Abstract
The development of tissue engineering therapies for the intervertebral disc is challenging due to ambiguities of disease and pain mechanisms in patients, and lack of consensus on preclinical models for safety and efficacy testing. Although the issues associated with model selection for studying orthopedic diseases or treatments have been discussed often, the multifaceted challenges associated with developing intervertebral disc tissue engineering therapies require special discussion. This review covers topics relevant to the clinical translation of tissue-engineered technologies: (1) the unmet clinical need, (2) appropriate models for safety and efficacy testing, (3) the need for standardized model systems, and (4) the translational pathways leading to a clinical trial. For preclinical evaluation of new therapies, we recommend establishing biologic plausibility of efficacy and safety using models of increasing complexity, starting with cell culture, small animals (rats and rabbits), and then large animals (goat and minipig) that more closely mimic nutritional, biomechanical, and surgical realities of human application. The use of standardized and reproducible experimental procedures and outcome measures is critical for judging relative efficacy. Finally, success will hinge on carefully designed clinical trials with well-defined patient selection criteria, gold-standard controls, and objective outcome metrics to assess performance in the early postoperative period.
Introduction
Pain of spinal origin afflicts most adults at some point in their lives: the annual U.S. incidence of acute and/or chronic back pain is approximately 100 million. Intervertebral disc degeneration underlies several painful low back disorders, including intervertebral disc herniation, degenerative spondylolisthesis, spinal stenosis, and degenerative disc disease (DDD). For the first three (intervertebral disc herniation, degenerative spondylolisthesis, and spinal stenosis), recent randomized clinical studies have demonstrated advantages of surgical care compared with nonoperative care.1,2 However, DDD management remains the most difficult challenge because the underlying source of pain is unclear, causing uncertainty when developing guidelines for operative and nonoperative care and therapies with improved efficacy.3 As a result, estimates suggest that there are between 1.5 and 4 million adults in the United States with DDD-related chronic low back pain (CLBP) who have failed conservative management and await therapeutic intervention, of which there are few options beyond spinal fusion. These DDD patients are potential candidates for a minimally invasive regenerative therapy.
The goal of this review is to describe the process of painful disc degeneration that forms the basis for therapy development. Next, we will explain the translational pathway for engineered intervertebral disc tissues that may successfully lead to clinical trial and therapeutic development. In the process, we will illustrate appropriate in vitro and in vivo models for efficacy and safety testing, followed by the current needs for preclinical model standardization.
Pathophysiology of CLBP
Because many asymptomatic individuals have image-based evidence of advanced disc degeneration, morphologic features of degeneration may be necessary but are not sufficient for CLBP.4 Consequently, a challenge for developing tissue-engineered disc therapies is that little is known about the cellular and matrix features unique to degenerate/painful (vs. degenerate/nonpainful) discs. These features define the context within which the tissue-engineered construct will function acutely and, equally important, provide the foundation for defining the desired treatment effect. For practical reasons, what little we do know mostly relates to how normal discs compare to degenerate ones in animal or cadaveric models where the identification of painful levels is unfeasible. In healthy tissues, relatively high collagen type I expression marks the anulus fibrosus (AF), whereas the nucleus pulposus (NP) is distinguished by very high levels of aggrecan and collagen type II.5 Other cellular markers that appear to be NP specific include glypican-3, keratin-19, pleiotrophin, heparin-binding factor, hypoxia-inducible factor-1α,6 and CD24.7 However, these more recent reports are limited by the use of rat tissues that contain notochordal cells and thus provide only clues as to what occurs in human, rather than offer definitive findings.
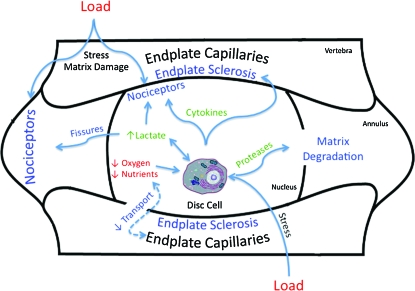
Age-related disc degeneration is a chronic matrix remodeling process that occurs in response to physical inputs, such as impaired transport and/or abnormal mechanical loading, with the reaction to both likely modulated by yet undefined familial risk factors.8,9 At an early age (before 10 years) there is a marked decrease in endplate vascularity and beginnings of structural disorganization. After age 20, the disc becomes sealed-off from the vertebral blood supply by the cartilage endplates and subchondral bone.10,11 Thereafter, disc cell survival is dependent on diffusion from capillaries in the adjacent vertebra (for NP cells) and surrounding vascularized tissues (for anular cells).12 The capillaries within the vertebra terminate just above the hyaline cartilage endplate, providing a continuous capillary bed across the bone–disc interface.13 Once nutrients reach the endplate, movement of small solutes (e.g., glucose and oxygen) pass through disc matrix primarily by diffusion12,14 (larger solutes may also be influenced by convective fluid flow created by mechanical disc compression and recovery). Cells compete for nutrition, making it difficult to sustain high cell densities at the long distances from the nutrition source typical of human lumbar discs (approximately 8 mm).15,16
This disc transport limitation has several negative consequences. NP cells produce energy through anaerobic glycolysis, which utilizes glucose and generates lactic acid as a byproduct.17 Accumulation of lactic acid decreases disc pH (to near pH 6.3) and is detrimental to the matrix as it decreases glycosaminoglycan production, tissue inhibitor of metalloproteinase (TIMP) production, and cell viability.16,18–20 Further, serum deprivation results in decreased cell proliferation and increased cell senescence.21 The direct effect of hypoxia is mixed.17 Some research indicates that hypoxia supports NP cell survival during serum withdrawal,22,23 whereas other data demonstrate that hypoxia causes decreased proteoglycan production and increased lactate production.24
In addition to transport modifications, disc pressure in response to spine loading can accelerate degeneration by causing cellular apoptosis, metalloprotease (MMP) production, and matrix damage.8,9 As a result, structural changes increase progressively with age, and include fibrocartilage replacement of the notochordal nucleus, loss of NP/AF distinction, endplate subchondral bone sclerosis, and focal endplate cartilage disorganization. Degeneration coincides strongly with elevated levels of a variety of inflammatory and degenerative factors, such as cytokines and MMPs.25 Loss of NP aggrecan, the backbone of most NP proteoglycan, is clearly a hallmark of degeneration.26 Not unrelated are observed increases in the aggrecanase, a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS-4), MMP-1, -3, -7, and -13 and reductions in TIMP-1 and TIMP-3 (the latter being a known aggrecanase inhibitor), all or some of which appear linked to increases in interleukin-1 (IL-1) combined with unchanged expression levels of the natural IL-1 receptor antagonist, IL-1Ra.27,28 Other inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and IL-6, are also elevated and likely indicative of degenerative effects.
The features that distinguish degenerate/painful from degenerate/painless discs are less clear. Recent reports suggest that painful discs have increased density of pain-transmitting neurons (nociceptors) at the vertebral endplate and outer AF, presumably as part of granulation tissue that is formed to heal matrix damage that is manifest with internal disc disruption.29–31 Data from other settings demonstrate how nociceptors can be sensitized by cytokines and lactate, thereby diminishing their activation threshold.32–35 Recently, data suggest that painful discs are distinguishable by having an increase in the ratio of lactate to proteoglycan.36 This observation fits well with the theoretical view regarding the nociceptor-stimulatory role of lactate, and the nerve growth-inhibitory role of proteoglycan.37 Based on these and other data, painful discs may be characterized by cellular dysfunction triggered by a confluence of stressful environmental inputs (principally diminished transport; Fig. 1).9
FIG. 1.
Disc cells are adversely influenced by load (pressure), hypoxia, and nutrient deprivation (red). In response, they can secrete lactate, cytokines, and proteases (green). These damage matrix, cause endplate sclerosis, sensitize nociceptors, and exacerbate the adverse effects of load and diminished transport (blue). Sensitized nociceptors can, in turn, be stimulated by tissue stress to cause pain. Color images available online at www.liebertonline.com/ten.
Painful discs in their early stages of degeneration are sensible candidates for regenerative therapies because collateral tissue deterioration (e.g., osteophytes, facet arthritis, and anular herniation) is minimal, increasing the likelihood that the disc is the sole pain generator. However, the disc's large size, ischemic and inflammatory milieu, and demanding biomechanical requirements make this tissue particularly challenging for biologically based therapies. Ideally, these features that are anticipated at the treatment site should be reproduced in preclinical models for therapy optimization.
