Abstract
Rationale: Milk fat globule epidermal growth factor 8 (MFG-E8) is a potent opsonin for the clearance of apoptotic cells and is produced by mononuclear cells of immune competent organs including the spleen and lungs. It attenuates chronic and acute inflammation such as autoimmune glomerulonephritis and bacterial sepsis by enhancing apoptotic cell clearance. Ischemia-reperfusion (I/R) injury of the gut results in severe inflammation, apoptosis, and remote organ damage, including acute lung injury (ALI).
Objectives: To determine whether MFG-E8 attenuates intestinal and pulmonary inflammation after gut I/R.
Methods: Wild-type (WT) and MFG-E8−/− mice underwent superior mesenteric artery occlusion for 90 minutes, followed by reperfusion for 4 hours. A group of WT mice was treated with 0.4 μg/20 g recombinant murine MFG-E8 (rmMFG-E8) at the beginning of reperfusion. Four hours after reperfusion, MFG-E8, cytokines, myeloperoxidase activity, apoptosis, and histopathology were assessed. A 24-hour survival study was conducted in rmMFG-E8– and vehicle-treated WT mice.
Measurements and Main Results: Mesenteric I/R caused severe widespread injury and inflammation of the small intestines and remote organs, including the lungs. MFG-E8 levels decreased in the spleen and lungs by 50 to 60%, suggesting impaired apoptotic cell clearance. Treatment with rmMFG-E8 significantly suppressed inflammation (TNF-α, IL-6, IL-1β, and myeloperoxidase) and injury of the lungs, liver, and kidneys. MFG-E8–deficient mice suffered from greatly increased inflammation and potentiated ALI, whereas treatment with rmMFG-E8 significantly improved the survival in WT mice.
Conclusions: MFG-E8 attenuates inflammation and ALI after gut I/R and may represent a novel therapeutic agent.
Keywords: inflammation, apoptosis, organ failure
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Ischemia-reperfusion injury of the gut results in severe inflammation, apoptosis, and remote organ damage, including acute lung injury.
What This Study Adds to the Field
Milk fat globule EGF-factor 8 attenuates inflammation and acute lung injury after gut ischemia-reperfusion and may represent a novel therapeutic agent.
Mesenteric ischemia remains a critical problem, resulting in mortality as high as 60 to 80% (1). Multiple organ failure, including acute lung injury (ALI), is a common complication of intestinal ischemia/reperfusion (I/R) injuries and contributes to its high mortality rate (2). ALI is caused by a systemic inflammatory response due to the release of proinflammatory cytokines and bacteria-derived endotoxins from reperfused ischemic tissue (3–6). Only a limited number pharmacologic treatment options have been found that provide some benefit in I/R and ALI, most of them targeting inflammatory mediators and oxidative stress pathways (7). A key aspect of I/R injury is the increased occurrence of apoptotic cell death of intestinal and bronchial epithelial cells and of type II alveolar epithelial cells (2, 8–11). Apoptosis is associated with a marked up-regulation of Fas and Fas-ligand and the activation of caspase-3 in lung epithelial cells (12, 13). Proinflammatory cytokines, such as IL-1β or TNF-α, play a major role in apoptosis induction, involving Bid, Bax up-regulation, and Bcl-2 down-regulation (9, 14, 15).
Deficient clearance of apoptotic cells after ischemia potentially leads to increased inflammation and impaired tissue repair (16, 17). Apoptotic cells expose phosphatidylserine (PS), which can be recognized by soluble molecules and receptors, thereby enabling their phagocytosis (18). One of these molecules is milk fat globule epidermal growth factor 8 (MFG-E8), which is crucial for apoptotic cell clearance (19). Hanayama and colleagues found that the effective clearance of apoptotic B cells in the spleen prevents proinflammatory immune responses and the development of autoantibodies (20). We have previously shown that MFG-E8 has a similar impact on outcome in acute inflammatory conditions. In a rat sepsis model using cecal ligation and puncture, we found that MFG-E8 is down-regulated in the spleen and liver. This was associated with impaired apoptotic cell clearance and increased mortality in these animals (21).
Similar to sepsis, gut I/R injury is accompanied by a systemic inflammatory response. We therefore hypothesize that MFG-E8 attenuates intestinal and pulmonary inflammation after gut I/R. In the present study, we investigated the effects of intestinal I/R on MFG-E8 expression and whether MFG-E8 beneficially affects associated organ failure, including ALI. We focused on the role of MFG-E8 in the modulation of inflammatory responses and tissue injury. Some of the results of this study were previously reported in the form of an abstract (22).
METHODS
Experimental Model
Ischemia was induced in male C57BL/6J wild-type (WT) mice (20–25 g; Taconic, Albany, NY) and male C57BL6/J MFG-E8 knockout (KO) mice (20–25 g; a generous gift from Dr. Shigekazu Nagata, Osaka University, Japan) by clamping the superior mesenteric artery (SMA) for 90 minutes under general anesthesia using isofluorane. The vascular clamp was released after 90 minutes to allow reperfusion. At the beginning of reperfusion, mice were resuscitated with 0.5 ml saline (intraperitoneally) and were treated with recombinant murine MFG-E8 (rmMFG-E8) (0.4 μg/20 g in 0.5 ml normal saline intraperitoneally; n = 6) or normal saline (vehicle; n = 6). The isofluorane was discontinued after intraperitoneal injection of rmMFG-E8 or saline. Control animals underwent the same operative procedure with the exception of the SMA clamping (sham I/R; n = 6). Four hours after reperfusion, animals were anesthetized, and blood (for plasma; 10 μl 0.3 M ethylenediaminetetraacetic acid per 1 ml blood was used as an anticoagulant) and tissue samples were harvested, frozen immediately in liquid nitrogen, and stored at −80°C until measurements. Additional experiments for observation of survival over the course of 24 hours were performed (n = 15/group). All experiments were performed in accordance with the guidelines for the use of experimental animals by the National Institutes of Health (Bethesda, MD) and were previously approved by the Institutional Animal Care and Use Committee of the Feinstein Institute for Medical Research (Manhasset, NY).
