Abstract
Staphylococcus aureus is a known major cause of foodborne illnesses, and milk and dairy products are often contaminated by enterotoxigenic strains of this bacterium. In the present study, 122 S. aureus isolates collected from different dairy products were characterised by phenotypic properties, by the distribution of genes encoding staphylococcal enterotoxins (sea, sec, sed, seg, seh, sei, sej, and sel) and by randomly amplified polymorphic DNA PCR (RAPD-PCR). Moreover, strain resistance to vancomycin and methicillin (oxacillin) was studied. The differences in the RAPD-PCR profiles obtained with the primers M13 and AP4 revealed the presence of a great genetic heterogeneity among the different S. aureus strains. Using the primer AP4 and M13, eight groups were distinguished by RAPD-PCR cluster analysis, although, except in few cases, it was not possible to correlate the isolates of different animal species (cow or ovine) with the presence of se genes. None of the isolates showed resistance to vancomycin or methicillin.
1. Introduction
Staphylococcus aureus is an important food-borne pathogen involved in a variety of invasive diseases. Of particular relevance is the ability of some S. aureus strains to produce heat stable enterotoxins that cause staphylococcal food poisoning, which ranks as one of the most prevalent worldwide causes of gastroenteritis [1].
Eleven major antigenic types of SEs have been recognised (SEA to SEJ) and their corresponding genes have been reported [2]. Recently, other SE toxins were identified (SEK, SEL, SEM, SEN, SEO, and SEU) and the corresponding genes (se) described [3–5], but their role in food poisoning is not clear.
S. aureus can gain access to milk either by direct excretion from udders with clinical and subclinical staphylococcal mastitis or by environmental contamination during the handling and processing of raw milk [6, 7].
S. aureus is also a frequent cause of human infections which can become especially serious if induced by strains resistant to antimicrobial drugs [8]. In fact, nowadays, antimicrobial resistance has become a major public health problem in many countries due to the constant circulation of resistant bacterial strains in the environment and possible foodstuff contamination. Indeed, it has already been suggested by several authors that the administration of antibiotics to food-producing animals, for therapeutic purposes or as growth promoters, could be a primary selection factor for antimicrobial-resistant bacterial pathogens. Furthermore, S. aureus has been reported to frequently show multiple antimicrobial resistance patterns, particularly to methicillin and vancomycin [9, 10].
Several molecular typing methods have been described in order to obtain an accurate and rapid characterization of S. aureus isolates, such as coagulase (coa) or protein A (spa) restriction fragment length polymorphism (RFLP), Multiple-Locus Variable-Number Tandem-Repeat (MLVA), Pulsed-Field Gel Electrophoresis (PFGE), Multilocus Sequence Typing (MLST), and amplified fragment length polymorphism (AFLP). Random amplification of polymorphic DNA (RAPD PCR) has been applied extensively to distinguish different isolates of S. aureus [11, 12]. However, there is little information on the RAPD method for typing S. aureus strains isolated from dairy products.
In the present study S. aureus strains isolated from different dairy products, collected in various Italian regions, were identified at the species level and characterised at the genetic level by means of RAPD-PCR. The isolates were also evaluated for the presence of enterotoxin genes (sea, sec, sed, seg, seh, sei, sej, and sel) and for phenotypic activities such as the presence of coagulase, thermonuclease, and hemolytic activity. In addition, the S. aureus strains were tested for resistance to methicillin and vancomycin.
2. Material and Methods
2.1. Source of Bacterial Isolates and S. aureus Identification
The study employed a total of 122 S. aureus strains. All the isolates were obtained from the ISPA (Institute of Science of Food Production) bacterial collection and came from different raw milk products (milk, curd, cheeses, butter, and whey) from different Italian regions and animal species. As reported in Table 1, 81 isolates originated from cow, 22 from goat, 17 from sheep, and 2 from buffalo.
Table 1.
