Abstract
Cysticercosis is an infection with larval cysts of the cestode Taenia solium. Through pathways that are incompletely understood, dying parasites initiate a granulomatous reaction that, in the brain, causes seizures. Substance P (SP), a neuropeptide involved in pain-transmission, contributes to inflammation and previously was detected in granulomas associated with dead T. crassiceps cysts. To determine if SP contributes to granuloma formation, we measured granuloma-size and levels of IL-1β, TNF-α, and IL-6 within granulomas in T. crassiceps-infected wild type (WT) mice and mice deficient in SP-precursor (SPP) or the SP-receptor (neurokinin 1, NK1). Granuloma volumes of infected SPP- and NK1-knockout mice were reduced by 31 and 36%, respectively, compared to WT mice (P < .05 for both) and produced up to 5-fold less IL-1β, TNF-α, and IL-6 protein. Thus, SP signaling contributes to granuloma development and proinflammatory cytokine production in T. crassiceps infection and suggests a potential role for this mediator in human cystercercosis.
1. Introduction
Neurocysticercosis is the most common parasitic disease of the central nervous system leading to seizures worldwide [1]. Humans develop cysticercosis when they ingest eggs of the tapeworm Taenia solium usually found in fecal-contaminated water or food [2]. Neurocysticercosis (NCC) is endemic to many parts of the world [3–7] and is becoming an increasingly important cause of seizures in the United States due to immigration from Mexico and Central and South America [8, 9]. Seizures in NCC most commonly arise as a result of the granulomatous responses to dead cysts in the brain. The granulomatous response is associated with production of several cytokines including T helper 1 (Th1) cytokines such as interferon gamma (IFN-γ), interleukin-2 (IL-2), and interleukin-12 (IL-12) [10].
T. crassiceps infection in mice is an experimental model for T. solium cysticercosis in man [11–15]. Intraperitoneal inoculation with 10 cysts of T. crassiceps results in the entire peritoneal cavity of the mouse demonstrating granulomatous inflammation within 3–6 months. Similar to human infection, minimal granulomatous inflammation is found surrounding live parasites; rather, granulomatous inflammation is initiated when the parasite dies. The mediators contributing to development of granulomas around the dead parasite and production of proinflammatory cytokines are not completely understood.
We previously detected substance P (SP) protein within granulomas associated with T. crassiceps infection [16, 17]. We also demonstrated that levels of IL-2, IFN-γ, IL-4, and IL-10 protein were significantly higher in granulomas from infected WT mice than granulomas from infected SPP-knockout or the SP-receptor (neurokinin 1, NK1) NK1-knockout mice [16, 17]. In addition, we detected mRNA for IL-1α, IL-1β, IL-1 receptor antagonist, and TNF-α in all granulomas derived from infected WT mice [18]. However, corresponding proteins levels were not assessed nor was the contribution of SP signaling to their mRNA and protein production and to granuloma formation.
The current studies were aimed at determining if SP and NK1 contributed to granuloma development and/or to production of IL-1β, TNF-α, and IL-6 in cysticercosis. SP stimulates production of proinflammatory cytokines such as of IL-1β, IL-6, and TNF-α by human peripheral mononuclear cells, bronchial cells, and astrocytes [19–29] SP also contributes to inflammatory processes associated with other infectious diseases. For example, granulomatous inflammation in murine schistosomiasis requires binding of SP to NK1 [30]. SP has been demonstrated to stimulate inflammatory cell infiltration. SP injection induced recruitment of leukocytes into the pleural cavity of mice and into the skin of humans [19–29] and stimulated the migration of human fibroblasts and peripheral blood lymphocytes in studies using modified Boyden chambers or micropore filter analysis, respectively [19–29]. In the current studies, we determined granuloma size and measured levels of IL-1β, TNF-α, and IL-6 protein within granuloma obtained from T. crassiceps-infected WT and mice deficient in SPP or NK1. These studies indicate that SP signaling contributes to granuloma development and proinflammatory cytokine production in T. crassiceps infection and suggests a potential role for this mediator in human cysticercosis.
