The aim of the current study was to provide normal reference values for the density of trabecular bone in the axial skeleton of male and female subjects from childhood to young adulthood.
Abstract
Purpose:
To determine normative reference values for vertebral trabecular bone density (TBD) obtained by using quantitative computed tomography (CT) in healthy white children, teenagers, and young adults of both sexes.
Materials and Methods:
The data presented in this HIPAA-compliant study are a compilation of data from multiple investigations on the determinants of bone acquisition in healthy children conducted at this institution from 1992 to 2006. The institutional review board for clinical investigations approved the protocols for each of these studies, and written informed consent was provided by all parents and/or participants. Quantitative CT measurements of TBD (in milligrams per cubic centimeter) were obtained at the first, second, and third lumbar vertebrae in 1222 healthy white male and female subjects aged 5–21 years (mean age for male subjects, 15.1 years ± 3.6 [standard deviation]; range, 5.6–21.9 years; mean age for female subjects, 14.2 years ± 3.9; range, 5.7–21.6 years; mean age for both sexes, 14.6 years ± 3.8). Mean and standard deviations for TBD were determined for each age group in 1-year intervals, and Student t tests for unpaired data were performed to compare male subjects with female subjects.
Results:
TBD increased equally during growth in male and female subjects. Although the percentage increase in TBD was similar for both sexes (23.7% [57 of 241] for male subjects, 22.2% [54 of 243] for female subjects), the rise began and reached peak values at an earlier age in female subjects; increases in TBD occurred from 10–15 years of age in female subjects, whereas in male subjects, these increases were not observed until age 12 years and were completed at 17 years.
Conclusion:
This study provides reference standards for quantitative CT bone measurements in children and young adults, which may aid in the diagnosis, prevention, and treatment of pediatric metabolic bone disorders.
© RSNA, 2008
Measurements of bone density during childhood are commonly obtained to assess the deficiencies in bone accumulation associated with a multitude of pediatric disorders. Moreover, the amount of bone that is gained during growth is an important determinant of the risk for osteoporosis later in life (1,2), and available data suggest that the genetic susceptibility to osteoporosis is detectable in childhood (3). Peak bone mass, a major determinant of the future risk of fractures in the elderly, is largely achieved by the end of sexual development (4).
Because of its availability, minimal radiation exposure, and relative ease of use, dual-energy x-ray absorptiometry (DXA) is the most commonly employed quantitative radiologic method to assess bone mass at any age (5). The interpretation of DXA bone studies is considerably more challenging in children than in adults. Although reference data and diagnostic algorithms are available for children, several investigators have stressed that DXA frequently leads to misdiagnoses of osteoporosis in the very young (6). This is caused, in part, by the two-dimensional nature of DXA, which cannot account for the dimension of the bone in the direction of the x-ray beams. DXA areal densities (in grams per square centimeter), therefore, depend on the size of the bone in addition to its volumetric density (in grams per cubic centimeter) (7,8). DXA values are also influenced by inhomogeneities in soft-tissue composition and fat distribution (9,10). The inability to account for growth-related variations in bone and body size and composition with this projection technique limits DXA bone determinations and leads to inaccurate measurements (7,11–14).
In contrast, quantitative computed tomography (CT) and peripheral quantitative CT are not subject to these limitations because they provide a three-dimensional assessment of bone structure. In addition, these techniques offer the ability to separate out trabecular bone, which is eight times more metabolically active than cortical bone (15–185), and provide a direct measure of volumetric bone density (19). Currently, although reference data are available for peripheral quantitative CT measurements of the appendicular skeleton in children, normative data for the axial skeleton are lacking (20). In the elderly, quantitative CT measurements of trabecular bone density (TBD) in the vertebral body have been shown to have the strongest capability to aid in the assessment of age-related bone loss and prediction of osteoporotic vertebral fractures (21).
Although treatment for osteoporosis has focused mainly on decreasing the rate of bone loss in the elderly, it is becoming increasingly clear that interventions to optimize bone accretion before it reaches its peak are key for the planning of early preventive strategies for this disease. The purpose of this study was to determine normative reference values for vertebral TBD obtained by using quantitative CT in healthy white children, teenagers, and young adults of both sexes.
