Abstract
Rationale: Radiotherapy (RT) is considered the standard treatment for patients with stage I or II non–small lung cancer who are not surgical candidates because of comorbities or preferences against surgery.
Objectives: To compare the outcomes of patients treated with RT alone with those who were untreated to assess the effect of RT on survival.
Methods: Using the Surveillance, Epidemiology and End Results (SEER) registry linked to Medicare files, we identified 6,065 unresected patients with histologically confirmed stage I and stage II non–small cell lung cancer, diagnosed between 1992 and 2002. We used propensity score methods and instrumental variable analysis to control for the possible effects of known as well as unmeasured confounders.
Measurements and Main Results: Overall, 59% of patients received RT. The overall and lung cancer–specific survival of unresected patients treated with RT was significantly better compared with the untreated cases (P < 0.0001 for both comparisons). RT was associated with a 6-month improvement in median overall survival. Propensity score analyses showed that RT was associated with improved overall (hazard ratio, 0.74; 95% confidence interval, 0.70–0.78) and lung cancer–specific survival (hazard ratio, 0.73; 95% confidence interval, 0.69–0.78). Instrumental variable analysis also indicated improved outcomes among patients treated with RT.
Conclusions: RT improves survival of elderly patients with unresected stage I or II lung cancer. These results should be confirmed in prospective trials.
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Radiation therapy is considered the standard of care for patients with unresected stage I and II lung cancer. Despite the frequent use of radiotherapy for these patients, limited data is available regarding its effectiveness.
What This Study Adds to the Field
Using several methods to control for selection bias, we showed that radiotherapy improves survival in a large population-based sample of elderly patients with stage I and II lung cancer who did not undergo resection due to comorbidities or preferences against surgery.
Surgical resection is the treatment of choice for patients with stage I and stage II non–small cell lung cancer (NSCLC) (1–3). However, nearly 25% of elderly of these patients with stage I and II disease do not undergo surgery (4). Radiotherapy (RT) is currently considered the standard treatment for NSCLC patients who, despite having resectable tumors, are not medically fit or who prefer not to undergo surgery (3).
Despite the frequent use of RT for patients with unresected early stage lung cancer, limited data is available about its effectiveness. Several retrospective analyses have reported the outcomes of patients treated with RT alone but did not include a control group (5–23). These studies showed that the prognosis of these patients is poor, with a 20% (range 13–39%) 5-year lung cancer–specific survival rate. Only one prior study compared survival of unresected patients treated with and without RT, showing that RT may improve survival of these patients (24). However, only standard regression was used to correct for potential selection bias and the analyses were not adjusted for use of chemotherapy or comorbidities. As patients with higher number of comorbidities are less likely to receive RT and/or chemotherapy, lack of adjustment for these factors may bias the analyses toward showing a significant RT effect on survival.
An important issue when evaluating treatment effectiveness using observational data is to adjust for observed and unknown differences in the baseline characteristics and prognostic factors of patients who were treated and those who were untreated (selection bias). Propensity scores and instrumental variable (IV) analysis are two techniques developed to minimize this potential bias (25, 26). A propensity score is a measure of the probability that a patient will receive an intervention (i.e., RT). Using propensity scores, potential bias due to treatment assignment is minimized if the assignment and the response being evaluated are conditionally independent given the measured pretreatment characteristics. IV analysis is a technique that attempts to replicate a randomized controlled trial using observational data. It has already been applied successfully to several other health-related questions and in studies using SEER-Medicare data (27, 28). The concept behind IV analysis is to identify a variable, the “instrument,” that is associated with a patient's likelihood of receiving the treatment but is independent of the patient's baseline characteristics or the therapy outcome. If a good IV is identified, both measured and unmeasured confounders can be accounted for in the analysis.
Using national population-based cancer data, we compared the survival of elderly patients with stage I and stage II NSCLC treated with RT alone to a concurrent cohort of patients of similar stage who did not receive treatment. We used IV analysis and propensity scores to reduce bias due to unbalances in the distribution of prognostic factors among treated and untreated patients.
METHODS
Patients were identified from the Surveillance, Epidemiology and End Results (SEER) Medicare registry. The SEER program collects data on all new cases of cancer from 17 population-based registries covering approximately 26% of the United States population (29). Our study sample consisted of 6,065 unresected patients older than 65 years of age, who did not receive chemotherapy, with histologically confirmed stage I-II NSCLC diagnosed between 1992 and 2002. See the online supplement for additional information regarding the identification of cases and statistical analyses.
