Abstract
Purpose
To investigate and compare the circadian pattern of blood pressure (BP), intraocular pressure (IOP) and mean ocular perfusion pressure (MOPP) while experiencing undisturbed sleep in normal-tension glaucoma (NTG) and non-glaucoma control patient groups.
Methods
Twenty-four eyes from 24 patients diagnosed with NTG and 22 eyes from 22 control group patients were enrolled. Systolic BP, diastolic BP and IOP were measured every two hours except for the period of time from 1 AM to 7 AM in the NTG group and from 11 PM to 7 AM in the control group over a one-day period. IOP and hemodynamic parameters were then compared between the two groups. NTG patients were subdivided according to the degree of morning BP dip and IOP, and hemodynamic parameters and visual field indices (mean deviation and pattern standard deviation) were also compared among these subgroups.
Results
There were no significant differences in mean systolic BP, mean diastolic BP and mean arterial pressure (MAP) between the NTG and the control groups. The NTG group showed a significantly large morning BP dip compared to the control group (7.1±4.2% vs. 3.8±3.4%, p=0.022). However, there were no significant differences in mean or fluctuation of MOPP between the two groups. Morning over-dippers showed significantly large MAP and MOPP fluctuations compared to non-dippers and dippers, while there were no significant differences in visual field indices among the three subgroups.
Conclusions
NTG patients showed significant morning BP dips compared to the control group. The marked morning BP dip was associated with significantly large MAP or MOPP fluctuations but was not associated with visual field indices.
Keywords: Blood pressure, Circadian rhythm, Intraocular pressure, Low tension glaucoma, Ocular perfusion pressure
Intraocular pressure (IOP) is a well-known significant risk factor in the development and progression of glaucoma, and clinical management of glaucoma patients mainly focuses on controlling glaucoma [1-4]. However, numerous studies have suggested that vascular factors also contribute to the development of glaucomatous optic neuropathy [5-9]. Ocular perfusion pressure (OPP) is calculated as two-thirds of mean arterial pressure (MAP) minus IOP [10]. Several large, population-based studies have demonstrated that decreased OPP is related to increased prevalence of glaucoma [7, 11, 12]. Just as both blood pressure (BP) and IOP follow a circadian rhythm, OPP, which is derived from BP and IOP, also has circadian variation [13-17]. Many studies have investigated circadian OPP fluctuation as a risk factor for glaucoma. Choi et al. [18, 19] noted that marked circadian mean ocular perfusion pressure (MOPP) fluctuation is associated with nocturnal BP reduction, and circadian MOPP fluctuation was the most consistent clinical risk factor for glaucoma severity in normal tension glaucoma (NTG) patients. However, in those studies, patients were awakened during sleep in the middle of the night (e.g., 3 AM) to obtain BP and IOP measurements, which disturbed their physiologic sleep patterns. In these studies, the potential for the influence of sleep disturbance on the true pattern of BP or IOP variation, which can affect a patient's MOPP fluctuation profile, has not been significantly considered.
The purpose of this study was to investigate and compare the circadian pattern of BP, IOP and MOPP while not disturbing sleep in NTG patients and a non-glaucoma control group.
Materials and Methods
Patients
We recruited patients who were initially diagnosed with NTG at Seoul National University Hospital glaucoma clinic from September 2007 to March 2009. NTG was diagnosed based on clinical and visual field evaluations as well as glaucomatous optic nerve changes with corresponding visual field defects and a peak IOP of less than 22 mmHg. Patients diagnosed with NTG were admitted overnight and underwent circadian BP and IOP measurements as described below. For unilateral NTG, the affected eye was evaluated; in bilateral NTG, we evaluated a randomly selected eye. Those patients with a previous or current history of anti-glaucoma medication use, severe heart or renal failure causing hemodynamic instability or any other ocular disease that might cause visual field loss and peak IOP exceeding 22 mmHg during a hospitalized circadian IOP measurement and subsequently lead to the diagnosis of primary open angle glaucoma (POAG) were excluded from this study.
During the same time period, we recruited a control group of age- and gender-matched patients who were not diagnosed with glaucoma but who had been admitted to Kangbuk Samsung Hospital for cataract surgery. These control patients also received circadian BP and IOP measurements as described below.