MATERIALS AND METHODS
The data presented in this study are a compilation of multiple investigations on the determinants of bone acquisition in healthy children, adolescents, and young adults at Childrens Hospital Los Angeles, Los Angeles, Calif, from 1992 to 2006 (22–3519,20). Included in this study were 1222 white subjects, aged 5–21 years, who were not taking any medications and were not known to have any illness. The subjects ranged in age from 5 to 21 years (mean age for male subjects, 15.1 years ± 3.6 [standard deviation], and range, 5.6–21.9 years; mean age for female subjects, 14.2 years ± 3.9, and range, 5.7–21.6 years; mean age for both sexes, 14.6 years ± 3.8). The Institutional Review Board for clinical investigations at Childrens Hospital Los Angeles approved the protocols for each of these studies, and written informed consent was obtained from all parents and/or participants. Compliance with Health Insurance Portability and Accountability Act requirements was also maintained, and authorization forms were signed by all participants examined after introduction of this standard. The quantitative CT protocol was designed to keep radiation exposure to a level roughly equivalent to the exposure during a round-trip airplane flight across North America (14,22), making its use in healthy subjects possible.
Data from one examination were included for each of the subjects. Values for height, weight, and body mass index and quantitative CT bone measurements were reviewed from available records. All subjects were assessed by using a quantitative CT scanner (Hilite Advantage; GE Medical Systems, Milwaukee, Wis) with the same mineral reference phantom (0, 125, and 250 mg/cm3 solid hydroxyapatite equivalent) for simultaneous calibration (CT-T bone densitometry package; GE Medical Systems). The sites to be scanned were identified with lateral scout views. Measurements of TBD (in milligrams per cubic centimeter) were obtained for a 10-mm section centered around the midpoint of each of the first, second, and third lumbar vertebrae by using 80 kVp, 70 mA, and scanning time of 2 seconds. Values from this two-dimensional scanning technique would be expected to correlate strongly with values obtained by using three-dimensional scanning, although values from the latter may be slightly higher (23,24). The coefficients of variation for repeated quantitative CT measurements of vertebral TBD ranged between 1% and 2% (25). The time required to complete the quantitative CT scans was approximately 10 minutes per subject. Radiation was 1.0–1.5 mSv localized to the midportions of the L1, L2, and L3 vertebrae; the effective radiation dose was approximately 0.1 mSv.
Subjects were grouped in 1-year age intervals (eg, all subjects aged ≥9 but <10 years were in the 9-year age group). All data are reported with mean and standard deviation for each age group. These reference data were used to calculate z scores, which indicate the number of standard deviations an individual's TBD is above or below the mean for his or her age. This is the standard method of evaluating bone density measurements in children with a z score below −2.0, which indicates low bone density for age (6,26). The data were graphed by using software (Excel; Microsoft, Redmond, Wash) and the default smoothing procedures of the software.
The difference in mean TBD values was compared among the various age groups in each sex. Values were considered to be increasing when they differed from the previous age group by at least 5 mg/cm3. They were considered to have peaked when they differed by less than this amount for all subsequent age groups and a linear regression analysis did not result in a significant increasing or decreasing slope over age. The Pearson correlation was used to examine the correlation between TBD and the anthropometric parameters. Student t tests for unpaired data were used to compare male subjects with female subjects in each age group. Statistical analysis was performed with software (StatView, version 5.0.1; SAS Institute, Cary, NC).
RESULTS
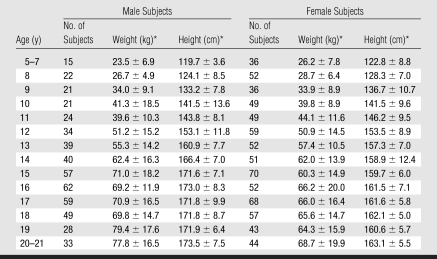
Tables 1 and 2 show weight, height, and TBD values according to age for both male and female subjects. The youngest (aged 5–7 years) and oldest (aged 20–21 years) subjects were grouped because of small sample sizes and lack of differences as a function of age within these groups. For both male and female subjects, moderate correlations were present between values for vertebral TBD and age, weight, and height (r = 0.38–0.46; all P < .0001) when all ages were considered together.