Origin of the 122 S. aureus strains examined in this study.
| Sample origin | Source | No strains | Regions |
|---|---|---|---|
| Cow (81 strains) | Raw milk | 29 | Lombardia |
| 16 | Piemonte | ||
| 5 | Emilia Romagna | ||
| 3 | Veneto | ||
| 2 | Valle d'Aosta | ||
| 1 | Trentino Alto Adige | ||
| 1 | Liguria | ||
| 1 | Puglia | ||
| 1 | Calabria | ||
| Curd | 6 | Lombardia | |
| 3 | Piemonte | ||
| Cheese | 4 | Lombardia | |
| 3 | Veneto | ||
| Butter | 5 | Lombardia | |
| 1 | Trentino Alto Adige | ||
| Goat (22 strains) | Raw milk | 17 | Lombardia |
| Cheese | 5 | Lombardia | |
| Sheep (17 strains) | Raw milk | 11 | Sardegna |
| 2 | Sicilia | ||
| 1 | Toscana | ||
| Curd | 1 | Sicilia | |
| Cheese | 1 | Sicilia | |
| Whey | 1 | Sicilia | |
| Water buffalo | Raw Milk | 2 | Lazio |
| (2 strains) |
A miniaturized biochemical system (Biolog GP Microplate, Biolog, Inc., Hayward, CA, USA) was used to confirm the staphylococcal species. The strains were maintained and propagated in Brain Heart Infusion broth (Oxoid, Milan, Italy) and incubated at 37°C overnight.
Strain identification was also confirmed by S. aureus specific primers for the 23S rRNA gene according to Cremonesi [13].
2.2. DNA Extraction and Detection of se Genes by Multiplex PCR
DNA was extracted, as described by Cremonesi [14], using one millilitre of the culture incubated in BHI broth overnight at 37°C, containing approximately 1 − 3 × 109 cells. In parallel, cell numbers were verified by total sample counts, following the ISO 6888 1/2:1999 procedure with Baird Parker RPF agar plate [15]. As several studies have described that none of the investigated strains isolated from bovine and goat milk, and related dairy products, harbour any of the seb, see, and sek genes, se genes, including sea, sec, sed, seg, seh, sei, sej, and sel were detected by multiplex PCR assay as described by Cremonesi [13]. This PCR assay also included species-specific primers for 23S rRNA, coagulase, and thermonuclease. The reference strains ATCC 700699 (harbouring sea, sec, seg, and sej genes), ATCC 23235 (sed, seg, sei, and sej), and ATCC 19095 (sec, seh, seg and sei) were included as positive controls for the PCR assay.
2.3. Investigation of the Phenotypes
The S. aureus strains were phenotyped by appraising the heat stable nuclease (TNase) test using Toluidine blu agar (Oxoid) according to ISO 8870:2006 [16] and coagulase determination according to ISO 6888 1/2:1999 [15].
2.4. Hemolysis on Blood Agar and Antibiotic Resistance
Hemolytic activity was determined on blood agar (defibrinated sheep blood) (Merck, Darmstad, Germany) at 37°C for 24 hours. The type of hemolysis was recorded as α-, β-, and double (α + β).
Antibiotic susceptibility was determined by the standardized agar diffusion test on Muller-Hinton (Biolife, Milan Italy) using the following disks: vancomycin bioDisc VA30 (30 μg/disk) and methicillin (oxacillin) OX1 (1 μg/disk) (bioMérieux, RCS Lyon, France) according to manufacturer instructions. S. aureus ATCC 29213 was used as the reference strain [17]. Isolates were categorized as susceptible and resistant based upon interpretative criteria developed by the National Committee of Clinical Laboratory Standards [18].
2.5. RAPD-PCR
RAPD-PCR reactions were performed with primers M13 and AP4. The amplification conditions, as well as electrophoresis and analysis of the amplification products, were the same as those described by Andrighetto [19], except for the amplification cycle of primer AP4 that was modified as follows: an initial step of 95°C for 90 seconds, followed by 35 cycles of 95°C for 30 seconds, 36°C for 60 seconds, and 72°C for 90 seconds. Grouping of the RAPD-PCR profiles was obtained with the Gel Compar 4.1 software package (Applied Maths, Kortrjik, Belgium), using the Pearson product moment correlation coefficient and UPGMA cluster analysis.
3. Results and Discussion
3.1. Identification of Microbial Isolates from Dairy Products
All 122 isolates were identified by PCR reaction as belonging to Staphylococcus aureus. The Biolog GP identification for 27 strains gave different identifications; 2 strains resulted S. delphini, 1 S. xylosus, 1 S. intermedius, and 1 S. haemolyticus; 1 was not identified, and for 21 strains the identification was only at the genus level (Staphylococcus spp.). The use of Biolog GP allowed the correct identification of 78% of S. aureus isolates, while for the remaining 22% of isolates, species-specific PCR was necessary. All 122 cultures were positive for the presence of coagulase and heat stable nuclease.
