Abstract
Background
Thymosin beta 4 (Tβ4) is a member of beta-thymosins, a family of peptides that play essential roles in many cellular functions. A recent study from our group suggested a role for Tβ4 in the development of human salivary glands. The aim of this study was to analyze the expression of Tβ4 in the human gut during development, and in the adult.
Methodology/Principal Findings
Immunolocalization of Tβ4 was studied in autoptic samples of tongue, oesophagus, stomach, ileum, colon, liver and pancreas obtained from two human foetuses and two adults. Tβ4 appeared unevenly distributed, with marked differences between foetuses and adults. In the stomach, superficial epithelium was positive in foetuses and negative in adults. Ileal enterocytes were strongly positive in the adult and weakly positive in the foetuses. An increase in reactivity for Tβ4 was observed in superficial colon epithelium of adults as compared with the foetuses. Striking differences were found between foetal and adult liver: the former showed a very low reactivity for Tβ4 while in the adult we observed a strong reactivity in the vast majority of the hepatocytes. A peculiar pattern was found in the pancreas, with the strongest reactivity observed in foetal and adult islet cells.
Significance
Our data show a strong expression of Tβ4 in the human gut and in endocrine pancreas during development. The observed differential expression of Tβ4 suggests specific roles of the peptide in the gut of foetuses and adults. The observed heterogeneity of Tβ4 expression in the foetal life, ranging from a very rare detection in liver cells up to a diffuse reactivity in endocrine pancreas, should be taken into account when the role of Tβ4 in the development of human embryo is assessed. Future studies are needed to shed light on the link between Tβ4 and organogenesis.
Introduction
Beta-thymosins (Tβs) constitute a highly conserved family of actin-binding polypeptides [1], presenting a well conserved four-aminoacid motif, corresponding to the sequence LKKT, which interacts with actin, promoting or inhibiting actin assembly [2]. Thymosin beta-4 (Tβ4) is the archetypal member of the beta-thymosins family: it is a 43-aminoacid peptide, isolated from human blood platelets [3] which forms a 1∶1 complex with actin, inhibits its polymerization [4], and acts as an extremely effective actin-monomer sequestering peptide [5]. Tβ4 has multiple functions: it moonlights to repair injured tissues [6], has anti-inflammatory efficacy in monocyte/macrophages [7], promotes wound healing [8] and mediates angiogenesis [9]. Tβ4 has been also shown to play a relevant role during the development of different neural cell types in the rat brain [10]. In particular, Tβ4 plays a neurotrophic and antiapoptotic role during the development of the nervous system [11]. In the embryo, reduced myocardial Tβ4 levels have been reported to cause a disrupted coronary vasculogenesis and a number of cardiac defects [12]. During coronary vessels development, Tβ4 should act on cardiac stem cells, also known as epicardially derived cells (EPDCs) [13] inducing their migration into the myocardium, where they differentiate into either endothelial or smooth muscle cells [9]. The theory on the putative role of Tβ4 in the physiological development of embryos, as well as in vascularization and tissue recovery in acute and chronic ischemia, has been reinforced by the discovery that Tβ4 is one of the most abundant factors secreted by embryonic endothelial progenitor cells [14]. Recently a study from our group evidenced a strong reactivity for Tβ4 in developing foetal salivary glands, with a switch from the acinar component to the ductal cells in the adult [15]. Tβ4 has also been identified as a predominant transcript in intraepithelial lymphocytes (IEL) in the murine gut [16], in which it could exert a relevant anti-inflammatory effect by inhibiting neutrophilic infiltration [17].
On the basis of these data, suggesting a relevant role for Tβ4 during the development of the foetus and the embryo, it seemed of some interest to investigate the expression of the peptide in the gastrointestinal tract of human fetuses and in adults, with the aim to gain insights into the expression of Tβ4 in the human gastrointestinal tract during development.