Table 1.
Weight and Height according to Age in 504 Male and 718 Female Subjects
* Data are the mean ± standard deviation.
Table 2.
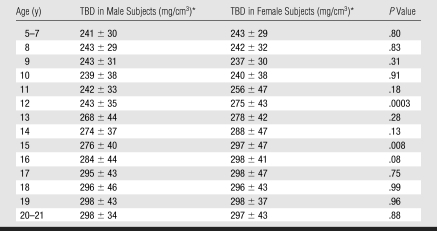
Vertebral TBD according to Age in 504 Male and 718 Female Subjects
* Data are the mean ± standard deviation.
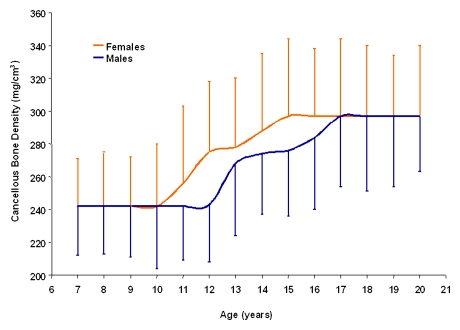
TBD increased equally during growth in male and female subjects. Although the percentage increase in TBD was similar for both sexes (23.7% for male subjects and 22.2% for female subjects), the increase began and reached peak values at an earlier age in female subjects; significant increases in TBD occurred from 10–15 years of age in female subjects, whereas, in male subjects, these increases were not observed until age 12 years and were completed at 17 years (Figure). There were no sex differences in TBD during the 1st decade of life (5–10 years of age) nor in older teenagers.
Mean and standard deviations of vertebral TBD in male and female subjects aged 7–20 years. TBD increased and reached peak values earlier in female than in male subjects. Because TBD did not correlate with age for male subjects aged 7–11 and 17–20 years and for female subjects aged 7–10 and 15–20 years, values for TBD in these age groups were equalized. Values at 7 and 20 years represent the mean for subjects 5–7 years of age and 20–21 years of age, respectively. Exact values are given in Table 2.
DISCUSSION
Bone density assessments are frequently performed in pediatric patients to facilitate the evaluation of the multiple conditions associated with a deficiency in bone acquisition during growth and to assess response to therapies (27–30). The aim of the current study was to provide normal reference values for the density of trabecular bone in the axial skeleton of male and female subjects from childhood to young adulthood. These values are not intended to be used as a sole diagnostic instrument but should contribute to forming an overall clinical impression for the child in whom these measurements are performed and are an improvement over current tools to evaluate bone in pediatric clinical and research settings.
The results of this study corroborate previous data indicating that vertebral bone density does not differ in male and female subjects before the onset of puberty or after sexual maturity but increases significantly during sexual development and achieves peak values by the end of the 2nd decade of life (31–34). Our findings are also concordant with those in studies that suggest that sex differences in peak bone mass in the axial skeleton, a major determinant of future susceptibility to vertebral fractures in the elderly, do not result from differences in TBD (31,35). Indeed, challenging the view that sex differences in bone mass are caused by differences in bone density are recent observations; these observations were made by using quantitative CT and indicate that, throughout life, female subjects have smaller vertebral bodies, but similar TBD, when compared with male subjects, even after accounting for differences in body size (34).
The factors that account for the increase in TBD observed in this study remain to be determined. It is reasonable to suspect, however, that they are associated with sexual development and are, in part, mediated by the actions of sex steroids. Regardless of mechanism, sex differences in the temporal sequence of TBD probably reflect sex differences in the appearance of sexual characteristics and the accelerated growth spurt. For both sexes, although growth acceleration begins in early adolescence, typically, peak growth velocity in boys is reached 2–3 years later, and boys continue growing for approximately 2–3 years longer than do girls (36). Interestingly, the differences between male and female subjects in the commencement of TBD increases parallel the differences in the tempo of peak height velocities. Our finding that quantitative CT measurements of TBD increase during the growth spurt contradicts the commonly held notion that an increase in fractures during the peripubertal growth spurt might correlate with a temporary decrease in bone density as new bone formation transiently outstrips mineral deposition (37).