Sociodemographic information was obtained from SEER and Medicare databases. To evaluate the burden of comorbidities, we used the Deyo adaptation of the Charlson comorbidity index, applying lung cancer–specific condition weights as described by Klabunde and colleagues (30, 31). Stage was classified according to the American Joint Committee on Cancer criteria (32). Data regarding tumor location, size, and extension was obtained from SEER.
RT use was ascertained from SEER and Medicare claims (33). Patients were considered as RT treated if they were coded by SEER as having received external beam radiation or if Medicare inpatient, outpatient, or physician claims contained any code indicating RT use. Survival was determined as the interval from the date of cancer diagnosis to the Medicare date of death. Those surviving past December 31, 2004 were classified as censored (alive at the end of follow-up).
Statistical Analysis
Differences in distribution of baseline characteristics between patients who received or did not receive RT were evaluated using the χ2 test. The Kaplan-Meier method was used to estimate survival rates among patients in the two treatment groups (34).
To perform the propensity score analyses, we estimated the probability that each patient would receive RT using logistic regression. The model included variables for the patients' sociodemographic characteristics, comorbidities, and cancer-related factors. Cox regression was used to compare survival of patients treated with and without RT while adjusting for propensity scores.
The IV was constructed by examining the intensity of RT use in the different regions, or Health Care Service Areas (HCSAs) in SEER. We classified patients as residing in a high- or low-use area based on the proportion of patients in the HCSA that received RT. Areas where the proportion of patients treated with RT was above the median were classified as high-use areas. We compared the characteristics of patients in high– and low–RT use areas, to evaluate if the two groups were reasonably matched for prognostic features.
As proposed by Earle and colleagues, the IV was calculated as the difference between the adjusted 1- and 2-year survival in the high- and low-use areas, divided by the probability of undergoing RT in those regions (27). Thus, the IV estimate represents the absolute difference in survival at these time points among patients treated with and without RT. Adjusted survival was estimated using Cox proportional hazards models, controlling for sociodemographic characteristics, comorbidities, and stage. The adjusted survival was then calculated for a 70-year-old white female with no comorbidities. The confidence interval (CI) of the IV estimate was obtained using bootstrap. All analyses were performed using SAS (SAS, Cary, NC) statistical package.
RESULTS
A total of 6,065 patients with unresected stage I or II NSCLC were identified. Overall, 3,588 (59%) of patients received RT. The baseline characteristics of these patients are shown in Table 1. Patients treated with RT were younger (P = 0.0006), more likely to be white (P = 0.0002), and to be married (P < 0.0001). There were also significant differences in the tumor characteristics (location, size, and histology) between patients who received RT compared with those who did not. Approximately 10% of patients treated with RT were of stage II NSCLC lung cancer compared with 5% of the untreated patients (P < 0.0001). As expected, untreated patients had higher burden of comorbidities (P = 0.01).
TABLE 1.
BASELINE CHARACTERISTICS OF UNRESECTED PATIENTS WITH STAGE I OR STAGE II NON–SMALL CELL LUNG CANCER IN SEER MEDICARE, 1992–2002
| Characteristic | Radiation Therapy (N = 3,588) | No Treatment (N = 2,477) | P Value | Adjusted P Value1 |
|---|---|---|---|---|
| Age, years, n (%) | 0.0006 | 0.95 | ||
| ≤69 | 723 (20) | 444 (18) | ||