2. Materials and Methods
2.1. Murine Cysticercosis Model
Female mice were infected by intraperitoneal inoculation with 10 cysts of the ORF strain of T. crassiceps, as described in [16, 17]. Three months following infection, the mice were euthanatized by cervical dislocation under anesthesia using a combination anesthetic sacrifice rodent cocktail ketamine, 25 mg/kg, acepromazine 0.8 mg/kg, xylazine 5 mg/kg intraperitoneally, at a dose of 0.5–0.7 mL/kg intramuscularly. Granulomas associated with dying cysts were removed from the peritoneal cavity of each of the infected mice that were euthanized. Three groups of mice were included in the experiments: (1) wild type C57BL/6 mice; (2) preprotachykinin or SPP-knockout mice (Jackson Laborotories, Maine, USA, bred >10 generations onto the C57BL/6 background); (3) NK1-knockout mice provided by Dr. Joel Weinstock, Tufts New England Medical Center, Boston, USA, bred >10 generations onto the C57BL/6 background. Three to 8 infected mice from each of the 3 groups were used for this study; 4–15 granulomas per mouse group were used for this study. Granulomas associated with parasites were identified visually, removed from the peritoneal cavity, and either used for quantifying cytokine proteins by ELISA or used for size determinations. This study was approved by the Animal Research Committee at Baylor College of Medicine.
2.2. Granuloma Size Determination
Intact granuloma was obtained from T. crassiceps-infected WT mice (2 granulomas), SPP-knockout mice (4 granulomas), and NK1-knockout mice (3 granulomas), fixed with 4% paraformaldehyde, paraffin imbedded and completely sectioned by microtome into 7 micron sections. Each section was stained with giemsa and examined microscopically. The area of granuloma within each section was measured using Image J software (NIH). The volume of granuloma within each section was calculated by multiplying the area times 7 microns and the volume of granuloma within each section totaled to give the total granuloma volume.
2.3. Sandwich ELISA for the Detection of Cytokines
Cytokine protein levels were determined in 12–15 granulomas derived from T. crassiceps infected WT mice, 4–6 granulomas derived from T. crassiceps infected SPP-knockout mice, and 9-10 granulomas derived from T. crassiceps-infected NK1-knockout mice. A portion of each granuloma was homogenized in PBS, followed by centrifugation at 16,000 g. Total protein in the supernatant was quantitated using the Bradford method (cat no. 500-0006, Bio-Rad, Hercules, CA). IL-1β, IL-6, and TNF-α protein levels were determined by sandwich ELISA (R&D Systems, San Diego, California) as per manufacturer's instruction. Results are expressed as pg cytokine/mg total protein.
2.4. Statistical Analysis
Differences between groups were compared using Student's t-test. Significance was set at P < .05.
3. Results
3.1. Granulomas from T. crassiceps-Infected SPP-Knockout and NK1-Knockout Mice Are Smaller than Granulomas Derived from Infected WT Mice
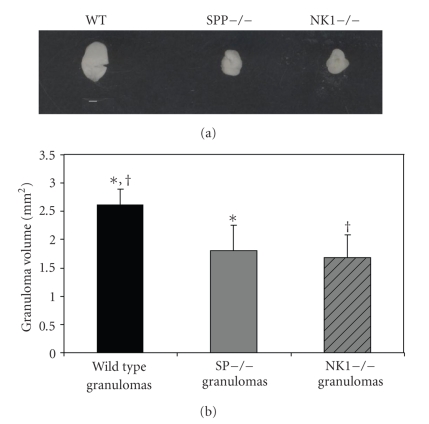
To begin to examine the contribution of SP signaling to granuloma formation in NCC, we measured granuloma volume in mice with normal and deficient SP signaling. The volumes of granulomas from Taenia crassiceps infected SPP-knockout mice (1.8 ± 0.45 mm2) and NK1-knockout mice (1.68 ± 0.40 mm2) were reduced by 31% and 36%, respectively, compared to granulomas derived from infected WT mice (2.62 ± 0.28 mm2; P < .05 for both; Student's t-test; see Figure 1).
Figure 1.
(a) Representative granuloma derived from infected WT, SPP-knockout, and NK1-knockout mice (white bar = 1 mm). (b) Volumes of granulomas derived from Taenia crassiceps infected, wild type (n = 2) versus SPP-knockout (n = 4) and NK1-knockout mice (n = 3). Data presented are mean ± SD; *P < 05, size of infected, SPP-knockout derived granulomas versus WT derived granulomas, †P < .05, size of infected, NK1-knockout derived granulomas versus WT derived granulomas.
