Abstract
Recently some have called for randomized controlled trials comparing RFA to hepatic resection, particularly for patients with only a few small metastases. The objectives were to compare local recurrence and survival following RFA and hepatic resection for colorectal liver metastases. This was a retrospective review of open RFA and hepatic resection for colorectal liver metastases between January 1998 and May 2007. All patients who had RFA were considered to have unresectable disease. 58 patients had hepatic resection and 43 had RFA. A 5-year survival after resection was 43% compared to 23% after RFA. For patients with solitary lesions, a 5-year survival was 48% after resection and 15% after RFA. Sixty percent of patients suffered local recurrences after RFA compared to 7% after hepatic resection. RFA is inferior to resection. The results observed in this study support the consensus that RFA cannot be considered an equivalent procedure to hepatic resection.
1. Introduction
Hepatic resection is a major surgical intervention with significant potential complications risk. Much effort has been placed on developing regional ablative techniques such as radiofrequency ablation (RFA) [1, 2] in hopes of achieving similar survival as with hepatic resection, but with less morbidity. However, the outcomes following RFA have not been firmly established. While some authors have reported that local recurrence rates with RFA are not significantly different than those with anatomic or wedge resections of the liver [3], the current literature reports a wide range of local recurrence rates for colorectal liver metastases treated with RFA. These rates range from 5% [4] to as high as 39% [5, 6]. Reports of low recurrence rates may be a function of patient selection (i.e., small lesions) or short follow-up in some instances, and many series have combined patients with primary and secondary hepatic malignancies. To date, long-term survival remains difficult to interpret. In all these studies, patients were considered to have unresectable disease, which limits comparisons to patients undergoing hepatic resection.
Several studies have reported favorable survival rates and this has prompted some authors to call for randomized controlled trials comparing RFA to hepatic resection [7–9]. Other results have been much less optimistic [10]. An updated report from the same center [11] showed that with longer follow-up, the proportion of patients treated with RFA that had recurrence at the ablation site had risen from 9% to 37%. Furthermore, the 5-year overall survival for patients who had RFA was significantly inferior to those who had resection, even though patient characteristics and the proportion of patients receiving adjuvant chemotherapy were similar between groups.
The effectiveness of RFA remains controversial, and in the absence of randomized studies several questions remain. Patient selection is an obvious concern and publication bias may be present.
The hypothesis of this study is that treatment with RFA leads to significantly higher recurrence rates and decreased survival compared to surgical resection. If this is confirmed, then this will provide important evidence that will be helpful in guiding treatment decisions for patients with potentially resectable colorectal liver metastases.
The objectives of this study are to report the local recurrence rates and overall survival rates following open RFA and following hepatic resection for the treatment of colorectal liver metastases in the Province of Manitoba.
2. Materials and Methods
2.1. Study Design
This was a retrospective review of the outcomes of RFA and hepatic resection for colorectal liver metastases. The primary outcome measures were recurrence rates and overall survival. The study was approved by the Health Research Ethics Board at the University of Manitoba.
2.2. Subjects
The study was conducted from January 1998, the year when RFA first became available in Manitoba, until June 2007. All patients who underwent open RFA and/or surgical resection for colorectal liver metastases at either Health Sciences Centre (HSC) or St. Boniface General Hospital (SBGH), the two University-affiliated teaching hospitals in the Province, were included in the study. All major hepatic surgery in the Province was performed at these two hospitals; thus, it was a population-based study. The population of the Province is just over 1.1 million people and the catchment area of the two hospitals is slightly larger than that. Patients received systemic chemotherapy at CancerCare Manitoba (CCMB), an outpatient oncology centre for the province.
2.3. Procedure
All patients had previously underwent hepatic resection or RFA. All patients who had hepatic resection first had intraoperative ultrasound performed. In the early years of the study period, parenchymal transection was done with the “clamp-crush” method and with the Cavitron Ultrasonic Surgical Aspirator (CUSA; Integra Life Sciences).
All patients who underwent RFA were considered to have unresectable disease. Due to the retrospective nature of this analysis, it is not possible to determine the exact reason why patients were considered to have unresectable disease in each case. However, in general patients were considered to have unresectable disease because of extensive disease that would result in an insufficient liver remnant, proximity to critical structures, prohibitive comorbidity, or patient refusal. RFA was performed as an open procedure in all cases. An open approach was chosen over a percutaneous approach because of the added benefit of intraoperative ultrasound to discover unsuspected disease [12, 13], the potentially lower recurrence with an open technique [14], the ability to protect adjacent structures, and the ability to perform simultaneous resection in select cases. RFA was done under real-time ultrasound guidance using the RF 3000 Radiofrequency Ablation System (Boston Scientific) with either a 3 cm or 4 cm probe. The RFA tract was routinely ablated as the probe was withdrawn.