| 70–75 | 964 (27) | 597 (24) | ||
| >75 | 1,901 (53) | 1,436 (58) | ||
| Female, n (%) | 1,642 (46) | 1,161 (47) | 0.39 | 0.99 |
| Race, n (%) | ||||
| White | 3,023 (84) | 1,997 (81) | 0.0002 | 0.07 |
| African American | 333 (9) | 275 (11) | ||
| Hispanic | 92 (3) | 105 (4) | ||
| Other | 140 (4) | 100 (4) | ||
| Marital status | ||||
| Married | 1,739 (48) | 1,011 (41) | <0.0001 | 0.98 |
| Not married | 1,849 (52) | 1,466 (59) | ||
| Median Income in ZIP code of residence, n (%) | ||||
| Highest three quartiles | 2,499 (69) | 1,676 (68) | 0.44 | 0.99 |
| Lowest quartile | 1,089 (31) | 801 (32) | ||
| Tumor location, n (%) | ||||
| Upper lobe | 2,008 (56) | 1,271 (51) | <0.0001 | 0.39 |
| Middle lobe | 184 (5) | 110 (4) | ||
| Lower lobe | 1,074 (30) | 779 (32) | ||
| Main bronchus | 149 (4) | 77 (3) | ||
| Other | 173 (5) | 240 (10) | ||
| Tumor size (mm), n (%) | ||||
| ≤20 | 486 (19) | 318 (20) | 0.002 | 0.99 |
| 21–30 | 695 (27) | 423 (27) | ||
| 31–50 | 925 (36) | 496 (32) | ||
| 51–70 | 329 (13) | 211 (14) | ||
| >70 | 133 (5) | 121 (8) | ||
| Stage | ||||
| I | 3,239 (90) | 2,349 (95) | <0.0001 | 0.41 |
| II | 349 (10) | 128 (5) | ||
| Histology, n (%) | ||||
| Adenocarcinoma | 1,045 (29) | 896 (36) | <0.0001 | 0.99 |
| Squamous cell carcinoma | 1,679 (47) | 964 (39) | ||
| Large cell carcinoma | 310 (9) | 168 (7) | ||
| Other | 554 (15) | 449 (18) | ||
| Charlson comorbidity score, n (%) | ||||
| 0 | 1,218 (34) | 846 (34) | 0.001 | 0.20 |
| 1 | 1,206 (34) | 761 (31) | ||
| ≥2 | 1,164 (32) | 870 (35) | ||
Shows P values for analysis adjusting for propensity scores.
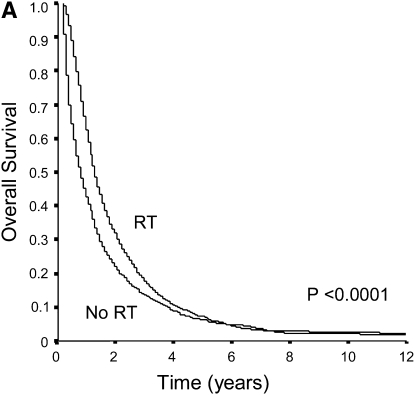
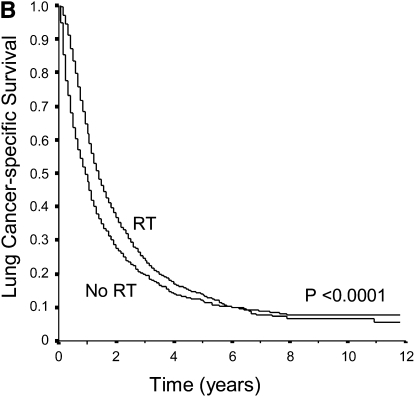
Unadjusted overall and lung cancer–specific survival curves of unresected stage I and II patients who did and did not receive RT therapy are shown in Figure 1. Overall, approximately 71% of patients died as a consequence of lung cancer progression. The overall survival of patients who were treated with RT was significantly better when compared with those who were untreated (P < 0.0001). Similarly, RT use was associated with significant improvements in lung cancer–specific survival. The median overall survival time of patients who received RT was 13 months (95% CI, 13–14 mo) compared with 7 months (95% CI, 6–8 mo) for patients who had not been treated with RT (increment in median survival time of 6 mo). Cox regression analysis also showed that RT was associated with improved overall survival (hazard ratio [HR], 0.71; 95% CI, 0.67–0.75) and lung cancer–specific survival (HR, 0.70; 95% CI, 0.66–0.75) after adjusting for potential confounders.
Figure 1.
(A) Unadjusted overall survival of elderly patients with unresected stage I and II NSCLC according to whether they were treated with radiotherapy (RT). Overall survival was significantly better among patients treated with RT (P < 0.0001). (B) Unadjusted lung cancer-specific survival of elderly patients with unresected stage I and II NSCLC treated with and without RT. Patients treated with RT had significantly better lung cancer specific survival compared with those who were not treated (P < 0.0001).
Propensity Score Analysis
A propensity score, indicating the probability of receiving RT, was constructed using the patient's age, sex, race/ethnicity, marital status, estimated income, tumor location, size and histology, stage at diagnosis, and comorbidity score. With adjustment for propensity score, all covariates were balanced among patients treated with and without RT (Table 1). The analyses evaluating the entire cohort showed that RT was associated with significantly improved overall survival (HR, 0.74; 95% CI, 0.70–0.78) and lung cancer–specific survival (HR, 0.74; 95% CI, 0.70–0.78; Table 2). When data was analyzed separately within propensity score quintiles, we found similar improvement in survival with RT use (HR ranging from 0.70 to 0.80).