3.2. Hemolysis Patterns of the S. aureus Isolates
All the tested S. aureus presented hemolysis on blood agar plates; 66 strains (54%) showed β-hemolysis, 49 (40%) double hemolysis (α + β), and 7 (6%) α-hemolysis. The majority of strains isolated from cow dairy products showed a prevalence of β-hemolysis (62%) while 29 strains (36%) gave double hemolysis. α-hemolysis was detected in only 2 cow isolates. β-hemolysis prevalence in bovine S. aureus strains is in full agreement with other research papers [20, 21], but contrary to what was shown in studies conducted by Stephan [22] who, in Switzerland, found double hemolysis in 23 of 34 S. aureus isolated from cow milk samples. Most of the S. aureus strains derived from goat dairy products (64%) showed double hemolysis, and in none of the isolates α− hemolysis was detected. For the strains isolated from sheep dairy products, there was the homogeneous distribution of α, β, and double-hemolysis (5, 6, and 6 strains). The two strains isolated from buffalo dairy products were β-hemolytic on blood agar.
3.3. Prevalence of the se Genes in the S. aureus Isolates
The frequency of the se genes and the relation between enterotoxins and sample origin are reported in Table 2. Of the 122 S. aureus isolates tested, 79 (65%) were found to be positive for one or more se gene. The most frequent gene was sed (n: 40) followed by sea, sej, sec, sel, and sei. The gene seh was the least frequent. The genes sec-sel (n: 16) were, in all cases, associated, but only one strain carried them with other genes. In the same way sej was always found in combination with sed, but sed was not necessarily always associated with sej. The most frequent se gene profiles were sec-sel (n: 15), sea-sed-sej (n: 14), sed-sej (n: 13), and sea alone (n: 13). Twenty-one S. aureus possessed only one type of toxin gene (13 sea, 3 sed, 1 seg, 3 seh, and 1 sei), while the remaining 58 strains harboured more than one toxin gene. Only 3 isolates harboured seg and sei that are comprised by the enterotoxin gene cluster (egc) [23].
Table 2.
Degree of heterogeneity among the se gene profiles of S. aureus.
| se gene | Strains | Origin of isolates | |||
|---|---|---|---|---|---|
| Cow | Goat | Sheep | Water buffalo | ||
| Not detected | 43 | 23 | 10 | 8 | 2 |
| a | 13 | 11 | 2 | ||
| ad | 6 | 6 | |||
| adj | 14 | 13 | 1 | ||
| adgj | 1 | 1 | |||
| adgij | 1 | 1 | |||
| ag | 1 | 1 | |||
| agi | 1 | 1 | |||
| aghi | 1 | 1 | |||
| cl | 15 | 1 | 7 | 7 | |
| cdjl | 1 | 1 | |||
| d | 3 | 3 | |||
| dj | 13 | 13 | |||
| djg | 1 | 1 | |||
| g | 1 | 1 | |||
| gi | 3 | 2 | 1 | ||
| h | 3 | 3 | |||
| i | 1 | 1 | |||
| Total | 122 | 81 | 22 | 17 | 2 |
The novel se genes (seg, seh, sei, sej, and sel) were often associated with the classical genes, except for 8 strains that were positive for only one of the newly described se or, in some cases, for just a few of them. From the multiplex PCR analysis it appears that there is a certain degree of heterogeneity among the se gene profiles; in fact it was possible to group them into 17 gene combinations.
Comparing the data relative to the strains isolated from cow, goat, sheep, and water buffalo dairy products, 58 of the 81 (72%) strains from cow were positive for se, and sea, sed, and sej were found more frequently. Only 2 strains (isolated from Trentino Alto Adige milk and Veneto cheese, two regions of North Italy) were found to have the sec gene. Twelve of the 22 S. aureus (55%) isolated from goat dairy products harboured se genes, and the enterotoxins sec and sel predominated, each being found in 7 strains. A similar toxin pattern was noted in S. aureus isolated from sheep. In fact 53% of the isolates produced enterotoxins, and sec and sel were the most widespread. The two strains isolated from buffalo did not produce staphylococcal enterotoxins.
This work shows that the sea and sed genes are dominant and are often associated with sej in S. aureus isolates. Sed and sej genes have been localized in the same plasmid [24].
