Abstract
Rationale: Vascular alteration of small pulmonary vessels is one of the characteristic features of pulmonary hypertension in chronic obstructive pulmonary disease. The in vivo relationship between pulmonary hypertension and morphological alteration of the small pulmonary vessels has not been assessed in patients with severe emphysema.
Objectives: We evaluated the correlation of total cross-sectional area of small pulmonary vessels (CSA) assessed on computed tomography (CT) scans with the degree of pulmonary hypertension estimated by right heart catheterization.
Methods: In 79 patients with severe emphysema enrolled in the National Emphysema Treatment Trial (NETT), we measured CSA less than 5 mm2 (CSA<5) and 5 to 10 mm2 (CSA5−10), and calculated the percentage of total CSA for the lung area (%CSA<5 and %CSA5–10, respectively). The correlations of %CSA<5 and %CSA5–10 with pulmonary arterial mean pressure (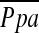 ) obtained by right heart catheterization were evaluated. Multiple linear regression analysis using
) obtained by right heart catheterization were evaluated. Multiple linear regression analysis using 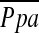 as the dependent outcome was also performed.
as the dependent outcome was also performed.
Measurements and Main Results: The %CSA<5 had a significant negative correlation with 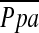 (r = −0.512, P < 0.0001), whereas the correlation between %CSA5–10 and
(r = −0.512, P < 0.0001), whereas the correlation between %CSA5–10 and 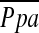 did not reach statistical significance (r = −0.196, P = 0.083). Multiple linear regression analysis showed that %CSA<5 and diffusing capacity of carbon monoxide (DlCO) % predicted were independent predictors of
did not reach statistical significance (r = −0.196, P = 0.083). Multiple linear regression analysis showed that %CSA<5 and diffusing capacity of carbon monoxide (DlCO) % predicted were independent predictors of 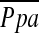 (r2 = 0.541): %CSA <5 (P < 0.0001), and DlCO % predicted (P = 0.022).
(r2 = 0.541): %CSA <5 (P < 0.0001), and DlCO % predicted (P = 0.022).
Conclusions: The %CSA<5 measured on CT images is significantly correlated to 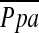 in severe emphysema and can estimate the degree of pulmonary hypertension.
in severe emphysema and can estimate the degree of pulmonary hypertension.
Keywords: chronic obstructive pulmonary disease, emphysema, pulmonary hypertension, CT
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Pulmonary hypertension in patients with chronic obstructive pulmonary disease (COPD) with predominate emphysema phenotype is likely caused by small pulmonary vessel vascular remodeling. However, the in vivo relationship between pulmonary hypertension and morphological alteration of the small pulmonary vessels has not been assessed.
What This Study Adds to the Field
Vascular alteration, measured from the cross-sectional area (CSA) of small pulmonary vessels by computed tomography correlates with the magnitude of pulmonary hypertension. These data suggest that CSA can be used to estimate pulmonary arterial pressure in subjects with severe emphysema.
Pulmonary hypertension is an important predictor of mortality in chronic obstructive pulmonary disease (COPD) (1–3). Various factors, including endothelial dysfunction, inflammation, and hypoxia, have been recognized as potential contributors to the development of secondary pulmonary hypertension in emphysema, but its pathogenesis has not been fully clarified. Nevertheless, it is generally recognized that vascular remodeling of small pulmonary arteries is an essential morphological feature of pulmonary hypertension; narrowing and diminution of the small pulmonary vessels have been shown on conventional angiography in emphysema (4–6). However, to our knowledge, the in vivo relationship between pulmonary hypertension and the magnitude of small pulmonary vessel morphological change has not been quantitatively assessed in severe emphysema.
A recent histological study suggested that vascular remodeling leads to reduced distensibility of small pulmonary vessels and that this remodeling is closely related to pulmonary hypertension (7). Such a decrease in distensibility of small pulmonary vessels may lead to increased pulmonary vascular resistance and elevated pulmonary vascular pressure. Therefore, we hypothesized that measurements of the total cross-sectional area of small pulmonary vessels (CSA) using noncontrast chest computed tomography (CT) scans (8) could be used to show a correlation of the relationship with the degree of pulmonary hypertension in patients with emphysema. The principal purpose of this study was to evaluate the relationship between the total CSA on CT images and the degree of pulmonary hypertension in patients with COPD. For comparison, we also evaluated the correlation of mean pulmonary arterial pressure (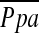 ) with other variables previously described useful in diagnosing pulmonary hypertension, such as the diameter of the main pulmonary artery (MPAD) measured by CT scan. Additional multiple linear regression analysis using
) with other variables previously described useful in diagnosing pulmonary hypertension, such as the diameter of the main pulmonary artery (MPAD) measured by CT scan. Additional multiple linear regression analysis using 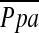 as the dependent outcome was also performed to evaluate the impact of the total CSA on CT images.
as the dependent outcome was also performed to evaluate the impact of the total CSA on CT images.
METHODS
Subjects
We retrospectively evaluated patients who were screened for participation in the National Emphysema Treatment Trial (NETT) at three institutions. The NETT was a prospective, multicenter, randomized, controlled study comparing optimal medical therapy to lung volume reduction surgery for the treatment of severe emphysema. Inclusion criteria for the NETT have been described elsewhere (9). In 3 of the 17 clinical centers, subjects underwent measurements of central pulmonary hemodynamics during right heart catheterization (RHC). Results have been reported elsewhere (10). All data reported were collected before randomization. The institutional review boards at each participating institution as well as the sponsor (National Institutes of Health) approved the main trial and the substudies. In accordance with the policies at each participating institution, all patients signed informed consent for the main trial and for each of the individual substudies. Exclusion criteria included (1) obvious abnormal lung parenchymal lesions other than emphysema, (2) pleural effusion or cardiomegaly that suggested cardiac failure, and (3) excessive image noise that prevented image analysis.
Pulmonary function tests (PFTs) as defined in NETT were performed using American Thoracic Society guidelines (11–14). PaO2 was measured with subjects breathing room air.
Multislice CT Scanning
CT scans were performed on one of three types of scanners as published previously (15). CT images were obtained with 120 kV, 150 to 300 mA, and 1- to 1.5-mm slice thickness for the high-resolution CT (HRCT) images, and 120 kV, 200 to 225mA, and 5- to 7-mm slice thickness for conventional CT images.
CT Measurement of Small Pulmonary Vessels and Main Pulmonary Artery
For the measurements of the pulmonary CSA, three CT slices were selected from HRCT images. The upper cranial slice was taken approximately 1 cm above the upper margin of the aortic arch, the middle slice was taken approximately 1 cm below the carina, and the lower caudal slice was taken approximately 1 cm below the right inferior pulmonary vein. These CT images were analyzed using a semiautomatic image-processing program (ImageJ Version 1.40 g, a public domain Java image processing program available at http://rsb.info.nih.gov/ij/).
Using the ImageJ software “Analyze Particles” function, which can count and measure objects on binary images, the number of vessels of a specified size and the CSA of each size range on every CT slice were obtained (8). Simultaneously, vessels that ran obliquely or parallel to the slice were excluded using the ImageJ software “Circularity” function (8) and only those vessels that ran closest to perpendicular to the CT slice based on their shape in the image were analyzed.