3.2. IL-1β Protein Levels Are Reduced within Granulomas from T. crassiceps Infected SPP-Knockout and Infected NK1-Knockout Mice Compared to Granulomas from Infected WT Mice
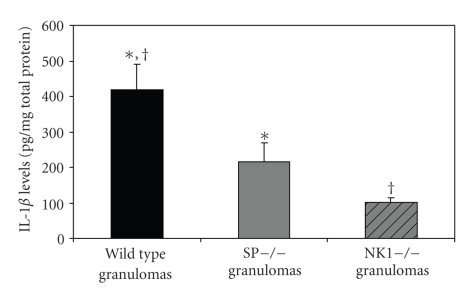
IL-1β is the primary mediator of granuloma formation in the S. mansoni pulmonary granuloma model [31]. Also, intratracheal injection of agarose beads coupled to recombinant IL-1β induced pulmonary granulomas in mice [32]. To determine if decreased production of IL-1β in SPP- and NK1-knockout mice contributed to reduced granuloma size in these animals, we measured IL-1β protein levels in the granulomas derived from each group of mice. IL-1β protein levels in granulomas from T. crassiceps infected SPP-knockout mice (216 ± 129 ng/mg total protein) and NK1-knockout mice (101 ± 43 ng/mg total protein) were reduced by 48% and 76%, respectively, compared to granulomas from infected WT mice (418 ± 278 ng/mg total protein; P < .05 for both; Student's t-test; see Figure 2). Thus, SP signaling contributes to IL-1β production within granulomas formed in response to dying T. crassiceps cysts. Furthermore, reduced levels of this cytokine likely contributed to reduced granuloma size in SPP- and NK1-knockout mice.
Figure 2.
Quantitative levels of IL-1β in granulomas derived from Taenia crassiceps infected, WT (n = 15), SPP-knockout mice (n = 6) and NK1-knockout mice (n = 9). Data presented are mean ± SEM; *P < 05, IL-1β levels in infected, SPP-knockout derived granulomas versus WT derived granulomas; †P < .05, IL-1β levels in infected, NK1-knockout derived granulomas versus WT derived granulomas.
3.3. TNF-α Protein Levels Are Decreased within Granulomas from T. crassiceps Infected SPP-Knockout and Infected NK1-Knockout Mice Compared to Granulomas from Infected WT Mice
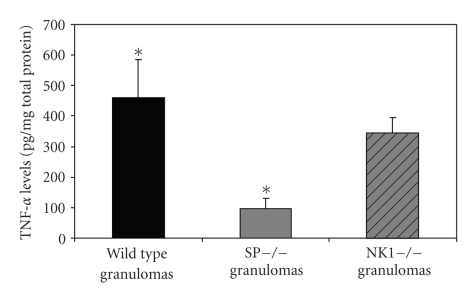
TNF-α is responsible for granuloma development in multiple settings. Intratracheal injection of agarose beads coupled to TNF-α induced pulmonary granulomas in mice [32]. TNF-α mediates granuloma growth in the S. mansoni pulmonary granuloma model [31] and is required for granuloma formation in a mouse model of tuberculosis [33]. Similar to our findings with IL-1β, TNF-α protein levels in granulomas from T. crassiceps infected SPP-knockout mice (96 ± 67 ng/mg total protein) were reduced by 79% compared to levels in granulomas derived from infected WT mice (460 ± 452 ng/mg total protein; P < .05; Student's t-test; see Figure 3). TNF-α protein levels within granulomas from NK1-knockout mice (345 ± 153 ng/mg total protein) were decreased by 25% compared to levels with granulomas of WT mice; however, this difference did not achieve statistical significance. Thus, SP contributes to TNF-production within granulomas formed in response to dying T. crassiceps cysts. Furthermore, reduced levels of this cytokine likely contributed to reduced granuloma size in SPP- and, perhaps, NK1-knockout mice. The failure to detect a significant difference in TNF-α protein levels between NK1-knockout and WT mice suggests the possibility that SP-mediated increases in TNF-α may occur through binding of SP to other members of the NK family, for example, NK2 or NK3.
Figure 3.
Quantitative levels of TNF-α in granulomas derived from Taenia crassiceps infected, WT (n = 13), SPP-knockout mice (n = 4), and NK1-knockout mice (n = 9). Data presented are mean ± SEM; *P < 05, TNF-α levels in infected, SPP-knockout derived granulomas versus WT derived granulomas.