Materials and Methods
Two human fetuses of 20 weeks (male) and 21 weeks (female) of gestational age, and two adults 65 and 74 year old respectively were the object of the present study. In each subject, we obtained, at autopsy, multiple samples from the following segments of the gut: tongue, oesophagus, stomach, ileum, colon. Samples were also obtained from liver and pancreas. Tissue samples were fixed in 10% formalin, routinely processed and paraffin-embedded. Immunohistochemistry was performed on 5 µm-thick sections, using the labeled streptavidin-biotin complex system (LSAB2, Dako) in a Dako Autostainer (DakoCytomation, Carpintera, CA, USA). Heat-induced antigen retrieval was carried out by steaming unstained sections in Target Retrieval Solution (Dako TRS pH 6.1) for 30 min. Tissue sections were incubated (30 min at room temperature) with the monoclonal anti-thymosin beta 4 antibody (Bachem, Bubendorf, Switzerland). Sections of a reactive human adult lymph node with activated macrophages were used as positive controls. As a negative control, we utilized sections of foetal oesophagus: immunoistochemistry was performed using isotype antibody (Fig. 1). The immunostaining was interpreted as positive when at least 10% of cells expressed the antigen. The positive expression was further categorized into focal (10–33%) and diffuse (>33%).
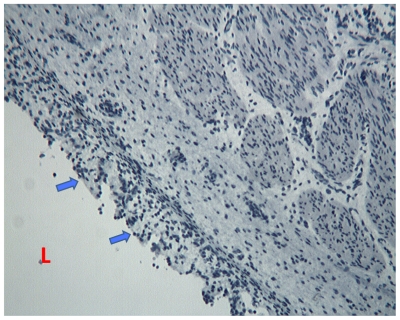
Figure 1. Negative control.
Foetal oesophagus immunostained by using isotype antibody. L = oesophageal lumen. Arrows indicate the surface epithelium.
All cases were independently reanalyzed by two pathologists specialized in gastrointestinal pathology (GF, SN).
Ethics Statements
The study protocol and written consent forms were approved by the Ethics Human Studies Committee of University Medical Centre of Cagliari (according to the instructions of the declaration of Helsinki). Full written consent forms were obtained from the parents of the newborns and all rules were respected. For the specimens from adults, we obtained written consent to revearch use by their next of kin.
Results
The immunostaining for Tβ4 appeared granular or homogeneously diffuse, always restricted to the cytoplasm of positive cells; no nuclear reactivity was observed in this study. Reactivity for the peptide was also observed, in some cases, in the interstitial spaces, as fine granules, mainly located around the epithelial structures. No significant differences were found in the immunoistochemical pattern for Tβ4 between the two foetuses and the two adults analyzed.
Tongue
Foetus (20 weeks of gestation)
The epithelium covering the tongue appeared constantly negative in its deeper layers. The superficial epithelial cells showed a diffuse immunoreactivity for Tβ4, localized in the cytoplasm (Fig. 2a). The underlying corion was negative. A weak positivity was also observed in the muscular cells.
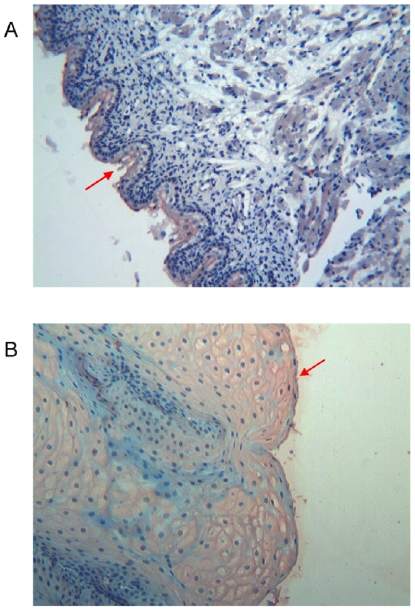
Figure 2. Immunohistochemical detection of thymosin β4 in foetal and adult tongue.
a) Foetal tongue: the superficial epithelial cells (arrow) shows a diffuse immunoreactivity for Tβ4, localized in the cytoplasm. The underlying corion is negative. (Original Magnification ×250) b) Adult tongue: the superficial layers of the stratified epithelium show a strong diffuse cytoplasmic reactivity for Tβ4 (arrow). (Original Magnification ×250)
Adult
The immunoistochemical pattern paralleled that detected in the foetuses. The superficial layers of the stratified epithelium of the tongue showed a diffuse cytoplasmic reactivity for Tβ4 (Fig. 2b).