The predominance of enterotoxins A and D contradicts reports from countries such as Brazil, Norway, France, and Japan [25–29], where enterotoxin C S. aureus producers were frequently isolated from milk and raw milk cheeses. However, Normanno [10] showed that in Italian dairy products most of the isolated strains produced SED, followed by SEA, SEC, and SEB; moreover in South Korea and in France the sea gene was dominant in strains linked to staphylococcal food poisoning studied from 1981 to 2002 [30, 31].
3.4. Antibiotic Resistance Profile of the Isolates
All the S. aureus strains studied were tested for resistance to antibiotics. The antibiotics selected for the study were vancomycin and methicillin, these being commonly used in the medical and veterinary fields. Of the 122 strains studied 120 were sensitive to vancomycin while the other 2 strains (1 from cow and 1 from sheep isolates) showed, according to NCCLS, intermediate resistance to this antibiotic. None of the strains isolated from dairy products showed resistance to methicillin.
3.5. RAPD-PCR Analysis of the Isolates
All 122 isolates considered in this study were characterized by means of RAPD-PCR, a technique used by many to type S. aureus isolated from different foodstuffs implicated in staphylococcal food poisoning [32–35], from individual quarter milk and human samples [36–40] and from mastitis milk samples [41]. The RAPD-PCR analyses on all the isolates were carried out with the primers M13 and AP4. The reproducibility value of the RAPD-PCR assay, calculated on the repetition of independent amplifications of S. aureus strains, was higher than 95% for both the M13 and AP4 primers.
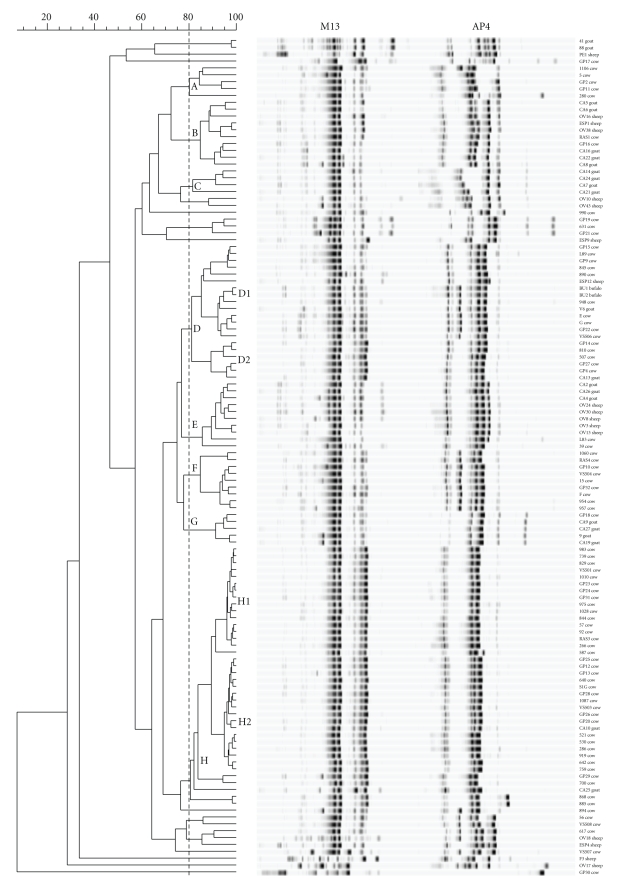
Genomic variability in the S. aureus strains became evident in the RAPD-PCR analysis (Figure 1). At 80% similarity, 8 distinct clusters were detected. Cluster A grouped 5 S. aureus strains isolated from cow dairy products: 4 of the 5 showed the presence of enterotoxin genes and 3 showed β-hemolytic activity. Most of the strains grouped in cluster B were isolated from ovine dairy products; this cluster contained 5 strains that came from goat, 3 from sheep, and 2 from cow. Six S. aureus isolates were not able to produce enterotoxins and 7 strains showed double hemolysis, 1 β and 2 α-hemolysis. Cluster C grouped 4 goat isolates, of which 2 strains harboured sec-sel and 2 were not enterotoxin producers. Double hemolysis was detected in 3 out of 4 strains. Cluster D contained 20 isolates (15 from cow, 2 from goat, 1 from sheep and 2 from water buffalo) and β- and double hemolysis were predominant, respectively, in 11 and 8 strains. Within this cluster, only one S. aureus strain isolated from sheep showed α-hemolysis. Cluster D can be divided into two subclusters (D1 and D2); D1 contained 14 strains (10 from cow, 1 from goat, 1 from sheep and 2 from water buffalo) of which 7 are not toxin producers, while the D2 subcluster grouped 6 isolates (5 from cow and 1 from goat) that all harboured the sed gene. Cluster E contained 8 strains that came from ovine dairy products (3 from goat and 5 from sheep) and 2 from cow. The 8 ovine strains showed the presence of enterotoxin genes, 7 harboured sec-sel and 1 seg-sei. The 2 strains isolated from cow were not enterotoxin producers. In cluster E β-hemolysis was predominant (7/10). All the strains belonging to cluster F were isolated from cow isolates, 6 out of 9 strains were not able to produce enterotoxins, and 5 isolates showed β-hemolysis. Cluster G grouped 5 strains (1 from cow and 4 from goat). All isolates were β-hemolytic and did not show the presence of enterotoxin genes. Cluster H