Translational Pathways for Engineered Intervertebral Disc Tissues
In general, an efficient pathway for a tissue-engineered therapy for low back pain consists of (1) selection of pain biomarkers that form the mechanistic basis for therapy development, (2) biologic plausibility studies in cell culture and small animals, (3) biomechanical studies in human cadavers, (4) safety studies, (5) large animal trials, (6) clinical trial planning, and (7) cost/effectiveness analyses. Because the ultimate clinical objective of spinal pain reduction is difficult to assess in nonhuman models, surrogate efficacy measures need to be developed, as well as those that establish safety.
Selection of pain biomarkers
The therapeutic target for a tissue-engineered strategy needs to be clarified at the outset. As discussed above, the pain generator and underlying mechanism associated with discogenic pain are unclear in most patients. Typically suspected factors include reduced proteoglycan quality leading to biomechanical instability and tissue damage accumulation, plus increased disc innervation and sensitization, all of which are exacerbated by poor transport and ineffective wound healing.9,38,39 Together, these can result in increased risk of nociceptor irritation due to hypoxia, pressure changes, and inflammation.40,41
Consequently, therapies are typically designed to target one or more axes of the triad of innervation, inflammation, and mechanical stability. For example, disc denervation as a basis for therapy has been implemented thermally (i.e., intradiscal electrothermal therapy42,43) or chemically (e.g., methylene blue44). Antiinflammatory therapies comprise inhibitors of several cytokines, including IL-1, IL-6, IL-8, TNF-α,45 and metalloproteinases that are part of the wound-healing cascade.46 These may include TNF antagonists such as Etanercept (Embrel®; Amgen, Thousand Oaks, CA), or Infliximab (Remicade®; J&J, Langhorne, PA), IL antagonists (Kineret®; Amgen), and other antiinflammatory nutraceuticals such as glucosamine.47 Mechanical stability may be augmented by stimulating disc matrix synthesis and/or delivering an intradiscal scaffold to re-establish nuclear pressure. A number of growth factors have been shown to stimulate proteoglycan synthesis by disc cells, including transforming growth factor-β, insulin-like growth factor, platelet-derived growth factor, bone morphogenetic protein-2 and -7, and growth and differentiation factor-5.48 Polymers of various types have been developed to replace or augment the NP and its ability to support spinal compression and synergize with the anular ligament.49 These implants can be designed as stand-alone devices, drug delivery vehicles, or cell-supporting carriers.50
Biologic plausibility studies—in vitro systems
The plausibility of biological efficacy should be established in models of increasing complexity (Table 1). To minimize the use of animals and to reduce therapy development costs, in vitro cell culture systems ought to be fully utilized. Two- and three-dimensional cell culture systems can be useful for initially demonstrating cellular effects, dosing, and toxicity. If the purpose is to screen a large number of compounds, a high-density monolayer culture of primary cells may be suitable because the format permits the use of robotic manipulation. Three-dimensional culture systems, such as alginate bead culture,70,71 are useful to study disc cells in a phenotypically stable state.64 Although other culture systems, such as collagen gel, or collagen-coated monolayer culture, may be suitable for AF cells, the use of two different culture systems in the same experiment may add complications for protocol development and data interpretation.
Table 1.
Preclinical Models for Intervertebral Disc Tissue Engineering
| In vitro |
|
|
||
|---|---|---|---|---|
| Cell culture | Organ culture | Biomechanical | Outcome measures | References |
| Bovine tail disc cells Human disc cells |
Cell viability, proliferation, matrix production, cytokine secretion |
51,52 53 |
||
| Bovine tail Sheep tail Rabbit |
Cell viability, proliferation, matrix production, cytokine secretion, water content, morphology, biomechanics | 54–57 | ||
| Human cadaver | Range of motion, neutral zone, stiffness, strength, implant migration, implant retention, fatigue resistance | 58,59 | ||
| In vivo |
|
|
|
|
|---|---|---|---|---|
| Small animal | Large animal | |||
| Rat, rabbit | X-ray measure of disc height, MRI and histologic grading, GAG and collagen content, gene expression, cytokine production | 60–66 | ||
| Goat, minipig | Biomechanics, disc height, MRI and histologic grading, GAG and collagen content, cytokine production | 67–69 | ||
GAG, glycosaminoglycan; MRI, magnetic resonance imaging.
As a cell source, the bovine coccygeal tail has been shown to be appropriate in terms of cellar phenotype.51,52 However, molecular probes and antibodies for bovine tissues are limited, and other species, such as the pig or rat, may be selected with caution for the presence of notochordal cells whose behavior may not reflect that of mature human NP cells.
Recent emphasis has been placed on the use of whole-disc organ culture models.54,57 This approach allows testing of effects of compounds, cells, and mechanical loading on host disc cells that are maintained in their native environment. However, only acute effects can be evaluated because the extent to which normal cell viability and cell function can be maintained with this approach needs further study. In addition, unless discs are harvested from animals used for food production (bovine) or sacrificed for other research uses (rat tail or rabbit spine), this technique will not reduce the numbers of animals used for research and consequently may not provide value beyond standard in vivo testing. The pros and cons on the use of this approach should be carefully considered.
After mode of effect studies on animal cells, it is recommended that the results be confirmed using human cells that may be harvested from surgical waste tissues. It may also be necessary to use human cells for the screening process when testing the effects of biologics that are human specific. Because there are significant variations in responses of human cells among donors, based on age and degree of degeneration, the experiments need to be repeated enough times to confirm the results.53 Additionally, there is risk of de-differentiation during serial passaging needed to increase cell numbers, and, consequently, expansion should be limited to passage three.72 Because disc tissues swell considerably, the development of a human disc organ culture systems for therapy screening may be impractical due to the need for complicated apparatus73 or procedures.74,75
Biomechanical studies in human cadavers
Several steps in the therapeutic trajectory of disc tissue engineering require biomechanical testing in a human cadaveric model. Challenging biomechanical features of the in vivo system influence successful therapy delivery, such as high interdiscal pressures,76 spatially varying tissue mechanical properties,77,78 and complex motion and load distribution patterns.79,80 Most disc tissue engineering approaches include surgical delivery with or without the use of carriers or devices. Consequently, biomechanical studies with human cadavers help refine surgical protocols that will be implemented as clinical trials. Also, implant migration and retention under physiologic cyclic loading is an important efficacy and safety consideration. The acute biomechanical kinematic response (e.g., range of motion, instant axis of rotation, and neutral zone) after treatment helps establish whether the treatment system supports mechanical stability important for proper disc healing.8 Equally important is to characterize the biomechanical properties of regenerated tissues. Newly synthesized matrix should be comparable to and integrated within native tissues so as to assure proper long-term biomechanics and minimize risk of extradiscal migration of scaffold or matrix.
Biologic plausibility studies—in vivo models
Ultimately, small animal studies are a critical next step because of the important in situ interactions between disc cells and spatially varying host features unique to the healing disc environment: pressure, hypoxia, degraded matrix, cytokines, other stromal and inflammatory cells, plus systemic factors. With this increasing model complexity comes challenges in response interpretation. Outcome measures should be coupled to the designed treatment mechanisms, but overall indices of disc quality, such as histology and biomechanics, are also desirable. Time dependence of the outcomes is critical to establish whether the therapeutic response is persistent above the background degenerative response typically triggered by the therapy delivery. Design-of-experiment techniques for study design and statistical analyses can help establish sample size and efficiently optimize treatment parameters.81
There have been a number of animal models developed with the aim of mimicking human DDD (see review in Refs.9,82,83). However, for the translational research required to develop clinical therapies, the role of animal models differs from that for basic science study of DDD mechanisms. Above all, it is important for researchers and regulatory agencies to understand animal model limitations—mainly that no animal model perfectly represents the pathological changes and cellular responses of the human due to differences in size, structure, cellular and matrix composition, biomechanical properties, and loading.82 Yet, useful information can be obtained when animal model selection is based on the mode of action of the therapy under investigation.
With these caveats in mind, how is an animal model selected? An animal model to assess the efficacy, safety, and therapy mechanism of action must reflect at least one, or more, clinically relevant aspects of human disc diseases, that is, a decrease in matrix component(s), disc height loss, biomechanical hypermobility, or expression of catabolic enzymes or inflammatory cytokines. Importantly, the model should be capable of supporting the desired changes and quantitative techniques utilized to assess posttherapy modifications. Currently, a reproducible, slowly progressive, and quantifiable large animal model of disc degeneration does not exist. That being said, this review will concentrate on those animal models that have been shown useful for identifying effective therapies for human disc diseases.