Oesophagus
Foetus (21 weeks of gestation)
The epithelium covering the oesophageal lumen appeared negative. The oesophageal lumen appeared coated with a thin Tβ4-immunoreactive layer, which resulted diffuse to the entire oesophageal lumen (Fig. 3a). A weak reactivity for the peptide was observed in the muscular cells.
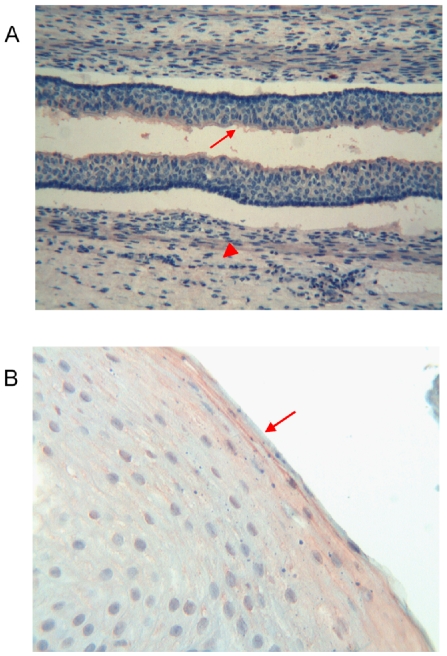
Figure 3. Immunohistochemical detection of thymosin β4 in foetal and adult oesophagus.
a) Foetal oesophagus: the oesophageal lumen is coated with a thin Tβ4-immunoreactive layer (arrow). A weak reactivity for the peptide is observed in the muscular cells (arrowhead). (Original Magnification ×400) b) Adult oesophagus: immunoreactivity for Tβ4 is restricted to epithelial cells of the superficial layers (arrow). (Original Magnification ×400)
Adult
Immunoreactivity for Tβ4 was restricted to epithelial cells of the superficial layers covering the esophageal lumen. No reactivity for Tβ4 was found in the intermediated and deep layers (Fig. 3b).
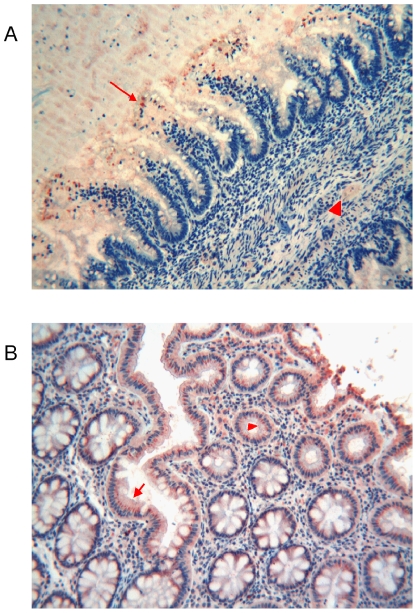
Stomach
Foetus (21 weeks of gestation)
The gastric epithelium showed the presence of Tβ4-immunoreactive granules in the cytoplasm of the majority of columnar cells. Immunoreactive deposits were mainly found aggregated in the perinuclear regions. Abundant deposits were also found dispersed throughout the mucous covering the gastric surface (Fig. 4a).
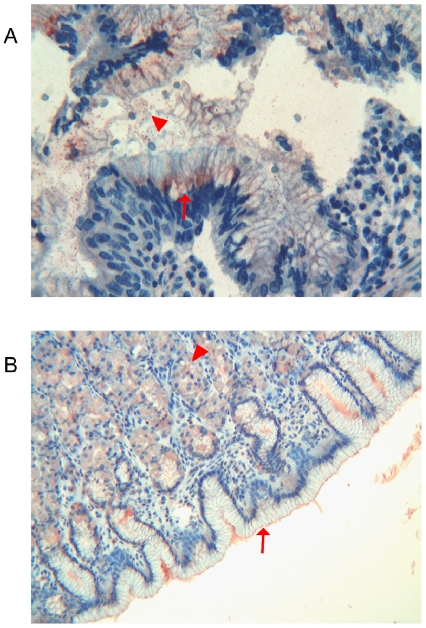
Figure 4. Immunohistochemical detection of thymosin β4 in foetal and adult stomach.