TABLE 2.
PROPENSITY SCORE ANALYSIS: RISK OF DEATH FOR PATIENTS TREATED WITH RADIATION THERAPY ACCORDING TO PROPENSITY SCORE QUINTILE
| Overall Survival |
Lung Cancer-specific Survival |
|||
|---|---|---|---|---|
| Quintile | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI |
| 1, lowest probability of RT | 0.70 | 0.62–0.79 | 0.73 | 0.63–0.84 |
| 2 | 0.72 | 0.64–0.81 | 0.71 | 0.62–0.81 |
| 3 | 0.75 | 0.66–0.85 | 0.73 | 0.64–0.84 |
| 4 | 0.72 | 0.63–0.81 | 0.71 | 0.61–0.81 |
| 5, highest probability of RT | 0.80 | 0.70–0.92 | 0.80 | 0.69–0.92 |
| Entire sample* | 0.74 | 0.70–0.78 | 0.73 | 0.69–0.78 |
Definition of abbreviations: CI = confidence interval; RT = radiation therapy.
Quintiles represent patients grouped on the basis of propensity scores.
The hazard ratio for the entire sample is adjusted for propensity score.
Instrumental Variable Analysis
Of the initial 72 HCSAs in our sample, we excluded 13 areas as there were less than 5 patients with unresected stage I or II NSCLC during the study period. Thus, the analyses were based on data for 59 HCSAs areas, which included 5,531 patients with unresected stage I or II NSCLC. We found considerable geographic variation in the use of RT, with rates ranging from 18 to 84% in the HCSAs included in the IV analysis. The baseline characteristics and key prognostic factors of patients in high and low RT–use areas suggest that the groups were reasonably matched for prognostic features including age, sex, race/ethnicity, socioeconomic status, tumor characteristics, and comorbidities (Table 3).
TABLE 3.
CHARACTERISTICS OF UNRESECTED PATIENTS WITH STAGE I OR II NON–SMALL CELL LUNG CANCER ACCORDING TO THE AREA OF RESIDENCE
| Characteristic | Low RT Utilization Areas | High RT Utilization Area |
|---|---|---|
| Age, years (%) | ||
| ≤69 | 20 | 19 |
| 70–75 | 26 | 25 |
| >75 | 54 | 56 |
| Female (%) | 46 | 48 |
| Race (%) | ||
| White | 83 | 81 |
| African American | 11 | 10 |
| Hispanic | 2 | 5 |
| Other | 4 | 4 |
| Median income in ZIP code of residence (%) | ||
| Highest three quartiles | 80 | 82 |
| Lowest quartile | 20 | 18 |
| Stage | ||
| I | 92 | 93 |
| II | 8 | 7 |
| Tumor size, mm (%) | ||
| ≤20 | 20 | 19 |
| 21–30 | 26 | 28 |
| 31–50 | 35 | 34 |
| 51–70 | 13 | 13 |
| >70 | 6 | 6 |
| Histology (%) | ||
| Adenocarcinoma | 31 | 32 |
| Squamous cell carcinoma | 44 | 43 |
| Large cell carcinoma | 8 | 9 |
| Other | 17 | 16 |
| Charlson comorbidity score (%) | ||
| 0 | 34 | 35 |
| 1 | 33 | 32 |
| ≥2 | 33 | 33 |
Definition of abbreviation: RT = radiation therapy.
Approximately 50.4% of the patients in the low-use areas received RT compared with 64.7% in the high-use areas (Table 4). The adjusted 1-year overall survival for a 70-year-old white woman without comorbidities in the low-use areas was 56.6 versus 58.8% in the high RT–use areas. Given that the probability of receiving RT was 14.2% higher among patients in the high-use areas, the IV analysis indicated a 15.6% increase in the 1-year survival rate (95% CI, 2.0–32.9%) with RT use. Similarly, the 2-year adjusted overall survival was 35.6 and 38.1% in the low and high RT–use areas, respectively. Consequently, IV estimate for the absolute difference in 2-year adjusted cumulative survival due to RT was 18.1% (95% CI, 2.2–38.0%). Consistent results were obtained for analyses using lung cancer–specific survival (Table 4).
TABLE 4.