MFG-E8 Western Blotting
Twenty-five micrograms of protein from spleen and lung samples was fractionated on a Bis-Tris gel and transferred to a 0.22-μm nitrocellulose membrane. Blots were blocked with 5% BSA in Tris-buffered saline containing 0.1% vol/vol Tween 20 and incubated with hamster antimouse MFG-E8 mAb (clone 2422; MBL, Nagoya, Japan). After the incubation with horseradish peroxidase–labeled goat-antihamster IgG (Santa Cruz Biotechnology Inc., Santa Cruz, CA) in 5% bovine serum alumin–Tris-buffered saline with Tween and washing with Tris-buffered saline with Tween, bands were detected using a chemiluminescent peroxidase substrate (ECLplus; Amersham, Little Chalfont, Buckinghamshire, UK) and exposure on a radiograph film.
MFG-E8 Gene Expression
RNA was extracted from spleen and lung tissue samples using TRIzol Reagent (Invitrogen, Carlsbad, CA). Five micrograms of RNA was reverse transcribed to cDNA using murine leukemia virus reverse transcriptase (Applied Biosystems, Foster City, CA) and amplified by qPCR using SYBR green PCR Master Mix (Applied Biosystems). The following primer sets were used: mouse MFG-E8 (forward, 5′-GGGCCTGAAGAATAACACGA-3′; reverse, 5′-AGGGCAACTTGGACAACAAC-3′) and mouse β-actin (endogenous control; forward 5′-TGTTACCAACTGGGACGACA-3′; reverse, 5′-GGGGTGTTGAAGGTCTCAAA-3′).
Cytokines and Organ Injury Variables
TNF-α, IL-1β, and IL-6, were quantified using specific mouse ELISA kits (BD Pharmingen, Franklin Lakes, NJ) in ethylenediaminetetraacetic acid plasma, small intestine, and lung tissues. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), lactate, and creatinine blood plasma levels were determined using commercial assay kits (Pointe Scientific, Canton, MI).
Histopathology
Samples of the small intestine (nonnecrotic areas selected based on the color of the small intestine segment) and lungs were fixed in 10% formalin and embedded in paraffin. Tissue blocks were sectioned at a thickness of 5 μm, transferred to glass slides, and stained with hematoxylin/eosin. Morphologic examinations were performed using light microscopy, and lung injury was analyzed by a blinded, experienced investigator for absent, mild, moderate, or severe injury (score 0–3) based on the presence of exudates, hyperemia/congestion, neutrophilic infiltrates, intraalveolar hemorrhage/debris, and cellular hyperplasia (23). The sum of scores of different animals was averaged. Intestinal injury was scored according to Stallion and colleagues, assessing villus-to-crypt ratio (normal ratio, 5:1), lymphocytic infiltrates, epithelial degeneration/necrosis, erosions, glandular dilatation, and transmural changes (score, 0–4) (24).
Apoptosis Assay
Tissue samples were de-waxed, incubated with proteinase K, stained using a green fluorescent-tagged TUNEL kit (Roche Diagnostics, Indianapolis, IN), counterstained with propidium iodide, and examined under a fluorescence microscope. Apoptotic cells appeared green fluorescent on a red background staining and were counted per visual field at a 200× magnification. Cleaved caspase-3 expression in the lungs was measured by Western blotting analysis as described above. Antibodies against cleaved caspase-3 were obtained from Cell Signaling Technology (Asp175, 1:1,000).
Tissue Myeloperoxidase Assay
Tissues were homogenized in KPO4 buffer containing 0.5% hexa-decyl-trimethyl-ammonium bromide (60°C for 2 hours). After centrifuging, the supernatant was diluted in reaction solution, and ΔOD (rate of change in optical density between 1 and 3 min) was measured at 460 nm to calculate myeloperoxidase (MPO) activity (25).
Statistics
Data were expressed as means ± SEM and compared by analysis of variance using Student-Newman-Keuls' test. Student's t test was used when only two groups were compared. The survival study was analyzed using the Kaplan-Meier log-rank test. Differences were considered significant if P < 0.05.
RESULTS
Suppression of MFG-E8 in the Spleen after Intestinal I/R Injury
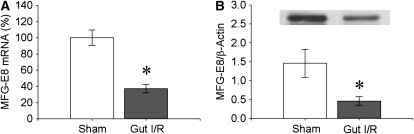
To investigate whether MFG-E8 levels are altered after intestinal I/R injury, we measured its mRNA and protein levels in WT mice 4 hours after reperfusion after 90-minute ischemia. The results indicate that splenic mRNA levels dropped significantly by an average 63% (Figure 1A) and protein levels by 68% (Figure 1B) after gut I/R.
Figure 1.
Suppression of splenic milk fat globule epidermal growth factor 8 (MFG-E8) after intestinal ischemia-reperfusion (I/R). The superior mesenteric artery (SMA) was occluded for 90 minutes, followed by 4 hours of reperfusion. (A) MFG-E8 mRNA levels in the spleen were measured by qPCR. (B) MFG-E8 protein levels were assessed by Western blot. A representative gel is presented. Data are expressed as means ± SEM. *P < 0.05 versus sham by Student's t test (n = 6 per group).