Systemic chemotherapy was administered at the discretion of the medical oncologists. Towards the end of the study period, chemotherapy was generally offered to all patients who were medically fit. Towards the end of the study period chemotherapy usually consisted of 5-fluourouracil and leucovorin in combination with either irinotecan or oxaliplatin, but a variety of regimens were used.
Eligible patients were later identified from the Medical Records Departments of both HSC and SBGH. The hospital charts and the outpatient charts at CCMB were then reviewed for demographic information, patient factors including comorbidities, pathological features of the primary tumors, dates of diagnoses, and the treatments received. The characteristics of the tumors including the number, distribution, and size of lesions were analyzed along with the nature and extent of the operative procedures. The outpatient charts were reviewed for dates and locations of disease recurrences. The Manitoba Health Population Registry is an administrative database belonging to Manitoba Health (the government agency that provides health insurance for all Manitobans). It lists up-to-date vital statistic, migration and loss to follow-up information for all people living in the Province of Manitoba, and was accessed in order to obtain the most accurate survival information possible.
Postoperative morbidity was graded according to a previously validated classification system [15]. Mortality was defined as either 30-day mortality or in-hospital mortality if patients died in hospital beyond 30 days. This is because mortality from postoperative liver failure in major hepatic surgery may occur well beyond 30 days [16], which is a commonly used end-point.
The outcomes for patients who underwent RFA, RFA plus hepatic resection, and hepatic resection were reported. The analysis focused mainly on comparing those who underwent hepatic resection to those who underwent RFA. Although, it was hypothesized that the limiting factor in terms of survival and recurrence in those who underwent simultaneous RFA and resection would be the effectiveness of the RFA, this group was excluded from the analyses to prevent any confounding effect. A subgroup analysis comparing outcomes of patients with solitary metastases who underwent RFA to those who underwent hepatic resection was planned a priori.
2.4. Sample Size
It was planned to include all eligible patients treated during the study period. A sample size calculation was performed to verify that the sample would be adequately powered for our primary objective of assessing local recurrence. It was anticipated that the local recurrence rate of lesions treated by RFA would be approximately 40%, while the recurrence rate for lesions treated by resection would be under 10% [11]. With a P-value of .05, 32 patients in each group were needed to detect this difference with a power of .80. The number of eligible patients in each group considerably exceeded this number, so the study was more than adequately powered to detect this endpoint.
2.5. Statistical Analysis
Overall survival and disease-free survival were calculated from the date of surgical intervention. Continuous variables were analyzed with Student's t test, and categorical variables were analyzed with a chi-square or Fisher's Exact Test where appropriate. Survival and recurrence rates were calculated using the Kaplan-Meier method and comparisons between groups were done with the logrank test. Predictors of overall and disease-free survival were analyzed by performing a Cox Proportional Hazards regression model using a backwards selection process. A P-value of .05 was used to define statistical significance.
3. Results
3.1. Patients
During the study period, 58 patients underwent hepatic resection, 43 underwent RFA, and 12 underwent simultaneous hepatic resection and RFA for colorectal liver metastases. Table 1 shows the patient characteristics in each group. Three patients were lost to follow-up, either because they lived out of province or moved out of province during the study period. The mean and median follow-up duration for all patients was 38 months and 33 months, respectively. The median follow-up duration for those who had resection, RFA, and both resection and RFA was 25 months (range 4 to 106), 42 months (range 15 to 85), and 20 months (range 15 to 45), respectively. There were no significant differences between groups.
Table 1.