For the accurate interpretation of our results, several technical issues in regard to the use of quantitative CT to determine TBD in the vertebral bodies should be addressed. Cancellous bone exists as a three-dimensional lattice of plates and columns (trabeculae). The trabeculae divide the interior volume of the bone into intercommunicating pores, which are filled with a variable mixture of red and yellow marrow (38). Because of the relatively small size of the trabeculae when compared with the pixel (the quantitative CT unit of measurement), quantitative CT values for TBD reflect not only the amount of bone (the number, the thickness, and the degree of mineralization of the trabeculae) but also the amount of marrow per pixel (5). The increases in the tissue density of cancellous bone observed throughout adolescence are probably the consequence of an increase in trabecular thickness and/or mineralization rather than in the number of trabeculae (39).
There were several limitations in this study. First, the subjects were not recruited from the community at large, and the cohort of white subjects may not reflect populations with different genetic or environmental backgrounds; future studies are needed to establish reference data for children of other racial groups. In addition, the only parameters considered in this study were age, height, weight, sex, and vertebral TBD. The data did not include important determinants of bone gains during growth, such as skeletal maturity or the degree of sexual development. To acquire these parameters in clinical practice is often not feasible or practical, however, and it is unlikely that they would be incorporated in the routine clinical practice of pediatric bone measurements. Last, the data did not include measures of cancellous bone at other skeletal sites, which are likely to exhibit different patterns of bone growth (19). In adults, the poor correlation between trabecular bone in the peripheral and axial skeleton suggests that trabecular bone, at least as measured by using quantitative CT and peripheral quantitative CT, should not be considered a single homogeneous system (21).
In conclusion, awareness that osteoporosis has its antecedents in childhood and the increasing demand for examinations of bone acquisition to diagnose and manage multiple pediatric diseases stress the need for reliable standards of bone measurements for children. For 2 decades, determinations of bone densitometry have relied on DXA. Although DXA values are commonly used to diagnose osteoporosis and predict fracture risk in the elderly, inaccuracies that result from growth-related variations in body composition limit their value in pediatric studies. This report of normative data for quantitative CT measures of vertebral TBD in a large pediatric population may help improve the diagnosis, prevention, and treatment of bone disorders in children.
Advance in Knowledge.
This study provides normative reference values for vertebral trabecular bone density obtained by using quantitative CT in healthy children, teenagers, and young adults of both sexes.
Implication for Patient Care.
Providing reference standards for quantitative CT bone measurements in children and young adults will aid in the diagnosis, prevention, and treatment of pediatric metabolic bone disorders.
Acknowledgments
The authors express their gratitude to Cara L. Wah, BBA, for her comments and technical assistance with this article.
Received January 30, 2008; revision requested April 5; revision received April 23; accepted May 30; final version accepted June 16.
Authors stated no financial relationship to disclose.
Funding: This work was supported by the National Institutes of Health (grants N01-HD0333, 1R01 AR-052744, 2R01 EB000298, 1R21 AR51564, R01 AR41853, 1R01 LM06270).
Supported by National Institutes of Health grants N01-HD0333, 1R01 AR-052744, 2R01 EB000298, 1R21 AR51564, R01 AR41853, and 1R01 LM06270; Department of the Army grant DAMD17-01-1-0817; the Gerber Foundation; and GlaxoSmithKline.