Administration of rmMFG-E8 Attenuates Multiple Organ Injury after Intestinal I/R
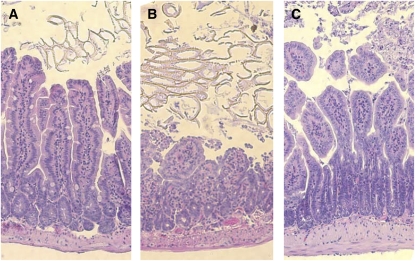
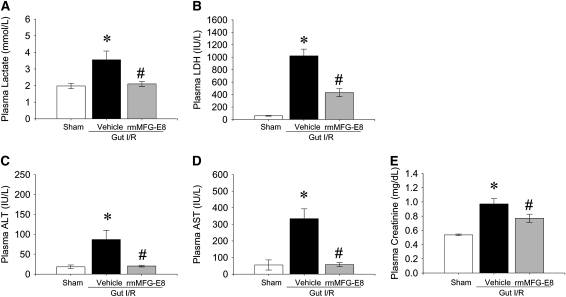
Gut I/R caused widespread macroscopic necrosis with severe enteric mucosal injury (average injury score, 2.25 ± 0.14) in intestinal areas juxtaposed to the macroscopically ischemic bowel (Figure 2B). Similarly, multiple blood markers of remote organ damage were significantly elevated, including lactate (80% increase), LDH (17.5-fold increase), ALT (4.8-fold increase), AST (18.3-fold increase), and creatinine (2-fold increase) compared with sham-operated animals (Figures 3A–3E), indicating the systemic scale of injury induced in this model. Treatment with one dose of rmMFG-E8 (0.4 μg/20 g BW intraperitoneally) at the beginning of reperfusion largely attenuated I/R-induced multiple organ injury. Histopathologically, even large parts of intestine were protected from secondary mucosal damage after treatment (average injury score, 1.25 ± 0.14) (Figure 2C). Treatment with rmMFG-E8 entirely blocked the elevation of lactate, AST, and ALT, whereas it suppressed LDH and creatinine levels by 58 and 21%, respectively (Figures 3A–3E).
Figure 2.
Attenuation of gut injury by recombinant murine milk fat globule epidermal growth factor 8 (rmMFG-E8) after intestinal ischemia-reperfusion (I/R). (A) Photomicrography of a small intestinal section from a sham-operated mouse. (B) Photomicrography of a small intestinal section from a gut I/R mouse treated with normal saline (vehicle). (C) Photomicrography of a small intestinal section from a gut I/R mouse treated with rmMFG-E8. The superior mesenteric artery (SMA) was occluded for 90 minutes, followed by 4 hours of reperfusion. H&E staining of the small intestine after intestinal I/R revealed widespread mucosal destruction, loss of villi and epithelial cells, and infiltration of inflammatory cells, whereas treatment with rmMFG-E8 showed beneficial local effects. Original magnification: ×100.
Figure 3.
Attenuation of organ injury by recombinant milk fat globule epidermal growth factor 8 (rmMFG-E8) after intestinal ischemia-reperfusion (I/R). The superior mesenteric artery was occluded for 90 minutes, followed by 4 hours of reperfusion. Plasma levels of (A) lactate, (B) lactate dehydrogenase (LDH), (C) alanine aminotransferase (ALT), (D) aspartate aminotransferase (AST), and (E) creatinine were measured 4 hours after reperfusion. Data are expressed as means ± SEM. *P < 0.05 versus sham; #P < 0.05 versus vehicle by one-way ANOVA and Student Newman Keul's test (n = 6 per group).
Administration of rmMFG-E8 Suppresses the Systemic Inflammatory Response after Intestinal I/R
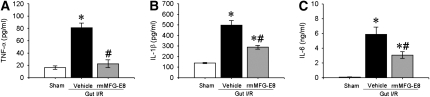
Proinflammatory cytokines are major contributors in the injury of remote organs after intestinal I/R. We investigated whether the cytokines TNF-α, IL-1β, and IL-6 were affected by the treatment with rmMFG-E8. Although blood levels of the cytokines increased significantly after gut I/R (TNF-α by 5.6-fold, IL-1β by 3.9-fold, and IL-6 by 96-fold), rmMFG-E8 dramatically reduced the proinflammatory response (by 72, 42, and 48%, respectively; Figures 4A–4C). To investigate whether MFG-E8 influences cytokine production in tissues, we analyzed the cytokine protein levels in the small intestine and the lungs using ELISA. Similar suppressive effects of rmMFG-E8 could be found on tissue cytokine levels (Table 1).
Figure 4.
Suppression of plasma cytokines by recombinant milk fat globule epidermal growth factor 8 (rmMFG-E8) after intestinal I/R. The superior mesenteric artery (SMA) was occluded for 90 minutes, followed by 4 hours of reperfusion. Blood cytokines were assessed by ELISA. Data are expressed as means ± SEM. *P < 0.05 versus sham; #P < 0.05 versus vehicle by one-way analysis of variance and Student Newman Keul's test (n = 6 per group).
TABLE 1.
LOCAL CYTOKINE SUPPRESSION BY RECOMBINANT MURINE MILK FAT GLOBULE EGF-FACTOR 8 AFTER INTESTINAL ISCHEMIA/REPERFUSION
| Cytokine (pg/mg protein) | Ischemia/Reperfusion |
|||
|---|---|---|---|---|
| Organ | Sham | Vehicle | rmMFG-E8 | |
| Gut | TNF-α | 6.5 ± 0.5 | 17.3 ± 3.2* | 6.7 ± 1.4# |
| IL-1β | 24.4 ± 2.6 | 62.5 ± 12.7* | 32.5 ± 8.4# | |
| IL-6 | 5.3 ± 0.4 | 14.3 ± 2.1* | 9.6 ± 2.6 | |
| Lung | TNF-α | 6.2 ± 0.5 | 7.7 ± 1.7 | 5.0 ± 0.2 |
| IL-1β | 33.7 ± 2.3 | 96.1 ± 15.8* | 36.6 ± 2.8# | |
| IL-6 | 15.9 ± 3.3 | 33.0 ± 4.5* | 14.7 ± 2.9# | |
Definition of abbreviation: rmMFG-E8 = recombinant murine milk fat globule epidermal growth factor 8.
C57BL6/J mice underwent intestinal ischemia/reperfusion for 90 min, followed by 4-h reperfusion. Tissue cytokine levels were determined by ELISA and are presented as means ± SEM.
* P < 0.05 vs. sham, and † P < 0.05 vs. vehicle by one-way analysis of variance and Student Newman Keul's test (n = 6).