contained 38 isolates (36 from cow and 2 from goat), and in this cluster we identified two subclusters, H1 (16 isolates) and H2 (17 isolates), characterized by a similarity coefficient of 90%. The 16 cow isolates belonging to the H1 subcluster showed the presence of enterotoxin genes (except one S. aureus strain), the strains harbouring singly, or in association with others, sea (9), sed (15), and sej (8), while of the strains grouped in the H2 subcluster (16 cow and 1 goat isolate) 14 showed the presence of the sea gene, 11 the sed, and 9 the sej. In H1 and H2 the β-hemolytic isolates predominated, respectively, 16 and 10 strains. Applying an 80% similarity value, 21 S. aureus isolates did not enter the 8 clusters.
Figure 1.
Dendrogram derived from the RAPD-PCR profiles generated with primers M13 and AP4.
The RAPD-PCR technique was shown to be efficient in typing the studied strains. The use of the primers allowed the subdivision of the isolates into eight major clusters within which, in some cases, the identified strains had similar characteristics (presence/absence of genes encoding enterotoxins, hemolysis type). As reported by other authors, the results of our research indicate that the presence of toxin genes is not associated with particular RAPD-PCR patterns [12, 42]. In addition, the RAPD-PCR and analyses of the genes encoding for the toxins showed no correlation with the geographical area of origin, whilst in many cases there was correlation with animal species.
With regard to resistance to antibiotics (vancomycin and methicillin), none of the strains isolated from the dairy products showed resistance, while a low frequency was reported by Normanno et al. [43] who found 3.75% of S. aureus resistant to methicillin. Indeed, also enterococci have shown similar results, different authors [44–46] having demonstrated that, in the dairy sector, most strains are sensitive to antibiotics.
4. Conclusions
The data acquired in the present work confirm the wide phenotype and genotype diversity of S. aureus from dairy products but such diversity was not always able to be intercorrelated. Furthermore, a similar enterotoxin strain incidence was confirmed in isolates from animals suffering mastitis [47]. It is interesting to note, however, that there was no evident correlation between the observed strain variability and the region from which the isolates originated.
References
- 1.Boerema JA, Clemens R, Brightwell G. Evaluation of molecular methods to determine enterotoxingenic status and molecular genotype of bovine, ovine, human and food isolates of Staphylococcus aureus . International Journal of Food Microbiology. 2006;107:192–201. doi: 10.1016/j.ijfoodmicro.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Balaban N, Rasooly A. Staphylococcal enterotoxins. International Journal of Food Microbiology. 2000;61(1):1–10. doi: 10.1016/s0168-1605(00)00377-9. [DOI] [PubMed] [Google Scholar]
- 3.Letertre C, Perelle S, Dilasser F, Fach P. Identification of a new putative enterotoxin SEU encoded by the egc cluster of Staphylococcus aureus . Journal of Applied Microbiology. 2003;95(1):38–43. doi: 10.1046/j.1365-2672.2003.01957.x. [DOI] [PubMed] [Google Scholar]
- 4.Becker K, Friedrich AW, Peters G, von Eiff C. Systematic survey on the prevalence of genes coding for staphylococcal enterotoxins SElM, SElO, and SElN. Molecular Nutrition and Food Research. 2004;48(7):488–495. doi: 10.1002/mnfr.200400044. [DOI] [PubMed] [Google Scholar]
- 5.Chiang Y-C, Chang L-T, Lin C-W, Yang C-Y, Tsen H-Y. PCR primers for the detection of staphylococcal enterotoxins K, L, and M and survey of staphylococcal enterotoxin types in Staphylococcus aureus isolates from food poisoning cases in Taiwan. Journal of Food Protection. 2006;69(5):1072–1079. doi: 10.4315/0362-028x-69.5.1072. [DOI] [PubMed] [Google Scholar]