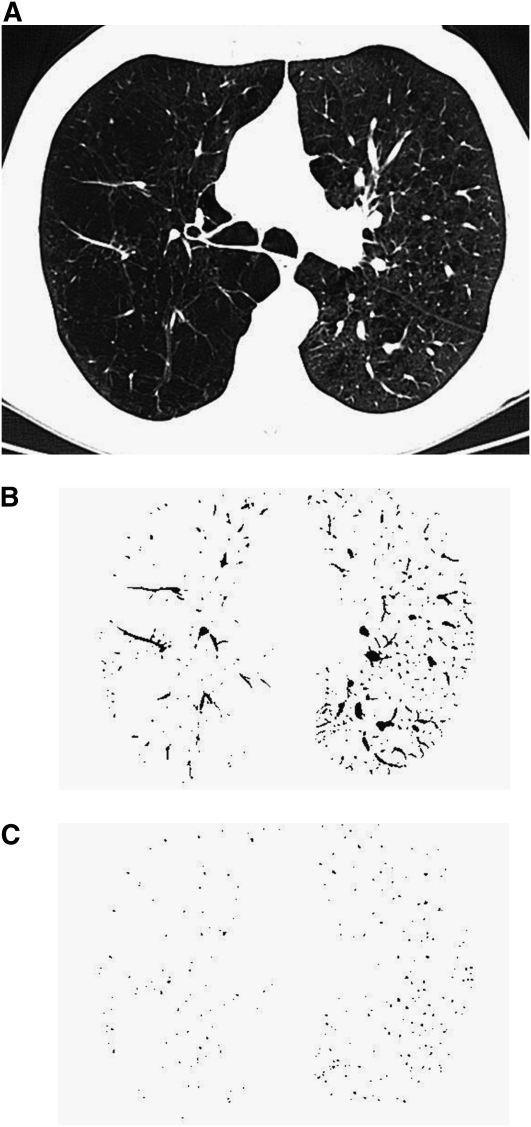
CSA measurements were conducted as follows: First, selected CT images were smoothed by performing Gaussian blurring to eliminate image noise because those CT images were reconstructed with a lung or bone algorithm. The lung field was then segmented using a threshold technique with all pixels between −500 and −1,024 Hounsfield units (HU) on each CT image (Figure 1A). Next, segmented images were converted into binary images with a window level of −720 HU. Vessels including pulmonary arteries and veins were displayed in black on the binary image (Figure 1B). We measured CSA at both the subsegmental and subsubsegmental levels separately, defined as those vessels with a cross-sectional area of 5 to 10 mm2 for subsegmental and less than 5 mm2 for subsubsegmental (16). After these settings, CSA of each vessel was calculated (Figure 1C). Finally, we totaled the CSA of vessels measured on each set of three CT slices, and those totals were abbreviated as follows: CSA<5 for the total cross-sectional area of the subsubsegmental vessels that ranged less than 5 mm2 and CSA5–10 for the total cross-sectional area of the subsegmental vessels that ranged from 5 to 10 mm2. Total lung area of the three selected slices was obtained using threshold values between −500 HU and −1,024 HU, and the percentages of CSA<5 (%CSA<5) and CSA5–10 (%CSA5–10) for the total lung area were calculated.
Figure 1.
The method of measuring the cross-sectional area of small pulmonary vessels using ImageJ software. (A) CT image of lung field segmented within the threshold values from −500 Hounsfield units (HU) to −1,024 HU. (B) Binary image converted from segmented image (A) with window level of −720 HU. Pulmonary vessels are displayed in black. (C) Mask image for particle analysis after setting vessel size parameters within 0 to 5 mm2 and the range of circularity within 0.9 to 1.0.
For the evaluation of the dimensions of the central pulmonary vessels, the diameter of the main pulmonary artery (MPAD) was measured at its widest portion within 3 cm of the bifurcation on the conventional CT image (17). On the same slice, the diameter of the ascending aorta (AoD) was measured. The ratio of MPAD to AoD (MPAD/AoD) was calculated. Measurements were performed by a single observer in a blinded fashion. All measurements were made three times, and the mean values were recorded.
The extent of emphysema was obtained by calculating the mean percentage of low attenuation values lower than −950 HU (%LAA−950) on each CT slice using the ImageJ software (18).
Pulmonary Arterial Pressure Measurements
Right heart catheterizations (RHC) were performed as detailed previously (10). All hemodynamic measurements are reported as the mean of three measurements taken at end-expiration. The 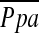 was calculated as the pulmonary artery diastolic pressure plus one-third of the pulse pressure.
was calculated as the pulmonary artery diastolic pressure plus one-third of the pulse pressure.
Statistical Analysis
For the main object of this study, the correlations of 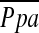 and CT measurements, including %CSA<5 and %CSA5–10, were tested using Spearman rank correlation analysis. We also used Spearman correlation coefficients to express the relationship between
and CT measurements, including %CSA<5 and %CSA5–10, were tested using Spearman rank correlation analysis. We also used Spearman correlation coefficients to express the relationship between 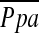 and PFTs, %LAA−950, PaO2, and other variables previously described as indicators of pulmonary arterial pressure, including MPAD and MPAD/AoD (17, 19–21).
and PFTs, %LAA−950, PaO2, and other variables previously described as indicators of pulmonary arterial pressure, including MPAD and MPAD/AoD (17, 19–21).
Multiple linear regression analysis using 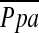 as the dependent outcome was performed to evaluate the impact of measured CT values, lung function, and subject characteristics including age, sex, and body mass index (BMI). Data were expressed as the mean ± standard deviation for all normally distributed variables. For all statistical analyses, the null hypothesis was rejected at the 5% level. All statistical analyses were performed using JMP 5.0 software (SAS Institute, Cary, NC).
as the dependent outcome was performed to evaluate the impact of measured CT values, lung function, and subject characteristics including age, sex, and body mass index (BMI). Data were expressed as the mean ± standard deviation for all normally distributed variables. For all statistical analyses, the null hypothesis was rejected at the 5% level. All statistical analyses were performed using JMP 5.0 software (SAS Institute, Cary, NC).
RESULTS
Characteristics of the Study Subjects
Characteristics of the patients, including the results of PFTs, are presented in Table 1. The cardiovascular substudy enrolled 163 patients from the NETT, including those who were screened but not found eligible for randomization. Ninety-three of the 163 patients had both analyzable chest CT and RHC data that were available for the current study. According to the CT criteria in this study, 14 subjects were excluded because of extensive old inflammatory changes (n = 3) or image noise (n = 11). Thus, 79 patients (mean age, 65 ± 7 yr; range, 47–80 yr; 33 women, 64 ± 6 yr; 46 men, 66 ± 7 yr) were included in this study. Table 2 shows the variables measured at RHC. 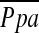 was 25.8 ± 5.0 mm Hg, and the range was from 12.7 to 40.7 mm Hg.
was 25.8 ± 5.0 mm Hg, and the range was from 12.7 to 40.7 mm Hg.
TABLE 1.
DEMOGRAPHICS, PULMONARY FUNCTION, AND THE EXTENT OF EMPHYSEMA (N = 79)
| Mean | SD | |
|---|---|---|
| Age, years | 65 | 7 |
| Sex, % female | 41.8 | |
| BMI | 25.3 | 4.3 |
| FEV1, % predicted | 28.0 | 6.5 |
| FVC, % predicted | 67.6 | 15.7 |
| TLC, % predicted | 125.6 | 12.2 |
| RV, % predicted | 220.4 | 45.6 |
| DlCO, % predicted | 27.2 | 8.2 |
| PaO2 | 64.5 | 9.9 |
| %LAA−950 | 26.1 | 11.7 |
Definition of abbreviations: BMI = body mass index; DlCO = diffusing capacity of the lung for carbon monoxide; RV = residual volume; %LAA−950 = CT measurement of the percentage of low attenuation area less than −950 Hounsfield units, defined as emphysema.
TABLE 2.
PULMONARY HEMODYNAMICS (N = 79)
| Mean | SD | |
|---|---|---|
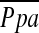 , mm Hg , mm Hg |
25.8 | 5.0 |
| Pulmonary artery systolic pressure, mm Hg | 37.5 | 6.7 |
| Pulmonary artery diastolic pressure, mm Hg | 20.0 | 4.8 |
| Pulmonary capillary wedge pressure, mm Hg | 14.2 | 5.1 |
| Right atrial pressure, mm Hg | 9.7 | 6.0 |
| Cardiac output, L/min | 5.3 | 1.4 |
Definition of abbreviation: Ppa = pulmonary arterial mean pressure.
All pressures were measured at end-expiration.
Correlations between 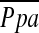 and CT Measurements
and CT Measurements
The results of CT measurements and correlations with 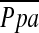 are shown in Table 3. Mean %CSA<5 was 0.55 ± 0.14%, and mean %CSA5–10 was 0.20 ± 0.05%. The %CSA<5 had a significant negative correlation with
are shown in Table 3. Mean %CSA<5 was 0.55 ± 0.14%, and mean %CSA5–10 was 0.20 ± 0.05%. The %CSA<5 had a significant negative correlation with 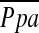 (r = −0.512, P < 0.0001), whereas the correlation between %CSA5–10 and
(r = −0.512, P < 0.0001), whereas the correlation between %CSA5–10 and 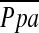 did not reach statistical significance (r = −0.196, P = 0.083) (Figure 2).
did not reach statistical significance (r = −0.196, P = 0.083) (Figure 2). 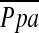 had a significant correlation with DlCO% predicted (r = −0.387, P = 0.0004), whereas
had a significant correlation with DlCO% predicted (r = −0.387, P = 0.0004), whereas 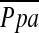 did not have significant correlations with PaO2 (r = −0.201, P = 0.075) or %LAA−950 (r = 0.12, P = 0.29).
did not have significant correlations with PaO2 (r = −0.201, P = 0.075) or %LAA−950 (r = 0.12, P = 0.29).
TABLE 3.