Administration of rmMFG-E8 Attenuates ALI after Intestinal I/R
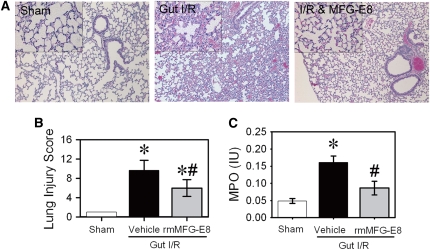
The lungs are among the organs that are most severely affected by intestinal I/R injury (26). Histopathological analysis of the lungs showed moderate to severe injury with exudates, congestion, cellular infiltrates, and intracellular hemorrhage (average histopathological score, 9.7 ± 0.8) (Figures 5A and 5B). The cell morphology was suggestive of a neutrophilic nature. We therefore assessed the MPO activity in the lungs, which was equally elevated by 3.3-fold after gut I/R (Figure 5C). Treatment with rmMFG-E8 significantly reduced ALI histopathologically and biochemically (Figures 5A–5C). The histopathology score was reduced by 38%, and MPO activity was suppressed by 47% (Figures 5B and 5C).
Figure 5.
Attenuation of acute lung injury (ALI) by recombinant murine milk fat globule epidermal growth factor 8 (rmMFG-E8). The superior mesenteric artery (SMA) was occluded for 90 minutes, followed by 4 hours of reperfusion. Lungs were fixed and stained with H&E. (A) Representative micrographs at original 100× and 400× magnification (inset). (B) Tissue injury was scored based on the presence of exudates, hyperemia/congestion, neutrophilic infiltrates, intraalveolar hemorrhage/debris, and cellular hyperplasia. (C) Neutrophil activity was assessed by myeloperoxidase assay. Data are expressed as means ± SEM. *P < 0.05 versus sham; #P < 0.05 versus vehicle by one-way analysis of variance and Student Newman Keul's test (n = 6 per group).
Pulmonary MFG-E8 Suppression and Apoptosis after Intestinal I/R
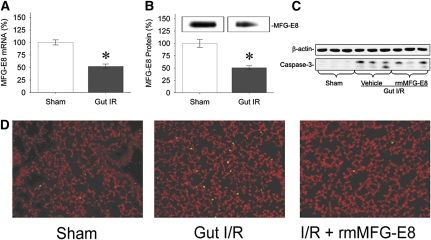
Gut I/R suppresses pulmonary MFG-E8 levels by 48% on an mRNA level and by 49% on a protein level (Figures 6A and 6B). As a measure of apoptosis, the expression of cleaved caspase-3 protein in the lungs was markedly increased after gut I/R (Figure 6C). Treatment with rmMFG-E8 decreased pulmonary cleaved caspase-3 levels dramatically (Figure 6C) to levels similar to those in sham animals. At the same time, we found fivefold increased numbers of apoptotic cells in the pulmonary tissue by TUNEL staining (Figure 6D). Treatment with rmMFG-E8, however, suppressed the number of detectable apoptotic cells in the lungs after gut I/R injury (Figure 6D).
Figure 6.
Decreased pulmonary murine milk fat globule epidermal growth factor 8 (MFG-E8) after intestinal ischemia-reperfusion (I/R) and restoration of apoptotic cell clearance by rmMFG-E8 treatment. (A) MFG-E8 mRNA levels were measured by qPCR. (B) MFG-E8 protein levels were assessed by Western blotting. Data are expressed as means ± SEM. *P < 0.05 versus sham by Student's t test (n = 6 per group). (C) Pulmonary levels of cleaved caspase-3. (D) Lungs were stained with TUNEL (green fluorescent) and counterstained with propidium iodide (red). TUNEL-positive cells exhibit green fluorescence.
MFG-E8 Deficiency Increases Pulmonary Inflammation and Potentiates ALI
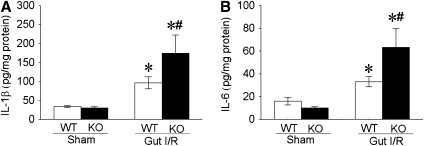
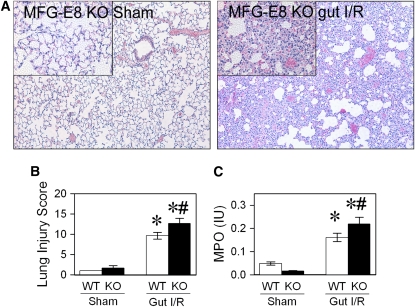
To further elucidate the role of MFG-E8 in gut I/R-mediated ALI, we investigated the inflammatory response and lung tissue injury in MFG-E8–deficient mice. Compared with WT mice, MFG-E8 KO mice produced a nearly twofold increase in IL-1β and IL-6 protein levels in the lungs after gut I/R (Figures 7A and 7B). This dramatic increase in proinflammatory cytokine production in the lungs was associated with a further potentiated ALI in MFG-E8 KO mice compared with their WT controls, including increased congestion, exudates, interstitial cellular infiltrates, and consolidation (Figures 8A and 8B). Pulmonary neutrophil activity was also significantly increased in MFG-E8 KO mice after gut I/R (Figure 8C).
Figure 7.
Murine milk fat globule epidermal growth factor 8 (MFG-E8) deficiency worsens the inflammatory response in the lung of mice after gut ischemia-reperfusion (I/R). Four hours postreperfusion after 90-minute superior mesenteric artery (SMA) occlusion, pulmonary tissues were collected and assessed for (A) IL-1β and (B) IL-6 (WT = wild type; KO = MFG-E8−/−). Data are expressed as means ± SEM. *P < 0.05 versus sham; #P < 0.05 versus WT-gut I/R by two-way analysis of variance and Student Newman Keul's test (n = 6 per group).
Figure 8.
Murine milk fat globule epidermal growth factor 8 (MFG-E8) deficiency potentiates acute lung injury (ALI). The superior mesenteric artery (SMA) was occluded for 90 minutes, followed by 4 hours of reperfusion. Lungs were fixed and stained with H&E. (A) Photomicrographs at original 100× and 400× magnification (inset). (B) Tissues were scored as described in Methods. (C) Neutrophil activity was assessed by myeloperoxidase assay. Data are expressed as means ± SEM. *P < 0.05 versus sham; #P < 0.05 versus WT-gut I/R by two-way analysis of variance and Student Newman Keul's test (n = 6 per group).