a) Foetal stomach: Tβ4-immunoreactive granules are shown in the cytoplasm of the majority of columnar cells (arrow). Tβ4 granular deposits are also found in mucous covering the gastric surface (arrowhead). (Original Magnification ×400) b) Adult stomach: gastric mucosa appears covered by a thin superficial layer intensely reactive for Tβ4 (arrow). Immunoreactivity for Tβ4 in gastric glands is restricted to chief and oxyntic cells (arrowhaed). (Original Magnification ×250)
Adult
Gastric mucosa was covered by a thin superficial layer intensely reactive for Tβ4 extending to gastric foveolae. Immunoreactivity for Tβ4 in gastric glands was restricted to chief and oxyntic cells (Fig. 4b).
Ileum
Foetus (21 weeks of gestation)
A granular reactivity for Tβ4 was detected in the epithelium covering the ileal villi, more evident in the cytoplasm of mucous cells (Fig. 5a). A reactivity for the peptide was also observed in the mucous occupying the intestinal lumen.
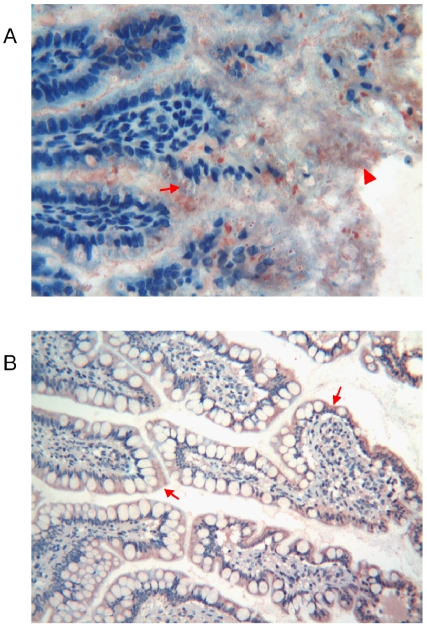
Figure 5. Immunohistochemical detection of thymosin β4 in foetal and adult ileum.
a) Foetal ileum: a granular reactivity for Tβ4 is present in the epithelium covering ileal villi (arrow). A reactivity for the peptide is also observed in the mucous occupying the intestinal lumen (arrowhead). (Original Magnification ×400) b) Adult ileum: a mild reactivity for Tβ4 is present in the cytoplasm of enterocytes covering villi (arrows). (Original Magnification ×250)
Adult
A mild but diffuse reactivity for Tβ4 was present in the cytoplasm of enterocytes covering villi (Fig. 5b). Fine granular deposits were also observed in the cytoplasm of mucous cells.
Colon
Foetus (21 weeks of gestation)
Coarse granules immunoreactive for Tβ4 were observed in the cytoplasm of superficial colon epithelial cells. No reactivity was found in the crypts (Fig. 6a). The muscular layer did not show any significant reactivity for the peptide. A weak positivity was detected in the nervous cells.
Figure 6. Immunohistochemical detection of thymosin β4 in foetal and adult colon.
a) Foetal colon: coarse granules immunoreactive for Tβ4 are observed in the cytoplasm of superficial enterocytes (arrow). A weak positivity is detected in nervous cells (arrowhead). (Original Magnification ×250) b) Adult colon: an intense and diffuse reactivity for Tβ4, characterized by coarse sovranuclear granules is present in the superficial (arrow) and crypt epithelium (arrowhead). (Original Magnification ×250)
Adult
An intense and diffuse reactivity with coarse sovranuclear granules immunoreactive for Tβ4 were present in the superficial and crypt epithelium. (Fig. 6b)
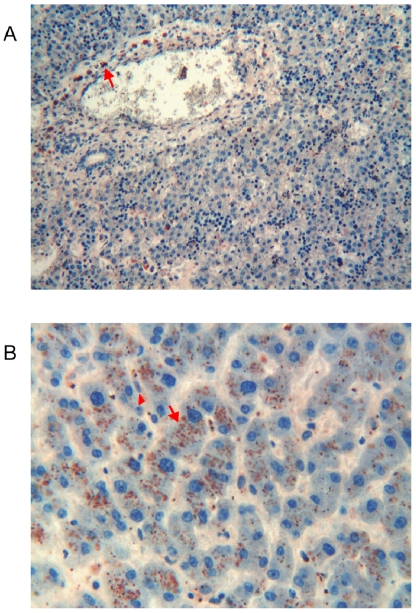
Liver
Foetus (21 weeks of gestation)
No significant reactivity for Tβ4 was found in hepatocytes and in biliary ducts nor in biliary epithelial cells of the ductal plate in the foetal liver examined in this study. A weak reactivity was frequently observed in the red blood cells in the dilated sinusoids. Only occasionally, scattered large cells in immature portal tracts showed a strong Tβ4 immunoreactivity (Fig. 7a).