CT MEASUREMENTS AND CORRELATION WITH PULMONARY MEAN ARTERIAL PRESSURE (N = 79)
|
|||||
|---|---|---|---|---|---|
| CT Parameters | Mean ± SD | Range | r | P Value | |
| %CSA<5, % | 0.55 ± 0.14 | 0.28–1.06 | −0.512 | <0.0001 | |
| %CSA5–10, % | 0.20 ± 0.05 | 0.10–0.41 | −0.196 | 0.083 | |
| MPAD, mm | 28.0 ± 3.3 | 20.6–38.1 | 0.210 | 0.063 | |
| MPAD/AoD | 0.81 ± 0.11 | 0.56–1.16 | 0.099 | 0.384 | |
Definition of abbreviations: AoD = diameter of ascending aorta adjacent to main pulmonary artery; MPAD = diameter of the main pulmonary artery; 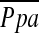 = pulmonary arterial mean pressure; %CSA<5 = percentage of total lung area taken up by the cross-sectional area of pulmonary vessels less than 5 mm2; %CSA5–10 = percentage of total lung area taken up by the cross-sectional area of pulmonary vessels between 5 and 10 mm2.
= pulmonary arterial mean pressure; %CSA<5 = percentage of total lung area taken up by the cross-sectional area of pulmonary vessels less than 5 mm2; %CSA5–10 = percentage of total lung area taken up by the cross-sectional area of pulmonary vessels between 5 and 10 mm2.
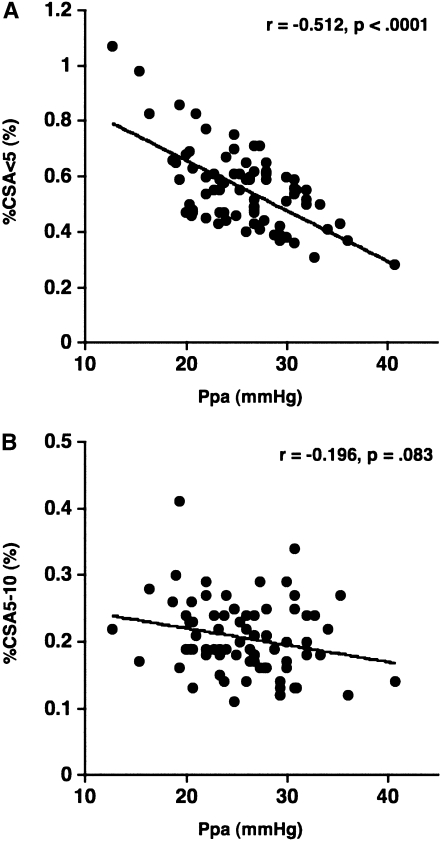
Figure 2.
The relationship between the pulmonary arterial mean pressure (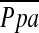 ) and (A) the percentage of the area taken up by the cross-sectional area (CSA) of pulmonary vessels smaller than 5 mm2 (%CSA<5), and (B) the percentage of the area taken up by the CSA of pulmonary vessels between 5 mm2 and 10 mm2 (%CSA5–10).
) and (A) the percentage of the area taken up by the cross-sectional area (CSA) of pulmonary vessels smaller than 5 mm2 (%CSA<5), and (B) the percentage of the area taken up by the CSA of pulmonary vessels between 5 mm2 and 10 mm2 (%CSA5–10). 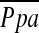 has a significant negative correlation with %CSA<5 (r = −0.512, P < 0.0001), whereas there is no significant correlation between
has a significant negative correlation with %CSA<5 (r = −0.512, P < 0.0001), whereas there is no significant correlation between 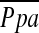 and %CSA5–10 (r = −0.196, P = 0.083).
and %CSA5–10 (r = −0.196, P = 0.083).
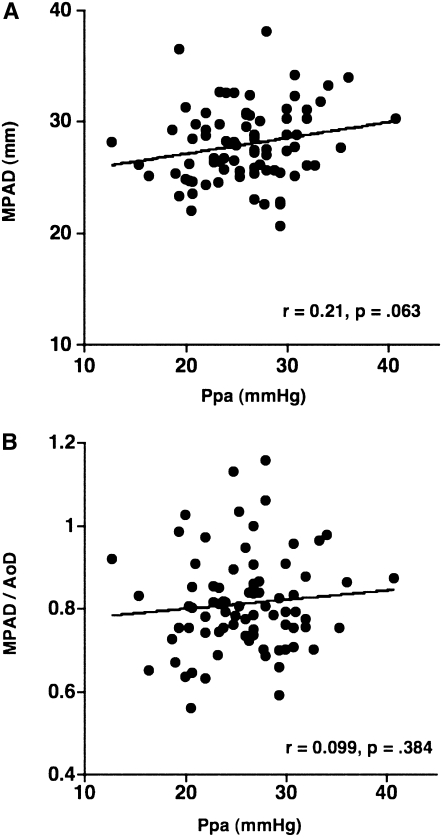
Mean MPAD was 28.6 ± 3.3 mm, and mean MPAD/AoD was 0.81 ± 0.11. The correlation between these CT dimensions of the central pulmonary vessels and 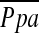 did not reach statistical significance (MPAD: r = 0.210, P = 0.063; MPAD/AoD: r = 0.099, P = 0.384) (Figure 3).
did not reach statistical significance (MPAD: r = 0.210, P = 0.063; MPAD/AoD: r = 0.099, P = 0.384) (Figure 3).
Figure 3.
The relationship between the pulmonary arterial mean pressure (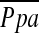 ) and (A) the diameter of main pulmonary artery (MPAD), and (B) the ratio of MPAD to the diameter of ascending aorta (MPAD/AoD). There is no significant correlation between
) and (A) the diameter of main pulmonary artery (MPAD), and (B) the ratio of MPAD to the diameter of ascending aorta (MPAD/AoD). There is no significant correlation between 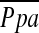 and MPAD or MPAD/AoD.
and MPAD or MPAD/AoD.
Multivariate Linear Regression Analysis
In multiple linear regression analysis with 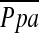 as the dependent variable, and age, sex, BMI, PaO2, FEV1% predicted, DlCO% predicted, %LAA−950, and %CSA<5 as the independent variables, only the following two variables were independent predictors of
as the dependent variable, and age, sex, BMI, PaO2, FEV1% predicted, DlCO% predicted, %LAA−950, and %CSA<5 as the independent variables, only the following two variables were independent predictors of 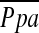 (r2 = 0.541): %CSA<5 (P < 0.0001) and predicted DlCO% (P = 0.022) (Table 4).
(r2 = 0.541): %CSA<5 (P < 0.0001) and predicted DlCO% (P = 0.022) (Table 4).
TABLE 4.
PREDICTORS OF PULMONARY MEAN ARTERIAL PRESSURE FROM MULTIPLE REGRESSION ANALYSIS (N = 79)
| Partial Regression Coefficient | P Value | |
|---|---|---|
| %CSA<5 (%) | −23.88 | <0.0001 |
| %LAA−950 | −0.09 | 0.054 |
| FEV1, % predicted | −0.04 | 0.549 |
| DlCO, % predicted | −0.13 | 0.022 |
| PaO2 | −0.08 | 0.067 |
| Age | −0.01 | 0.936 |
| Sex | −0.37 | 0.453 |
| BMI | 0.01 | 0.268 |
Definition of abbreviations: BMI = body mass index; DLCO = diffusing capacity of the lung for carbon monoxide; 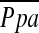 = pulmonary arterial mean pressure; %LAA−950 = CT measurement of the percentage of low attenuation area less than −950 HU, defined as emphysema.
= pulmonary arterial mean pressure; %LAA−950 = CT measurement of the percentage of low attenuation area less than −950 HU, defined as emphysema.
Additional Analysis
We evaluated the relationships between %CSA<5 and the extent of emphysema, pulmonary function, and Pao2 using Spearman rank correlation analysis. Table 5 shows the correlations between %CSA<5 and PFT results, PaO2, and %LAA−950. These correlations are graphed in the online data supplement, Figure E1. The %CSA<5 had significant correlations with %LAA−950 (r = −0.507, P < 0.0001), FEV1% (% predicted) (r = 0.330, P = 0.0029), and DlCO (% predicted) (r = 0.389, P = 0.0004), whereas no significant correlation with PaO2 (r = 0.0064, P = 0.955) was found.
TABLE 5.
CORRELATION BETWEEN CROSS-SECTIONAL AREA OF PULMONARY VESSELS AND PULMONARY FUNCTION TESTS (N = 79)
| %CSA<5 |
||
|---|---|---|
| r | P Value | |
| FEV1, % predicted | 0.330 | 0.0029 |
| DlCO, % predicted | 0.389 | 0.0004 |
| PaO2 | 0.006 | 0.9554 |
| %LAA−950 | −0.507 | <0.0001 |
Definition of abbreviations: DlCO = diffusing capacity of the lung for carbon monoxide; %CSA<5 = percentage of total lung area taken up by the cross-sectional area of pulmonary vessels less than 5 mm2; %LAA−950 = CT measurement of the percentage of low attenuation area less than −950 HU, defined as emphysema.