INSTRUMENTAL VARIABLE ANALYSIS OF THE EFFECTIVENESS OF RADIATION THERAPY FOR UNRESECTED STAGE I AND II NON–SMALL CELL LUNG CANCER
| Health Care Service Area |
Probability of RT Use |
||||
|---|---|---|---|---|---|
| Adjusted Survival* | High Utilization† | Low Utilization | High Utilization | Low Utilization | IV Estimator‡ (95% CI) |
| Overall | |||||
| 12-mo | 58.8% | 56.6% | 64.7% | 50.4% | 15.6% (2.0%–33.9%) |
| 24-mo | 38.1% | 35.6% | 18.1% (2.2%–38.0%) | ||
| Lung cancer-specific | |||||
| 12-mo | 64.4% | 61.2% | 64.7% | 50.4% | 21.6% (0%–29.6%) |
| 24-mo | 45.0% | 40.9% | 26.3% (0.2%–36.4%) | ||
Definition of abbreviation: IV = instrumental variable; RT = radiation therapy.
Adjusted survival was calculated using Cox regression adjusting for sociodemographic characteristics, comorbidities, and stage.
Represent Health Care Service areas with high use of radiation therapy.
The IV estimator was calculated as the difference between adjusted survival in the highest and lowest utilization areas, divided by the difference in the probability of undergoing radiation therapy among lung cancer patients in these regions.
DISCUSSION
As shown by SEER data, almost 25% of potentially operable stage I and II NSCLCs in the United States are not resected due to patient preferences or because of coexistent illnesses that preclude surgery (4, 35, 36). Using population-based data and two different robust methods to control for selection bias, we found that RT compared with no treatment is associated with a significant improvement in survival of elderly patients with early stage NSCLC. However, our results indicate that RT is associated with a modest magnitude of benefit; thus, the long-term outcomes of these stage I and II lung cancer patients remain poor despite the use of this treatment.
Several retrospective case series and two recent systematic reviews have summarized the outcomes of patients with stage I or II NSCLC treated with RT alone (5, 6). These studies showed that the majority of patients ultimately die of disseminated lung carcinoma rather than comorbid conditions, and that treatment-related complications are uncommon with doses of 60 to 65 Gy, even among elderly patients (37). Although some studies suggested better survival rates with increasing doses of radiation (10, 14, 20), others did not (7, 11). Some studies showed that histologic type (22) and initial response to radiation (12) were associated with improved patient survival. None of the studies however, included an untreated control group. Thus, it is not possible to determine the effectiveness of RT based on the findings of these data. Conversely, in this study we used a large population-based sample of patients with NSCLC and showed that RT improved survival of unresected patients. These results should help clinicians indicating RT and counseling patients with early stage NSCLC who are not candidates for surgery about the potential advantages of this treatment.
Observational data cannot provide definitive evidence about whether a certain treatment improves outcomes, because nonrandomized studies cannot fully control for the distribution of important covariates among treatment groups. However, propensity scores and IV analysis allow researchers to control for a larger number of variables, self-selection, and the potential effect of unmeasured confounders. Using these methods, we observed a consistent association between RT use and improved overall and lung cancer–specific survival rates. However, it is important to recognize that propensity score methods do not adjust for unmeasured confounders. The SEER Medicare registry does not contain data on patient's pulmonary function, a factor that may be associated with RT use as well as survival. Thus, the results of the propensity score analyses did not control for potential differences in lung function among patients in the two groups. Cormorbities can also influence use of RT. Although we included the Charlson's index as a covariate in our propensity score analyses, this is a crude measure of comorbidity that may not have fully accounted for differences in the burden of other diseases among patients treated with and without RT. Additionally, IV analysis estimates the effectiveness of RT for marginal patients, the sample subgroup whose treatment status depends on the value of the IV (38). Thus, if the effect of RT were heterogeneous, the IV estimates may not be generalizable to all patients with unresected stage I and II NSCLC. Given that unresected elderly patients usually have multiple comorbidities and a limited life expectancy, it is unlikely that a randomized control trial comparing RT versus placebo will be conducted in the near future. Thus, in the absence of information from a randomized controlled trial, as is the case for this clinical scenario, data from a large, population-based cohort is probably the best source for evaluating the effectiveness of RT for these patients (39).
The propensity score analyses showed that RT is associated with a 20 to 30% decreased hazard of mortality. Moreover, the analyses stratified by propensity score quintiles showed that RT had a similar survival benefit among patients with lower propensity as in those with a high propensity for receiving RT. These findings suggest that the benefit from RT may not be limited to those patients who are more likely to receive the treatment in actual practice. Thus, all unresected stage I and II unresected NSCLC patients should be given the option of receiving this treatment unless they have a definitive contraindication for RT.