Overall patient characteristics.
| All patients | Resection | RFA | Both | P-value (Res versus RFA)* | ||
|---|---|---|---|---|---|---|
|
| ||||||
| n | 113 | 58 | 43 | 12 | ||
| Age | Median | 67 | 67 | 67 | 63 | NS |
| Range | 28 to 83 | 28 to 83 | 37 to 83 | 45 to 82 | ||
| Gender | M | 61 | 29 | 25 | 7 | NS |
| F | 52 | 29 | 18 | 5 | ||
| Primary site | Colon | 80 | 44 | 29 | 7 | NS |
| Rectal | 33 | 14 | 14 | 5 | ||
| ASA score | Median | 2 | 2 | 2 | 2 | NS |
| Range | 1 to 3 | 1 to 3 | 2 to 3 | 2 to 3 | ||
| Timing of primary | Synchronous | 51 | 24 | 21 | 6 | NS |
| Metachronous | 62 | 34 | 22 | 6 | ||
| Node Pos primary† | Yes | 69 | 33 | 27 | 9 | NS |
| No | 34 | 20 | 13 | 1 | ||
| No. lesions | Median | 1 | 1 | 2 | 3.5 | NS |
| Range | 1 to 7 | 1 to 7 | 1 to 6 | 1 to 5 | ||
| Solitary lesion | Yes | 58 | 37 | 19 | 0 | <.001 |
| No | 54 | 20 | 24 | 12 | ||
| Size (cm) | Median | 4 | 4.1 | 3 | 4.8 | .012 |
| Range | 1 to 14.5 | 1.5 to 14.5 | 1 to 7.5 | 1.2 to 7 | ||
| Bilateral disease† | Yes | 22 | 2 | 13 | 7 | <.001 |
| No | 31 | 17 | 11 | 5 | ||
| Preop CEA (mg/L)† | Median | 18.1 | 24 | 18.1 | 6.1 | NS |
| Range | 0 to 699 | 0 to 279 | 1 to 699 | 2 to 58 | ||
*P-value for comparison between patients undergoing resection alone compared to those undergoing RFA alone.
†There are missing values for some patients for the marked variables.
3.2. Procedure and Complications
Sixty-eight percent of patients in the group who had liver resection alone had major resections (a lobectomy or greater). The operative time was significantly longer for the resection group compared to the RFA group (median of 269 minutes (range 118 to 452) versus 204 minutes (range 113 to 316); P < .0005). Operative blood loss (median 1400 mL (range 100 to 9000) versus 150 mL (range 50 to 2300); P < .0005) and transfusion requirements (44% of patients versus 5%; P < .0005) were higher for resection than for RFA. Rates of ICU admission (6.5% of patients overall), and length of stay (median of 7 days for all patients; range 1 to 48) were not significantly different between patients having hepatic resection and RFA. More patients developed complications with resection compared to RFA (59% compared to 43%), but the difference was not statistically significant. Overall, 32% of all complications were major (Grade III or higher), and there was no difference between groups. There was only one postoperative death, which occurred in a patient who underwent RFA alone.
3.3. Overall Survival
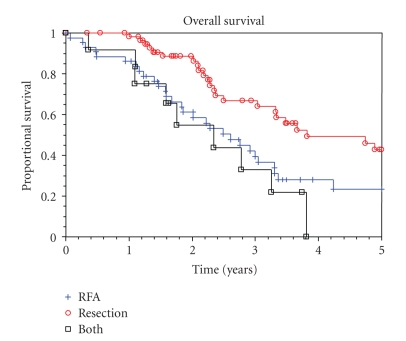
The median survival for patients who had resection, RFA, and resection in combination with RFA of their CRC metastases was 3.8 years (95% CI = 3.0 to 5.9 years), 2.6 years (95% CI = 1.8 to 3.3 years), and 2.3 (95% CI = 1.6 to 3.2 years), respectively. The 5-year overall survival after resection alone was 43% (95% CI = 26 to 58%), while the 5-year survival after RFA alone was 23% (95% CI = 11 to 39%), as shown in Figure 1. This difference was statistically significant (P = .02). When comparing the overall survival in patients who underwent surgical resection to those who had RFA alone, the following variables were significant on univariate analysis (see Table 2): which procedure was performed, age (less than 70 versus 70 or greater), size of metastasis (under 5 cm versus 5 cm or greater), number of lesions (less than 5 versus 5 or more), and the timing of the lesion (patients with synchronous lesions had better survival compared to patients with metachronous disease). In the multivariate analysis, age was no longer a significant predictor of survival. The other variables remained significant (Table 3). The majority of patients (67%) received postoperative chemotherapy, but this was not significantly associated with increased survival.
Figure 1.
Overall survival.
Table 2.