DISCUSSION
In the present study, we found that %CSA<5 correlated inversely with 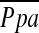 in severe emphysema. To our knowledge, this is the first report to quantitatively evaluate the in vivo relationship between pulmonary arterial pressure and small vessel morphology in severe emphysema. Vascular remodeling of small pulmonary vessels, which mainly consists of intimal thickening of the pulmonary muscular artery, is believed to be the most important factor in the development of pulmonary hypertension in severe emphysema, although the relationship between vascular alteration and pulmonary hypertension is not yet proved. However, pulmonary vascular remodeling is not exclusively a characteristic of severe emphysema; it has also been shown in patients with mild COPD and in smokers with normal pulmonary function (22–28). Previous authors have not found a significant correlation between vascular remodeling and
in severe emphysema. To our knowledge, this is the first report to quantitatively evaluate the in vivo relationship between pulmonary arterial pressure and small vessel morphology in severe emphysema. Vascular remodeling of small pulmonary vessels, which mainly consists of intimal thickening of the pulmonary muscular artery, is believed to be the most important factor in the development of pulmonary hypertension in severe emphysema, although the relationship between vascular alteration and pulmonary hypertension is not yet proved. However, pulmonary vascular remodeling is not exclusively a characteristic of severe emphysema; it has also been shown in patients with mild COPD and in smokers with normal pulmonary function (22–28). Previous authors have not found a significant correlation between vascular remodeling and 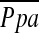 at rest (29, 30). In contrast, Kubo and colleagues (7) found that pulmonary vascular remodeling assessed histologically in severe emphysema led to reduced distensibility of pulmonary vessels and was closely correlated to
at rest (29, 30). In contrast, Kubo and colleagues (7) found that pulmonary vascular remodeling assessed histologically in severe emphysema led to reduced distensibility of pulmonary vessels and was closely correlated to 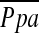 during exercise, but not at rest. Wright and colleagues (31) demonstrated that
during exercise, but not at rest. Wright and colleagues (31) demonstrated that 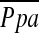 correlates with vascular mediators that control vasoconstriction or vasodilation of pulmonary vessels. Thus, the degree of pulmonary arterial pressure in COPD may be related to the dynamic morphological change in the pulmonary vascular bed rather than static or histological vascular alteration. The %CSA<5 may reflect the effects of the vascular distensibility and vasoactive mediators because it is measured in vivo; this may explain the significant correlation between %CSA<5 and
correlates with vascular mediators that control vasoconstriction or vasodilation of pulmonary vessels. Thus, the degree of pulmonary arterial pressure in COPD may be related to the dynamic morphological change in the pulmonary vascular bed rather than static or histological vascular alteration. The %CSA<5 may reflect the effects of the vascular distensibility and vasoactive mediators because it is measured in vivo; this may explain the significant correlation between %CSA<5 and 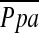 in our study, although decrease in total capillary bed may be associated with
in our study, although decrease in total capillary bed may be associated with 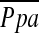 because
because 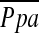 had a significant correlation with DlCO% predicted.
had a significant correlation with DlCO% predicted.
We confirmed that the relationship between vascular alteration and 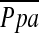 depends on vessel size. A significant correlation was found between
depends on vessel size. A significant correlation was found between 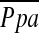 and %CSA<5, but not %CSA5–10. The degree of histological vascular alteration in COPD varies according to vessel size (22). In patients with pulmonary hypertension in COPD, histological vascular alteration can be found mainly in muscular pulmonary arteries (21–24). In this study, the lower limit of detection of cross-sectional area of pulmonary vessel is defined by the size of the CT pixel (approximately 0.6 mm in this study). Thus, %CSA<5 included both elastic and relatively large muscular vessels, whereas %CSA5–10 was composed of mostly elastic vessels. Therefore, the difference in correlations between the two CSA groups and
and %CSA<5, but not %CSA5–10. The degree of histological vascular alteration in COPD varies according to vessel size (22). In patients with pulmonary hypertension in COPD, histological vascular alteration can be found mainly in muscular pulmonary arteries (21–24). In this study, the lower limit of detection of cross-sectional area of pulmonary vessel is defined by the size of the CT pixel (approximately 0.6 mm in this study). Thus, %CSA<5 included both elastic and relatively large muscular vessels, whereas %CSA5–10 was composed of mostly elastic vessels. Therefore, the difference in correlations between the two CSA groups and 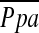 may reflect the difference between the proportions of elastic versus muscular pulmonary vessels in each group. Characteristics of central large vessels, such as the measured MPAD or MPAD/AoD, correlated poorly if at all with
may reflect the difference between the proportions of elastic versus muscular pulmonary vessels in each group. Characteristics of central large vessels, such as the measured MPAD or MPAD/AoD, correlated poorly if at all with 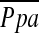 , a finding in agreement with previous work (17).
, a finding in agreement with previous work (17).
Although earlier studies suggested that emphysema, and consequent tissue destruction, leads to pulmonary hypertension, this contention is not supported by recent studies (10, 32, 33). Likewise, we found no significant correlation between the extent of emphysema and 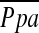 at least in patients with severe emphysema. However, there was a significant correlation between the extent of emphysema and %CSA<5 as well as the relationship between
at least in patients with severe emphysema. However, there was a significant correlation between the extent of emphysema and %CSA<5 as well as the relationship between 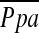 and %CSA<5. The correlations among the extent of emphysema, %CSA<5, and
and %CSA<5. The correlations among the extent of emphysema, %CSA<5, and 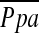 suggest that the increase in emphysema itself does not induce the decrease in %CSA<5. Recently, several researchers have demonstrated the relationship between vascular alteration and emphysema from the viewpoint of endothelial dysfunction (34–42). Thus, the negative correlation between the decrease in %CSA<5 and the extent of emphysema could be related to endothelial dysfunction. Meanwhile, the decrease in %CSA<5 might result from the passive vascular compression by emphysema. However, previous study has shown that a significant correlation between %CSA<5 and the extent of emphysema was found even in patients with relatively mild emphysema. Thus, the association of the passive vascular compression by emphysema to %CSA<5 may be relatively minimal.
suggest that the increase in emphysema itself does not induce the decrease in %CSA<5. Recently, several researchers have demonstrated the relationship between vascular alteration and emphysema from the viewpoint of endothelial dysfunction (34–42). Thus, the negative correlation between the decrease in %CSA<5 and the extent of emphysema could be related to endothelial dysfunction. Meanwhile, the decrease in %CSA<5 might result from the passive vascular compression by emphysema. However, previous study has shown that a significant correlation between %CSA<5 and the extent of emphysema was found even in patients with relatively mild emphysema. Thus, the association of the passive vascular compression by emphysema to %CSA<5 may be relatively minimal.
Various other factors have been recognized as potential association to the development of pulmonary hypertension in emphysema. Hypoxia-induced vasoconstriction and vascular remodeling have been considered as causes of pulmonary hypertension in COPD. However, when accounting for other factors, previous study (10) showed that PaO2 was not a significant predictor for 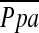 , although there was a limitation with a narrow range of PaO2 in this study. Likewise, although we found significant correlations between
, although there was a limitation with a narrow range of PaO2 in this study. Likewise, although we found significant correlations between 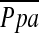 and DlCO% predicted, or FEV1% predicted as reported in previous studies (10, 43, 44), these significant correlations were not corroborated by another previous study (45). In multiple linear regression analysis, DlCO% predicted was a significant but weak predictor of
and DlCO% predicted, or FEV1% predicted as reported in previous studies (10, 43, 44), these significant correlations were not corroborated by another previous study (45). In multiple linear regression analysis, DlCO% predicted was a significant but weak predictor of 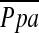 , which may be due to destruction of pulmonary microvessels. The %CSA<5, which can reflect morphological vascular alteration, was by far the best predictor of
, which may be due to destruction of pulmonary microvessels. The %CSA<5, which can reflect morphological vascular alteration, was by far the best predictor of 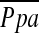 . However, further evaluation, including reproducibility of the relationship between %CSA and
. However, further evaluation, including reproducibility of the relationship between %CSA and 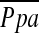 , may be required to use this method to estimate
, may be required to use this method to estimate 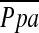 .
.