If the therapeutic aim is to alter the metabolic status of disc cells and thereby induce structural repair, several species can be used. Rat models using mechanically stressed tails are convenient to study the effects of cell supplementation60 or to screen target molecules.84 The efficacy of cell injection has also been demonstrated in a rabbit disc degeneration model induced by NP aspiration.61–63 The presence of the NP defect created by matrix aspiration simulates a nucleotomy, and may be appropriate to test the feasibility of tissue-engineered NP regeneration strategies. For example, this model has been used to study the effects of platelet-rich plasma encapsulated in gelatin microspheres.85
In small animals, mild and progressive disc degeneration can be induced by surgical needle puncture of the AF. This model type has been used to test the efficacy of growth factor therapies.65,86,87 However, care has to be used in interpreting the cellular response because the NP of rats and rabbits contain a large number of notochordal cells that have been shown to have a metabolic activity different from adult human NP cells.88 Yet, when disc degeneration is first induced (by means such as a disc puncture, enzyme injection, or compression), notochordal cells may eventually be replaced with fibrochondrocytic and chondrocytic cells,89,90 generating a phenotype that may be more representative of human adult discs. By contrast, characteristics of animal and human AF are not drastically different, and therefore the effects of therapy on the AF can be effectively assessed in animals.
Because pain is the major reason patients with DDD seek treatment, alleviation of pain is considered to be the primary desirable outcome. To date, a quantitative assessment for back pain in animals has not yet been developed. The fact that the specific pain mechanism from DDD has not been characterized represents a large hurdle to the development surrogate pain measures in animals and to detect the direct effect of therapies aimed at reducing pain. For sciatic nerve pain or dorsal root ganglion–related pain, there have been successful reports of pain assessment, notably on mechanical allodynia.91–94 However, the assessment of discogenic pain is very limited.66 Recently, Olmarker has reported that rats receiving anular puncture showed a significant increase in grooming and in wet-dog shakes.95 Likewise, Rousseau et al. quantified physical disability in rats after lumbar disc stab injury.66 The sensitivity of these measurements to assess the efficacy of drugs or treatment should be further investigated, and the standardization of methodology will be required to compare results from different groups.
Safety studies
Safety needs to be established in preclinical models. The avascular nature of the disc environment can lead to persistence of active therapeutic agents, secreted cytokines, and carrier degradation products. Consequently, even though a growth factor or carrier has an established use track record in other tissues, they need to be evaluated in the unique NP environment. Adverse reactions can manifest through interdiscal toxicity, inflammatory cell recruitment, and matrix erosion. For example, cytokines and scaffold degradation products can diffuse from the disc and incite a sclerotic reaction in the adjacent vertebral endplates, along the delivery wound site, or outside the AF.96 Equally important is to establish the reaction to extradiscal placement of the therapeutic materials and delivery vehicles. It is likely that these can escape from the disc during surgery or early in the postsurgical period. Inflammation and the mass effect induced by these materials can adversely affect adjacent nerve roots and other paraspinal tissues.97 The safety of injected materials or cells should also be confirmed under the worst-case scenario to avoid a catastrophic event, such as the one seen for chemonucleolysis.98
Large animal trials
After efficacy and safety are established in vitro and in small animals, large animal studies are required to motivate clinical use, principally because of size effects on disc transport17 and biomechanics.99 The animals should be skeletally mature (so as not to grow during the study period), and have disc size and biomechanics as close as possible to those of the human. For the lumbar spine, these include the goat,67 minipig,68,69 and primates100 that have disc heights ranging from 5 to 7 mm.99 However, for ethical and cost reasons primates are less desirable. As with other preclinical models, efficacy may be difficult to establish due to a lack of relevant starting points (e.g., degeneration and pain) and clinical metrics that match the intended patient population (e.g., disability). Yet, biologic plausibility should be supported as well as safety through radiographical, histological, biochemical, and biomechanical assays. Comparisons to negative controls (surgical procedure without treatment delivery) and untreated levels can help judge effect size and potential clinical relevance.
Standardization of animal models and experimental procedures
Currently, there is no consensus on which animal species must be used for preclinical studies before initiating human clinical trials. Clinical studies on growth factors were initiated with efficacy studies using the rabbit anular needle puncture model of disc degeneration. However, there are no defining standard about the models and species that are considered sufficient, for example, American Society for Testing and Materials standards. The establishment of some form of standard, which delineates the species recommendation, age, injection volume, and follow-up period, may be helpful to develop new therapeutic approaches. It is essential to carefully consider the experimental levels used within spinal columns. In rabbits, the disc sizes and mechanical properties are similar in the upper lumbar spine (L2/3, L3/4, and L4/5), but L5/6 and L6/7 are significantly larger and show different biomechanical properties. When multiple level experiments are performed, either the absence of adjacent level effects must be confirmed, or the placement of a nontreatment control disc between the treatment levels is recommended. In each species, the injection volume, which is calculated based on the disc volume, should be translatable to the human disc.
Depending on the material and drugs applied, it is essential to perform proper safety studies (systemic and local); these can be done in a rodent or small animal (i.e., the rabbit). The effects on other organs (liver and kidney) should also be assessed. Because the disc is avascular, diffusion and transport may significantly influence the kinetics of injected growth factors, cells, and scaffolds.17 For example, polymer degradation product accumulation and decreased pH may have deleterious effects on disc cells. This may not be adequately tested in small animals because of the differences in disc size with the human.
To develop new therapeutic approaches, experiments need to be performed with proper documentation and procedures. The reproducibility of disc degeneration is one of the most important points in establishing the testing protocol. Because animal models generally involve a surgical procedure, it is difficult to perform the exact same procedure, even at the same institution. The reasons for this include different procedures by surgeons (intrasurgeon variability), differences in anesthesia, and age, sex, and strain of animals. Written standard operation protocols and education systems are essential. The learning curve of surgical procedures must be taken into consideration, and frequent checks by the principal investigator on procedures are required. Slight variations in each procedure over time need detailed documentation.
Because the degree of degeneration is different in each animal, experiment groups need to be randomized before the treatment procedure, with the result that control and experiment groups have a similar distribution of degeneration. Radiographic assessment of disc degeneration may be adequate, but magnetic resonance imaging (MRI) for live animals is best if the cost and environment are appropriate.
Records of the size of cage and animal source, age, and weight are essential because these factors influence the results. The availability of animals of a specific age is a concern when the experiment size is large. If the difference in degree of degeneration and response to the therapy is confirmed, younger (less expensive and more available) animals can be used. For example, 5–6-month-old rabbits were shown to have changes similar to 2-year-old rabbits in an anular puncture model.65,101
Radiographic analyses need to be carefully performed. Mal-alignment of the X-ray beam and positioning error of the spine are major sources of error. Importantly, the depth of anesthesia influences disc height; therefore, it is critical to maintain the same depth of anesthesia during X-rays throughout the course of a study. For rat experiments, the use of microCT may provide an accurate disc height measurement and structural analysis. The quantitative measurement of disc height is best performed in a blind fashion with the inter- and intraobserver error checked.
Histology and MRI grading also must be performed by individuals blinded to the study. Because there are significant differences in histology during development and aging, experimental levels of each animal should always be assessed together with an internal control level. There are several histological grading systems65,102 and MRI grading systems,103,104 and it is important that some standardization be established by the research community. Recently developed techniques, such as T2 mapping and T1rho, can be used to quantify the quality of matrix in both the NP and AF of small animals if the higher magnet, 3T MRI, is used.105
The quantification of biochemical components is relatively straightforward. Established methods to determine glycosaminoglycan and collagen contents can be used. It is best to avoid the use of ELISA to determine collagen content because the extraction efficiency of collagen is problematic. The assessment of mRNA in the NP and AF requires careful extraction and purification. The use of real-time polymerase chain reaction with standards is recommended because of multiple samples and differences in quality of mRNA extracted from tissues.