All patients underwent measurement of central corneal thickness using ultrasonic pachymetry. IOP was adjusted by 2.5 mmHg for every 50 µm that deviated from 525 µm [20]. All procedures conformed to the Declaration of Helsinki, and our study was approved by the institutional review board of the clinical research institute. Informed consent was obtained from all patients before the study began.
Measurement of circadian BP and IOP
NTG patients were admitted in the afternoon (between 1 and 2 PM), and they underwent BP and IOP measurements at 3, 5, 7, 9, and 11 PM; 1, 7, 9, and 11 AM; and at 1 PM the following day. The patients slept without any disturbance between 1 and 7 AM. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) measurements was taken using an automated BP monitor (PA350; Mediana, Wonju, Korea) placed on the patient's upper arm after at least three minutes of rest. IOP was subsequently measured using Goldmann applanation tonometry three times alternatively for each eye, and the average of the three values was recorded as the IOP.
Control patients were hospitalized in the morning (at 7 AM) the day before the cataract surgery and underwent BP and IOP measurement in the same manner as our NTG patients every two hours until 11 PM. The 1 AM measurement was omitted in the control group to guarantee enough sleep time since most of the patients had cataract surgery scheduled early the next day. They slept uninterrupted from 11 PM to 6 AM.
Visual field examination
All NTG patients had visual field examinations with the 30-2 full-threshold Humphrey field analyzer (Carl Zeiss; Meditec Inc., Dublin, CA, USA) within two weeks after discharge. Only those patients with reliable results (false-positive error less than 20%, false-negative error less than 20% and a fixation loss less than 20%) were included in our study. Visual field indices for analysis were mean deviation (MD) and pattern standard deviation (PSD).
Hemodynamic parameters and nocturnal dip and morning dip indices
Mean arterial pressure (MAP) and mean ocular perfusion pressure (MOPP) were calculated as follows: MAP=DBP+1/3×(SBP-DBP); MOPP=2/3×MAP-IOP. The fluctuation of each parameter was defined as the difference between the peak and the trough measurement of each parameter. Additionally, we measured the degree of BP reduction during the night or morning using the following indices: Nocturnal dip index=(diurnal average MAP-nocturnal lowest MAP)/diurnal average MAP×100 (%); Morning dip index=(24-hour average MAP-morning lowest MAP)/24-hour average MAP×100 (%). The nocturnal period was defined as from 8 PM to 6 AM the following day, and the diurnal period included the remaining times of the day [13, 21]. The morning period was defined as 7 AM to 11 AM.
NTG patients were divided into three groups according to the degree of morning BP reduction as follows: non-dipper, morning dip index<5%; dipper, morning dip index ≥5% but <10%; and over-dipper, morning dip index ≥10%.
Statistical analysis
SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Patient demographics and hemodynamic and IOP parameters were compared between the NTG and control groups using Mann-Whitney U test and Chi-square tests. Patient demographics, hemodynamic and IOP parameters and visual field indices were compared among three NTG subgroups using Kruskal-Wallis H test and Chi-square tests. A p-value<0.05 was considered statistically significant.