Abbreviations:
- DXA
- dual-energy x-ray absorptiometry
- TBD
- trabecular bone density
References
- 1.Mora S, Gilsanz V.Establishment of peak bone mass. Endocrinol Metab Clin North Am 2003;32:39–63 [DOI] [PubMed] [Google Scholar]
- 2.Fares JE, Choucair M, Nabulsi M, Salamoun M, Shahine CH, Fuleihan Gel-H.Effect of gender, puberty, and vitamin D status on biochemical markers of bone remodeling. Bone 2003;33:242–247 [DOI] [PubMed] [Google Scholar]
- 3.Stewart TL, Ralston SH.Role of genetic factors in the pathogenesis of osteoporosis. J Endocrinol 2000;166:235–245 [DOI] [PubMed] [Google Scholar]
- 4.Root AW.Bone strength and the adolescent. Adolesc Med 2002;13:53–72, vi [PubMed] [Google Scholar]
- 5.Genant HK, Engelke K, Fuerst T, et al. Noninvasive assessment of bone mineral and structure: state of the art. J Bone Miner Res 1996;11:707–730 [DOI] [PubMed] [Google Scholar]
- 6.Gafni RI, Baron J.Overdiagnosis of osteoporosis in children due to misinterpretation of dual-energy x-ray absorptiometry (DEXA). J Pediatr 2004;144:253–257 [DOI] [PubMed] [Google Scholar]
- 7.Carter DR, Bouxsein ML, Marcus R.New approaches for interpreting projected bone densitometry data. J Bone Miner Res 1992;7:137–145 [DOI] [PubMed] [Google Scholar]
- 8.Engelke K, Gluer CC.Quality and performance measures in bone densitometry. I. Errors and diagnosis. Osteoporos Int 2006;17:1283–1292 [DOI] [PubMed] [Google Scholar]
- 9.Formica C, Loro ML, Gilsanz V, Seeman E.Inhomogeneity in body fat distribution may result in inaccuracy in the measurement of vertebral bone mass. J Bone Miner Res 1995;10:1504–1511 [DOI] [PubMed] [Google Scholar]
- 10.Hangartner TN.Influence of fat on bone measurements with dual-energy absorptiometry. Bone Miner 1990;9:71–78 [DOI] [PubMed] [Google Scholar]
- 11.Katzman DK, Bachrach LK, Carter DR, Marcus R.Clinical and anthropometric correlates of bone mineral acquisition in healthy adolescent girls. J Clin Endocrinol Metab 1991;73:1332–1339 [DOI] [PubMed] [Google Scholar]
- 12.Kröger H, Kotaniemi A, Vainio P, Alhava E.Bone densitometry of the spine and femur in children by dual-energy x-ray absorptiometry. Bone Miner 1992;17:75–85 [DOI] [PubMed] [Google Scholar]
- 13.Lewis MK, Blake GM, Fogelman I.Patient dose in dual x-ray absorptiometry. Osteoporos Int 1994;4:11–15 [DOI] [PubMed] [Google Scholar]
- 14.Kalender WA.Effective dose values in bone mineral measurements by photon absorptiometry and computed tomography. Osteoporos Int 1992;2:82–87 [DOI] [PubMed] [Google Scholar]
- 15.Cann CE, Genant HK.Precise measurement of vertebral mineral content using computed tomography. J Comput Assist Tomogr 1980;4:493–500 [DOI] [PubMed] [Google Scholar]
- 16.Hangartner TN, Gilsanz V.Evaluation of cortical bone by computed tomography. J Bone Miner Res 1996;11:1518–1525 [DOI] [PubMed] [Google Scholar]
- 17.Hangartner TN, Overton TR.Quantitative measurement of bone density using gamma-ray computed tomography. J Comput Assist Tomogr 1982;6:1156–1162 [DOI] [PubMed] [Google Scholar]
- 18.Rüegsegger P, Elsasser U, Anliker M, Gnehm H, Kind H, Prader A.Quantification of bone mineralization using computed tomography. Radiology 1976;121:93–97 [DOI] [PubMed] [Google Scholar]
- 19.Bachrach LK, Hastie T, Wang MC, Narasimhan B, Marcus R.Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. J Clin Endocrinol Metab 1999;84:4702–4712 [DOI] [PubMed] [Google Scholar]
- 20.Specker BL, Schoenau E.Quantitative bone analysis in children: current methods and recommendations. J Pediatr 2005;146:726–731 [DOI] [PubMed] [Google Scholar]
