G. Frank C.B. Dynamic in vivo three-dimensional (3D) kinematics of the anterior cruciate ligament/medial collateral ligament transected ovine stifle joint. J Orthop Res. 2008;26:660. doi: 10.1002/jor.20557. [DOI] [PubMed] [Google Scholar]
- 105.Waltrip R.L. Zheng N. Dugas J.R. Andrews J.R. Rotator cuff repair. A biomechanical comparison of three techniques. Am J Sports Med. 2003;31:493. doi: 10.1177/03635465030310040301. [DOI] [PubMed] [Google Scholar]
- 106.Bicknell R.T. Harwood C. Ferreira L. King G.J. Johnson J.A. Faber K. Drosdowech D. Cyclic loading of rotator cuff repairs: an in vitro biomechanical comparison of bioabsorbable tacks with transosseous sutures. Arthroscopy. 2005;21:875. doi: 10.1016/j.arthro.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 107.Rossouw D.J. McElroy B.J. Amis A.A. Emery R.J. A biomechanical evaluation of suture anchors in repair of the rotator cuff. J Bone Joint Surg Br. 1997;79:458. doi: 10.1302/0301-620x.79b3.6983. [DOI] [PubMed] [Google Scholar]
- 108.Mahar A. Allred D.W. Wedemeyer M. Abbi G. Pedowitz R. A biomechanical and radiographic analysis of standard and intracortical suture anchors for arthroscopic rotator cuff repair. Arthroscopy. 2006;22:130. doi: 10.1016/j.arthro.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 109.Mazzocca A.D. Millett P.J. Guanche C.A. Santangelo S.A. Arciero R.A. Arthroscopic single-row versus double-row suture anchor rotator cuff repair. Am J Sports Med. 2005;33:1861. doi: 10.1177/0363546505279575. [DOI] [PubMed] [Google Scholar]
- 110.Ma C.B. Comerford L. Wilson J. Puttlitz C.M. Biomechanical evaluation of arthroscopic rotator cuff repairs: double-row compared with single-row fixation. J Bone Joint Surg Am. 2006;88:403. doi: 10.2106/JBJS.D.02887. [DOI] [PubMed] [Google Scholar]
- 111.Burkhart S.S. Diaz Pagan J.L. Wirth M.A. Athanasiou K.A. Cyclic loading of anchor-based rotator cuff repairs: confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy. 1997;13:720. doi: 10.1016/s0749-8063(97)90006-2. [DOI] [PubMed] [Google Scholar]
- 112.Tashjian R.Z. Levanthal E. Spenciner D.B. Green A. Fleming B.C. Initial fixation strength of massive rotator cuff tears: in vitro comparison of single-row suture anchor and transosseous tunnel constructs. Arthroscopy. 2007;23:710. doi: 10.1016/j.arthro.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 113.Zheng N. Harris H.W. Andrews J.R. Failure analysis of rotator cuff repair: a comparison of three double-row techniques. J Bone Joint Surg Am. 2008;90:1034. doi: 10.2106/JBJS.G.00049. [DOI] [PubMed] [Google Scholar]
- 114.Barber F.A. Herbert M.A. Boothby M.H. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24:20. doi: 10.1016/j.arthro.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 115.Iannotti J.P. Codsi M.J. Kwon Y.W. Derwin K. Ciccone J. Brems J.J. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88:1238. doi: 10.2106/JBJS.E.00524. [DOI] [PubMed] [Google Scholar]
- 116.Walton J.R. Bowman N.K. Khatib Y. Linklater J. Murrell G.A. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am. 2007;89:786. doi: 10.2106/JBJS.F.00315. [DOI] [PubMed] [Google Scholar]