3.4. IL-6 Levels Are Decreased within Granulomas from T. crassiceps Infected SPP-Knockout and Infected NK1-Knockout Mice Compared to Granulomas from Infected WT Mice
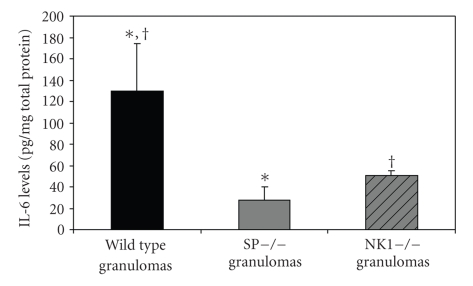
IL-6 production in human peripheral blood mononuclear cells, bronchial cells, and astrocytes is increased directly by SP through the action of nuclear factor IL-6 (NF-IL-6) and p38 MAPK, as well as indirectly in response to IL-1β and TNF-α through the activation of NF-κB [26, 27, 34, 35]. IL-6 mediates its acute proinflammatory effects within infected or injured tissues, in part, through upregulation of CXC chemokines, which leads to recruitment of the first wave of inflammatory cells. As we expected from the IL-1β and TNF-α results summarized above, IL-6 protein levels in the granulomas derived from infected SPP-knockout mice (28 ± 30 ng/mg total protein) and from infected NK1-knockout mice (50 ± 16 ng/mg total protein) were reduced by 79 and 62%, respectively, compared to levels in granulomas from infected WT mice (130 ± 153 ng/mg total protein; P < .05 for both; Student's t-test; see Figure 4). Thus, SP signaling either directly or indirectly through the actions of IL-1β and TNF-α contributes to IL-6 production within granulomas formed in response to dying T. crassiceps cysts.
Figure 4.
Quantitative levels of IL-6 in granulomas derived from Taenia crassiceps infected, WT (n = 12), SPP-knockout mice (n = 6), and NK1-knockout mice (n = 9). Data presented are mean ± SEM; *P < 05, IL-6 levels in infected, SPP-knockout derived granulomas versus WT derived granulomas, †P < .05, IL-6 levels in infected, NK1-knockout derived granulomas versus WT derived granulomas.
4. Discussion
The current studies were performed to determine the contribution of SP and its specific receptor, NK1, to granuloma development and proinflammatory cytokine production within granulomas arising in mice infected with T. crassiceps. We demonstrated that the size of granulomas from the T. crassiceps-infected SPP-knockout mice and infected NK1-knockout mice were significantly smaller than granulomas from the infected WT mice. Furthermore, proteins levels of IL-1β, a key mediator of granuloma formation, were significantly lower within granuloma from SPP- and NK1- knockout mice compared to granuloma from mice. In addition, compared to granulomas from WT mice, protein levels of TNF-α, another key mediator of granuloma formation, were significantly lower in SPP-knockout mice and trended in the same direction in NK1-knockout mice. Thus, SP signaling contributes to granuloma formation, in part, through induction of IL-1β and TNF-α, key mediators of granuloma formation.
Substance P and its high affinity receptor, NK1, are known to play an important role in inflammatory responses. Granuloma formation in response to murine schistosomiasis requires binding of SP to its specific receptor [30]. SP is known to stimulate inflammatory cell infiltration and to induce the production of proinflammatory cytokines such as IL-1β, IL-6, and TNF-α by human peripheral mononuclear cells, bronchial cells, or astrocytoma cells [19–29]. Our findings extend these observations and indicate that SP signaling contributes to granuloma formation and production of IL-1β, TNF-α, and IL-6 protein within granuloma formed in response to T. crassiceps infection. The mechanism by which SP stimulates the production of these cytokines may be by mediating inflammatory cell influx [19–24]. Nerves, endothelial cells, and cells of the immune system produce SP [36–38]. All of these cells have receptors for SP and are known to respond to SP [38–40]. SP is known to stimulate influx of lymphocytes, monocytes, macrophages, and other immune cells that produce proinflammatory cytokines such as IL-1β, IL-6, and TNF-α [19–29]. Although there are various studies on the molecular mechanisms by which SP stimulates the production of IL-6 and TNF-α, there is limited information on the molecular mechanisms by which SP stimulates IL-1β production. A study by Martin et al. determined that SP stimulated IL-1 production by astrocytes via increasing intracellular calcium. These studies demonstrated that treatment with an intracellular calcium chelator blocked SP-induced IL-1β production [41]. Other studies have demonstrated that SP induces TNF-alpha and IL-6 production through NF kappa B in peritoneal mast cells [42]. Substance P production of TNF-α in peritoneal mast cells is also known to be mediated via P38 and JNK Map kinases [43]. Also IL-6 production in human peripheral blood mononuclear cells, bronchial cells, and astrocytes is increased directly by SP through the action of nuclear factor IL-6 (NF-IL-6) and p38 MAPK [26, 27, 34, 35].