- 21.Grampp S, Jergas M, Lang P, et al. Quantitative CT assessment of the lumbar spine and radius in patients with osteoporosis. AJR Am J Roentgenol 1996;167:133–140 [DOI] [PubMed] [Google Scholar]
- 22.Cann CE.Why, when and how to measure bone mass: a guide for the beginning user. In: Frey GD, Yester MV, eds. Expanding the role of medical physics in nuclear medicine. Washington, DC: American Physics Institute, 1991;250–279. [Google Scholar]
- 23.Link TM, Koppers BB, Licht T, Bauer J, Lu Y, Rummeny EJ.In vitro and in vivo spiral CT to determine bone mineral density: initial experience in patients at risk for osteoporosis. Radiology 2004;231:805–811 [DOI] [PubMed] [Google Scholar]
- 24.Theodorou DJ, Theodorou SJ, Andre MP, Kubota D, Weigert JM, Sartoris DJ.Quantitative computed tomography of spine: comparison of three-dimensional and two-dimensional imaging approaches in clinical practice. J Clin Densitom 2001;4:57–62 [DOI] [PubMed] [Google Scholar]
- 25.Gilsanz V.Quantitative computed tomography. In: Siegel M, ed. Pediatric body CT. New York, NY: Churchill Livingstone, 1988;349–369. [Google Scholar]
- 26.Lewiecki EM, Watts NB, McClung MR, et al. Official positions of the international society for clinical densitometry. J Clin Endocrinol Metab 2004;89:3651–3655 [DOI] [PubMed] [Google Scholar]
- 27.Genant HK, Lang TF, Engelke K, et al. Advances in the noninvasive assessment of bone density, quality, and structure. Calcif Tissue Int 1996;59(suppl 1):S10–S15 [DOI] [PubMed] [Google Scholar]
- 28.Kovanlikaya A, Loro ML, Hangartner TN, Reynolds RA, Roe TF, Gilsanz V.Osteopenia in children: CT assessment. Radiology 1996;198:781–784 [DOI] [PubMed] [Google Scholar]
- 29.Kanis JA.Osteoporosis and osteopenia. J Bone Miner Res 1990;5:209–211 [DOI] [PubMed] [Google Scholar]
- 30.Boskey AL, Posner AS.Bone structure, composition, and mineralization. Orthop Clin North Am 1984;15:597–612 [PubMed] [Google Scholar]
- 31.Gilsanz V, Gibbens DT, Roe TF, et al. Vertebral bone density in children: effect of puberty. Radiology 1988;166:847–850 [DOI] [PubMed] [Google Scholar]
- 32.Gilsanz V, Roe TF, Mora S, Costin G, Goodman WG.Changes in vertebral bone density in black girls and white girls during childhood and puberty. N Engl J Med 1991;325:1597–1600 [DOI] [PubMed] [Google Scholar]
- 33.Arabi A, Nabulsi M, Maalouf J, et al. Bone mineral density by age, gender, pubertal stages, and socioeconomic status in healthy Lebanese children and adolescents. Bone 2004;35:1169–1179 [DOI] [PubMed] [Google Scholar]
- 34.Gilsanz V, Kovanlikaya A, Costin G, Roe TF, Sayre J, Kaufman F.Differential effect of gender on the size of the bones in the axial and appendicular skeletons. J Clin Endocrinol Metab 1997;82:1603–1607 [DOI] [PubMed] [Google Scholar]
- 35.Gilsanz V, Boechat MI, Roe TF, Loro ML, Sayre JW, Goodman WG.Gender differences in vertebral body sizes in children and adolescents. Radiology 1994;190:673–677 [DOI] [PubMed] [Google Scholar]
- 36.Marcell AV.Adolescence. In: Kliegman RM, Jenson HB, Behrman RE, Stanton BF, eds. Nelson textbook of pediatrics. 18th ed. Philadelphia, Pa: Saunders Elsevier, 2007;60–65. [Google Scholar]
- 37.Kleerekoper M, Tolia K, Parfitt AM.Nutritional, endocrine, and demographic aspects of osteoporosis. Orthop Clin North Am 1981;12:547–558 [PubMed] [Google Scholar]
- 38.Dyson ED, Jackson CK, Whitehouse WJ.Scanning electron microscope studies of human trabecular bone. Nature 1970;225:957–959 [DOI] [PubMed] [Google Scholar]
- 39.Gilsanz V.Accumulation of bone mass during childhood and adolescence. In: Orwoll E, ed. Osteoporosis in men San Diego, Calif: Academic Press, 1999; 65–85 [Google Scholar]