Results of univariate analysis of predictors of overall survival.
| Med OS (Yr) | 5-yr OS (%) | Hazard ratio | 95% confidence interval | P-value | |||
|---|---|---|---|---|---|---|---|
| Procedure | RFA | 2.6 | 23 | 1.00 | |||
| Resection | 3.8 | 43 | 0.54 | 0.32 | .91 | .021 | |
| Age | <70 | 3.5 | 38 | 1.00 | |||
| ≥70 | 2.5 | 26 | 1.81 | 1.08 | 3.05 | .025 | |
| Gender | Male | 3.1 | 30 | 1.00 | |||
| Female | 3.4 | 38 | 0.87 | 0.51 | 1.51 | .630 | |
| Hospital | HSC | 3.3 | 30 | 1.00 | |||
| SBGH | 3.3 | 40 | 1.04 | 0.60 | 1.78 | .900 | |
| Primary tumor | Colon | 3.3 | 36 | 1.00 | |||
| Rectum | 2.5 | 28 | 1.13 | 0.63 | 2.00 | .660 | |
| Node positive primary | No | 3.1 | 44 | 1.00 | |||
| Yes | 3.3 | 30 | 0.97 | 0.54 | 1.72 | .910 | |
| Timing of lesion(s) | Synchronous | 3.8 | 50 | 1.00 | |||
| Metachronous | 2.4 | 19 | 1.99 | 1.17 | 3.39 | .012 | |
| Bilateral disease | Yes | 2.4 | 36 | 1.00 | |||
| No | 3.3 | 32 | 0.78 | 0.40 | 1.57 | .460 | |
| No. lesions | <5 | 3.3 | 37 | 1.00 | |||
| ≥5 | 2.1 | 0 | 2.93 | 1.30 | 6.60 | .009 | |
| Size of lesion(s) | <5 cm | 3.5 | 44 | 1.00 | |||
| ≥5 cm | 2.5 | 11 | 1.85 | 1.09 | 3.17 | .024 | |
| Postoperative chemo | Yes | 3.0 | 24 | 1.00 | |||
| No | 3.3 | 39 | 0.85 | 0.49 | 1.45 | .540 | |
Table 3.
Results of multivariate Cox Proportional Hazards regression model of predictors of overall survival.
| Hazard ratio | 95% confidence | P-value | |||
|---|---|---|---|---|---|
| interval | |||||
| Procedure | RFA | 1.00 | |||
| Resection | 0.36 | 0.19 | 0.70 | .002 | |
| Size of lesion(s) | <5 cm | 1.00 | |||
| ≥5 cm | 2.43 | 1.26 | 4.67 | .008 | |
| No. lesions | <5 | 1.00 | |||
| ≥5 | 6.08 | 2.21 | 16.70 | <.001 | |
| Timing of lesion(s) | Synchronous | 1.00 | |||
| Metachronous | 2.92 | 1.50 | 5.70 | .002 | |
Thirty-two patients underwent repeat surgical procedures for recurrent disease in their liver and/or lungs. Seven patients underwent a subsequent hepatic resection and 13 patients underwent a subsequent RFA procedure. Three of the patients who underwent an additional RFA for recurrence also underwent a pulmonary resection for metastases. Another 12 patients underwent pulmonary resections without repeat liver procedures.
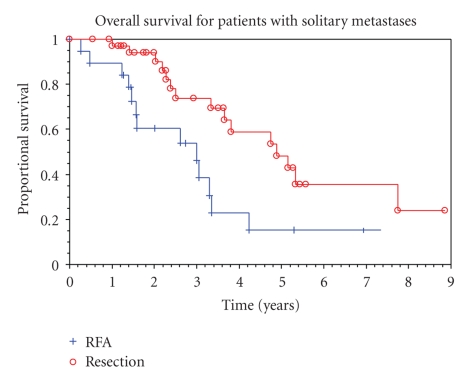
A preplanned subgroup analysis was performed for patients with solitary lesions treated by resection versus RFA. The median overall survival times in this subset for resection and for RFA were 4.9 years (95% CI = 3.7 to 7.7 years) and 3.0 years (95% CI 1.6 to 3.4 years), respectively. The 5-year survival rates were 48% (95% CI = 26 to 67%) and 15% (95% CI = 2.6 to 38%), respectively. The overall survival for patients with solitary liver lesions treated by resection compared to RFA is shown in Figure 2. The size of the solitary lesion (under 5 cm versus 5 cm or greater) and the procedure performed were independent predictors of overall survival (Table 4). Even when limited to solitary lesions less than 3 cm in diameter, the survival associated with resection was significantly greater than with RFA.