Results
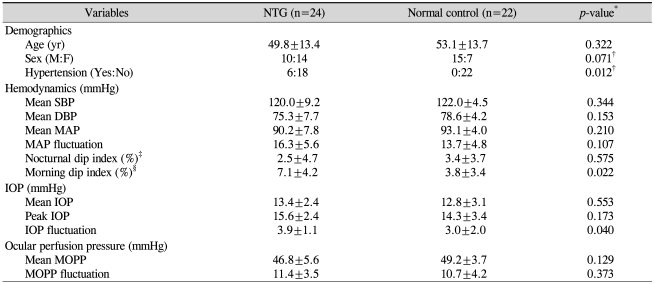
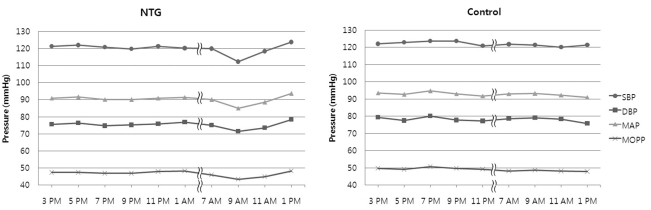
Twenty-four patients were included in the NTG group (mean age, 49.8±13.4 years; 10 males and 14 females), and 22 patients were included in the control group (mean age, 53.1±13.7 years; 15 males and 7 females). Two patients admitted for BP and IOP measurement after initial diagnosis of NTG revealed peak IOP values over 22 mmHg, and these patients were excluded from the study. Table 1 summarizes patient demographics and hemodynamic and IOP parameters of both NTG patients and normal controls. There were no significant differences in age and gender between the two groups. Six of the 24 patients in the NTG group had hypertension and were under medical management for this condition, while none of the control group patients had underlying hypertension. Fig. 1 represents the circadian hemodynamic patterns in the two groups. There were no significant differences in the hemodynamic parameters between the two groups in terms of mean SBP, mean DBP, mean MAP, and MAP fluctuation (Table 1). We found no definite nocturnal BP dip in either group, but the NTG group showed a prominent morning BP dip. Therefore, we made a new index, the morning dip index, exhibiting the degree of morning BP dip as represented in our Methods section. The NTG group showed a significantly large morning BP dip compared to the control group (7.1±4.2% vs. 3.8±3.4%, p=0.022)
Table 1.
Patient demographics and hemodynamic and IOP parameters of NTG and normal control groups
NTG=normal tension glaucoma; SBP=systolic blood pressure; DBP=diastolic blood pressure; MAP=mean arterial pressure; IOP=intraocular pressure; MOPP=mean ocular perfusion pressure.
*All p-values by Mann-Whitney U test except; †Chi-square test; ‡Nocturnal dip index=(diurnal average MAP-nocturnal lowest MAP)/diurnal average MAP×100 (%); §Morning dip index=(24-hr average MAP-morning lowest MAP)/24-hr average MAP)×100 (%).
Fig. 1.
Circadian hemodynamic patterns in the normal tension glaucoma (NTG) and the non-glaucoma control group.
SBP=systolic blood pressure; DBP=diastolic blood pressure; MAP=mean arterial pressure; MOPP=mean ocular perfusion pressure.
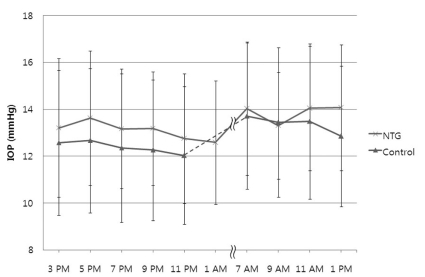
Fig. 2 shows the circadian IOP curve in the NTG and control groups. The NTG group usually showed a higher IOP level than the control group, but this difference was not statistically significant (Fig. 2 and Table 1). In both groups, the mean IOP was low during the nighttime and high during the daytime, especially in the early morning. There were no significant differences in peak IOP between the two groups, but the NTG group showed a significantly large IOP fluctuation compared to the control group (3.9±1.1 mmHg vs. 3.0±2.0 mmHg, p=0.040).
Fig. 2.
Circadian intraocular pressure (IOP) patterns in the normal tension glaucoma (NTG) and the non-glaucoma control group.
The NTG group also showed a lower MOPP than the control group at all times, and the difference was statistically significant at 9 AM and 7 PM (Fig.1). However, there were no significant differences in OPP between the two groups in terms of mean MOPP and MOPP fluctuations (Table 1).