Because the disc is an important structural tissue for supporting spinal compression and intervertebral movement,106 efficacy and safety should also be judged using biomechanical criteria. These tests are important to establish the mechanical properties and integration of regenerated tissues; risk for herniation or implant migration; and restoration of physiologic kinetics. A simple axial compression test can be useful to investigate the therapy's ability to reconstitute tissues appropriate for supporting spinal loads. Yet, because the disc is poroviscoelastic, the specifics of testing can confound interpretation and therefore requires standardization. Cyclic loading is typical, with several cycles (between 5 and 20) of preconditioning performed before collecting analyzable data.99,107 Because of the extended duration of specimen preparation and testing, samples need to be hydrated to maintain physiologic behavior.87,108 Data from these experiments can be used to gauge the material properties of regenerated tissues, overall strength of the treated disc, and migration/expulsion propensity. The animal species appropriate for compression test evaluation is mostly dependent on size and the investigator's ability to reasonably replicate the anticipated clinical protocols. Therefore, it may be difficult to perform the salient surgical technique in small animals. That being said, the results can be scaled to human by accounting for differences in disc height and cross-sectional area.99 Similar features may be gleaned from pressure/volume testing,109 with more emphasis on healing of anular fissures.
The treatment's ability to re-establish biomechanical stability can be evaluated using flexibility testing (bending or torsion). These tests usually involve application of pure moments as a reproducible boundary condition, although it is acknowledged that this does not replicate in vivo loads.110 Yet, superposition of compression and/or shear forces along with moments significantly complicates the testing regimen,111 and may not be required when doing comparison tests with control samples. Parameters such as range-of-motion, bending stiffness, and neutral zone are typically used to quantify the specimen's performance.112 Fatigue tests should be performed when nondegrading engineered materials are used for long-term defect filling. In this regard, it is necessary to establish a standard method to assess material durability, perhaps using synthetic or organ culture systems.82
Clinical trial planning
Given that clinical outcomes have a circular reliance on diagnosis accuracy and treatment efficacy, a principal challenge ultimately with intervertebral disc therapies is establishing criteria to define spinal levels appropriate for treatment and metrics to judge success in the early postoperative period. There is currently significant debate regarding the methods used for selecting patients for lumbar surgery to treat discogenic pain. The two most commonly used methods are MRI113 and discography, which are considered by many to be the gold standards to localize the pain generator,114–116 but recent concerns about specificity, sensitivity, and safety117 have raised concerns about their use. There are a number of alternate approaches currently under investigation that include MR spectroscopy,36 T1-rho imaging,118,119 MR diffusion imaging,120,121 and kinematic imaging.122,123 Yet, until these methods can be validated, subjectivity in selecting treatment levels can confound the ability to critically evaluate the short-term clinical effect of novel therapies.
Cost effectiveness analyses and outcomes
The clinical adoption of tissue engineering strategies for disc repair will ultimately rely on treatment outcomes,124 their comparison to current gold standards, cost, and payer reimbursement.125–127 Clinical trials to establish superiority, or at least noninferiority, to currently approved treatments, such as fusion, require regulatory approval and study-site IRB review.128 An understanding of the regulatory process is critical to planning the efficient introduction of important new technologies. However, this arduous process is expensive, and raising sufficient funds to execute a well-conceived clinical trial typically relies on solid intellectual property protection, and corporate or venture capital support.129,130
However, a great deal of commercial risk can remain even after scientific and clinical risks have been overcome,131 underscoring the need for a broad team of experts who understand all aspects of the scientific, clinical, and development issues associated with medical implants (Figs. 2 and 3). These teams often span industries and universities that are not natural partners given organizational and cultural differences.131,132 Ultimately, the goal of improving health is shared by both groups, as it is the stated mission of health-focused universities and is also the result most likely to produce commercial success.
FIG. 2.
Development pathway and time line for cell-based therapy.
FIG. 3.
Development pathway for drug and growth factor.
Summary and Authors' Opinions
This review has outlined the major opportunities and challenges associated with intervertebral disc tissue engineering. Clearly, there is a need for standardization of animal models, outcome measures, and success metrics. Although there is no ideal model, small animals, such as rats and rabbits, are useful for screening purposes. Ultimately, large animals such as goats and minipigs are preferred so as to mimic nutritional and surgical constraints of human application. Typical success metrics include measures of morphology (e.g., disc height, annular delamination, and disc degeneration grade via histology and MRI), cellularity, matrix quality/quantity, cytokine levels, and biomechanics (e.g., compressive strength, pressure/volume testing, and range of motion). Yet, the pace of therapy development would benefit from consensus on tools, techniques, and target outcome values that would be generally adoptable to specific technologies. Toward that end, we recommend that training centers be established where those experienced with validated model systems disseminate valuable techniques (e.g., animal surgical protocols) and tools (e.g., antibodies and primers) to facilitate efficient progress, and allow straightforward comparisons of results across research sites. Dissemination of negative results, which are normally not publishable, would be beneficial so that mistakes are not repeated. Funding should be directed toward establishing and validating these models, ideally coming from combined federal and industrial sources. To monitor success noninvasively and facilitate clinical trials, advanced imaging techniques need to be developed and validated in animal models and translated to humans because it is not feasible to perform disc biopsies. Finally, efforts need to be focused on improved diagnosis and patient selection criteria. Ultimately, even the best therapy will not be clinically successful if applied to the wrong patient. These efforts stress close interactions between basic scientists, clinicians, and industrial partners.
Disclosure Statement
No competing financial interests exist.
References
- 1.Weinstein J.N. Lurie J.D. Tosteson T.D. Hanscom B. Tosteson A.N. Blood E.A. Birkmeyer N.J. Hilibrand A.S. Herkowitz H. Cammisa F.P. Albert T.J. Emery S.E. Lenke L.G. Abdu W.A. Longley M. Errico T.J. Hu S.S. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007;356:2257. doi: 10.1056/NEJMoa070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinstein J.N. Lurie J.D. Tosteson T.D. Skinner J.S. Hanscom B. Tosteson A.N. Herkowitz H. Fischgrund J. Cammisa F.P. Albert T. Deyo R.A. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT) observational cohort. JAMA. 2006;296:2451. doi: 10.1001/jama.296.20.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schafer J. O'Connor D. Feinglass S. Salive M. Medicare Evidence Development and Coverage Advisory Committee Meeting on lumbar fusion surgery for treatment of chronic back pain from degenerative disc disease. Spine. 2007;32:2403. doi: 10.1097/BRS.0b013e3181573841. [DOI] [PubMed] [Google Scholar]