Figure 2.
Overall survival for patients with solitary metastases.
Table 4.
Results of multivariate Cox Proportional Hazards regression model of predictors of overall survival in patient with solitary liver metastases.
| Hazard ratio | 95% confidence | P-value | |||
|---|---|---|---|---|---|
| interval | |||||
| Procedure | RFA | 1.00 | |||
| Resection | 0.38 | 0.18 | 0.81 | .013 | |
| Size of lesion(s) | <5 cm | 1.00 | |||
| ≥5 cm | 3.06 | 1.43 | 6.55 | .004 | |
| Timing of Lesion(s) | Synchronous | 1.00 | |||
| Metachronous | 3.36 | 1.39 | 8.14 | .007 | |
3.4. Disease-Free Survival and Recurrence
The median times to recurrence for patients who had hepatic resection, RFA, and resection plus RFA were 11 months (range 2 to 49), 7 months (range 1 to 26), and 8 months (range 2 to 30), respectively. The 5-year disease-free survival (DFS) for patients who underwent hepatic resection was 17% (95% CI = 7 to 29%), compared to 15% (95% CI = 6 to 28%) for patients who underwent RFA alone (P = .06). In a multivariate regression the procedure performed, the size of lesion, the number of lesions, and the hospital where the surgery was performed were independent predictors of disease-free survival.
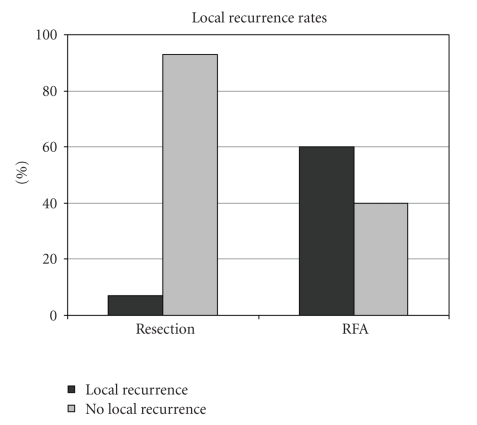
The rates of local recurrence were dramatically different (Figure 3). Over the course of the study (mean follow-up of 46 months for patients undergoing RFA), 60% of patients who had only open RFA suffered local recurrences compared to 7% of patients who underwent hepatic resection (P < .0005). Over the study period, the local recurrence rate dropped, and in the last 4 years of the study period it was 43%. The local recurrence rate in 10 patients with small (3 cm or smaller), solitary lesions treated by RFA was still 50%.
Figure 3.
Local recurrence rates.
4. Discussion
This is a population-based study reporting the experience with RFA and hepatic resection in the Province of Manitoba, Canada. By accessing provincial vital statistics information from the Government, the survival figures in this study are thought to be quite accurate. The 5-year survival was 43% (95% CI = 26% to 58%) and the median survival was 3.8 years (95% CI = 3.0 to 5.9 years) following hepatic resection in this study. This generally compares favorably to other reports in the literature [17–19], suggesting that these results are generalizable. The number of metastases and the size of metastases were found to be independent predictors of survival, as seen in other reports [17]. Chemotherapy use was not standardized in the study, but was not found to be a predictor of increased survival on univariate or multivariate analysis.
The unexpectedly high local recurrence rate with RFA is an alarming finding. With a median follow-up of 42 months, patients who underwent RFA alone had a local recurrence rate of 60%. This is higher than what is reported elsewhere in the literature and may be due to several causes. Firstly, the median length of follow-up in this study is longer than most other reports. Other studies report recurrence rates from 6% to 39% with follow-up ranging from 6 to 28 months [3, 4, 6, 7, 10, 20–28]. Abdalla et al. reported a local recurrence rate of 9% after RFA for colorectal liver metastases with a follow-up of 21 months [10]. When they updated their experience with a subgroup of patients with solitary lesions receiving RFA, the local recurrence rate rose to 37% when the follow-up had lengthened to a median of 31 months [11]. Perhaps with longer follow-up, the local recurrence rate in their series may have been even higher.