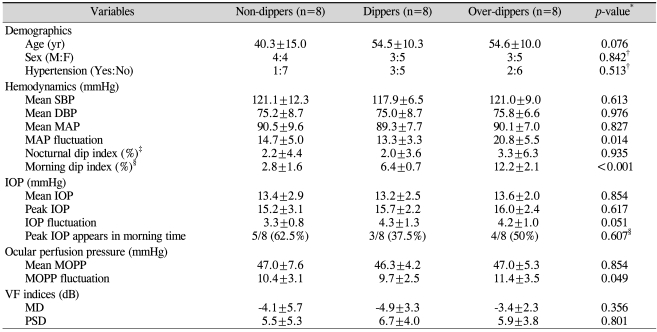
The NTG group was subdivided into three groups according to the degree of morning BP dip: non-dipper, dipper and over-dipper groups. Table 2 presents patient demographics, hemodynamic and IOP parameters and visual field indices of each subgroup. Morning non-dippers were younger than dippers or over-dippers, but this difference did not reach statistical significance. There were no significant differences in mean SBP, mean DBP or mean MAP among the three groups, while the over-dipper group showed a significantly large MAP fluctuation. The mean and peak and fluctuation of IOP among the subgroups also were not significantly different. While the mean MOPP was not significantly different, the MOPP fluctuation showed significant differences among the groups. Finally, there were no significant differences in MD and PSD among the groups (Table 2).
Table 2.
Patient demographics and hemodynamic and IOP parameters of morning non-dippers, dippers, and over-dippers
SBP=systolic blood pressure; DBP=diastolic blood pressure; MAP=mean arterial pressure; IOP=intraocular pressure; MOPP=mean ocular perfusion pressure; VF=visual field; MD=mean deviation; PSD=pattern standard deviation.
*All p-values by Kruskal-Wallis H test except; †Chi-square test; ‡Nocturnal dip index=(diurnal average MAP-nocturnal lowest MAP)/diurnal average MAP×100 (%); §Morning dip index=(24-hr average MAP-morning lowest MAP)/24-hr average MAP)×100 (%).
Discussion
Both the NTG and the non-glaucoma control group did not show a significant nocturnal BP dip (2.5±4.7% in NTG, 3.4±3.7% in control group) compared to previous studies. Plange et al. [22] investigated circadian BP patterns using ambulatory blood pressure monitoring in 51 NTG patients and 28 age-matched controls, and their study showed that the mean nocturnal arterial dip was 14.1±6.4% in NTG patients and 15.9±8.6% in controls, which was higher than our results, although their method of BP measurement differed somewhat from ours. Several studies have suggested a correlation between nocturnal hypotension and glaucoma [5, 17, 23, 24]. Others have reported that there are no significant differences in nocturnal BP dips between glaucoma and control groups [25, 26]. In our study, we did not find any significant differences in nocturnal BP dips between the NTG and control groups.
However, NTG patients revealed a marked BP dip during the morning (7 AM to 11 AM). Sehi et al. [10] reported that SBP and DBP are low at 7 AM and also after lunch (1 PM) in both untreated POAG patients and age-matched normal control groups, while MOPP showed a significantly different pattern between the two groups. The minimum MOPP in the untreated POAG group was recorded at 7 AM when IOP is at its highest. Therefore, we evaluated the degree of BP dip in the morning compared to the whole day. The NTG group showed a larger morning BP dip compared to the control group (7.1±4.2% in NTG and 3.8±3.4% in controls), although the mean SBP, DBP and MAP were similar in the two groups. The MAP fluctuation was larger in the NTG group, but it did not reach statistical significance; there were also no significant differences in mean MOPP and MOPP fluctuations between the two groups. Therefore, in this study, a larger morning BP dip in NTG patients was not linked to significant MAP or MOPP fluctuations. However, it is possible that a morning BP dip compromises ocular perfusion status along with the increase in morning IOP; 50% of NTG patients experienced peak IOP in the morning. When the NTG group was divided into three subgroups according to the degree of morning BP dip, over-dippers showed significantly large MAP and MOPP fluctuations compared to non-dippers or dippers. However, there were no significant differences in visual field indices (MD and PSD) among the three subgroups (Table 2). These results disagree with previous studies that emphasized the correlation between MOPP fluctuations and visual field indices or glaucoma severity [18, 19]. These discrepancies might be due to our study's small sample size and short follow-up period. Long-term serial follow-up of visual field tests with larger number of cases might reveal the true relationship between MOPP or fluctuations between it and visual field indices.