Limitations of the CSA Method
Echocardiography has been used to evaluate pulmonary hypertension; however, a recent study showed that the estimation of pulmonary hypertension with echocardiography was not reliable in patients with COPD (46). The measurement of the peripheral vessel CSA may be an alternative diagnostic method that can evaluate pulmonary vascular alteration and pulmonary hypertension without a particular CT scanning technique or injection of contrast material. However, there are still several issues that should be resolved: First, we used the threshold value of −720 HU to identify vascular structure on CT images because using a threshold lower than −720 HU led to an increase in image noise. In the future, the appropriate threshold value should be assessed. Second, this method cannot evaluate the pulmonary arteries and veins separately; however, we believe that three-dimensional reconstruction from multislice CT images in conjunction with innovative image analysis software might overcome this limitation. In addition, with recent developments in CT technology, each CT image can be obtained in less than 1 second. Thus, CSA may be influenced by the cardiac cycle. Respiratory phases, including inspiration and expiration, also may affect CSA measurements. These effects should be evaluated with each respiratory phase and cardiac gating in the future. Other confounding variables that may affect the correlations of lung function and CT morphological measurements of small vessel dimensions could be the influence of posture and lung volume on measurements made in the upright and supine postures, respectively. Decreased lung volume and increased venous return in the supine posture during CT performance could limit the ability to correlate measurements of small pulmonary vessel dimensions with lung function measures made during relative conditions of a higher lung volume and decreased venous return in the upright posture.
Study Limitations
There are some limitations of this study, first, because this is a retrospective study, and because of the characteristics of this cohort, the majority of patients have severe emphysema. In some patients with COPD, pulmonary hypertension can also develop without emphysema. Thus, the relationship between pulmonary vascular alteration and pulmonary hypertension remains unclear in subjects with COPD who do not have such severe parenchymal disease as found in the NETT cohort. Likewise, the majority of patients have mild to moderate pulmonary hypertension. Thus, the relationship between pulmonary vascular alteration and pulmonary hypertension remains unclear in severe pulmonary hypertension. Further evaluation in patients with severe pulmonary hypertension is necessary. Second, we did not measure the cross-sectional area of pulmonary vessels histologically; therefore, there might be some differences between CSA measured on CT image and actual cross-sectional area of pulmonary vessels. Further evaluation is necessary.
Conclusion
We found that 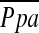 in severe emphysema is significantly correlated to a decrease in the %CSA less than 5 mm2. In addition, this parameter is a strong predictor of the degree of pulmonary hypertension. The measurement of CSA using CT scans may be an alternative noninvasive technique to estimate the pulmonary hypertension in patients with COPD.
in severe emphysema is significantly correlated to a decrease in the %CSA less than 5 mm2. In addition, this parameter is a strong predictor of the degree of pulmonary hypertension. The measurement of CSA using CT scans may be an alternative noninvasive technique to estimate the pulmonary hypertension in patients with COPD.
Supplementary Material
Acknowledgments
The authors thank Janice Cook-Granroth for organizing the CT images for analysis, and Alba Cid for editorial assistance.
Members of the NETT Research Group: Office of the Chair of the Steering Committee, University of Pennsylvania, Philadelphia, PA: Alfred P. Fishman, MD (Chair); Betsy Ann Bozzarello; Ameena Al-Amin. Clinical centers: Baylor College of Medicine, Houston, TX: Marcia Katz, MD (Principal Investigator); Carolyn Wheeler, RN, BSN (Principal Clinic Coordinator); Elaine Baker, RRT, RPFT; Peter Barnard, PhD, RPFT; Phil Cagle, MD; James Carter, MD; Sophia Chatziioannou, MD; Karla Conejo-Gonzales; Kimberly Dubose, RRT; John Haddad, MD; David Hicks, RRT, RPFT; Neal Kleiman, MD; Mary Milburn-Barnes, CRTT; Chinh Nguyen, RPFT; Michael Reardon, MD; Joseph Reeves-Viets, MD; Steven Sax, MD; Amir Sharafkhaneh, MD; Owen Wilson, PhD; Christine Young PT; Rafael Espada, MD (Principal Investigator 1996–2002); Rose Butanda (1999–2001); Minnie Ellisor (2002); Pamela Fox, MD (1999–2001); Katherine Hale, MD (1998–2000); Everett Hood, RPFT (1998–2000); Amy Jahn (1998–2000); Satish Jhingran, MD (1998–2001); Karen King, RPFT (1998–1999); Charles Miller III, PhD (1996–1999); Imran Nizami, MD (Co-Principal Investigator, 2000–2001); Todd Officer (1998–2000); Jeannie Ricketts (1998–2000); Joe Rodarte, MD (Co-Principal Investigator 1996–2000); Robert Teague, MD (Co-Principal Investigator 1999–2000); Kedren Williams (1998–1999). Brigham and Women's Hospital, Boston, MA: John Reilly, MD (Principal Investigator); David Sugarbaker, MD (Co-Principal Investigator); Carol Fanning, RRT (Principal Clinic Coordinator); Simon Body, MD; Sabine Duffy, MD; Vladmir Formanek, MD; Anne Fuhlbrigge, MD; Philip Hartigan, MD; Sarah Hooper, EP; Andetta Hunsaker, MD; Francine Jacobson, MD; Marilyn Moy, MD; Susan Peterson, RRT; Roger Russell, MD; Diane Saunders; Scott Swanson, MD (Co-Principal Investigator, 1996–2001). Cedars-Sinai Medical Center, Los Angeles, CA: Rob McKenna, MD (Principal Investigator); Zab Mohsenifar, MD (Co-Principal Investigator); Carol Geaga, RN (Principal Clinic Coordinator); Manmohan Biring, MD; Susan Clark, RN, MN; Jennifer Cutler, MD; Robert Frantz, MD; Peter Julien, MD; Michael Lewis, MD; Jennifer Minkoff-Rau, MSW; Valentina Yegyan, BS, CPFT; Milton Joyner, BA (1996–2002). Cleveland Clinic Foundation, Cleveland, OH: Malcolm DeCamp, MD (Principal Investigator); James Stoller, MD (Co-Principal Investigator); Yvonne Meli, RN,C (Principal Clinic Coordinator); John Apostolakis, MD; Darryl Atwell, MD; Jeffrey Chapman, MD; Pierre DeVilliers, MD; Raed Dweik, MD; Erik Kraenzler, MD; Rosemary Lann, LISW; Nancy Kurokawa, RRT, CPFT; Scott Marlow, RRT; Kevin McCarthy, RCPT; Priscilla McCreight, RRT, CPFT; Atul Mehta, MD; Moulay Meziane, MD; Omar Minai, MD; Mindi Steiger, RRT; Kenneth White, RPFT; Janet Maurer, MD (Principal Investigator, 1996–2001); Terri Durr, RN (2000–2001); Charles Hearn, DO (1998–2001); Susan Lubell, PA-C (1999–2000); Peter O'Donovan, MD (1998–2003); Robert Schilz, DO (1998–2002). Columbia University, New York, NY in consortium with Long Island Jewish Medical Center, New Hyde Park, NY: Mark Ginsburg, MD (Principal Investigator); Byron Thomashow, MD (Co-Principal Investigator); Patricia Jellen, MSN, RN (Principal Clinic Coordinator); John Austin, MD; Matthew Bartels, MD; Yahya Berkmen, MD; Patricia Berkoski, MS, RRT (Site coordinator, LIJ); Frances Brogan, MSN, RN; Amy Chong, BS, CRT; Glenda DeMercado, BSN; Angela DiMango, MD; Sandy Do, MS, PT; Bessie Kachulis, MD; Arfa Khan, MD; Berend Mets, MD; Mitchell O'Shea, BS, RT, CPFT; Gregory Pearson, MD; Leonard Rossoff, MD; Steven Scharf, MD, PhD (Co-Principal Investigator, 1998–2002); Maria Shiau, MD; Paul Simonelli, MD; Kim Stavrolakes, MS, PT; Donna Tsang, BS; Denise Vilotijevic, MS, PT; Chun Yip, MD; Mike Mantinaos, MD (1998–2001); Kerri McKeon, BS, RRT, RN (1998–1999); Jacqueline Pfeffer, MPH, PT (1997–2002). Duke University Medical Center, Durham, NC: Neil MacIntyre, MD (Principal Investigator); R. Duane Davis, MD (Co-Principal Investigator); John Howe, RN (Principal Clinic Coordinator); R. Edward Coleman, MD; Rebecca Crouch, RPT; Dora Greene; Katherine Grichnik, MD; David Harpole, Jr., MD; Abby Krichman, RRT; Brian Lawlor, RRT; Holman McAdams, MD; John Plankeel, MD; Susan Rinaldo-Gallo, MED; Sheila Shearer, RRT; Jeanne Smith, ACSW; Mark Stafford-Smith, MD; Victor Tapson, MD; Mark Steele, MD (1998–1999); Jennifer Norten, MD (1998–1999). Mayo Foundation, Rochester, MN: James Utz, MD (Principal Investigator); Claude Deschamps, MD (Co-Principal Investigator); Kathy Mieras, CCRP (Principal Clinic Coordinator); Martin Abel, MD; Mark Allen, MD; Deb Andrist, RN; Gregory Aughenbaugh, MD; Sharon Bendel, RN; Eric Edell, MD; Marlene Edgar; Bonnie Edwards; Beth Elliot, MD; James Garrett, RRT; Delmar Gillespie, MD; Judd Gurney, MD; Boleyn Hammel; Karen Hanson, RRT; Lori Hanson, RRT; Gordon Harms, MD; June Hart; Thomas Hartman, MD; Robert Hyatt, MD; Eric Jensen, MD; Nicole Jenson, RRT; Sanjay Kalra, MD; Philip Karsell, MD; Jennifer Lamb; David Midthun, MD; Carl Mottram, RRT; Stephen Swensen, MD; Anne-Marie Sykes, MD; Karen Taylor; Norman Torres, MD; Rolf Hubmayr, MD (1998–2000); Daniel Miller, MD (1999–2002); Sara Bartling, RN (1998–2000); Kris Bradt (1998–2002). National Jewish Medical and Research Center, Denver, CO: Barry Make, MD (Principal Investigator); Marvin Pomerantz, MD (Co-Principal Investigator); Mary Gilmartin, RN, RRT (Principal Clinic Coordinator); Joyce Canterbury; Martin Carlos; Phyllis Dibbern, PT; Enrique Fernandez, MD; Lisa Geyman, MSPT; Connie Hudson; David Lynch, MD; John Newell, MD; Robert Quaife, MD; Jennifer Propst, RN; Cynthia Raymond, MS; Jane Whalen-Price, PT; Kathy Winner, OTR; Martin Zamora, MD; Reuben Cherniack, MD (Principal Investigator, 1997–2000). Ohio State University, Columbus, OH: Philip Diaz, MD (Principal Investigator); Patrick Ross, MD (Co-Principal Investigator); Tina Bees (Principal Clinic Coordinator); Jan Drake; Charles Emery, PhD; Mark Gerhardt, MD, PhD; Mark King, MD; David Rittinger; Mahasti Rittinger. Saint Louis University, Saint Louis, MO: Keith Naunheim, MD (Principal Investigator); Robert Gerber, MD (Co-Principal Investigator); Joan Osterloh, RN, MSN (Principal Clinic Coordinator); Susan Borosh; Willard Chamberlain, DO; Sally Frese; Alan Hibbit; Mary Ellen Kleinhenz, MD; Gregg Ruppel; Cary Stolar, MD; Janice Willey; Francisco Alvarez, MD (Co-Principal Investigator, 1999–2002); Cesar Keller, MD (Co-Principal Investigator, 1996–2000). Temple University, Philadelphia, PA: Gerard Criner, MD (Principal Investigator); Satoshi Furukawa, MD (Co-Principal Investigator); Anne Marie Kuzma, RN, MSN (Principal Clinic Coordinator); Roger Barnette, MD; Neil Brister, MD; Kevin Carney, RN, CCTC; Wissam Chatila, MD; Francis Cordova, MD; Gilbert D'Alonzo, DO; Michael Keresztury, MD; Karen Kirsch; Chul Kwak, MD; Kathy Lautensack, RN, BSN; Madelina Lorenzon, CPFT; Ubaldo Martin, MD; Peter Rising, MS; Scott Schartel, MD; John Travaline, MD; Gwendolyn Vance, RN, CCTC; Phillip Boiselle, MD (1997–2000); Gerald O'Brien, MD (1997–2000). University of California, San Diego, San Diego, CA: Andrew Ries, MD, MPH (Principal Investigator); Robert Kaplan, PhD (Co-Principal Investigator); Catherine Ramirez, BS, RCP (Principal Clinic Coordinator); David Frankville, MD; Paul Friedman, MD; James Harrell, MD; Jeffery Johnson; David Kapelanski, MD; David Kupferberg, MD, MPH; Catherine Larsen, MPH; Trina Limberg, RRT; Michael Magliocca, RN, CNP; Frank J. Papatheofanis, MD, PhD; Dawn Sassi-Dambron, RN; Melissa Weeks. University of Maryland at Baltimore, Baltimore, MD in consortium with Johns Hopkins Hospital, Baltimore, MD: Mark Krasna, MD (Principal Investigator); Henry Fessler, MD (Co-Principal Investigator); Iris Moskowitz (Principal Clinic Coordinator); Timothy Gilbert, MD; Jonathan Orens, MD; Steven Scharf, MD, PhD; David Shade; Stanley Siegelman, MD; Kenneth Silver, MD; Clarence Weir; Charles White, MD. University of Michigan, Ann Arbor, MI: Fernando Martinez, MD (Principal Investigator); Mark Iannettoni, MD (Co-Principal Investigator); Catherine Meldrum, BSN, RN, CCRN (Principal Clinic Coordinator); William Bria, MD; Kelly Campbell; Paul Christensen, MD; Kevin Flaherty, MD; Steven Gay, MD; Paramjit Gill, RN; Paul Kazanjian, MD; Ella Kazerooni, MD; Vivian Knieper; Tammy Ojo, MD; Lewis Poole; Leslie Quint, MD; Paul Rysso; Thomas Sisson, MD; Mercedes True; Brian Woodcock, MD; Lori Zaremba, RN. University of Pennsylvania, Philadelphia, PA: Larry Kaiser, MD (Principal Investigator); John Hansen-Flaschen, MD (Co-Principal Investigator); Mary Louise Dempsey, BSN, RN (Principal Clinic Coordinator); Abass Alavi, MD; Theresa Alcorn, Selim Arcasoy, MD; Judith Aronchick, MD; Stanley Aukberg, MD; Bryan Benedict, RRT; Susan Craemer, BS, RRT, CPFT; Ron Daniele, MD; Jeffrey Edelman, MD; Warren Gefter, MD; Laura Kotler-Klein, MSS; Robert Kotloff, MD; David Lipson, MD; Wallace Miller, Jr., MD; Richard O'Connell, RPFT; Staci Opelman, MSW; Harold Palevsky, MD; William Russell, RPFT; Heather Sheaffer, MSW; Rodney Simcox, BSRT, RRT; Susanne Snedeker, RRT, CPFT; Jennifer Stone-Wynne, MSW; Gregory Tino, MD; Peter Wahl; James Walter, RPFT; Patricia Ward; David Zisman, MD; James Mendez, MSN, CRNP (1997–2001); Angela Wurster, MSN, CRNP (1997–1999). University of Pittsburgh, Pittsburgh, PA: Frank Sciurba, MD (Principal Investigator); James Luketich, MD (Co-Principal Investigator); Colleen Witt, MS (Principal Clinic Coordinator); Gerald