Another reason for this high local recurrence rate is that this study represents the initial experience with this technology in the Province of Manitoba. Part of the high rate may be due to a learning curve, which seems to be present over the first 40 to 50 cases [14, 29, 30]. Furthermore, the selection criteria over the time frame of this study changed. In the early years of the study, lesions as large as 6 to 7.5 cm in diameter were being ablated. Treatment of such large lesions is associated with very high recurrence [5, 14, 31] and most centers would restrict RFA to smaller lesions. In fact, there was a trend to ablation of smaller lesions over the course of the study, but the trend was not statistically significant (P = .077). Similarly, there was a nonsignificant trend to treat lower number of lesions per single patient over time in the present study, since a high number of lesions are a risk factor for local recurrence [2]. Both the number and size of metastases were significant predictors of overall survival and disease-free survival in the multivariate analyses in this study. Patients with lesions over 5 cm in size and/or with more than 4 or 5 lesions would generally no longer be treated by RFA in Manitoba. In addition, many patients were considered to have unresectable disease due to proximity to critical vascular structures, which may have acted as a “heat-sink.” A more aggressive surgical approach has been adopted in the recent years and it is likely that many such patients would now undergo resection. It is likely that with more experience and improvements in selection criteria, the recurrence rates will fall in the future. Towards the end of the study period, the local recurrence rate did drop to 43%, which is much closer to what has been reported from other larger centers [11, 32]. This is likely due to more prudent patient selection, although the shorter follow-up for these later patients may also play a role.
There are other reports of very high local recurrence following RFA of colorectal liver metastases in the recent literature as well. In one of the largest series, Berber and Siperstein [32] recently reported a local recurrence rate for colorectal lesions of 34% with a median of 12 months of follow-up. Other reports suggest the recurrence rate may be close to 40% [5, 6, 11]. Therefore, while a local recurrence rate of 60% seems inappropriately high, the changes in patient selection and techniques that have evolved are not expected to reduce recurrence to the range seen following hepatic resection.
RFA was associated with worse survival and recurrence compared to hepatic resection in all analyses in this study. Others have also shown inferior survival with RFA compared to resection. In a series of 418 patients with colorectal metastases, Abdalla et al. [10] reported a 4-year survival following hepatic resection of 65%. This was significantly higher than the 36% 4-year survival following resection plus RFA and the 22% 4-year survival following RFA alone.
Some studies have shown more promising results. In a series of 45 consecutive patients with solitary colorectal liver metastases Oshowo et al. [8] reported almost identical 3-year survival following RFA compared to resection (53% and 55%, resp.). A recent report from Berber and Siperstein [33] described their experience with 158 patients who underwent laparoscopic RFA and 90 patients who underwent open resection of solitary colorectal liver metastases. The actual 5-year survival was 30% for RFA and 40% for resection, which was not statistically different. However, the 3-year survival for RFA in patients without extrahepatic disease was 35% compared to 70% for those who had liver resection.
All patients in the current series and in the other series listed above who underwent RFA were considered to have unresectable disease, and consequently there must be some selection bias present. It is very likely that there were inherent differences in the biology and aggressiveness of the tumors' behavior between the two groups. Because the study is retrospective, it is impossible to completely control for these differences. In this study patients who underwent RFA more often had multiple lesions and bilateral lesions. In addition, RFA is offered more commonly to patients with multiple medical comorbidities, which may also bias survival in favor of resection. These fundamental differences will always be a limitation in interpreting the results of such studies that show inferior results with RFA in patients with unresectable disease compared to hepatic resection in patients with resectable disease. A randomized trial with very strict inclusion criteria would be needed to eliminate this weakness [7–9]. However, the high local recurrence rates following RFA observed in this study and others [10, 11] are difficult to ignore. Even with solitary lesions less than 3 cm in diameter, the recurrence rate with hepatic resection appears to be superior to that of RFA.
5. Conclusions
With the 0% operative mortality rate for hepatic resection in this study and the very low mortality reported by others in the literature [17, 18, 34], the improved safety of liver surgery is well established. Until current RFA technology improves or alternative ablation technology is developed with much improved local recurrence rates, it would be very difficult to support a randomized trial. The results observed in this study support the consensus that RFA cannot be considered an equivalent procedure to hepatic resection. Resection must be considered the standard of care for colorectal metastases.
Acknowledgments
This paper was presented as a poster at the American HepatoPancreatoBiliary Association's 2009 Annual Meeting in Miami, Florida. The results and conclusions presented are those of the authors. No official endorsement by Manitoba Health and Healthy Living is intended or should be inferred.
Abbreviations
- RFA:
Radiofrequency Ablation
- HSC:
Health Sciences Centre
- SBGH:
St. Boniface General Hospital
- CCMB:
CancerCare Manitoba.