- 4.Boden S.D. Davis D.O. Dina T.S. Patronas N.J. Wiesel S.W. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403. [PubMed] [Google Scholar]
- 5.Adams P. Eyre D.R. Muir H. Biochemical aspects of development and ageing of human lumbar intervertebral discs. Rheumatol Rehabil. 1977;16:22. doi: 10.1093/rheumatology/16.1.22. [DOI] [PubMed] [Google Scholar]
- 6.Lee C.R. Sakai D. Nakai T. Toyama K. Mochida J. Alini M. Grad S. A phenotypic comparison of intervertebral disc and articular cartilage cells in the rat. Eur Spine J. 2007;16:2174. doi: 10.1007/s00586-007-0475-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujita N. Miyamoto T. Imai J. Hosogane N. Suzuki T. Yagi M. Morita K. Ninomiya K. Miyamoto K. Takaishi H. Matsumoto M. Morioka H. Yabe H. Chiba K. Watanabe S. Toyama Y. Suda T. CD24 is expressed specifically in the nucleus pulposus of intervertebral discs. Biochem Biophys Res Commun. 2005;338:1890. doi: 10.1016/j.bbrc.2005.10.166. [DOI] [PubMed] [Google Scholar]
- 8.Lotz J.C. Animal models of intervertebral disc degeneration: lessons learned. Spine. 2004;29:2742. doi: 10.1097/01.brs.0000146498.04628.f9. [DOI] [PubMed] [Google Scholar]
- 9.Lotz J.C. Ulrich J.A. Innervation, inflammation, and hypermobility may characterize pathologic disc degeneration: review of animal model data. J Bone Joint Surg Am. 2006;88(Suppl 2):76. doi: 10.2106/JBJS.E.01448. [DOI] [PubMed] [Google Scholar]
- 10.Nachemson A. Lewin T. Maroudas A. Freeman M.A.R. In Vitro Diffusion of dye through the end-plates and the annulus fibrosus of human lumbar intervertebral discs. AOS. 1970;41:589. doi: 10.3109/17453677008991550. [DOI] [PubMed] [Google Scholar]
- 11.Bernick S. Cailliet R. Vertebral end-plate changes with aging of human vertebrae. Spine. 1982;7:97. doi: 10.1097/00007632-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Bartels E.M. Fairbank J.C. Winlove C.P. Urban J.P. Oxygen and lactate concentrations measured in vivo in the intervertebral discs of patients with scoliosis and back pain. Spine. 1998;23:1. doi: 10.1097/00007632-199801010-00001. discussion 8. [DOI] [PubMed] [Google Scholar]
- 13.Crock H.V. Goldwasser M. Yoshizawa H. Vascular anatomy related to the intervertebral disc. In: Ghosh P., editor. The Biology of the Intervertebral Disc. Vol. 1. Boca Raton, FL: CRC Press; 1988. p. 109. [Google Scholar]
- 14.Urban M.R. Fairbank J.C. Etherington P.J. Loh F.L. Winlove C.P. Urban J.P. Electrochemical measurement of transport into scoliotic intervertebral discs in vivo using nitrous oxide as a tracer. Spine. 2001;26:984. doi: 10.1097/00007632-200104150-00028. [DOI] [PubMed] [Google Scholar]
- 15.Stairmand J.W. Holm S. Urban J.P.G. Factors influencing oxygen concentration gradients in the intervertebral disc. Spine. 1991;16:444. doi: 10.1097/00007632-199104000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Horner H.A. Urban J.P. 2001 Volvo Award Winner in Basic Science Studies: effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine. 2001;26:2543. doi: 10.1097/00007632-200112010-00006. [DOI] [PubMed] [Google Scholar]
- 17.Urban J.P. Smith S. Fairbank J.C. Nutrition of the intervertebral disc. Spine. 2004;29:2700. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 18.Bibby S.R. Urban J.P. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur Spine J. 2004;13:695. doi: 10.1007/s00586-003-0616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Razaq S. Wilkins R.J. Urban J.P. The effect of extracellular pH on matrix turnover by cells of the bovine nucleus pulposus. Eur Spine J. 2003;12:341. doi: 10.1007/s00586-003-0582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohshima H. Urban J.P.G. The effect of lactate and pH on proteoglycan and protein synthesis rates in the intervertebral disc. Spine. 1992;17:1079. doi: 10.1097/00007632-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Johnson W.E. Stephan S. Roberts S. The influence of serum, glucose and oxygen on intervertebral disc cell growth in vitro: implications for degenerative disc disease. Arthritis Res Ther. 2008;10:R46. doi: 10.1186/ar2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Risbud M.V. Albert T.J. Guttapalli A. Vresilovic E.J. Hillibrand A.S. Vaccaro A.R. Shapiro I.M. Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro: implications for cell-based transplantation therapy. Spine. 2004;29:2627. doi: 10.1097/01.brs.0000146462.92171.7f. [DOI] [PubMed] [Google Scholar]
- 23.Risbud M.V. Fertala J. Vresilovic E.J. Albert T.J. Shapiro I.M. Nucleus pulposus cells upregulate PI3K/Akt and MEK/ERK signaling pathways under hypoxic conditions and resist apoptosis induced by serum withdrawal. Spine. 2005;30:882. doi: 10.1097/01.brs.0000159096.11248.6d. [DOI] [PubMed] [Google Scholar]
- 24.Ishihara H. Urban J.P. Effects of low oxygen concentrations and metabolic inhibitors on proteoglycan and protein synthesis rates in the intervertebral disc. J Orthop Res. 1999;17:829. doi: 10.1002/jor.1100170607. [DOI] [PubMed] [Google Scholar]
- 25.Burke J.G. Watson R.W. McCormack D. Dowling F.E. Walsh M.G. Fitzpatrick J.M. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84:196. doi: 10.1302/0301-620x.84b2.12511. [DOI] [PubMed] [Google Scholar]
- 26.Mwale F. Roughley P. Antoniou J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc. Eur Cell Mater. 2004;8:58. doi: 10.22203/ecm.v008a06. discussion 63–64. [DOI] [PubMed] [Google Scholar]
- 27.Tsuji T. Chiba K. Imabayashi H. Fujita Y. Hosogane N. Okada Y. Toyama Y. Age-related changes in expression of tissue inhibitor of metalloproteinases-3 associated with transition from the notochordal nucleus pulposus to the fibrocartilaginous nucleus pulposus in rabbit intervertebral disc. Spine. 2007;32:849. doi: 10.1097/01.brs.0000259804.39881.62. [DOI] [PubMed] [Google Scholar]
- 28.Le Maitre C.L. Freemont A.J. Hoyland J.A. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown M.F. Hukkanen M.V. McCarthy I.D. Redfern D.R. Batten J.J. Crock H.V. Hughes S.P. Polak J.M. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Joint Surg Br. 1997;79:147. doi: 10.1302/0301-620x.79b1.6814. [DOI] [PubMed] [Google Scholar]
- 30.Freemont A.J. Watkins A. Le Maitre C. Baird P. Jeziorska M. Knight M.T. Ross E.R. O'Brien J.P. Hoyland J.A. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197:286. doi: 10.1002/path.1108. [DOI] [PubMed] [Google Scholar]
- 31.Freemont A.J. Peacock T.E. Goupille P. Hoyland J.A. O'Brien J. Jayson M.I. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178. doi: 10.1016/s0140-6736(97)02135-1. [DOI] [PubMed] [Google Scholar]
- 32.Naves L.A. McCleskey E.W. An acid-sensing ion channel that detects ischemic pain. Braz J Med Biol Res. 2005;38:1561. doi: 10.1590/s0100-879x2005001100001. [DOI] [PubMed] [Google Scholar]
- 33.Sutherland S.P. Benson C.J. Adelman J.P. McCleskey E.W. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc Natl Acad Sci U S A. 2001;98:711. doi: 10.1073/pnas.011404498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMahon S.B. Cafferty W.B. Neurotrophic influences on neuropathic pain. Novartis Found Symp. 2004;261:68. discussion 92–102, 149–154. [PubMed] [Google Scholar]
- 35.Cavanaugh J.M. Ozaktay A.C. Yamashita T. Avramov A. Getchell T.V. King A.I. Mechanisms of low back pain: a neurophysiologic and neuroanatomic study. Clin Orthop Relat Res. 1997;335:166. [PubMed] [Google Scholar]
- 36.Keshari K.R. Lotz J.C. Link T.M. Hu S.S. Majumdar S. Kurhanewicz J. Lactic acid and proteoglycans as metabolic markers for discogenic back pain. Spine. 2008;33:312. doi: 10.1097/BRS.0b013e31816201c3. [DOI] [PubMed] [Google Scholar]