Preprotachykinin protein is cleaved to form two active neuropeptides, SP and neurokinin A. Therefore, SP precursor (preprotachykinin) knockout mice produce neither SP nor neurokinin A. Consequently, our results in SPP-knockout mice can be attributed to a deficiency in either SP or neurokinin A or both. However, since NK1 binds only SP and not neurokinin A and the results in NK1-knockout mice mirror the findings in SP-knockout mice, we are confident in attributing reduced granuloma formation and proinflammatory cytokine production to the absence of SP signaling and not to reduced or absent neurokinin A signaling.
In the current studies, we demonstrated that the granuloma size and the levels of the proinflammatory cytokine, IL-1β, are lower in the infected NK1-knockout mice compared to those of the infected SPP-knockout mice. Besides SP, peptide hormones such as hemokinin can also bind and activate NK1 at sites of chronic inflammation [44]. Therefore, although the current studies suggest that SP may be an important mediator associated with cytokine production, there may be other peptide hormones like hemokinin that also bind and activate the NK1 receptor that may be associated with granuloma and cytokine production. Therefore, it may be possible that in the NK1-knockout mice, the synergistic lack of activity of both SP and hemokinin may have resulted in lower IL-1β levels and granuloma size as compared to SPP-knockout mice.
Granuloma formation by the host in response to agents causing chronic infections is thought to be essential for limiting and eventually clearing infection. However, recent work in zebra fish infected with Mycobacterium marinum suggests that granuloma formation contributes to early bacterial growth [45]. Intraparenchymal cysts of NCC are thought to die spontaneously and to elicit a granulomatous response that does not in itself contribute to the demise of the cyst. Rather, we have previously demonstrated that early granuloma formed in response to dying cysts contributes to NCC disease manifestations by producing mediators that induce seizures [46]. Other groups have demonstrated that SP is epileptogenic [47]. The current studies demonstrating that SP signaling contributes to granuloma formation in Taenia crassiceps infection, together with other published observations, suggest the possibility that diminishing granuloma formation in NCC by blocking SP, which contributes to granuloma formation and epileptogenic responses, may be beneficial in the treatment of this disease.
Acknowledgments
These studies were supported by the National Institutes of Health, 1 R01 NS042604-01A2 to P. Robinson. Taenia crassiceps cysts were provided by Dr. Raymond Kuhn, Wake Forest University, Winston-Salem, North Carolina. SPP-knockout mice were provided by Dr. Julio Pérez Fontán, University of Texas Southwestern School of Medicine, Houston, Texas.