Several strengths and limitations regarding our study should be noted. The SEER database is the best-known source of population-based cancer data in the United States and is less affected by referral patterns and other sources of bias that might be associated with hospital-based case series. Levels of ascertainment within participating areas have been reported to be has high as 98%, showing that most eligible cases are captured in the registry. Thus, the generalizability of our results should be strong. Additionally, the large number of patients with unresected stage I and II lung cancer in SEER allowed us to perform the IV analyses (which requires a large sample size) and for a precise estimation of survival rates in patients treated with and without RT.
We focused on Medicare beneficiaries who were older than 65 years of age, thus we could not explore whether the effectiveness of RT is similar among younger patients with unresected NSCLC. Exclusion of patients in SEER who received diagnosis of lung cancer before 65 years of age, however, allowed us to adjust for comorbid conditions, socioeconomic status, and eliminated the potential confounding effect of insurance.
Although RT may improve survival of patients with stage I and II NSCLC, this treatment may be associated with side effects. Given that these patient have a limited life expectancy, survival gains should be weighted against the potential negative impact of RT on the quality of life. Unfortunately, the SEER Medicare registry does not include a quality of life measure. Thus, this issue should be further explored in future studies.
No data regarding the total radiation dose, fractionation schedule, and RT technique used to treat each patient is provided in SEER. Some patients treated in latter periods may have not received three-dimensional conformal RT or intensity-modulated RT, techniques that have been recently introduced in some centers in the United States. Thus, it is possible that newer RT techniques may achieve better outcomes than those observed in the study. Additionally, we may have underestimated the effect of RT on lung cancer survival because some of the patients could have received radiation doses less than 60 to 65 Gy. The Radiotherapy Patterns of Care Study showed however, that full-dose RT was the standard of care in most US centers, particularly in the late 1990s (40). The 1-, 3-, and 5-year survival rates for patients treated with RT in our study are well within the range of those reported in prior studies using curative doses, suggesting that most patients in SEER were treated according to the standard of care. In addition, information regarding RT provided by SEER Medicare has been shown to be approximately 90% accurate (33, 41).
In summary, our study suggests that RT alone is associated with improved survival of patients with unresected stage I or II NSCLC. The observed increment in survival was modest and RT did not appear to offer the possibility of long-term survival. However, this improvement in survival is comparable to the gains achieved with the use of accepted chemotherapy regimens for advanced stage NSCLC (42). Changes in the current RT protocols and or new therapeutic strategies may help improve the outcome of unresected NSCLC patients.
Supplementary Material
Acknowledgments
The authors thank the Applied Research Branch, Division of Cancer Prevention and Population Science, National Cancer Institute; the Office of Information Services, and the Office of Strategic Planning, Center for Medicare and Medicaid Services; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results Program tumor registries in the creation of the SEER-Medicare Database. The interpretation and reporting of these data are the sole responsibility of the authors.
Supported by the National Cancer Institute (1R01CA125447-01A1).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournal.org
Originally Published in Press as DOI: 10.1164/rccm.200907-1064OC on November 5, 2009
Conflict of Interest Statement: J.P.W. has served on an advisory board for EHE International ($5,001–$10,000), has received lecture fees paid by Novartis ($1,001–$5,000), industry sponsored grant from GlaxoSmithKline ($10,001–$50,000). E.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.P. has received a patent pending from Columbia University. E.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Spiro SG, Porter JC. Lung cancer–where are we today? Current advances in staging and nonsurgical treatment. Am J Respir Crit Care Med 2002;166:1166–1196. [DOI] [PubMed] [Google Scholar]
- 2.Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med 2004;350:379–392. [DOI] [PubMed] [Google Scholar]
- 3.National comprehensive cancer network. Practice guidelines in oncology. Lung cancer-v.1.2008. Available from: http://www.Nccn.Org/professionals/physician_gls/pdf/esophageal.Pdf. Accessed August 14, 2008.