In this study, we measured circadian IOP and BP profiles without disturbing nocturnal sleep, and we found that there were no significant differences in nocturnal BP dips between the NTG patients and the normal control group. However, NTG patients revealed a significant morning BP dip compared to the control group. Our study is the first report that found that NTG patients showed a more prominent morning BP dip than a control group, but it is still inconclusive whether this resulted from undisturbed sleep that is not considered in other studies or if it is just a representation of the hemodynamic instability of NTG patients. Further studies with a larger number of patients may clarify the relationship between undisturbed sleep and morning BP dips in NTG patients. Previous studies on circadian BP measurement with ambulatory blood pressure monitoring have shown that BP declines during sleep, reaching a trough level roughly between 2 and 4 AM [27]. As we did not measure BP during these hours, our study could have underestimated a nocturnal BP dip.
NTG patients were significantly more likely to have underlying hypertension than members of the control group. We compared IOP parameters (including mean and peak and fluctuation of IOP) and hemodynamic parameters (including mean SBP, mean DBP, mean MAP, MAP fluctuation, and nocturnal and morning dip indices) between those with and without hypertension in NTG patients. We found that there were no significant IOP or hemodynamic parameter differences between the two groups (results not shown). Therefore, the significant morning BP dip seen in the NTG group could be attributed more to glaucoma than to hypertension. Regardless of whether BP dips occur at night or in the morning, ocular perfusion status compromise is possible in NTG patients as both morning and night are well known for having the highest IOP level. The morning BP dip might represent the hemodynamic instability of NTG patients and support the vascular theory of glaucoma development.
In conclusion, NTG patients revealed a significant morning BP dip compared to the control group. A marked morning BP dip was associated with significantly large MAP or MOPP fluctuations, but it was not associated with visual field indices. Long-term and serial follow-up of these patients are needed to further investigate the association between hemodynamic changes and the severity and functional aspects of glaucoma.
Acknowledgements
This work was partially supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2009-0091931).
References
- 1.Collaborative Normal-Rension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126:498–505. doi: 10.1016/s0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]
- 2.Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 3.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 4.Higginbotham EJ, Gordon MO, Beiser JA, et al. The Ocular Hypertension Treatment Study: topical medication delays or prevents primary open-angle glaucoma in African American individuals. Arch Ophthalmol. 2004;122:813–820. doi: 10.1001/archopht.122.6.813. [DOI] [PubMed] [Google Scholar]
- 5.Hayreh SS, Zimmerman MB, Podhajsky P, Alward WL. Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am J Ophthalmol. 1994;117:603–624. doi: 10.1016/s0002-9394(14)70067-4. [DOI] [PubMed] [Google Scholar]
- 6.Flammer J, Orgul S. Optic nerve blood-flow abnormalities in glaucoma. Prog Retin Eye Res. 1998;17:267–289. doi: 10.1016/s1350-9462(97)00006-2. [DOI] [PubMed] [Google Scholar]
- 7.Bonomi L, Marchini G, Marraffa M, et al. Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt Study. Ophthalmology. 2000;107:1287–1293. doi: 10.1016/s0161-6420(00)00138-x. [DOI] [PubMed] [Google Scholar]
- 8.Flammer J, Orgul S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–393. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 9.Fuchsjager-Mayrl G, Wally B, Georgopoulos M, et al. Ocular blood flow and systemic blood pressure in patients with primary open-angle glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci. 2004;45:834–839. doi: 10.1167/iovs.03-0461. [DOI] [PubMed] [Google Scholar]