References
- 1.García HH, Evans CAW, Nash TE, et al. Current consensus guidelines for treatment of neurocysticercosis. Clinical Microbiology Reviews. 2002;15(4):747–756. doi: 10.1128/CMR.15.4.747-756.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carpio A. Neurocysticercosis: an update. The Lancet Infectious Diseases. 2002;2(12):751–762. doi: 10.1016/s1473-3099(02)00454-1. [DOI] [PubMed] [Google Scholar]
- 3.Bharucha NE. Epidemiology of epilepsy in India. Epilepsia. 2003;44(supplement 1):9–11. doi: 10.1046/j.1528-1157.44.s.1.5.x. [DOI] [PubMed] [Google Scholar]
- 4.de Bittencourt PRM, Adamolekum B, Bharucha N, et al. Epilepsy in the tropics: II. Clinical presentations, pathophysiology, immunologic diagnosis, economics, and therapy. Epilepsia. 1996;37(11):1128–1137. doi: 10.1111/j.1528-1157.1996.tb01036.x. [DOI] [PubMed] [Google Scholar]
- 5.Jallon P. Epilepsy in developing countries. Epilepsia. 1997;38(10):1143–1151. doi: 10.1111/j.1528-1157.1997.tb01205.x. [DOI] [PubMed] [Google Scholar]
- 6.Rajshekhar V, Joshi DD, Doanh NQ, van De N, Xiaonong Z. Taenia solium taeniosis/cysticercosis in Asia: epidemiology, impact and issues. Acta Tropica. 2003;87(1):53–60. doi: 10.1016/s0001-706x(03)00055-x. [DOI] [PubMed] [Google Scholar]
- 7.Sánchez AL, Lindbäck J, Schantz PM, et al. A population-based, case-control study of Taenia solium taeniasis and cysticercosis. Annals of Tropical Medicine and Parasitology. 1999;93(3):247–258. [PubMed] [Google Scholar]
- 8.Ong S, Talan DA, Moran GJ, et al. Neurocysticercosis in radiographically imaged seizure patients in U.S. emergency departments. Emerging Infectious Diseases. 2002;8(6):608–613. doi: 10.3201/eid0806.010377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorvillo FJ, Waterman SH, Richards FO, Schantz PM. Cysticercosis surveillance: locally acquired and travel-related infections and detection of intestinal tapeworm carriers in Los Angeles County. American Journal of Tropical Medicine and Hygiene. 1992;47(3):365–371. doi: 10.4269/ajtmh.1992.47.365. [DOI] [PubMed] [Google Scholar]
- 10.Restrepo BI, Llaguno P, Sandoval MA, Enciso JA, Teale JM. Analysis of immune lesions in neurocysticercosis patients: central nervous system response to helminth appears Th1-like instead of Th2. Journal of Neuroimmunology. 1998;89(1-2):64–72. doi: 10.1016/s0165-5728(98)00112-x. [DOI] [PubMed] [Google Scholar]
- 11.Villa OF, Kuhn RE. Mice infected with the larvae of Taenia crassiceps exhibit a Th2-like immune response with concomitant anergy and downregulation of Th1-associated phenomena. Parasitology. 1996;112(6):561–570. doi: 10.1017/s0031182000066142. [DOI] [PubMed] [Google Scholar]
- 12.Larralde C, Montoya RM, Sotelo J, et al. Murine T. crassiceps antigens in immunodiagnosis of T. solium human neurocysticercosis, T. saginata bovine cysticercosis, and human E. granulosus hydatidosis. Bulletin de la Societe Francaise de Parasitology. 1990;8:p. S8B. [Google Scholar]
- 13.Villa O, Kuhn R. Antigenic and immunogenic analysis of Taenia crassiceps and Taenia solium using sera from their natural intermediate hosts. ASM Bulletin. 1991;39:99–103. [Google Scholar]
- 14.Kunz J, Kalinna B, Watschke V, Geyer E. Taenia crassiceps metacestode vesicular fluid antigens shared with the Taenia solium larval stage and reactive with serum antibodies from patients with neurocysticercosis. Zentralblatt für Bakteriologie. 1989;271(4):510–520. doi: 10.1016/s0934-8840(89)80113-6. [DOI] [PubMed] [Google Scholar]