Administration of rmMFG-E8 Is Protective after Intestinal I/R in MFG-E8−/− Mice
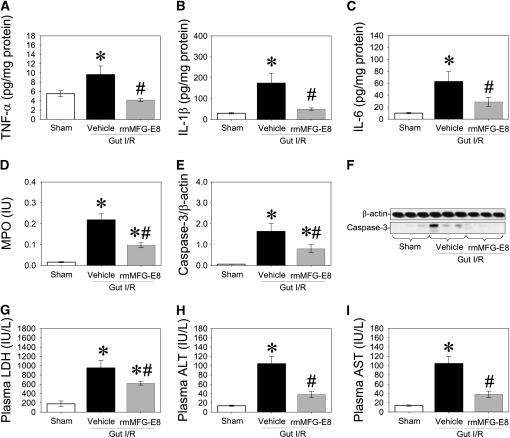
To assess the effect of rmMFG-E8 on intestinal I/R-induced organ injury in MFG-E8−/− mice, rmMFG-E8 (0.4 μg/20 g in 0.5 ml normal saline) or normal saline (Vehicle) were injected intraperitoneally at the end of 90-minute SMA clamping. Lung and blood samples were collected at 4 hours after reperfusion. Proinflammatory cytokine (i.e., TNF-α, IL-1β, and IL-6) levels, MPO activities, cleaved caspase-3 levels in the lung, and circulating levels of LDH, ALT, and AST were measured. Administration of rmMFG-E8 decreased pulmonary proinflammatory cytokine (i.e., TNF-α, IL-1β, and IL-6) levels, inhibited neutrophil infiltration (MPO activities) and apoptosis (cleaved caspase-3 levels) in the lung, and reduced circulating levels of LDH, ALT, and AST after intestinal I/R in MFG-E8−/− mice (Figures 9A–9I).
Figure 9.
Beneficial effects of recombinant murine milk fat globule epidermal growth factor 8 (rmMFG-E8) after intestinal ischemia-reperfusion (I/R) in MFG-E8−/− mice. The superior mesenteric artery (SMA) was occluded for 90 minutes, followed by 4 hours of reperfusion. Pulmonary levels of (A) TNF-α, (B) IL-1β, (C) IL-6, (D) MPO, and (E) cleaved caspase-3. (F) Representative blots for (E). Plasma levels of (G) lactate dehydrogenase (LDH), (H) alanine aminotransferase (ALT), and (I) aspartate aminotransferase (AST) were measured 4 hours after reperfusion. Data are expressed as means ± SEM. *P < 0.05 versus sham; #P < 0.05 versus vehicle by one-way analysis of variance and Student Newman Keul's test (n = 6 per group).
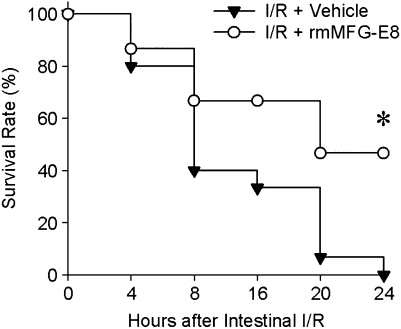
Treatment with rmMFG-E8 Improves Survival after Intestinal I/R Injury
The above results suggest that MFG-E8 is beneficial in I/R-mediated injury. We therefore performed a survival study in mice receiving rmMFG-E8 at the beginning of reperfusion and compared them with control mice treated with normal saline. All of the control mice died within 24 hours (median survival time, 8 h; 95% confidence interval, 5.5–10.5; Figure 10). Seven of the 15 animals treated with rmMFG-E8, however, were alive 24 hour after gut I/R (median survival time, 20 h; 95% confidence interval, 15.0–32.5; Figure 10). Ninety-minute SMA clamping is a severe model of gut ischemia in mice. Therefore, because all the animals (treated and nontreated) died within 48 hours after gut I/R injury, we could not reasonably show longer than 24-hour survival.
Figure 10.
Survival benefit in recombinant murine milk fat globule epidermal growth factor 8 (rmMFG-E8)–treated mice after gut I/R injury. The superior mesenteric artery (SMA) was occluded for 90 minutes, followed by 4 hours of reperfusion. Each group of mice received one dose of rmMFG-E8 or normal saline intraperitoneally at the beginning of reperfusion and was observed for 24 hours. Each point in the figure shows the mean survival rate at each time point. *P < 0.05 versus vehicle by Kaplan Meyer log-rank test (n = 15 per group).
DISCUSSION
We have shown that intestinal I/R negatively affects lung morphology and increases MPO and local cytokine production consistent with ALI. Treatment with rmMFG-E8 attenuates organ injury and inflammation and improves survival. Treated animals also displayed less apoptosis in their lungs. MFG-E8 KO mice showed a dramatically potentiated ALI and inflammation, providing further evidence for the crucial role of MFG-E8 in I/R-mediated remote organ injury.
ALI is a syndrome of respiratory failure, resulting from acute pulmonary edema and inflammation (2). Intestinal ischemia due to transient obliteration of the SMA causes vast local tissue injury, and the reperfusion of the ischemic bowel leads to a tremendous activation of the inflammatory response, leading to a very severe clinical picture with multiple organ failure, including ALI (27). Pathophysiologically, ALI is associated with alveolar exudates and bleeding and with influx and activation of immune cells with the release of abundant cytokines and enzymes, which can be further complicated by infection and ventilation-induced injury (3, 26). Impaired barrier function of the lung epithelium and endothelium plays a major role in the development of ALI and ARDS, and one key aspect of this failure is the loss of cells through apoptosis (2). In addition, apoptosis plays a role beyond the initial phase of tissue injury because it is central to the imbalances between inflammatory resolution and the progression of ALI later in the disease (28). Controlled tissue repair through the modulation of apoptosis or apoptotic cell phagocytosis may prevent lung fibrosis, the progression of the disease, and its overall severity (2, 12, 26, 28).