Figure 7. Immunohistochemical detection of thymosin β4 in foetal and adult liver.
a) Foetal liver: no significant reactivity for Tβ4 is found in hepatocytes and in ductal cells. Scattered large cells in immature portal tracts show a strong immunoreactivity for the peptide (arrow). (Original Magnification ×250) b) Adult liver: a strong granular immunoreactivity for Tβ4 is found in the cytoplasm of the majority of hepatocytes in all acinar zones (arrow) and in activated Kupffer cells (arrowhead). Portal tracts, including bile ducts are negative. (Original Magnification ×400)
Adult
The expression pattern for Tβ4 changed completely in immunostained adult livers. A strong granular immunoreactivity was found in the cytoplasm of the majority of hepatocytes in all acinar zones and in activated Kupffer cells (Fig. 7b). Portal tracts, including bile ducts were constantly negative.
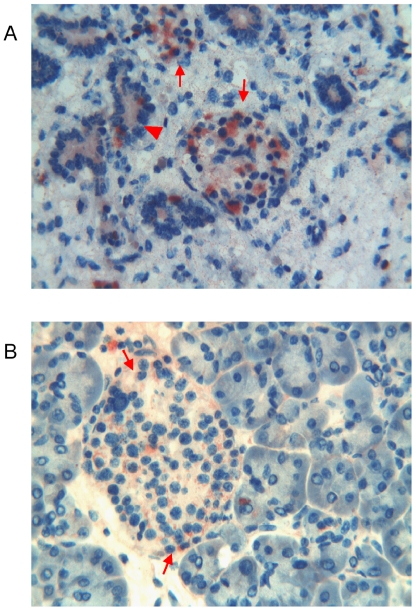
Pancreas
Foetus (20 weeks of gestation)
Immunoreactivity for Tβ4 appeared focal, mainly localized inside the islets of Langerhans. A part of the endocrine cells showed the entire cytoplasm strongly immunoreactive for the peptide. Few Tβ4-reactive cells were also present in the exocrine pancreas, inside the tubular structures, intermingled among the tubular epithelium. A weak granular reactivity for the peptide was also found inside the cytoplasm of the tubular cells, a pattern suggestive for a secretion of Tβ4 in the pancreatic juice (Fig. 8a).
Figure 8. Immunohistochemical detection of thymosin β4 in foetal and adult pancreas.
a) Foetal pancreas: immunoreactivity for Tβ4 is localized inside the islets of Langherans. Some of endocrine cells show the entire cytoplasm strongly immunoreactive for the peptide (arrows). Few Tβ4-reactive cells are also present in the exocrine pancreas, inside the tubular structures (arrowhead). (Original Magnification ×400). b) Adult pancreas: the highest reactivity for Tβ4 is observed in Langherans islet cells, which show diffuse fine granular deposits in their cytoplasm (arrows). (Original Magnification ×400).
Adult
The distribution of Tβ4 immunoreactivity paralleled that observed in the foetus: the highest reactivity was observed in the islet cells which showed diffuse fine cytoplasmic granular deposits in the majority of endocrine cells. Only rare acinar cells contained cytoplasmic vacuoles reactive for Tβ4 (Fig. 8b).
Discussion
Tβ4, the most abundant member in human cells of the thymosin family, has been initially embraced as the ideal actin monomer-sequestering peptide [18], and its function was restricted to regulate actin polymerization of non-muscle cells [19]. Further data, suggesting a role of Tβ4 in modulating stem cell migration [9], activation [20] and inhibition [21], as well a relation between Tβ4 and integrin signalling [22] induced some authors to speak about “the β-thymosin enigma” [9]. The oxidized form of Tβ4, thymosin beta 4 sulfoxide, has been demonstrated to have an important anti-inflammatory activity inhibiting neutrophil chemotaxis in vitro [23] and in vivo [7].