- 4.Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. N Engl J Med 1999;341:1198–1205. [DOI] [PubMed] [Google Scholar]
- 5.Qiao X, Tullgren O, Lax I, Sirzen F, Lewensohn R. The role of radiotherapy in treatment of stage I non-small cell lung cancer. Lung Cancer 2003;41:1–11. [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann FB, Bamberg M, Molls M, Jeremic B. Radiation therapy alone in early stage non-small cell lung cancer. Semin Surg Oncol 2003;21:91–97. [DOI] [PubMed] [Google Scholar]
- 7.Sandler HM, Curran WJ Jr, Turrisi AT III. The influence of tumor size and pre-treatment staging on outcome following radiation therapy alone for stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 1990;19:9–13. [DOI] [PubMed] [Google Scholar]
- 8.Haffty BG, Goldberg NB, Gerstley J, Fischer DB, Peschel RE. Results of radical radiation therapy in clinical stage I, technically operable non-small cell lung cancer. Int J Radiat Oncol Biol Phys 1988;15:69–73. [DOI] [PubMed] [Google Scholar]
- 9.Perez CA, Stanley K, Grundy G, Hanson W, Rubin P, Kramer S, Brady LW, Marks JE, Perez-Tamayo R, Brown GS, et al. Impact of irradiation technique and tumor extent in tumor control and survival of patients with unresectable non-oat cell carcinoma of the lung: report by the radiation therapy oncology group. Cancer 1982;50:1091–1099. [DOI] [PubMed] [Google Scholar]
- 10.Cooper JD, Pearson G, Todd TR, Patterson GA, Ginsberg RJ, Basiuk J, Blair V, Cass W. Radiotherapy alone for patients with operable carcinoma of the lung. Chest 1985;87:289–292. [DOI] [PubMed] [Google Scholar]
- 11.Morita K, Fuwa N, Suzuki Y, Nishio M, Sakai K, Tamaki Y, Niibe H, Chujo M, Wada S, Sugawara T, et al. Radical radiotherapy for medically inoperable non-small cell lung cancer in clinical stage I: a retrospective analysis of 149 patients. Radiother Oncol 1997;42:31–36. [DOI] [PubMed] [Google Scholar]
- 12.Zhang HX, Yin WB, Zhang LJ, Yang ZY, Zhang ZX, Wang M, Chen DF, Gu XZ. Curative radiotherapy of early operable non-small cell lung cancer. Radiother Oncol 1989;14:89–94. [DOI] [PubMed] [Google Scholar]
- 13.Noordijk EM, vd Poest Clement E, Hermans J, Wever AM, Leer JW. Radiotherapy as an alternative to surgery in elderly patients with resectable lung cancer. Radiother Oncol 1988;13:83–89. [DOI] [PubMed] [Google Scholar]
- 14.Dosoretz DE, Katin MJ, Blitzer PH, Rubenstein JH, Salenius S, Rashid M, Dosani RA, Mestas G, Siegel AD, Chadha TT, et al. Radiation therapy in the management of medically inoperable carcinoma of the lung: results and implications for future treatment strategies. Int J Radiat Oncol Biol Phys 1992;24:3–9. [DOI] [PubMed] [Google Scholar]
- 15.Green N, Weinstein H. Reassessment of radiation therapy for the management of lung cancer in patients with chronic pulmonary disease. Int J Radiat Oncol Biol Phys 1983;9:1891–1896. [DOI] [PubMed] [Google Scholar]
- 16.Talton BM, Constable WC, Kersh CR. Curative radiotherapy in non-small cell carcinoma of the lung. Int J Radiat Oncol Biol Phys 1990;19:15–21. [DOI] [PubMed] [Google Scholar]
- 17.Coy P, Kennelly GM. The role of curative radiotherapy in the treatment of lung cancer. Cancer 1980;45:698–702. [DOI] [PubMed] [Google Scholar]
- 18.Krol AD, Aussems P, Noordijk EM, Hermans J, Leer JW. Local irradiation alone for peripheral stage I lung cancer: could we omit the elective regional nodal irradiation? Int J Radiat Oncol Biol Phys 1996;34:297–302. [DOI] [PubMed] [Google Scholar]
- 19.Hayakawa K, Mitsuhashi N, Saito Y, Nakayama Y, Furuta M, Sakurai H, Kawashima M, Ohno T, Nasu S, Niibe H. Limited field irradiation for medically inoperable patients with peripheral stage I non-small cell lung cancer. Lung Cancer 1999;26:137–142. [DOI] [PubMed] [Google Scholar]
- 20.Kaskowitz L, Graham MV, Emami B, Halverson KJ, Rush C. Radiation therapy alone for stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 1993;27:517–523. [DOI] [PubMed] [Google Scholar]
- 21.Slotman BJ, Njo KH, Karim AB. Curative radiotherapy for technically operable stage I nonsmall cell lung cancer. Int J Radiat Oncol Biol Phys 1994;29:33–37. [DOI] [PubMed] [Google Scholar]