MFG-E8 is a secretory molecule that is mainly produced in the spleen. It can also be found in significant amounts in the lymph nodes and lungs (20, 29). It is mainly produced by macrophages and dendritic cells and has been linked to the opsonization of apoptotic cells. Hanayama and colleagues discovered that MFG-E8 plays a major role in the clearance of apoptotic B cells in the spleen, which prevents the development of autoimmune diseases (16, 19, 20). In mice, MFG-E8 is a 64-kD glycoprotein with two EGF-like domains (E1 and E2) containing an RGD-motif that can bind certain integrins (vitronectin receptor, αvβ3, or αvβ5) that are highly expressed on macrophages and other phagocytic cells. These domains are separated by a P/T-rich region from two coagulation factor V/VIII–like domains (C1 and C2) that have a strong affinity to PS. These properties make it an important factor in binding apoptotic cells that express high amounts of PS on their surface to phagocytes (19). Although PS-expressing apoptotic cells can bind to other receptors on macrophages, including the putative PS receptor and CD36, the binding to αvβ3 or αvβ5-integrins via MFG-E8 is required to induce their engulfment (19). At very high concentrations, MFG-E8 has been shown to modulate the intrinsic coagulation cascade due to its competition for PS binding cites and to increase the prothrombin time by 50% (30). It is also involved in the VEGF-dependent neovascularization (31) and in the migration of enterocytes and intestinal repair (32). It has been proposed to be beneficial in atherosclerosis (33) and Alzheimer's disease (34), although the mechanism is still unclear. MFG-E8 plays an important role at the interface between phagocytosis of apoptotic cells and inflammation in chronic and acute inflammatory diseases. We have recently shown that a MFG-E8–dependent increase in apoptotic cell clearance can prevent deaths from sepsis (21).
Both sepsis and mesenteric I/R lead to a systemic inflammatory response (24). Gut I/R has also been shown to lead to increased microbial translocation and the release of bacterial toxins into the blood stream (4, 5). Activation of Toll-like receptors has indeed been shown to suppress MFG-E8 levels in vitro and in vivo (29, 35). Only granulocyte/monocyte colony-stimulating factor is known to modulate MFG-E8 expression (29). Whether other cytokines have the potential to change MFG-E8 expression and at what kinetics is unknown. We have shown a clear suppression of MFG-E8 in the spleen and lungs 4 hours after reperfusion of the 90-minute ischemic gut. The suppression of MFG-E8 by 50%, as we have found in the lungs of gut I/R mice, appears to be sufficient to impair phagocytosis of apoptotic cells, as previously reported in a sepsis model (21). Treatment with rmMFG-E8 reduced the number of apoptotic cells and suppressed local inflammation. Decreased apoptosis by rmMFG-E8 after gut I/R is not mediated by a direct antiapoptotic effect but through the stimulation of apoptotic cell clearance (21). We do not have direct data showing the clearance of apoptotic cells is impaired after gut I/R; however, our previous studies (21) and others' reports (16, 19, 20) have clearly demonstrated that the decreased MFG-E8 level is associated with impaired apoptotic cell clearance. Our current study also showed that the decreased MFG-E8 level and accumulation of apoptotic cells in the lungs present after gut I/R. Therefore, the beneficial effects of MFG-E8 were possibly due to enhanced clearance of apoptotic cells. We also found that the pulmonary injury after gut I/R was attenuated after treatment with rmMFG-E8, as evidenced by decreased tissue injury and decreased neutrophil activity. Although the proinflammatory cytokines were generally suppressed in the small intestine, lungs, and blood after treatment with rmMFG-E8, we did not find any difference in the cytokine levels of the intestine and the blood between MFG-E8 KO mice and the WT control mice 4 hours after reperfusion of the ischemic bowel. The lungs, however, demonstrated a twofold increase of IL-6 and IL-1β in the MFG-E8 KO mice, indicating that in this model deficiency of MFG-E8 mostly affects the lungs. This implies that the lungs are not only a victim of the systemic inflammatory response but to a great extent contribute to the inflammation.
How the antiinflammatory effect of MFG-E8 works in this ALI model remains unknown. However, it presents an immense potential for exploration in the near future. The clearance of apoptotic cells clearly suppresses the inflammatory responsiveness of macrophages (17, 28). Our recent study has shown that MFG-E8–mediated apoptotic cell phagocytosis results in an inhibition of MAPK and NFκB signaling pathways and therefore down-regulates proinflammatory responses (36). Because alveolar macrophages are potentially the cells that lose MFG-E8 after I/R, these may be primed by the deficit of the suppressive effect of apoptotic cell phagocytosis to produce and release more inflammatory cytokines (17). This could explain the increased inflammation and injury in the lungs and the release of inflammatory mediators into the circulation. The mere removal of dying cells that have the potential to release toxic and proinflammatory contents may be another potential mechanism by which MFG-E8 confers its beneficial effect (37). All this needs to be elucidated in the future.
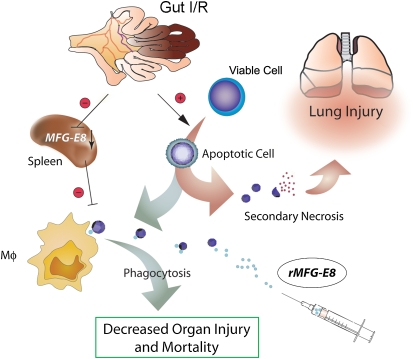
Under physiological conditions, a secondary (postapoptotic) necrosis of apoptotic cells can be prevented through their fast removal by phagocytes in tissues and circulation (38, 39). Hence, the potential harm from apoptotic cells by leakage of their dangerous contents (e.g., cytokines, enzymes, etc.) due to secondary necrosis can be abrogated (39–41). Several studies have shown that excessive apoptosis has pathological consequences on the immune system (42–46). Apoptotic cells undergoing secondary necrosis may contribute to the proinflammatory response. In a study by Hotchkiss and colleagues (47), pretreatment of animals with apoptotic splenocytes worsens the outcome in a mouse model of sepsis, pointing out the detrimental effect of accumulated apoptotic cells in the body. As summarized in Figure 11, gut I/R induces apoptosis in various cells and decreases apoptotic cell clearance through down-regulation of MFG-E8 at the same time. Accumulated apoptotic cells may undergo secondary necrosis, potentiating lung injury under such a condition. The administration of rMFG-E8 enhances apoptotic cell clearance and therefore attenuates lung injury after gut I/R. Thus, MFG-E8 may serve as a novel treatment option for ALI after I/R, or of other etiology, by promoting tissue repair and positively affecting morbidity and mortality in affected patients.