- 37.Dou C.L. Levine J.M. Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J Neurosci. 1994;14:7616. doi: 10.1523/JNEUROSCI.14-12-07616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeLeo J.A. Winkelstein B.A. Physiology of chronic spinal pain syndromes: from animal models to biomechanics. Spine. 2002;27:2526. doi: 10.1097/00007632-200211150-00026. [DOI] [PubMed] [Google Scholar]
- 39.Zhou Y. Abdi S. Diagnosis and minimally invasive treatment of lumbar discogenic pain—a review of the literature. Clin J Pain. 2006;22:468. doi: 10.1097/01.ajp.0000208244.33498.05. [DOI] [PubMed] [Google Scholar]
- 40.Brisby H. Pathology and possible mechanisms of nervous system response to disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):68. doi: 10.2106/JBJS.E.01282. [DOI] [PubMed] [Google Scholar]
- 41.Edgar M.A. The nerve supply of the lumbar intervertebral disc. J Bone Joint Surg Br. 2007;89:1135. doi: 10.1302/0301-620X.89B9.18939. [DOI] [PubMed] [Google Scholar]
- 42.Maurer P. Block J.E. Squillante D. Intradiscal electrothermal therapy (IDET) provides effective symptom relief in patients with discogenic low back pain. J Spinal Disord Tech. 2008;21:55. doi: 10.1097/BSD.0b013e31812f4f29. [DOI] [PubMed] [Google Scholar]
- 43.Saal J.A. Saal J.S. Intradiscal electrothermal therapy for the treatment of chronic discogenic low back pain. Clin Sports Med. 2002;21:167. doi: 10.1016/s0278-5919(03)00064-4. [DOI] [PubMed] [Google Scholar]
- 44.Peng B. Zhang Y. Hou S. Wu W. Fu X. Intradiscal methylene blue injection for the treatment of chronic discogenic low back pain. Eur Spine J. 2007;16:33. doi: 10.1007/s00586-006-0076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maeda S. Kokubun S. Changes with age in proteoglycan synthesis in cells cultured in vitro from the inner and outer rabbit annulus fibrosus. Responses to interleukin-1 and interleukin-1 receptor antagonist protein. Spine. 2000;25:166. doi: 10.1097/00007632-200001150-00005. [DOI] [PubMed] [Google Scholar]
- 46.Olmarker K. Neovascularization and neoinnervation of subcutaneously placed nucleus pulposus and the inhibitory effects of certain drugs. Spine. 2005;30:1501. doi: 10.1097/01.brs.0000167823.17687.ec. [DOI] [PubMed] [Google Scholar]
- 47.Walsh A.J. O'Neill C.W. Lotz J.C. Glucosamine HCl alters production of inflammatory mediators by rat intervertebral disc cells in vitro. Spine J. 2007;7:601. doi: 10.1016/j.spinee.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 48.Masuda K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur Spine J. 2008;17(Suppl 4):441. doi: 10.1007/s00586-008-0749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goins M.L. Wimberley D.W. Yuan P.S. Fitzhenry L.N. Vaccaro A.R. Nucleus pulposus replacement: an emerging technology. Spine J. 2005;5(6 Suppl):317S. doi: 10.1016/j.spinee.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 50.Kandel R. Roberts S. Urban J.P. Tissue engineering and the intervertebral disc: the challenges. Eur Spine J. 2008;17(Suppl 4):480. doi: 10.1007/s00586-008-0746-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oshima H. Ishihara H. Urban J.P. Tsuji H. The use of coccygeal discs to study intervertebral disc metabolism. J Orthop Res. 1993;11:332. doi: 10.1002/jor.1100110304. [DOI] [PubMed] [Google Scholar]
- 52.Demers C.N. Antoniou J. Mwale F. Value and limitations of using the bovine tail as a model for the human lumbar spine. Spine. 2004;29:2793. doi: 10.1097/01.brs.0000147744.74215.b0. [DOI] [PubMed] [Google Scholar]
- 53.Imai Y. Miyamoto K. An H.S. Thonar E.J. Andersson G.B. Masuda K. Recombinant human osteogenic protein-1 upregulates proteoglycan metabolism of human anulus fibrosus and nucleus pulposus cells. Spine. 2007;32:1303. doi: 10.1097/BRS.0b013e3180593238. discussion 1310. [DOI] [PubMed] [Google Scholar]
- 54.Roberts S. Menage J. Sivan S. Urban J.P. Bovine explant model of degeneration of the intervertebral disc. BMC Musculoskelet Disord. 2008;9:24. doi: 10.1186/1471-2474-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Korecki C.L. MacLean J.J. Iatridis J.C. Characterization of an in vitro intervertebral disc organ culture system. Eur Spine J. 2007;16:1029. doi: 10.1007/s00586-007-0327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haschtmann D. Stoyanov J.V. Ettinger L. Nolte L.P. Ferguson S.J. Establishment of a novel intervertebral disc/endplate culture model: analysis of an ex vivo in vitro whole-organ rabbit culture system. Spine. 2006;31:2918. doi: 10.1097/01.brs.0000247954.69438.ae. [DOI] [PubMed] [Google Scholar]
- 57.Gantenbein B. Grunhagen T. Lee C.R. van Donkelaar C.C. Alini M. Ito K. An in vitro organ culturing system for intervertebral disc explants with vertebral endplates: a feasibility study with ovine caudal discs. Spine. 2006;31:2665. doi: 10.1097/01.brs.0000244620.15386.df. [DOI] [PubMed] [Google Scholar]
- 58.Wilke H.J. Kavanagh S. Neller S. Haid C. Claes L.E. Effect of a prosthetic disc nucleus on the mobility and disc height of the L4-5 intervertebral disc postnucleotomy. J Neurosurg. 2001;95((2 Suppl)):208. doi: 10.3171/spi.2001.95.2.0208. [DOI] [PubMed] [Google Scholar]
- 59.Bertagnoli R. Sabatino C.T. Edwards J.T. Gontarz G.A. Prewett A. Parsons J.R. Mechanical testing of a novel hydrogel nucleus replacement implant. Spine J. 2005;5:672. doi: 10.1016/j.spinee.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 60.Nishimura K. Mochida J. Percutaneous reinsertion of the nucleus pulposus. An experimental study. Spine. 1998;23:1531. doi: 10.1097/00007632-199807150-00006. [DOI] [PubMed] [Google Scholar]
- 61.Sakai D. Mochida J. Yamamoto Y. Nomura T. Okuma M. Nishimura K. Nakai T. Ando K. Hotta T. Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc: a potential therapeutic model for disc degeneration. Biomaterials. 2003;24:3531. doi: 10.1016/s0142-9612(03)00222-9. [DOI] [PubMed] [Google Scholar]
- 62.Nomura T. Mochida J. Okuma M. Nishimura K. Sakabe K. Nucleus pulposus allograft retards intervertebral disc degeneration. Clin Orthop. 2001;389:94. doi: 10.1097/00003086-200108000-00015. [DOI] [PubMed] [Google Scholar]
- 63.Okuma M. Mochida J. Nishimura K. Sakabe K. Seiki K. Reinsertion of stimulated nucleus pulposus cells retards intervertebral disc degeneration: an in vitro and in vivo experimental study. J Orthop Res. 2000;18:988. doi: 10.1002/jor.1100180620. [DOI] [PubMed] [Google Scholar]
- 64.An H.S. Masuda K. Relevance of in vitro and in vivo models for intervertebral disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):88. doi: 10.2106/JBJS.E.01272. [DOI] [PubMed] [Google Scholar]
- 65.Chujo T. An H.S. Akeda K. Miyamoto K. Muehleman C. Attawia M. Andersson G. Masuda K. Effects of growth differentiation factor-5 on the intervertebral disc—in vitro bovine study and in vivo rabbit disc degeneration model study. Spine. 2006;31:2909. doi: 10.1097/01.brs.0000248428.22823.86. [DOI] [PubMed] [Google Scholar]
- 66.Rousseau M.A. Ulrich J.A. Bass E.C. Rodriguez A.G. Liu J.J. Lotz J.C. Stab incision for inducing intervertebral disc degeneration in the rat. Spine. 2007;32:17. doi: 10.1097/01.brs.0000251013.07656.45. [DOI] [PubMed] [Google Scholar]
- 67.Hoogendoorn R.J. Helder M.N. Kroeze R.J. Bank R.A. Smit T.H. Wuisman P.I. Reproducible long-term disc degeneration in a large animal model. Spine. 2008;33:949. doi: 10.1097/BRS.0b013e31816c90f0. [DOI] [PubMed] [Google Scholar]
- 68.Yoon S.H. Miyazaki M. Hong S.W. Tow B. Morishita Y. Hu M. Ahn S.J. Wang J.C. A porcine model of intervertebral disc degeneration induced by annular injury characterized with magnetic resonance imaging and histopathological findings. Laboratory investigation. J Neurosurg Spine. 2008;8:450. doi: 10.3171/SPI/2008/8/5/450. [DOI] [PubMed] [Google Scholar]