Ayres; Michael Donahoe, MD; Carl Fuhrman, MD; Robert Hoffman, MD; Joan Lacomis, MD; Joan Sexton; William Slivka; Diane Strollo, MD; Erin Sullivan, MD; Tomeka Simon; Catherine Wrona, RN, BSN; Gerene Bauldoff, RN, MSN (1997–2000); Manuel Brown, MD (1997–2002); Elisabeth George, RN, MSN (Principal Clinic Coordinator 1997–2001); Robert Keenan, MD (Co-Principal Investigator 1997–2000); Theodore Kopp, MS (1997–1999); Laurie Silfies (1997–2001). University of Washington, Seattle, WA: Joshua Benditt, MD (Principal Investigator), Douglas Wood, MD (Co-Principal Investigator); Margaret Snyder, MN (Principal Clinic Coordinator); Kymberley Anable; Nancy Battaglia; Louie Boitano; Andrew Bowdle, MD; Leighton Chan, MD; Cindy Chwalik; Bruce Culver, MD; Thurman Gillespy, MD; David Godwin, MD; Jeanne Hoffman; Andra Ibrahim, MD; Diane Lockhart; Stephen Marglin, MD; Kenneth Martay, MD; Patricia McDowell; Donald Oxorn, MD; Liz Roessler; Michelle Toshima; Susan Golden (1998–2000). Other participants: Agency for Healthcare Research and Quality, Rockville, MD: Lynn Bosco, MD, MPH; Yen-Pin Chiang, PhD; Carolyn Clancy, MD; Harry Handelsman, DO. Centers for Medicare and Medicaid Services, Baltimore, MD: Steven M Berkowitz, PhD; Tanisha Carino, PhD; Joe Chin, MD; JoAnna Baldwin; Karen McVearry; Anthony Norris; Sarah Shirey; Claudette Sikora Steven Sheingold, PhD (1997–2004). Coordinating Center, The Johns Hopkins University, Baltimore, MD: Steven Piantadosi, MD, PhD (Principal Investigator); James Tonascia, PhD (Co-Principal Investigator); Patricia Belt; Amanda Blackford, ScM; Karen Collins; Betty Collison; Ryan Colvin, MPH; John Dodge; Michele Donithan, MHS; Vera Edmonds; Gregory L. Foster, MA; Julie Fuller; Judith Harle; Rosetta Jackson; Shing Lee, ScM; Charlene Levine; Hope Livingston; Jill Meinert; Jennifer Meyers; Deborah Nowakowski; Kapreena Owens; Shangqian Qi, MD; Michael Smith; Brett Simon, MD; Paul Smith; Alice Sternberg, ScM; Mark Van Natta, MHS; Laura Wilson, ScM; Robert Wise, MD. Cost Effectiveness Subcommittee: Robert M. Kaplan, PhD (Chair); J. Sanford Schwartz, MD (Co-Chair); Yen-Pin Chiang, PhD; Marianne C. Fahs, PhD; A. Mark Fendrick, MD; Alan J. Moskowitz, MD; Dev Pathak, PhD; Scott Ramsey, MD, PhD; Steven Sheingold, PhD; A. Laurie Shroyer, PhD; Judith Wagner, PhD; Roger Yusen, MD. Cost Effectiveness Data Center, Fred Hutchinson Cancer Research Center, Seattle, WA: Scott Ramsey, MD, PhD (Principal Investigator); Ruth Etzioni, PhD; Sean Sullivan, PhD; Douglas Wood, MD; Thomas Schroeder, MA; Karma Kreizenbeck; Kristin Berry, MS; Nadia Howlader, MS. CT Scan Image Storage and Analysis Center, University of Iowa, Iowa City, IA: Eric Hoffman, PhD (Principal Investigator); Janice Cook-Granroth, BS; Angela Delsing, RT; Junfeng Guo, PhD; Geoffrey McLennan, MD; Brian Mullan, MD; Chris Piker, BS; Joseph Reinhardt, PhD; Blake Wood; Jered Sieren, RTR; William Stanford, MD. Data and Safety Monitoring Board: John A. Waldhausen, MD (Chair); Gordon Bernard, MD; David DeMets, PhD; Mark Ferguson, MD; Eddie Hoover, MD; Robert Levine, MD; Donald Mahler, MD; A. John McSweeny, PhD; Jeanine Wiener-Kronish, MD; O. Dale Williams, PhD; Magdy Younes, MD. Marketing Center, Temple University, Philadelphia, PA: Gerard Criner, MD (Principal Investigator); Charles Soltoff, MBA. Project Office, National Heart, Lung, and Blood Institute, Bethesda, MD: Gail Weinmann, MD (Project Officer); Joanne Deshler (Contracting Officer); Dean Follmann, PhD; James Kiley, PhD; Margaret Wu, PhD (1996–2001).
Other acknowledgments: Arthur Gelb, MD, Lakewood Regional Medical Center, Lakewood, CA.
The National Emphysema Treatment Trial (NETT) is supported by contracts with the National Heart, Lung, and Blood Institute (N01HR76101, N01HR76102, N01HR76103, N01HR76104, N01HR76105, N01HR76106, N01HR76107, N01HR76108, N01HR76109, N01HR76110, N01HR76111, N01HR76112, N01HR76113, N01HR76114, N01HR76115, N01HR76116, N01HR76118, and N01HR76119), the Centers for Medicare and Medicaid Services (CMS); and the Agency for Healthcare Research and Quality (AHRQ). G.R.W. was supported by National Institutes of Health grant K23HL089353-01A1 and a grant from the Parker B. Francis Foundation.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200908-1189OC on October 29, 2009
Conflict of Interest Statement: S.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.R.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.Y. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.S.J.E. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.K.S. received $10,001–$50,000 from GlaxoSmithKline and $10,001–$50,000 from AstraZeneca in consultancy fees; $1,001–$5,000 from GlaxoSmithKline, $5,001– $10,000 from AstraZeneca, and $1,001–$5,000 from Bayer in lecture fees; and more than $100,001 from GlaxoSmithKline in industry-sponsored grants. E.H received $5,001–$10,000 from QI2 for image protocol advice and oversight; $1,001–$5,000 from AstraZeneca, $1,001–$5,000 from Sanofi-Aventis, and $1,001–$5,000 from Grifols in travel and hotel support, an honoraria for a lecture, or both; up to $1,000 from Siemens Medical Systems for travel expenses only; $1,001–$5,000 from Chiesi Pharmaceuticals, $1,001–$5,000 from AstraZeneca, and $1,001–$5,000 from GlaxoSmithKline in lecture fees; holds a patent from the University of Iowa for texture analysis of medical images and a patent from Marval Diagnostics for nano-scale liposomal blood-pool contrast agent; holds more than $100,001 in stock ownership or options from VIDA Diagnostics as the founder and shareholder and $100,001 from Marval Diagnostics in stock ownership or options as the founder and shareholder. E.H. is a founder and shareholder of VIDA Diagnostics, which is commercializing image analysis software, developed in E.H.'s university laboratory, which has been used in this study. H.E.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.J.C. received $1,001–$5,000 from Boehringer Ingelheim, $1,001–$5,000 from GlaxoSmithKline, and $1,001–$5,000 from Aeris in industry-sponsored grants for a research study. N.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.M.S. received $1,001–$5,000 from Spirocor for consultation—technical—heart lung physiology and $1,001–$5,000 from TCI Enterprises consultation—technical—pulmonary physiology. F.J.M does not have a financial relationship with commercial entity that has an interest in the subject of this manuscript. J.J.R. received more than $100,001 for the human clinical trial of bronchoscopic lung volume reduction, funded from 2004 to 2007, from Aeris, Inc. H.H. received more than $100,000 from Toshiba Medical Inc. in industry-sponsored grants.