- 15.Sciutto E, Fragoso G, Baca M, De la Cruz V, Lemus L, Lamoyi E. Depressed T-cell proliferation associated with susceptibility to experimental Taenia crassiceps infection. Infection and Immunity. 1995;63(6):2277–2281. doi: 10.1128/iai.63.6.2277-2281.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garza A, Weinstock J, Robinson P. Absence of the SP/SP receptor circuitry in the substance P-precursor knockout mice or SP receptor, neurokinin (NK)1 knockout mice leads to an inhibited cytokine response in granulomas associated with murine Taenia crassiceps infection. Journal of Parasitology. 2008;94(6):1253–1258. doi: 10.1645/GE-1481.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson P, White AC, Lewis DE, Thornby J, David E, Weinstock J. Sequential expression of the neuropeptides substance P and somatostatin in granulomas associated with murine cysticercosis. Infection and Immunity. 2002;70(8):4534–4538. doi: 10.1128/IAI.70.8.4534-4538.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patil S, Actor J, Robinson P, Baig S, White AJ. Proinflammatory cytokines in murine cysticercal granulomas. In: Proceedings of the 48th Annual Meeting of the American Society of Tropical Medicine and Hygiene (ASTMH ’99); November-December 1999; Washington, DC, USA. [Google Scholar]
- 19.Fröde-Saleh TS, Calixto JB, Medeiros YS. Analysis of the inflammatory response induced by substance P in the mouse pleural cavity. Peptides. 1999;20(2):259–265. doi: 10.1016/s0196-9781(98)00170-3. [DOI] [PubMed] [Google Scholar]
- 20.Hegde A, Bhatia M. Neurogenic inflammation in acute pancreatitis. Journal of the Pancreas. 2005;6(5):417–421. [PubMed] [Google Scholar]
- 21.Haines KA, Kolasinski SL, Cronstein BN, Reibman J, Gold LI, Weissmann G. Chemoattraction of neutrophils by substance P and transforming growth factor-β1 is inadequately explained by current models of lipid remodeling. The Journal of Immunology. 1993;151(3):1491–1499. [PubMed] [Google Scholar]
- 22.Kahler CM, Sitte BA, Reinisch N, Wiedermann CJ. Stimulation of the chemotactic migration of human fibroblastsfd by substance P. European Journal of Pharmacology. 1993;249(3):281–286. doi: 10.1016/0014-2999(93)90523-k. [DOI] [PubMed] [Google Scholar]
- 23.Schratzberger P, Reinisch N, Prodinger WM, et al. Differential chemotactic activities of sensory neuropeptides for human peripheral blood mononuclear cells. The Journal of Immunology. 1997;158(8):3895–3901. [PubMed] [Google Scholar]
- 24.Smith CH, Barker JNWN, Morris RW, MacDonald DM, Lee TH. Neuropeptides induce rapid expression of endothelial cell adhesion molecules and elicit granulocytic infiltration in human skin. The Journal of Immunology. 1993;151(6):3274–3282. [PubMed] [Google Scholar]
- 25.Veronesi B, Carter JD, Devlin RB, Simon SA, Oortgiesen M. Neuropeptides and capsaicin stimulate the release of inflammatory cytokines in a human bronchial epithelial cell line. Neuropeptides. 1999;33(6):447–456. doi: 10.1054/npep.1999.0761. [DOI] [PubMed] [Google Scholar]
- 26.Lieb K, Schaller H, Bauer J, Berger M, Schulze-Osthoff K, Fiebich BL. Substance P and histamine induce interleukin-6 expression in human astrocytoma cells by a mechanism involving protein kinase C and nuclear factor-IL-6. Journal of Neurochemistry. 1998;70(4):1577–1583. doi: 10.1046/j.1471-4159.1998.70041577.x. [DOI] [PubMed] [Google Scholar]
- 27.Fiebich BL, Schleicher S, Butcher RD, Craig A, Lieb K. The neuropeptide substance P activates p38 mitogen-activated protein kinase resulting in IL-6 expression independently from NF-κB. The Journal of Immunology. 2000;165(10):5606–5611. doi: 10.4049/jimmunol.165.10.5606. [DOI] [PubMed] [Google Scholar]
- 28.Cuesta MC, Quintero L, Pons H, Suarez-Roca H. Substance P and calcitonin gene-related peptide increase IL-1β, IL-6 and TNFα secretion from human peripheral blood mononuclear cells. Neurochemistry International. 2002;40(4):301–306. doi: 10.1016/s0197-0186(01)00094-8. [DOI] [PubMed] [Google Scholar]
- 29.Lotz M, Vaughan JH, Carson DA. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science. 1988;241(4870):1218–1221. doi: 10.1126/science.2457950. [DOI] [PubMed] [Google Scholar]
- 30.Blum AM, Metwali A, Kim-Miller M, et al. The substance P receptor is necessary for a normal granulomatous response in murine schistosomiasis mansoni. The Journal of Immunology. 1999;162(10):6080–6085. [PubMed] [Google Scholar]
- 31.Boros DL. The role of cytokines in the formation of the schistosome egg granuloma. Immunobiology. 1994;191(4-5):441–450. doi: 10.1016/S0171-2985(11)80450-X. [DOI] [PubMed] [Google Scholar]