This study represents the first comprehensive analysis of Tβ4 immunoreactivity in the human gastrointestinal tract, pancreas and liver of human foetus in comparison to adult. Interesting differences were found between foetus and adult Tβ4 immunoreactivity: in some organs, such as liver, the immunoreactivity of Tβ4 was higher in the adult, while in others, such as endocrine pancreas, it was lower. Differences in Tβ4 expression between the foetus and the adult have been previously reported by our group in human salivary glands [15]. In that study developing major and minor salivary glands showed a marked reactivity for Tβ4, which contrasted with the low levels of immunoreactivity observed in the adult glands. In this study liver showed an opposite pattern, characterized by low reactivity for the peptide in the intrauterine life and much higher levels in the adult life. All together, these data seem to indicate the existence of an exquisite cell type- and differentiation stage-specific regulation and expression pattern of Tβ4 in the human gut and annexed glands during development. As a consequence, Tβ4 could play different roles in different organs during the organogenesis, and these roles should change during adult life.
Immunoreactivity for Tβ4 was detected in all different intestinal segments and in all glands examined, but with striking differences among different sites: pancreas and liver of adults showed the highest levels of reactivity for Tβ4, while the lowest reactivity was observed in the developing liver. A remarkable heterogeneity of Tβ4 immunoreactivity within the developing gastrointestinal tract was observed, ranging from diffuse immunoreactivity in pancreas and enterocytes, to peptide absence in the foetal hepatocytes. It should be outlined that some differences observed in Tβ4 immunoreactivity between fetuses and adult organs may be related to the different degree of cell differentiation. For example, chief and oxyntic cells, the site of Tβ4 storage in the adult stomach, are not well differentiated yet in the foetal stomach glands. Noticeable interindividual differences during gut development, regarding the intensity of the positivity for Tβ4 and its subcellular localization, were also observed (data not reported).
On the whole, the high amounts of Tβ4 here reported in the human gut during development are in agreement with previous experimental studies on the role of Tβ4 in organogenesis. High levels of Tβ4 mRNA had been indeed reported in early mouse postimplantation embryos [24] and in the ventricular myocardium of mice at embryonic day 10 [25]. The essential role of Tβ4 in heart development had been demonstrated by the generation of mice with RNAi-mediated cardiac specific knockdown of Tβ4.
Tβ4-mutant hearts showed severe impairment in the mobilization of cardiac progenitor cells, resulting in impaired cardiac development and survival [26]. Our data clearly indicate a major expression of Tβ4 during the development of the human gastrointestinal tract. Such a strong expression should be related to relevant roles in gut development. The well known function of Tβ4 in the regulation of the equilibrium betwen globular and filamentous actin [27] could partly explain the strong Tβ4 immunoreactivity found in the cytoplasm of enterocytes in this study. Tβ4 has been hypothesized to also function as a sentinel of the cell oxidative stress, its oxidation reflecting an oxidizing environment that often correlates with cell damage [7]. Lymphoid Tβ4, a splice variant of Tβ4 produced by intraepithelial lymphocytes normally present in the cytoplasm of enterocytes, has been shown to have a great capacity to reduce oxidative stress [17]. Following this hypothesis, a strong immunohistochemical reaction for Tβ4 could be interpreted as a cell's response to stress, also considering that antibodies utilized in this study cannot discriminate the oxidized Tβ4 from the non-oxidized one. Recently, Tβ4 has been shown to be involved in regulating phagocytosis of apoptotic cells mediated by stabilin-2 [28]. Given the fundamental role of apoptosis in embryology, our finding of a strong reactivity for Tβ4 in the developing gut as well as in developing salivary glands [15] reinforces the hypothesis of an important role of Tβ4 in organ development during embryogenesis. Future studies will be necessary to shed light on the link between Tβ4 and organogenesis, with particular emphasis on the role plaied by the peptide in cell response to oxidative stress, in apoptosis, in actin metabolism in the different phases of fetal, embryo and adult life. The heterogeneity of Tβ4 immunoreactivity detected in different organs of gastrointestinal tract and the differences observed between intrauterine and adult life should be taken into account when the role of Tβ4 in human physiology is assessed.