- 22.Gauden S, Ramsay J, Tripcony L. The curative treatment by radiotherapy alone of stage I non-small cell carcinoma of the lung. Chest 1995;108:1278–1282. [DOI] [PubMed] [Google Scholar]
- 23.Jeremic B, Shibamoto Y, Acimovic L, Milisavljevic S. Hyperfractionated radiotherapy alone for clinical stage I nonsmall cell lung cancer. Int J Radiat Oncol Biol Phys 1997;38:521–525. [DOI] [PubMed] [Google Scholar]
- 24.Wisnivesky JP, Bonomi M, Henschke C, Iannuzzi M, McGinn T. Radiation therapy for the treatment of unresected stage I–II non-small cell lung cancer. Chest 2005;128:1461–1467. [DOI] [PubMed] [Google Scholar]
- 25.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med 1997;127:757–763. [DOI] [PubMed] [Google Scholar]
- 26.Zohoori N, Savitz DA. Econometric approaches to epidemiologic data: Relating endogeneity and unobserved heterogeneity to confounding. Ann Epidemiol 1997;7:251–257. [DOI] [PubMed] [Google Scholar]
- 27.Earle CC, Tsai JS, Gelber RD, Weinstein MC, Neumann PJ, Weeks JC. Effectiveness of chemotherapy for advanced lung cancer in the elderly: Instrumental variable and propensity analysis. J Clin Oncol 2001;19:1064–1070. [DOI] [PubMed] [Google Scholar]
- 28.Zeliadt SB, Potosky AL, Penson DF, Etzioni R. Survival benefit associated with adjuvant androgen deprivation therapy combined with radiotherapy for high- and low-risk patients with nonmetastatic prostate cancer. Int J Radiat Oncol Biol Phys 2006. [DOI] [PubMed]
- 29.National Cancer Institute. Surveillance, epidemiology, and end results (SEER) program populations (1969–2006). Bethesda, MD: National Cancer Institute, DCCPS, surveillance research program, cancer statistics branch, February 2009. Accessed April 31, 2009. Available from: www.Seer.Cancer.Gov/popdata
- 30.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000;53:1258–1267. [DOI] [PubMed] [Google Scholar]
- 31.Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol 2007;17:584–590. [DOI] [PubMed] [Google Scholar]
- 32.Mountain CF. Revisions in the international system for staging lung cancer. Chest 1997;111:1710–1717. [DOI] [PubMed] [Google Scholar]
- 33.Virnig BA, Warren JL, Cooper GS, Klabunde CN, Schussler N, Freeman J. Studying radiation therapy using SEER-medicare-linked data. Med Care 2002;40:IV-49–IV-54. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan EL, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc 1958;53:457–481. [Google Scholar]
- 35.Wisnivesky JP, Halm EA. Sex differences in lung cancer survival: do tumors behave differently in elderly women? J Clin Oncol 2007;25:1705–1712. [DOI] [PubMed] [Google Scholar]
- 36.Wisnivesky JP, McGinn T, Henschke C, Hebert P, Iannuzzi MC, Halm EA. Ethnic disparities in the treatment of stage I non-small cell lung cancer. Am J Respir Crit Care Med 2005;171:1158–1163. [DOI] [PubMed] [Google Scholar]
- 37.Hayakawa K, Mitsuhashi N, Katano S, Saito Y, Nakayama Y, Sakurai H, Akimoto T, Hasegawa M, Yamakawa M, Niibe H. High-dose radiation therapy for elderly patients with inoperable or unresectable non-small cell lung cancer. Lung Cancer 2001;32:81–88. [DOI] [PubMed] [Google Scholar]
- 38.Harris KM, Remler DK. Who is the marginal patient? Understanding instrumental variables estimates of treatment effects. Health Serv Res 1998;33:1337–1360. [PMC free article] [PubMed] [Google Scholar]
- 39.Black N. Why we need observational studies to evaluate the effectiveness of health care. BMJ 1996;312:1215–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Movsas B, Moughan J, Komaki R, Choy H, Byhardt R, Langer C, Goldberg M, Graham M, Ettinger D, Johnstone D, et al. Radiotherapy patterns of care study in lung carcinoma. J Clin Oncol 2003;21:4553–4559. [DOI] [PubMed] [Google Scholar]
- 41.Du X, Freeman JL, Goodwin JS. Information on radiation treatment in patients with breast cancer: the advantages of the linked medicare and SEER data. Surveillance, epidemiology and end results. J Clin Epidemiol 1999;52:463–470. [DOI] [PubMed] [Google Scholar]
- 42.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.