- 6.Scherrer D, Corti S, Muehlherr JE, Zweifel C, Stephan R. Phenotypic and genotypic characteristics of Staphylococcus aureus isolates from raw bulk-tank milk samples of goats and sheep. Veterinary Microbiology. 2004;101(2):101–107. doi: 10.1016/j.vetmic.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Jørgensen HJ, Mørk T, Rørvik LM. The occurrence of Staphylococcus aureus on a farm with small-scale production of raw milk cheese. Journal of Dairy Science. 2005;88(11):3810–3817. doi: 10.3168/jds.S0022-0302(05)73066-6. [DOI] [PubMed] [Google Scholar]
- 8.Schlegelova J, Dendis M, Benedik J, Babak V, Rysanek D. Staphylococcus aureus isolates from dairy cows and humans on a farm differ in coagulase genotype. Veterinary Microbiology. 2003;92(4):327–334. doi: 10.1016/s0378-1135(02)00409-1. [DOI] [PubMed] [Google Scholar]
- 9.Reinoso EB, Ibanez F, Raspanti C, Odierno L, Bogni CI. Characterization of Staphylococcus aureus strains isolated from humans in Argentina. Journal of Basic Microbiology. 2006;46(4):286–293. doi: 10.1002/jobm.200510100. [DOI] [PubMed] [Google Scholar]
- 10.Normanno G, La Salandra G, Dambrosio A, et al. Occurrence, characterization and antimicrobial resistance of enterotoxigenic Staphylococcus aureus isolated from meat and dairy products. International Journal of Food Microbiology. 2007;115(3):290–296. doi: 10.1016/j.ijfoodmicro.2006.10.049. [DOI] [PubMed] [Google Scholar]
- 11.Van Belkum A, Kluytmans J, Van Leeuwen W, et al. Multicenter evaluation of arbitrarily primed PCR for typing of Staphylococcus aureus strains. Journal of Clinical Microbiology. 1995;33(6):1537–1547. doi: 10.1128/jcm.33.6.1537-1547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naffa RG, Bdour SM, Migdadi HM, Shehabi AA. Enterotoxicity and genetic variation among clinical Staphylococcus aureus isolates in Jordan. Journal of Medical Microbiology. 2006;55(2):183–187. doi: 10.1099/jmm.0.46183-0. [DOI] [PubMed] [Google Scholar]
- 13.Cremonesi P, Luzzana M, Brasca M, et al. Development of a multiplex PCR assay for the identification of Staphylococcus aureus enterotoxigenic strains isolated from milk and dairy products. Molecular and Cellular Probes. 2005;19(5):299–305. doi: 10.1016/j.mcp.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Cremonesi P, Castiglioni B, Malferrari G, et al. Technical note: improved method for rapid DNA extraction of mastitis pathogens directly from milk. Journal of Dairy Science. 2006;89(1):163–169. doi: 10.3168/jds.S0022-0302(06)72080-X. [DOI] [PubMed] [Google Scholar]
- 15.International Standard Organization (ISO), EN ISO 6888:1. Microbiology of food and animal feeding stuffs: horizontal method for the enumeration of coagulase-positive staphylococci (Staphylococcus aureus and other species). Part. 1: technique using Baird-Parker agar medium. ISO Geneva, 1999.
- 16.International Standard Organization (ISO), EN ISO 8870. Milk and milk-based products. Detection of thermonuclease produced by coagulase-positive staphylococci. ISO Geneva, 2006.
- 17.Moroni P, Pisoni G, Vimercati C, et al. Characterization of Staphylococcus aureus isolated from chronically infected dairy goats. Journal of Dairy Science. 2005;88(10):3500–3509. doi: 10.3168/jds.S0022-0302(05)73035-6. [DOI] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards (NCCLS), NCCLS M31-A2. Performance standards for antimicrobial disk and dilution susceptibility test for bacteria isolated from animals; approved standard. NCCLS, Wayne, Pa, USA, 2002.