Acknowledgments
We thank Mr. Iganzio Ferru for the secretarial assistance and Mrs Sandra Serra and Mrs Simonetta Paderi for technical support.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors acknowledge the financial support of Fondazione Banco di Sardegna, Cagliari, Sardinia, Italy. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Low TL, Thurmann GB, McAdoo M, McClure J, Rossio JL, et al. The chemistry and biology of thymosin. I. Isolation, characterization, and biological activities of thymosin alpha1 and polypeptide beta1 from calf thymus. J Biol Chem. 1979;254:981–986. [PubMed] [Google Scholar]
- 2.Hertzog M, van Heijenoort C, Didry D, Gaudier M, Countant J, et al. The beta-thymosin/WH2 domain; structural basis for the switch from inhibition to promotion of actin assembly. Cell. 2004;117:611–623. doi: 10.1016/s0092-8674(04)00403-9. [DOI] [PubMed] [Google Scholar]
- 3.Safer D, Golla R, Nachmias VT. Isolation of a 5-kilodalton actin-sequestering peptide from human blood platelets. PNAS USA. 1990;87:2536–2540. doi: 10.1073/pnas.87.7.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Safer D, Sosnick TR, Elzinga M. Thymosin beta 4 binds actin in an extended conformation and contacts both the barbed and pointed ends. Biochemistry. 1997;36:5806–5816. doi: 10.1021/bi970185v. [DOI] [PubMed] [Google Scholar]
- 5.Dominguez R. The β-Thymosin/WH2 fold. Multifunctionality and structure. Ann N.Y. Acad Sci. 2007;1112:86–94. doi: 10.1196/annals.1415.011. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein AL, Hannappel E, Kleinman HK. Thymosin beta-4: actin sequestering protein moonlights to repair injured tissues. Trends Mol Med. 2005;11:421–429. doi: 10.1016/j.molmed.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Young JD, Lawrence AJ, MacLean AG, Leung BP, McInnes IB, et al. Thymosin beta-4 sulfoxide is an anti-inflammatory agent generated by monocytes in the presence of glucocorticoids. Nat Med. 1999;5:1424–1427. doi: 10.1038/71002. [DOI] [PubMed] [Google Scholar]
- 8.Sosne G, Szliter EA, Barrett R, Kernacki KA, Kleinman H, et al. Thymosin beta-4 promotes corneal wound healing and decreases inflammation in vivo following alkali injury. Exp Eye Res. 2002;74:293–299. doi: 10.1006/exer.2001.1125. [DOI] [PubMed] [Google Scholar]
- 9.Smart N, Rossdeutsch A, Riley PR. Thymosin β4 and angiogenesis: modes of action and therapeutic potential. Angiogenesis. 2007;10:229–241. doi: 10.1007/s10456-007-9077-x. [DOI] [PubMed] [Google Scholar]
- 10.Lin SC, Morrison-Bogorad M. Developmental expression of mRNAs encoding thymosins beta-4 and beta 10 in rat brain and other tissues. J Mol Neurosci. 1990;2:35–44. doi: 10.1007/BF02896924. [DOI] [PubMed] [Google Scholar]
- 11.Sun W, Kim H. Neurotrophic roles of the Beta-thymosins in the development and regeneration of the nervous system. Ann N.Y. Acad Sci. 2007;1112:210–218. doi: 10.1196/annals.1415.013. [DOI] [PubMed] [Google Scholar]
- 12.von Kodolitsch Y, Franzen O, Lund GK, Koschyk DH, Ito WD, et al. Coronary artery anomalies Part I: recent insights from molecular embryology. Z Kardiol. 2004;93:929–937. doi: 10.1007/s00392-004-0152-7. [DOI] [PubMed] [Google Scholar]
- 13.Wessels A, Perez-Pomares JM. The epicardium and epicardially derived cells (EPDCs) as cardiac stem cells. Anat Rec A Discov Mol Cell Evol Biol. 2004;276:43–57. doi: 10.1002/ar.a.10129. [DOI] [PubMed] [Google Scholar]