- 10.Sehi M, Flanagan JG, Zeng L, et al. Relative change in diurnal mean ocular perfusion pressure: a risk factor for the diagnosis of primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2005;46:561–567. doi: 10.1167/iovs.04-1033. [DOI] [PubMed] [Google Scholar]
- 11.Tielsch JM, Katz J, Sommer A, et al. Hypertension, perfusion pressure, and primary open-angle glaucoma: a population-based assessment. Arch Ophthalmol. 1995;113:216–221. doi: 10.1001/archopht.1995.01100020100038. [DOI] [PubMed] [Google Scholar]
- 12.Leske MC, Wu SY, Nemesure B, Hennis A. Incident open-angle glaucoma and blood pressure. Arch Ophthalmol. 2002;120:954–959. doi: 10.1001/archopht.120.7.954. [DOI] [PubMed] [Google Scholar]
- 13.Verdecchia P, Schillaci G, Guerrieri M, et al. Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation. 1990;81:528–536. doi: 10.1161/01.cir.81.2.528. [DOI] [PubMed] [Google Scholar]
- 14.Veerman DP, Imholz BP, Wieling W, et al. Circadian profile of systemic hemodynamics. Hypertension. 1995;26:55–59. doi: 10.1161/01.hyp.26.1.55. [DOI] [PubMed] [Google Scholar]
- 15.Liu JH. Circadian rhythm of intraocular pressure. J Glaucoma. 1998;7:141–147. [PubMed] [Google Scholar]
- 16.Liu JH, Kripke DF, Hoffman RE, et al. Nocturnal elevation of intraocular pressure in young adults. Invest Ophthalmol Vis Sci. 1998;39:2707–2712. [PubMed] [Google Scholar]
- 17.Liu JH, Zhang X, Kripke DF, Weinreb RN. Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Invest Ophthalmol Vis Sci. 2003;44:1586–1590. doi: 10.1167/iovs.02-0666. [DOI] [PubMed] [Google Scholar]
- 18.Choi J, Jeong J, Cho HS, Kook MS. Effect of nocturnal blood pressure reduction on circadian fluctuation of mean ocular perfusion pressure: a risk factor for normal tension glaucoma. Invest Ophthalmol Vis Sci. 2006;47:831–836. doi: 10.1167/iovs.05-1053. [DOI] [PubMed] [Google Scholar]
- 19.Choi J, Kim KH, Jeong J, et al. Circadian fluctuation of mean ocular perfusion pressure is a consistent risk factor for normal-tension glaucoma. Invest Ophthalmol Vis Sci. 2007;48:104–111. doi: 10.1167/iovs.06-0615. [DOI] [PubMed] [Google Scholar]
- 20.Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: a review and meta-analysis approach. Surv Ophthalmol. 2000;44:367–408. doi: 10.1016/s0039-6257(00)00110-7. [DOI] [PubMed] [Google Scholar]
- 21.Kario K, Matsuo T, Kobayashi H, et al. Nocturnal fall of blood pressure and silent cerebrovascular damage in elderly hypertensive patients. Advanced silent cerebrovascular damage in extreme dippers. Hypertension. 1996;27:130–135. doi: 10.1161/01.hyp.27.1.130. [DOI] [PubMed] [Google Scholar]
- 22.Plange N, Kaup M, Daneljan L, et al. 24-h blood pressure monitoring in normal tension glaucoma: night-time blood pressure variability. J Hum Hypertens. 2006;20:137–142. doi: 10.1038/sj.jhh.1001959. [DOI] [PubMed] [Google Scholar]
- 23.Kaiser HJ, Flammer J, Graf T, Stumpfig D. Systemic blood pressure in glaucoma patients. Graefes Arch Clin Exp Ophthalmol. 1993;231:677–680. doi: 10.1007/BF00919280. [DOI] [PubMed] [Google Scholar]
- 24.Graham SL, Drance SM. Nocturnal hypotension: role in glaucoma progression. Surv Ophthalmol. 1999;43(Suppl 1):S10–S16. doi: 10.1016/s0039-6257(99)00016-8. [DOI] [PubMed] [Google Scholar]
- 25.Kashiwagi K, Hosaka O, Kashiwagi F, et al. Systemic circulatory parameters. comparison between patients with normal tension glaucoma and normal subjects using ambulatory monitoring. Jpn J Ophthalmol. 2001;45:388–396. doi: 10.1016/s0021-5155(01)00364-1. [DOI] [PubMed] [Google Scholar]
- 26.Harris A, Evans D, Martin B, et al. Nocturnal blood pressure reduction: effect on retrobulbar hemodynamics in glaucoma. Graefes Arch Clin Exp Ophthalmol. 2002;240:372–378. doi: 10.1007/s00417-002-0466-y. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien E, Murphy J, Tyndall A, et al. Twenty-four-hour ambulatory blood pressure in men and women aged 17 to 80 years: the Allied Irish Bank Study. J Hypertens. 1991;9:355–360. doi: 10.1097/00004872-199104000-00007. [DOI] [PubMed] [Google Scholar]