- 69.Henriksson H.B. Svanvik T. Jonsson M. Hagman M. Horn M. Lindahl A. Brisby H. Transplantation of human mesenchymal stems cells into intervertebral discs in a xenogeneic porcine model. Spine. 2009;34:141. doi: 10.1097/BRS.0b013e31818f8c20. [DOI] [PubMed] [Google Scholar]
- 70.Chiba K. Andersson G.B. Masuda K. Thonar E.J. Metabolism of the extracellular matrix formed by intervertebral disc cells cultured in alginate. Spine. 1997;22:2885. doi: 10.1097/00007632-199712150-00011. [DOI] [PubMed] [Google Scholar]
- 71.Masuda K. Takegami K. An H. Kumano F. Chiba K. Andersson G.B. Schmid T. Thonar E. Recombinant osteogenic protein-1 upregulates extracellular matrix metabolism by rabbit annulus fibrosus and nucleus pulposus cells cultured in alginate beads. J Orthop Res. 2003;21:922. doi: 10.1016/S0736-0266(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 72.Preradovic A. Kleinpeter G. Feichtinger H. Balaun E. Krugluger W. Quantitation of collagen I, collagen II and aggrecan mRNA and expression of the corresponding proteins in human nucleus pulposus cells in monolayer cultures. Cell Tissue Res. 2005;321:459. doi: 10.1007/s00441-005-1116-6. [DOI] [PubMed] [Google Scholar]
- 73.Le Maitre C.L. Hoyland J.A. Freemont A.J. Studies of human intervertebral disc cell function in a constrained in vitro tissue culture system. Spine. 2004;29:1187. doi: 10.1097/00007632-200406010-00006. [DOI] [PubMed] [Google Scholar]
- 74.Bayliss M.T. Urban J.P.G. Johnstone B. Holm S. In vitro method for measuring synthesis rates in the intervertebral disc. J Orthop Res. 1986;4:10. doi: 10.1002/jor.1100040102. [DOI] [PubMed] [Google Scholar]
- 75.Chiba K. Andersson G.B. Masuda K. Momohara S. Williams J.M. Thonar E.J. A new culture system to study the metabolism of the intervertebral disc in vitro. Spine. 1998;23:1821. doi: 10.1097/00007632-199809010-00002. [DOI] [PubMed] [Google Scholar]
- 76.Wilke H.J. Neef P. Caimi M. Hoogland T. Claes L.E. New in vivo measurements of pressures in the intervertebral disc in daily life. Spine. 1999;24:755. doi: 10.1097/00007632-199904150-00005. [DOI] [PubMed] [Google Scholar]
- 77.Feng H. Danfelter M. Stromqvist B. Heinegard D. Extracellular matrix in disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):25. doi: 10.2106/JBJS.E.01341. [DOI] [PubMed] [Google Scholar]
- 78.Roughley P.J. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine. 2004;29:2691. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- 79.Niosi C.A. Oxland T.R. Degenerative mechanics of the lumbar spine. Spine J. 2004;4(6 Suppl):202S. doi: 10.1016/j.spinee.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 80.Schnake K.J. Putzier M. Haas N.P. Kandziora F. Mechanical concepts for disc regeneration. Eur Spine J. 2006;15(Suppl 3):S354. doi: 10.1007/s00586-006-0176-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tye H. Application of statistical “design of experiments” methods in drug discovery. Drug Discov Today. 2004;9:485. doi: 10.1016/S1359-6446(04)03086-7. [DOI] [PubMed] [Google Scholar]
- 82.Alini M. Eisenstein S.M. Ito K. Little C. Kettler A.A. Masuda K. Melrose J. Ralphs J. Stokes I. Wilke H.J. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17:2. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Singh K. Masuda K. An H.S. Animal models for human disc degeneration. Spine J. 2005;5(6 Suppl):267S. doi: 10.1016/j.spinee.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 84.Walsh A.J. Bradford D.S. Lotz J.C. In vivo growth factor treatment of degenerated intervertebral discs. Spine. 2004;29:156. doi: 10.1097/01.BRS.0000107231.67854.9F. [DOI] [PubMed] [Google Scholar]
- 85.Nagae M. Ikeda T. Mikami Y. Hase H. Ozawa H. Matsuda K. Sakamoto H. Tabata Y. Kawata M. Kubo T. Intervertebral disc regeneration using platelet-rich plasma and biodegradable gelatin hydrogel microspheres. Tissue Eng. 2007;13:147. doi: 10.1089/ten.2006.0042. [DOI] [PubMed] [Google Scholar]
- 86.Masuda K. Imai Y. Okuma M. Muehleman C. Nakagawa K. Akeda K. Thonar E. Andersson G. An H.S. Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine. 2006;31:742. doi: 10.1097/01.brs.0000206358.66412.7b. [DOI] [PubMed] [Google Scholar]
- 87.Miyamoto K. Masuda K. Kim J.G. Inoue N. Akeda K. Andersson G.B. An H.S. Intradiscal injections of osteogenic protein-1 restore the viscoelastic properties of degenerated intervertebral discs. Spine J. 2006;6:692. doi: 10.1016/j.spinee.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 88.Aguiar D.J. Johnson S.L. Oegema T.R. Notochordal cells interact with nucleus pulposus cells: regulation of proteoglycan synthesis. Exp Cell Res. 1999;246:129. doi: 10.1006/excr.1998.4287. [DOI] [PubMed] [Google Scholar]
- 89.Lotz J.C. Colliou O.K. Chin J.R. Duncan N.A. Liebenberg E. Compression-induced degeneration of the intervertebral disc: an in vivo mouse model and finite-element study. Spine. 1998;23:2493. doi: 10.1097/00007632-199812010-00004. [DOI] [PubMed] [Google Scholar]
- 90.Masuda K. Aota Y. Muehleman C. Imai Y. Okuma M. Thonar E.J. Andersson G.B. An H.S. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine. 2005;30:5. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- 91.Suzuki M. Inoue G. Gemba T. Watanabe T. Ito T. Koshi T. Yamauchi K. Yamashita M. Orita S. Eguchi Y. Ochiai N. Kishida S. Takaso M. Aoki Y. Takahashi K. Ohtori S. Nuclear factor-kappa B decoy suppresses nerve injury and improves mechanical allodynia and thermal hyperalgesia in a rat lumbar disc herniation model. Eur Spine J. 2009;18:1001. doi: 10.1007/s00586-009-0940-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sasaki N. Kikuchi S. Konno S. Sekiguchi M. Watanabe K. Anti-TNF-alpha antibody reduces pain-behavioral changes induced by epidural application of nucleus pulposus in a rat model depending on the timing of administration. Spine. 2007;32:413. doi: 10.1097/01.brs.0000255097.18246.bc. [DOI] [PubMed] [Google Scholar]
- 93.Kawakami M. Matsumoto T. Hashizume H. Kuribayashi K. Chubinskaya S. Yoshida M. Osteogenic protein-1 (osteogenic protein-1/bone morphogenetic protein-7) inhibits degeneration and pain-related behavior induced by chronically compressed nucleus pulposus in the rat. Spine. 2005;30:1933. doi: 10.1097/01.brs.0000176319.78887.64. [DOI] [PubMed] [Google Scholar]
- 94.Norimoto M. Ohtori S. Yamashita M. Inoue G. Yamauchi K. Koshi T. Suzuki M. Orita S. Eguchi Y. Sugiura A. Ochiai N. Takaso M. Takahashi K. Direct application of the TNF-alpha inhibitor, etanercept, does not affect CGRP expression and phenotypic change of DRG neurons following application of nucleus pulposus onto injured sciatic nerves in rats. Spine. 2008;33:2403. doi: 10.1097/BRS.0b013e31818441a2. [DOI] [PubMed] [Google Scholar]
- 95.Olmarker K. Puncture of a lumbar intervertebral disc induces changes in spontaneous pain behavior: an experimental study in rats. Spine. 2008;33:850. doi: 10.1097/BRS.0b013e31816b46ca. [DOI] [PubMed] [Google Scholar]
- 96.Ulrich J.A. Liebenberg E.C. Thuillier D.U. Lotz J.C. ISSLS prize winner: repeated disc injury causes persistent inflammation. Spine. 2007;32:2812. doi: 10.1097/BRS.0b013e31815b9850. [DOI] [PubMed] [Google Scholar]
- 97.Olmarker K. Larsson K. Tumor necrosis factor alpha and nucleus-pulposus-induced nerve root injury. Spine. 1998;23:2538. doi: 10.1097/00007632-199812010-00008. [DOI] [PubMed] [Google Scholar]
- 98.Brown M.D. Update on chemonucleolysis. Spine. 1996;21(24 Suppl):62S. doi: 10.1097/00007632-199612151-00007. [DOI] [PubMed] [Google Scholar]
- 99.Beckstein J.C. Sen S. Schaer T.P. Vresilovic E.J. Elliott D.M. Comparison of animal discs used in disc research to human lumbar disc: axial compression mechanics and glycosaminoglycan content. Spine (Phila Pa 1976) 2008;33:E166. doi: 10.1097/BRS.0b013e318166e001. [DOI] [PubMed] [Google Scholar]