- 32.Kasahara K, Kobayashi K, Shikama Y, et al. The role of monokines in granuloma formation in mice: the ability of interleukin 1 and tumor necrosis factor-α to induce lung granulomas. Clinical Immunology and Immunopathology. 1989;51(3):419–425. doi: 10.1016/0090-1229(89)90040-8. [DOI] [PubMed] [Google Scholar]
- 33.Algood HMS, Lin PL, Flynn JL. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clinical Infectious Diseases. 2005;41(supplement 3):S189–S193. doi: 10.1086/429994. [DOI] [PubMed] [Google Scholar]
- 34.Parikh AA, Salzman AL, Kane CD, Fischer JE, Hasselgren P-O. IL-6 production in human intestinal epithelial cells following stimulation with IL-1β is associated with activation of the transcription factor NF-κB. Journal of Surgical Research. 1997;69(1):139–144. doi: 10.1006/jsre.1997.5061. [DOI] [PubMed] [Google Scholar]
- 35.Kurokouchi K, Kambe F, Yasukawa K, et al. TNF-α increases expression of IL-6 and ICAM-1 genes through activation of NF-κB in osteoblast-like ROS17/2.8 cells. Journal of Bone and Mineral Research. 1998;13(8):1290–1299. doi: 10.1359/jbmr.1998.13.8.1290. [DOI] [PubMed] [Google Scholar]
- 36.Weinstock JV, Blum A, Walder J, Walder R. Eosinophils from granulomas in murine schistosomiasis mansoni produce substance P. The Journal of Immunology. 1988;141(3):961–966. [PubMed] [Google Scholar]
- 37.Maggi CA. The effects of tachykinins on inflammatory and immune cells. Regulatory Peptides. 1997;70(2-3):75–90. doi: 10.1016/s0167-0115(97)00029-3. [DOI] [PubMed] [Google Scholar]
- 38.Ho W-Z, Lai J-P, Zhu X-H, Uvaydova M, Douglas SD. Human monocytes and macrophages express substance P and neurokinin-1 receptor. The Journal of Immunology. 1997;159(11):5654–5660. [PubMed] [Google Scholar]
- 39.Goode T, O’Connell J, Sternini C, et al. Substance P (neurokinin-1) receptor is a marker of human mucosal but not peripheral mononulear cells: molecular quantitation and localization. The Journal of Immunology. 1998;161(5):2232–2240. [PubMed] [Google Scholar]
- 40.Cook GA, Elliott D, Metwali A, et al. Molecular evidence that granuloma T lymphocytes in murine schistosomiasis mansoni express an authentic substance P (NK-1) receptor. The Journal of Immunology. 1994;152(4):1830–1835. [PubMed] [Google Scholar]
- 41.Martin FC, Charles AC, Sanderson MJ, Merrill JE. Substance P stimulates IL-1 production by astrocytes via intracellular calcium. Brain Research. 1992;599(1):13–18. doi: 10.1016/0006-8993(92)90846-2. [DOI] [PubMed] [Google Scholar]
- 42.Azzolina A, Bongiovanni A, Lampiasi N. Substance P induces TNF-α and IL-6 production through NFκB in peritoneal mast cells. Biochimica et Biophysica Acta. 2003;1643(1–3):75–83. doi: 10.1016/j.bbamcr.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Azzolina A, Guarneri P, Lampiasi N. Involvement of p38 and JNK MAPKs pathways in substance P-induced production of TNF-α by peritoneal mast cells. Cytokine. 2002;18(2):72–80. doi: 10.1006/cyto.2002.0879. [DOI] [PubMed] [Google Scholar]
- 44.Metwali A, Blum AM, Elliott DE, Setiawan T, Weinstock JV. Cutting edge: hemokinin has substance P-like function and expression in inflammation. The Journal of Immunology. 2004;172(11):6528–6532. doi: 10.4049/jimmunol.172.11.6528. [DOI] [PubMed] [Google Scholar]
- 45.Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136(1):37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stringer JL, Marks LM, White AC, Jr., Robinson P. Epileptogenic activity of granulomas associated with murine cysticercosis. Experimental Neurology. 2003;183(2):532–536. doi: 10.1016/s0014-4886(03)00179-1. [DOI] [PubMed] [Google Scholar]
- 47.Liu H, Mazarati AM, Katsumori H, Sankar R, Wasterlain CG. Substance P is expressed in hippocampal principal neurons during status epilepticus and plays a critical role in the maintenance of status epilepticus. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(9):5286–5291. doi: 10.1073/pnas.96.9.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]