Figure 11.
Gut ischemia-reperfusion (I/R) induces apoptosis in various cells and decreases apoptotic cell clearance by macrophages (Mφ) through down-regulation of murine milk fat globule epidermal growth factor 8 (MFG-E8) at the same time. Accumulated apoptotic cells may undergo secondary necrosis, potentiating lung injury under such a condition. Administration of recombinant MFG-E8 enhances apoptotic cell clearance and therefore attenuates lung injury after gut ischemia-reperfusion (I/R).
Supported by National Institutes of Health grants R01 GM057468, HL076179, and GM053008 (P.W.).
Originally Published in Press as DOI: 10.1164/rccm.200804-625OC on November 5, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Tendler DA. Acute intestinal ischemia and infarction. Semin Gastrointest Dis 2003;14:66–76. [PubMed] [Google Scholar]
- 2.Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA Jr, Hoffman E, Hubmayr RD, et al. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med 2003;167:1027–1035. [DOI] [PubMed] [Google Scholar]
- 3.Bellingan GJ. The pulmonary physician in critical care * 6: The pathogenesis of ALI/ARDS. Thorax 2002;57:540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gathiram P, Gaffin SL, Wells MT, Brock-Utne JG. Superior mesenteric artery occlusion shock in cats: modification of the endotoxemia by antilipopolysaccharide antibodies (anti-LPS). Circ Shock 1986;19:231–237. [PubMed] [Google Scholar]
- 5.Souza DG, Vieira AT, Soares AC, Pinho V, Nicoli JR, Vieira LQ, Teixeira MM. The essential role of the intestinal microbiota in facilitating acute inflammatory responses. J Immunol 2004;173:4137–4146. [DOI] [PubMed] [Google Scholar]
- 6.Tamion F, Richard V, Lyoumi S, Daveau M, Bonmarchand G, Leroy J, Thuillez C, Lebreton JP. Gut ischemia and mesenteric synthesis of inflammatory cytokines after hemorrhagic or endotoxic shock. Am J Physiol 1997;273:G314–G321. [DOI] [PubMed] [Google Scholar]
- 7.Artigas A, Bernard GR, Carlet J, Dreyfuss D, Gattinoni L, Hudson L, Lamy M, Marini JJ, Matthay MA, Pinsky MR, et al. The American-European Consensus Conference on ARDS, part 2: ventilatory, pharmacologic, supportive therapy, study design strategies, and issues related to recovery and remodeling. Acute respiratory distress syndrome. Am J Respir Crit Care Med 1998;157:1332–1347. [DOI] [PubMed] [Google Scholar]
- 8.Shah KA, Shurey S, Green CJ. Apoptosis after intestinal ischemia-reperfusion injury: a morphological study. Transplantation 1997;64:1393–1397. [DOI] [PubMed] [Google Scholar]
- 9.An S, Hishikawa Y, Liu J, Koji T. Lung injury after ischemia-reperfusion of small intestine in rats involves apoptosis of type II alveolar epithelial cells mediated by TNF-alpha and activation of Bid pathway. Apoptosis 2007;12:1989–2001. [DOI] [PubMed] [Google Scholar]
- 10.Collange O, Fabienne T, Nathalie R, Christian T, Vincent R, Bertrand D, Didier P. Pulmonary apoptosis after supraceliac aorta clamping in a rat model. J Surg Res 2005;129:190–195. [DOI] [PubMed] [Google Scholar]
- 11.Mura M, Andrade CF, Han B, Seth R, Zhang Y, Bai XH, Waddell TK, Hwang D, Keshavjee S, Liu M. Intestinal ischemia-reperfusion-induced acute lung injury and oncotic cell death in multiple organs. Shock 2007;28:227–238. [DOI] [PubMed] [Google Scholar]
- 12.Perl M, Chung CS, Perl U, Lomas-Neira J, de Paepe M, Cioffi WG, Ayala A. Fas-induced pulmonary apoptosis and inflammation during indirect acute lung injury. Am J Respir Crit Care Med 2007;176:591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perl M, Lomas-Neira J, Chung CS, Ayala A. Epithelial cell apoptosis and neutrophil recruitment in acute lung injury-a unifying hypothesis? What we have learned from small interfering RNAs. Mol Med 2008;14:465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seitz DH, Perl M, Mangold S, Neddermann A, Braumuller ST, Zhou S, Bachem MG, Huber-Lang MS, Knoferl MW. Pulmonary contusion induces alveolar type 2 epithelial cell apoptosis: role of alveolar macrophages and neutrophils. Shock 2008;30:537–544. [DOI] [PubMed] [Google Scholar]
- 15.Flierl MA, Perl M, Rittirsch D, Bartl C, Schreiber H, Fleig V, Schlaf G, Liener U, Brueckner UB, Gebhard F, et al. The role of C5a in the innate immune response after experimental blunt chest trauma. Shock 2008;29:25–31. [DOI] [PubMed] [Google Scholar]
- 16.Hanayama R, Miyasaka K, Nakaya M, Nagata S. MFG-E8-dependent clearance of apoptotic cells, and autoimmunity caused by its failure. Curr Dir Autoimmun 2006;9:162–172. [DOI] [PubMed] [Google Scholar]
- 17.Hart SP, Dransfield I, Rossi AG. Phagocytosis of apoptotic cells. Methods 2008;44:280–285. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Tibrewal N, Birge RB. Phosphatidylserine recognition by phagocytes: a view to a kill. Trends Cell Biol 2006;16:189–197. [DOI] [PubMed] [Google Scholar]
- 19.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature 2002;417:182–187. [DOI] [PubMed] [Google Scholar]
- 20.Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science 2004;304:1147–1150. [DOI] [PubMed] [Google Scholar]
- 21.Miksa M, Wu R, Dong W, Das P, Yang D, Wang P. Dendritic cell-derived exosomes containing milk fat globule epidermal growth factor-factor VIII attenuate proinflammatory responses in sepsis. Shock 2006;25:586–593. [DOI] [PubMed] [Google Scholar]
- 22.Cui T, Miksa M, Wu R, Zhou M, Dong W, Komura H, Higuchi S, Blau SA, Marini CP, Ravikumar TS, et al. Recombinant murine MFG-E8 (rmMFG-E8) prevents inflammation and organ injury after gut ischemia/reperfusion. Crit Care Med 2007;35(Suppl)A34.