References
- 1.Yoon SS, Tanabe KK. Surgical treatment and other regional treatments for colorectal cancer liver metastases. Oncologist. 1999;4(3):197–208. [PubMed] [Google Scholar]
- 2.Curley SA. Radiofrequency ablation of malignant liver tumors. Oncologist. 2001;6(1):14–23. doi: 10.1634/theoncologist.6-1-14. [DOI] [PubMed] [Google Scholar]
- 3.Elias D, Baton O, Sideris L, Matsuhisa T, Pocard M, Lasser P. Local recurrences after intraoperative radiofrequency ablation of liver metastases: a comparative study with anatomic and wedge resections. Annals of Surgical Oncology. 2004;11(5):500–505. doi: 10.1245/ASO.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 4.Iannitti DA, Dupuy DE, Mayo-Smith WW, Murphy B. Hepatic radiofrequency ablation. Archives of Surgery. 2002;137(4):422–427. doi: 10.1001/archsurg.137.4.422. [DOI] [PubMed] [Google Scholar]
- 5.Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221(1):159–166. doi: 10.1148/radiol.2211001624. [DOI] [PubMed] [Google Scholar]
- 6.White TJ, Roy-Choudhury SH, Breen DJ, et al. Percutaneous radiofrequency ablation of colorectal hepatic metastases—initial experience: an adjunct technique to systemic chemotherapy for those with inoperable colorectal hepatic metastases. Digestive Surgery. 2004;21(4):314–320. doi: 10.1159/000080886. [DOI] [PubMed] [Google Scholar]
- 7.Gillams AR, Lees WR. Radiofrequency ablation of colorectal liver metastases. Abdominal Imaging. 2005;30(4):419–426. doi: 10.1007/s00261-004-0256-6. [DOI] [PubMed] [Google Scholar]
- 8.Oshowo A, Gillams A, Harrison E, Lees WR, Taylor I. Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. British Journal of Surgery. 2003;90(10):1240–1243. doi: 10.1002/bjs.4264. [DOI] [PubMed] [Google Scholar]
- 9.Mulier S, Ni Y, Jamart J, Michel L, Marchal G, Ruers T. Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? Annals of Surgical Oncology. 2008;15(1):144–157. doi: 10.1245/s10434-007-9478-5. [DOI] [PubMed] [Google Scholar]
- 10.Abdalla EK, Vauthey J-N, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Annals of Surgery. 2004;239(6):818–827. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aloia TA, Vauthey J-N, Loyer EM, et al. Solitary colorectal liver metastasis: resection determines outcome. Archives of Surgery. 2006;141(5):460–467. doi: 10.1001/archsurg.141.5.460. [DOI] [PubMed] [Google Scholar]
- 12.Conlon R, Jacobs M, Dasgupta D, Lodge JPA. The value of intraoperative ultrasound during hepatic resection compared with improved preoperative magnetic resonance imaging. European Journal of Ultrasound. 2003;16(3):211–216. doi: 10.1016/s0929-8266(02)00075-7. [DOI] [PubMed] [Google Scholar]
- 13.Zacherl J, Scheuba C, Imhof M, et al. Current value of intraoperative sonography during surgery for hepatic neoplasms. World Journal of Surgery. 2002;26(5):550–554. doi: 10.1007/s00268-001-0266-2. [DOI] [PubMed] [Google Scholar]
- 14.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Annals of Surgery. 2005;242(2):158–171. doi: 10.1097/01.sla.0000171032.99149.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of Surgery. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagino M, Kamiya J, Nishio H, Ebata T, Arai T, Nimura Y. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Annals of Surgery. 2006;243(3):364–372. doi: 10.1097/01.sla.0000201482.11876.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Annals of Surgery. 1999;230(3):309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Annals of Surgery. 2002;235(6):759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belli G, D’Agostino A, Ciciliano F, Fantini C, Russolillo N, Belli A. Liver resection for hepatic metastases: 15 years of experience. Journal of Hepato-Biliary-Pancreatic Surgery. 2002;9(5):607–613. doi: 10.1007/s005340200082. [DOI] [PubMed] [Google Scholar]