- 14.Kupatt C, Horstkotte J, Vlastos GA, Pfosser A, Leberz C, et al. Embryonic endothelial progenotor cells expressing a broad range of proangiogenic and remodeling factors enhance vascularization and tissue recovery in acute and chronic ischemia. FASEB J. 2005;19:1576–1578. doi: 10.1096/fj.04-3282fje. [DOI] [PubMed] [Google Scholar]
- 15.Nemolato S, Messana I, Cabras T, Manconi B, Inzitari R, et al. Thymosin beta (4) and beta (10) levels in preterm newborn oral cavity and foetal salivary glands evidence a switch of secretion during foetal development. PLoS One. 2009;4(4):e5109. doi: 10.1371/journal.pone.0005109. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shires J, Theodoridis E, Hayday AC. Biological insights into murine TCR γδ and TCR αβ intraepithelial lymphocytes provided by the serial analysis of gene expression. Immunity. 2001;15:419–434. doi: 10.1016/s1074-7613(01)00192-3. [DOI] [PubMed] [Google Scholar]
- 17.Girardi M, Sherling MA, Filler RB, Shires J, Theodoridis E, et al. Anti-inflammatory effects in the skin of thymosin-β4-splice-variants. Immunology. 2003;109:1–7. doi: 10.1046/j.1365-2567.2003.01616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun HQ, Kwiatkowska K, Yin HL. Actin Monomer binding proteins. Curr Opin Cell Biol. 1995;7:102–110. doi: 10.1016/0955-0674(95)80051-4. [DOI] [PubMed] [Google Scholar]
- 19.Stossel TP, Fenteany G, Hartwig JH. Cell surface actin remodeling. J Cell Sci. 2006;119:3261–3264. doi: 10.1242/jcs.02994. [DOI] [PubMed] [Google Scholar]
- 20.Philp D, Nguyen M, Scheremeta B, St-Surin S, Villa AM, et al. Thymosin beta 4 increases hair growth by activation of hair follicle stem cells. FASEB J. 2004;18:385–387. doi: 10.1096/fj.03-0244fje. [DOI] [PubMed] [Google Scholar]
- 21.Bonnet D, Lemoine FM, Frobert Y, Bonnet ML, Baillou C, et al. Thymosin beta 4 inhibitor for normal hematopoietic progenitor cells. Exp Hematol. 1996;24:776–782. [PubMed] [Google Scholar]
- 22.Moon H-S, Even-Ram S, Kleinman HK, Cha HJ. Zyxin is upregulated in the nucleus by thymosin beta 4 in SiHa cells. Exp Cell Res. 2006;312:3425–3431. doi: 10.1016/j.yexcr.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 23.Huff T, Muller CS, Otto AM, Netzker R, Hannappel E. Β thymosins, small acidic peptides with multiple functions. Int J Biochem Cell Biol. 2001;33:205–220. doi: 10.1016/s1357-2725(00)00087-x. [DOI] [PubMed] [Google Scholar]
- 24.Gomez-Marquez J, del Amo F, Carpintero P, Anadon R. High levels of mouse thymosin β4 mRNA in differentiating P19 embryonic cells and during development of cardiovascular tissues. Biochim Biophys Acta. 1996;1306:187–193. doi: 10.1016/0167-4781(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 25.Smart N, Hill AA, Cross JC, Riley PR. A differential screen for putative targets of the bHLH transcription factor Hand1 in cardiac morphogenesis. Mech Dev. 2002;119:S65–S71. doi: 10.1016/s0925-4773(03)00093-5. [DOI] [PubMed] [Google Scholar]
- 26.Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, et al. Thymosin beta 4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 27.De La Cruz EM, Pollard TD. Structural biology: actin'up. Science. 2001;293:616–618. doi: 10.1126/science.1063558. [DOI] [PubMed] [Google Scholar]
- 28.Lee SJ, So I-S, Park S-Y, Kim I-S. Thymosin beta 4 is involved in stabilin-2-mediated apoptotic cell engulfment. FEBS Letters. 2008;582:2161–2166. doi: 10.1016/j.febslet.2008.03.058. [DOI] [PubMed] [Google Scholar]