References
- 1.Burrows B, Kettel LJ, Niden AH, Rabinowitz M, Diener CF. Patterns of cardiovascular dysfunction in chronic obstructive lung disease. N Engl J Med 1972;286:912–918. [DOI] [PubMed] [Google Scholar]
- 2.Traver GA, Cline MG, Burrows B. Predictors of mortality in chronic obstructive pulmonary disease. Am Rev Respir Dis 1979;119:895–902. [DOI] [PubMed] [Google Scholar]
- 3.Oswald-Mammosser M, Weitzenblum E, Quoix E, Moser G, Chaouat A, Charpentier C, Kessler R. Prognostic factors in COPD patients receiving long-term oxygen therapy. Importance of pulmonary artery pressure. Chest 1995;107:1193–1198. [DOI] [PubMed] [Google Scholar]
- 4.Cordasco EM, Beerel FR, Vance JW, Wende RW, Toffolo RR. Newer aspects of the pulmonary vasculature in chronic lung disease. A comparative study. Angiology 1968;19:399–407. [DOI] [PubMed] [Google Scholar]
- 5.Scarrow GD. The pulmonary angiogram in chronic bronchitis and emphysema. Proc R Soc Med 1965;58:684–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobson G, Turner AF, Balchum OJ, Jung R. Vascular changes in pulmonary emphysema. The radiologic evaluation by selective and peripheral pulmonary wedge angiography. Am J Roentgenol Radium Ther Nucl Med 1967;100:374–396. [PubMed] [Google Scholar]
- 7.Kubo K, Ge RL, Koizumi T, Fujimoto K, Yamanda T, Haniuda M, Honda T. Pulmonary artery remodeling modifies pulmonary hypertension during exercise in severe emphysema. Respir Physiol 2000;120:71–79. [DOI] [PubMed] [Google Scholar]
- 8.Matsuoka S, Washko GR, Dransfield MT, Yamashiro T, San José Estépar R, Diaz A, Silverman EK, Patz S, Hatabu H. Quantitative CT measurement of cross-sectional area of small pulmonary vessel in COPD: correlations with emphysema and airflow limitation. Acad Radiol 2010;17:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The National Emphysema Treatment Trial Research Group. Rationale and design of the national emphysema treatment trial (NETT): a prospective randomized trial of lung volume reduction surgery. J Thorac Cardiovasc Surg 1999;118:518–528. [DOI] [PubMed] [Google Scholar]
- 10.Scharf SM, Iqbal M, Keller C, Criner G, Lee S, Fessler HE; National Emphysema Treatment Trial (NETT) Group. Hemodynamic characterization of patients with severe emphysema. Am J Respir Crit Care Med 2002;166:314–322. [DOI] [PubMed] [Google Scholar]
- 11.American Thoracic Society. Standardization of spirometry: 1994 update. Am J Respir Crit Care Med 1995;152:1107. [DOI] [PubMed] [Google Scholar]
- 12.American Thoracic Society. Lung function testing selection of reference values and interpretative strategies. Am Rev Respir Dis 1991;144:1202–1218. [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic Society. Single-breath carbon monoxide diffusing capacity (transfer factor): recommendations for a standard technique—1995 update. Am J Respir Crit Care Med 1995;152:2185–2198. [DOI] [PubMed] [Google Scholar]
- 14.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis 1981;123:659–664. [DOI] [PubMed] [Google Scholar]
- 15.Washko GR, Criner GJ, Mohsenifar Z, Sciurba FC, Sharafkhaneh A, Make BJ, Hoffman EA, Reilly JJ. Computed tomographic-based quantification of emphysema and correlation to pulmonary function and mechanics. COPD 2008;5:177–186. [DOI] [PubMed] [Google Scholar]
- 16.Coche E, Pawlak S, Dechambre S, Maldague B. Peripheral pulmonary arteries: identification at multi-slice spiral CT with 3D reconstruction. Eur Radiol 2003;13:815–822. [DOI] [PubMed] [Google Scholar]
- 17.Tan RT, Kuzo R, Goodman LR, Siegel R, Haasler GB, Presberg KW. Utility of CT scan evaluation for predicting pulmonary hypertension in patients with parenchymal lung disease. Medical College of Wisconsin Lung Transplant Group. Chest 1998;113:1250–1256. [DOI] [PubMed] [Google Scholar]
- 18.Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med 1995;152:653–657. [DOI] [PubMed] [Google Scholar]
- 19.Kuriyama K, Gamsu G, Stern RG, Cann CE, Herfkens RJ, Brundage BH. CT-determined pulmonary artery diameters in predicting pulmonary hypertension. Invest Radiol 1984;19:16–22. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt HC, Kauczor HU, Schild HH, Renner C, Kirchhoff E, Lang P, Iversen S, Thelen M. Pulmonary hypertension in patients with chronic pulmonary thromboembolism: chest radiograph and CT evaluation before and after surgery. Eur Radiol 1996;6:817–825. [DOI] [PubMed] [Google Scholar]
- 21.Pérez-Enguix D, Morales P, Tomás JM, Vera F, Lloret RM. Computed tomographic screening of pulmonary arterial hypertension in candidates for lung transplantation. Transplant Proc 2007;39:2405–2408. [DOI] [PubMed] [Google Scholar]
- 22.Hale KA, Niewoehner DE, Cosio MG. Morphologic changes in the muscular pulmonary arteries: relationship to cigarette smoking, airway disease, and emphysema. Am Rev Respir Dis 1980;122:273–278. [DOI] [PubMed] [Google Scholar]
- 23.Wright JL, Lawson L, Pare PD, Hooper RO, Peretz DI, Nelems JM, Schulzer M, Hogg JC. The structure and function of the pulmonary vasculature in mild chronic obstructive pulmonary disease. The effect of oxygen and exercise. Am Rev Respir Dis 1983;128:702–707. [DOI] [PubMed] [Google Scholar]
- 24.Magee F, Wright JL, Wiggs BR, Pare PD, Hogg JC. Pulmonary vascular structure and function in chronic obstructive pulmonary disease. Thorax 1988;43:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbera JA, Riverola A, Roca J, Ramirez J, Wagner PD, Ros D, Wiggs BR, Rodriguez-Roisin R. Pulmonary vascular abnormalities and ventilation perfusion relationships in mild chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1994;149:423–429. [DOI] [PubMed] [Google Scholar]
- 26.Santos S, Peinado VI, Ramirez J, Melgosa T, Roca J, Rodriguez-Roisin R, Barbera JA. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Respir J 2002;19:632–638. [DOI] [PubMed] [Google Scholar]
- 27.Peinado VI, Barbera JA, Abate P, Ramirez J, Roca J, Santos S, Rodriguez-Roisin R. Inflammatory reaction in pulmonary muscular arteries of patients with mild chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;159:1605–1611. [DOI] [PubMed] [Google Scholar]
- 28.Peinado VI, Barbera JA, Ramirez J, Gomez FP, Roca J, Jover L, Gimferrer JM, Rodriguez-Roisin R. Endothelial dysfunction in pulmonary arteries of patients with mild COPD. Am J Physiol 1998;274:L908–L913. [DOI] [PubMed] [Google Scholar]
- 29.Wright JL, Petty T, Thurlbeck WM. Analysis of the structure of the muscular pulmonary arteries in patients with pulmonary hypertension and COPD: National Institutes of Health Nocturnal Oxygen Therapy Trial. Lung 1992;170:109–124. [DOI] [PubMed] [Google Scholar]
- 30.Yamato H, Sun JP, Churg A, Wright JL. Guinea pig pulmonary hypertension caused by cigarette smoke cannot be explained by capillary bed destruction. J Appl Physiol 1997;82:1644–1653. [DOI] [PubMed] [Google Scholar]
- 31.Wright JL, Tai H, Churg A. Vasoactive mediators and pulmonary hypertension after cigarette smoke exposure in the guinea pig. J Appl Physiol 2006;100:672–678. [DOI] [PubMed] [Google Scholar]
- 32.Boushy SF, North LB. Hemodynamic changes in chronic obstructive pulmonary disease. Chest 1977;72:565–570. [DOI] [PubMed] [Google Scholar]
- 33.Wright JL. Relationship of pulmonary arterial pressure and airflow obstruction to emphysema. J Appl Physiol 1993;74:1320–1324. [DOI] [PubMed] [Google Scholar]
- 34.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 2000;106:1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santos S, Peinado VI, Ramirez J, Morales-Blanhir J, Bastos R, Roca J, Rodriguez-Roisin R, Barbera JA. Enhanced expression of vascular endothelial growth factor in pulmonary arteries of smokers and patients with moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003;167:1250–1256. [DOI] [PubMed] [Google Scholar]
- 36.Barr RG, Mesia-Vela S, Austin JH, Basner RC, Keller BM, Reeves AP, Shimbo D, Stevenson L. Impaired flow-mediated dilation is associated with low pulmonary function and emphysema in ex-smokers: the Emphysema and Cancer Action Project (EMCAP) Study. Am J Respir Crit Care Med 2007;176:1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med 2001;163:737–744. [DOI] [PubMed] [Google Scholar]
- 38.Kanazawa H, Asai K, Hirata K, Yoshikawa J. Possible effects of vascular endothelial growth factor in the pathogenesis of chronic obstructive pulmonary disease. Am J Med 2003;114:354–358. [DOI] [PubMed] [Google Scholar]
- 39.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med 2005;11:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taraseviciene-Stewart L, Scerbavicius R, Choe KH, Moore M, Sullivan A, Nicolls MR, Fontenot AP, Tuder RM, Voelkel NF. An animal model of autoimmune emphysema. Am J Respir Crit Care Med 2005;171:734–742. [DOI] [PubMed] [Google Scholar]
- 41.Tang K, Rossiter HB, Wagner PD, Breen EC. Lung-targeted VEGF inactivation leads to an emphysema phenotype in mice. J Appl Physiol 2004;97:1559–1566. [DOI] [PubMed] [Google Scholar]
- 42.Tuder RM, Zhen L, Cho CY, Taraseviciene-Stewart L, Kasahara Y, Salvemini D, Voelkel NF, Flores SC. Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockade. Am J Respir Cell Mol Biol 2003;29:88–97. [DOI] [PubMed] [Google Scholar]
- 43.Thabut G, Dauriat G, Stern JB, Logeart D, Lévy A, Marrash-Chahla R, Mal H. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest 2005;127:1531–1536. [DOI] [PubMed] [Google Scholar]
- 44.Chaouat A, Bugnet AS, Kadaoui N, Schott R, Enache I, Ducoloné A, Ehrhart M, Kessler R, Weitzenblum E. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;172:189–194. [DOI] [PubMed] [Google Scholar]
- 45.Falk JA, Martin UJ, Scharf S, Criner GJ. Lung elastic recoil does not correlate with pulmonary hemodynamics in severe emphysema. Chest 2007;132:1476–1484. [DOI] [PubMed] [Google Scholar]
- 46.Fisher MR, Criner GJ, Fishman AP, Hassoun PM, Minai OA, Scharf SM, Fessler AH; NETT Research Group. Estimating pulmonary artery pressures by echocardiography in patients with emphysema. Eur Respir J 2007;30:914–921. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.