- 23.Bachofen M, Weibel ER. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med 1982;3:35–56. [PubMed] [Google Scholar]
- 24.Stallion A, Kou TD, Latifi SQ, Miller KA, Dahms BB, Dudgeon DL, Levine AD. Ischemia/reperfusion: a clinically relevant model of intestinal injury yielding systemic inflammation. J Pediatr Surg 2005;40:470–477. [DOI] [PubMed] [Google Scholar]
- 25.Day YJ, Marshall MA, Huang L, McDuffie MJ, Okusa MD, Linden J. Protection from ischemic liver injury by activation of A2A adenosine receptors during reperfusion: inhibition of chemokine induction. Am J Physiol Gastrointest Liver Physiol 2004;286:G285–G293. [DOI] [PubMed] [Google Scholar]
- 26.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349. [DOI] [PubMed] [Google Scholar]
- 27.Hart ML, Ceonzo KA, Shaffer LA, Takahashi K, Rother RP, Reenstra WR, Buras JA, Stahl GL. Gastrointestinal ischemia-reperfusion injury is lectin complement pathway dependent without involving C1q. J Immunol 2005;174:6373–6380. [DOI] [PubMed] [Google Scholar]
- 28.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest 2002;109:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jinushi M, Nakazaki Y, Dougan M, Carrasco DR, Mihm M, Dranoff G. MFG-E8-mediated uptake of apoptotic cells by APCs links the pro- and antiinflammatory activities of GM-CSF. J Clin Invest 2007;117:1902–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi J, Gilbert GE. Lactadherin inhibits enzyme complexes of blood coagulation by competing for phospholipid-binding sites. Blood 2003;101:2628–2636. [DOI] [PubMed] [Google Scholar]
- 31.Silvestre JS, Thery C, Hamard G, Boddaert J, Aguilar B, Delcayre A, Houbron C, Tamarat R, Blanc-Brude O, Heeneman S, et al. Lactadherin promotes VEGF-dependent neovascularization. Nat Med 2005;11:499–506. [DOI] [PubMed] [Google Scholar]
- 32.Bu HF, Zuo XL, Wang X, Ensslin MA, Koti V, Hsueh W, Raymond AS, Shur BD, Tan XD. Milk fat globule-EGF factor 8/lactadherin plays a crucial role in maintenance and repair of murine intestinal epithelium. J Clin Invest 2007;117:3673–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.it-Oufella H, Kinugawa K, Zoll J, Simon T, Boddaert J, Heeneman S, Blanc-Brude O, Barateau V, Potteaux S, Merval R, et al. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation 2007;115:2168–2177. [DOI] [PubMed] [Google Scholar]
- 34.Boddaert J, Kinugawa K, Lambert JC, Boukhtouche F, Zoll J, Merval R, Blanc-Brude O, Mann D, Berr C, Vilar J, et al. Evidence of a role for lactadherin in Alzheimer's disease. Am J Pathol 2007;170:921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Komura H, Miksa M, Wu R, Goyert SM, Wang P. Milk fat globule epidermal growth factor-factor VIII is down-regulated in sepsis via the lipopolysaccharide-CD14 pathway. J Immunol 2009;182:581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miksa M, Amin D, Wu R, Jacob A, Zhou M, Dong W, Yang WL, Ravikumar TS, Wang P. Maturation-induced down-regulation of MFG-E8 impairs apoptotic cell clearance and enhances endotoxin response. Int J Mol Med 2008;22:743–748. [PMC free article] [PubMed] [Google Scholar]
- 37.Krysko DV, Vanden BT, D'Herde K, Vandenabeele P. Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods 2008;44:205–221. [DOI] [PubMed] [Google Scholar]
- 38.Zullig S, Hengartner MO. Cell biology: tickling macrophages, a serious business. Science 2004;304:1123–1124. [DOI] [PubMed] [Google Scholar]
- 39.Lauber K, Blumenthal SG, Waibel M, Wesselborg S. Clearance of apoptotic cells: getting rid of the corpses. Mol Cell 2004;14:277–287. [DOI] [PubMed] [Google Scholar]
- 40.Wu Y, Singh S, Georgescu MM, Birge RB. A role for Mer tyrosine kinase in alphavbeta5 integrin-mediated phagocytosis of apoptotic cells. J Cell Sci 2005;118:539–553. [DOI] [PubMed] [Google Scholar]
- 41.Gershov D, Kim S, Brot N, Elkon KB. C-Reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J Exp Med 2000;192:1353–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oberholzer A, Oberholzer C, Minter RM, Moldawer LL. Considering immunomodulatory therapies in the septic patient: should apoptosis be a potential therapeutic target? Immunol Lett 2001;75:221–224. [DOI] [PubMed] [Google Scholar]
- 43.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE Jr, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol 2001;166:6952–6963. [DOI] [PubMed] [Google Scholar]
- 44.Hotchkiss RS, Coopersmith CM, Karl IE. Prevention of lymphocyte apoptosis–a potential treatment of sepsis? Clin Infect Dis 2005;41:S465–S469. [DOI] [PubMed] [Google Scholar]
- 45.Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J Immunol 2005;174:5110–5118. [DOI] [PubMed] [Google Scholar]
- 46.Ayala A, Xin XY, Ayala CA, Sonefeld DE, Karr SM, Evans TA, Chaudry IH. Increased mucosal B-lymphocyte apoptosis during polymicrobial sepsis is a Fas ligand but not an endotoxin-mediated process. Blood 1998;91:1362–1372. [PubMed] [Google Scholar]
- 47.Hotchkiss RS, Chang KC, Grayson MH, Tinsley KW, Dunne BS, Davis CG, Osborne DF, Karl IE. Adoptive transfer of apoptotic splenocytes worsens survival, whereas adoptive transfer of necrotic splenocytes improves survival in sepsis. Proc Natl Acad Sci USA 2003;100:6724–6729. [DOI] [PMC free article] [PubMed] [Google Scholar]