- 20.Bleicher RJ, Allegra DP, Nora DT, Wood TF, Foshag LJ, Bilchik AJ. Radiofrequency ablation in 447 complex unresectable liver tumors: lessons learned. Annals of Surgical Oncology. 2003;10(1):52–58. doi: 10.1245/aso.2003.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Bowles BJ, Machi J, Limm WML, et al. Safety and efficacy of radiofrequency thermal ablation in advanced liver tumors. Archives of Surgery. 2001;136(8):864–869. doi: 10.1001/archsurg.136.8.864. [DOI] [PubMed] [Google Scholar]
- 22.de Baere T, Elias D, Dromain C, et al. Radiofrequency ablation of 100 metastases with a mean follow-up of more than 1 year. American Journal of Roentgenology. 2000;175(6):1619–1625. doi: 10.2214/ajr.175.6.1751619. [DOI] [PubMed] [Google Scholar]
- 23.Joosten J, Jager G, Oyen W, Wobbes Th, Ruers T. Cryosurgery and radiofrequency ablation for unresectable colorectal liver metastases. European Journal of Surgical Oncology. 2005;31(10):1152–1159. doi: 10.1016/j.ejso.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Kosari K, Gomes M, Hunter D, Hess DJ, Greeno E, Sielaff TD. Local, intrahepatic, and systemic recurrence patterns after radiofrequency ablation of hepatic malignancies. Journal of Gastrointestinal Surgery. 2002;6(2):255–263. doi: 10.1016/s1091-255x(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 25.Machi J, Uchida S, Sumida K, et al. Ultrasound-guided radiofrequency thermal ablation of liver tumors: percutaneous, laparoscopic, and open surgical approaches. Journal of Gastrointestinal Surgery. 2001;5(5):477–489. doi: 10.1016/s1091-255x(01)80085-8. [DOI] [PubMed] [Google Scholar]
- 26.Siperstein A, Garland A, Engle K, et al. Local recurrence after laparoscopic radiofrequency thermal ablation of hepatic tumors. Annals of Surgical Oncology. 2000;7(2):106–113. doi: 10.1007/s10434-000-0106-x. [DOI] [PubMed] [Google Scholar]
- 27.Solbiati L, Goldberg SN, Ierace T, et al. Hepatic metastases: percutaneous radio-frequency ablation with cooled-tip electrodes. Radiology. 1997;205(2):367–373. doi: 10.1148/radiology.205.2.9356616. [DOI] [PubMed] [Google Scholar]
- 28.Solbiati L, Ierace T, Goldberg SN, et al. Percutaneous US-guided radio-frequency tissue ablation of liver metastases: treatment and follow-up in 16 patients. Radiology. 1997;202(1):195–203. doi: 10.1148/radiology.202.1.8988211. [DOI] [PubMed] [Google Scholar]
- 29.Poon RT, Ng KK, Lam CM, et al. Learning curve for radiofrequency ablation of liver tumors: prospective analysis of initial 100 patients in a tertiary institution. Annals of Surgery. 2004;239(4):441–449. doi: 10.1097/01.sla.0000118565.21298.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hildebrand P, Leibecke T, Kleemann M, et al. Influence of operator experience in radiofrequency ablation of malignant liver tumours on treatment outcome. European Journal of Surgical Oncology. 2006;32(4):430–434. doi: 10.1016/j.ejso.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Kuvshinoff BW, Ota DM. Radiofrequency ablation of liver tumors: influence of technique and tumor size. Surgery. 2002;132(4):605–612. doi: 10.1067/msy.2002.127545. [DOI] [PubMed] [Google Scholar]
- 32.Berber E, Siperstein A. Local recurrence after laparoscopic radiofrequency ablation of liver tumors: an analysis of 1032 tumors. Annals of Surgical Oncology. 2008;15(10):2757–2764. doi: 10.1245/s10434-008-0043-7. [DOI] [PubMed] [Google Scholar]
- 33.Berber E, Tsinberg M, Tellioglu G, Simpfendorfer CH, Siperstein AE. Resection versus laparoscopic radiofrequency thermal ablation of solitary colorectal liver metastasis. Journal of Gastrointestinal Surgery. 2008;12(11):1967–1972. doi: 10.1007/s11605-008-0622-8. [DOI] [PubMed] [Google Scholar]
- 34.Taylor M, Forster J, Langer B, Taylor BR, Greig PD, Mahut C. A study of prognostic factors for hepatic resection for colorectal metastases. American Journal of Surgery. 1997;173(6):467–471. doi: 10.1016/s0002-9610(97)00020-2. [DOI] [PubMed] [Google Scholar]