- 100.Nuckley D.J. Kramer P.A. Del Rosario A. Fabro N. Baran S. Ching R.P. Intervertebral disc degeneration in a naturally occurring primate model: radiographic and biomechanical evidence. J Orthop Res. 2008;26:1283. doi: 10.1002/jor.20526. [DOI] [PubMed] [Google Scholar]
- 101.Chujo T. An H. Asanuma K. Takatori R. Inoue N. Attawia M. Lee C. Muehleman C. Masuda K. A single injection of recombinant human GDF-5 effectively restores mature rabbit of intervertebral discs degenerated by anular puncture. Trans Orthop Res Soc. 2007;32:267. [Google Scholar]
- 102.Boos N. Weissbach S. Rohrbach H. Weiler C. Spratt K.F. Nerlich A.G. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine. 2002;27:2631. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 103.Thompson J.P. Pearce R.H. Schechter M.T. Adams M.E. Tsang I.K. Bishop P.B. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine. 1990;15:411. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 104.Pfirrmann C.W. Metzdorf A. Zanetti M. Hodler J. Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 105.Bae W.C. Yoshikawa T. Kakutani K. Hammed A. Znamirowski R. Chung C.B. Bydder G.M. Masuda K. Effect of rhGDF-5 on the thrombin model of rabbit intervertebral disc degeneration; T2 quantification using 3T MRI. Ortho Res Soc Trans. 2009;34:2129. [Google Scholar]
- 106.Adams M.A. Dolan P. Spine biomechanics. J Biomech. 2005;38:1972. doi: 10.1016/j.jbiomech.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 107.Bass E.C. Duncan N.A. Hariharan J.S. Dusick J. Bueff H.U. Lotz J.C. Frozen storage affects the compressive creep behavior of the porcine intervertebral disc. Spine. 1997;22:2867. doi: 10.1097/00007632-199712150-00009. [DOI] [PubMed] [Google Scholar]
- 108.Huber G. Morlock M.M. Ito K. Consistent hydration of intervertebral discs during in vitro testing. Med Eng Phys. 2007;29:808. doi: 10.1016/j.medengphy.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 109.Ahlgren B.D. Lui W. Herkowitz H.N. Panjabi M.M. Guiboux J.P. Effect of anular repair on the healing strength of the intervertebral disc: a sheep model. Spine. 2000;25:2165. doi: 10.1097/00007632-200009010-00004. [DOI] [PubMed] [Google Scholar]
- 110.Adams M.A. Mechanical testing of the spine. An appraisal of methodology, results, and conclusions. Spine. 1995;20:2151. doi: 10.1097/00007632-199510000-00015. [DOI] [PubMed] [Google Scholar]
- 111.Cripton P.A. Bruehlmann S.B. Orr T.E. Oxland T.R. Nolte L.P. In vitro axial preload application during spine flexibility testing: towards reduced apparatus-related artefacts. J Biomech. 2000;33:1559. doi: 10.1016/s0021-9290(00)00145-7. [DOI] [PubMed] [Google Scholar]
- 112.Mimura M. Panjabi M.M. Oxland T.R. Crisco J.J. Yamamoto I. Vasavada A. Disc degeneration affects the multidirectional flexibility of the lumbar spine. Spine. 1994;19:1371. doi: 10.1097/00007632-199406000-00011. [DOI] [PubMed] [Google Scholar]
- 113.O'Neill C. Kurgansky M. Kaiser J. Lau W. Accuracy of MRI for diagnosis of discogenic pain. Pain Physician. 2008;11:311. [PubMed] [Google Scholar]
- 114.Cohen S.P. Larkin T.M. Barna S.A. Palmer W.E. Hecht A.C. Stojanovic M.P. Lumbar discography: a comprehensive review of outcome studies, diagnostic accuracy, and principles. Reg Anesth Pain Med. 2005;30:163. doi: 10.1016/j.rapm.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 115.Sandhu H.S. Sanchez-Caso L.P. Parvataneni H.K. Cammisa F.P., Jr. Girardi F.P. Ghelman B. Association between findings of provocative discography and vertebral endplate signal changes as seen on MRI. J Spinal Disord. 2000;13:438. doi: 10.1097/00002517-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 116.Wolfer L.R. Derby R. Lee J.E. Lee S.H. Systematic review of lumbar provocation discography in asymptomatic subjects with a meta-analysis of false-positive rates. Pain Physician. 2008;11:513. [PubMed] [Google Scholar]
- 117.Carragee E.J. Don A. Hurwitz E. Cuellar J. Carrino J. Herzog R. Does Discography Cause Accelerated Progression of Degeneration Changes in the Lumbar Disc: A Ten-Year Cohort-Controlled Study. Miami, FL: International Society for the Study of the Lumbar Spine; 2009. p. 57. [DOI] [PubMed] [Google Scholar]
- 118.Nguyen A.M. Johannessen W. Yoder J.H. Wheaton A.J. Vresilovic E.J. Borthakur A. Elliott D.M. Noninvasive quantification of human nucleus pulposus pressure with use of T1rho-weighted magnetic resonance imaging. J Bone Joint Surg Am. 2008;90:796. doi: 10.2106/JBJS.G.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Blumenkrantz G. Li X. Han E.T. Newitt D.C. Crane J.C. Link T.M. Majumdar S. A feasibility study of in vivo T1rho imaging of the intervertebral disc. Magn Reson Imaging. 2006;24:1001. doi: 10.1016/j.mri.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 120.Rajasekaran S. Babu J.N. Arun R. Armstrong B.R. Shetty A.P. Murugan S. ISSLS prize winner: a study of diffusion in human lumbar discs: a serial magnetic resonance imaging study documenting the influence of the endplate on diffusion in normal and degenerate discs. Spine. 2004;29:2654. doi: 10.1097/01.brs.0000148014.15210.64. [DOI] [PubMed] [Google Scholar]
- 121.Arun R. Freeman B. Scammell B. McNally D. Gowland P. An in-vivo study using serial post-contract magnetic resonance imaging. Miami, FL: International Society for the Study of the Lumbar Spine; 2009. What influence does sustained mechanical load have on trasport of small solutes into the human intervertebral discs? p. 56. [DOI] [PubMed] [Google Scholar]
- 122.McGregor A.H. Anderton L. Gedroyc W.M. Johnson J. Hughes S.P. Assessment of spinal kinematics using open interventional magnetic resonance imaging. Clin Orthop Relat Res. 2001;392:341. doi: 10.1097/00003086-200111000-00044. [DOI] [PubMed] [Google Scholar]
- 123.Kuniyoshi K. Ohtori S. Ochiai N. Murata R. Matsudo T. Yamada T. Ochiai S.S. Moriya H. Takahashi K. Characteristics of sensory DRG neurons innervating the wrist joint in rats. Eur J Pain. 2007;11:323. doi: 10.1016/j.ejpain.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 124.Zanoli G. Outcome assessment in lumbar spine surgery. Acta Orthop Suppl. 2005;76:5. [PubMed] [Google Scholar]
- 125.Miller C. Bringing a new medical device to market. Minnesota Physician. 2008;XXI(8) [Google Scholar]
- 126.Whang P.G. Lim M.R. Sasso R.C. Skelton A. Brown Z.B. Anderson D.G. Albert T.J. Hilibrand A.S. Vaccaro A.R. Financial incentives for lumbar surgery: a critical analysis of physician reimbursement for decompression and fusion procedures. J Spinal Disord Tech. 2008;21:381. doi: 10.1097/BSD.0b013e31814d4e1b. [DOI] [PubMed] [Google Scholar]
- 127.Polly D.W., Jr. Glassman S.D. Schwender J.D. Shaffrey C.I. Branch C. Burkus J.K. Gornet M.F. SF-36 PCS benefit-cost ratio of lumbar fusion comparison to other surgical interventions: a thought experiment. Spine. 2007;32(11 Suppl):S20. doi: 10.1097/BRS.0b013e318053d4e5. [DOI] [PubMed] [Google Scholar]
- 128.Kaplan A.V. Baim D.S. Smith J.J. Feigal D.A. Simons M. Jefferys D. Fogarty T.J. Kuntz R.E. Leon M.B. Medical device development: from prototype to regulatory approval. Circulation. 2004;109:3068. doi: 10.1161/01.CIR.0000134695.65733.64. [DOI] [PubMed] [Google Scholar]
- 129.Moazzam F. Bednarek M.D. Intellectual property protection for medical devices. In: Becker K.M., editor; Whyte J.J., editor. Clinical Evaluation of Medical Devices. Totowa, NJ: Humana Press; 2006. pp. 117–139. [Google Scholar]
- 130.Gertner M. You have an idea, now what? Semin Pediatr Surg. 2006;15:302. doi: 10.1053/j.sempedsurg.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 131.Glenna L.L. Welsh R. Lacy W.B. Biscotti D. Industry perceptions of university-industry relationships related to agricultural biotechnology research. Rural Sociol. 2007;72:608. [Google Scholar]
- 132.Santoro M.D. Chakrabarti A.K. Corporate strategic objectives for establishing relationship with university research centers. IEEE Trans Eng Manage. 2001;48:157. [Google Scholar]