- 19.Andrighetto C, Knijff E, Lombardi A, et al. Phenotypic and genetic diversity of enterococci isolated from Italian cheeses. Journal of Dairy Research. 2001;68(2):303–316. doi: 10.1017/s0022029901004800. [DOI] [PubMed] [Google Scholar]
- 20.Aarestrup FM, Larsen HD, Eriksen NHR, Elsberg CS, Jensen NE. Frequency of α- and β-haemolysin in Staphylococcus aureus of bovine and human origin. A comparison between pheno- and genotype and variation in phenotypic expression. Acta Pathologica, Microbiologica et Immunologica Scandinavica. 1999;107(4):425–430. [PubMed] [Google Scholar]
- 21.Larsen HD, Aarestrup FM, Jensen NE. Geographical variation in the presence of genes encoding superantigenic exotoxins and β-hemolysin among Staphylococcus aureus isolated from bovine mastitis in Europe and USA. Veterinary Microbiology. 2002;85(1):61–67. doi: 10.1016/s0378-1135(01)00478-3. [DOI] [PubMed] [Google Scholar]
- 22.Stephan R, Annemüller C, Hassan AA, Lämmler Ch. Characterization of enterotoxigenic Staphylococcus aureus strains isolated from bovine mastitis in north-east Switzerland. Veterinary Microbiology. 2001;78(4):373–382. doi: 10.1016/s0378-1135(00)00341-2. [DOI] [PubMed] [Google Scholar]
- 23.Jarraud S, Peyrat MA, Lim A, et al. egc, a highly prevalent operon of enterotoxin gene, forms a putative nursery of superantigens in Staphylococcus aureus . Journal of Immunology. 2001;166(1):669–677. doi: 10.4049/jimmunol.166.1.669. [DOI] [PubMed] [Google Scholar]
- 24.Zhang S, Iandolo JJ, Stewart GC. The enterotoxin D plasmid of Staphylococcus aureus encodes a second enterotoxin determinant (sej) FEMS Microbiology Letters. 1998;168(2):227–233. doi: 10.1111/j.1574-6968.1998.tb13278.x. [DOI] [PubMed] [Google Scholar]
- 25.da Silva ER, do Carmo LS, da Silva N. Detection of the enterotoxins A, B, and C genes in Staphylococcus aureus from goat and bovine mastitis in Brazilian dairy herds. Veterinary Microbiology. 2005;106(1-2):103–107. doi: 10.1016/j.vetmic.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Loncarevic S, Jørgensen HJ, Løvseth A, Mathisen T, Rørvik LM. Diversity of Staphylococcus aureus enterotoxin types within single samples of raw milk and raw milk products. Journal of Applied Microbiology. 2005;98(2):344–350. doi: 10.1111/j.1365-2672.2004.02467.x. [DOI] [PubMed] [Google Scholar]
- 27.Jørgensen HJ, Mørk T, Caugant DA, Kearns A, Rørvik LM. Genetic variation among Staphylococcus aureus strains from Norwegian bulk milk. Applied and Environmental Microbiology. 2005;71(12):8352–8361. doi: 10.1128/AEM.71.12.8352-8361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villard L, Lamprell H, Borges E, et al. Enterotoxin D producing strains of Staphylococcus aureus are typeable by pulsed-field gel electrophoresis (PFGE) Food Microbiology. 2005;22(2-3):261–265. [Google Scholar]
- 29.Katsuda K, Hata E, Kobayashi H, et al. Molecular typing of Staphylococcus aureus isolated from bovine mastitic milk on the basis of toxin genes and coagulase gene polymorphisms. Veterinary Microbiology. 2005;105:301–305. doi: 10.1016/j.vetmic.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Cha JO, Lee JK, Jung YH, et al. Molecular analysis of Staphylococcus aureus isolates associated with staphylococcal food poisoning in South Korea. Journal of Applied Microbiology. 2006;101(4):864–871. doi: 10.1111/j.1365-2672.2006.02957.x. [DOI] [PubMed] [Google Scholar]
- 31.Kérouanton A, Hennekinne JA, Letertre C, et al. Characterization of Staphylococcus aureus strains associated with food poisoning outbreaks in France. International Journal of Food Microbiology. 2007;115(3):369–375. doi: 10.1016/j.ijfoodmicro.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 32.Esteves A, Patarata L, Aymerich T, Garriga M, Martins C. Multiple correspondence analysis and random amplified polymorphic DNA molecular typing to assess the sources of Staphylococcus aureus contamination in Alheira production lines. Journal of Food Protection. 2007;70(3):685–691. doi: 10.4315/0362-028x-70.3.685. [DOI] [PubMed] [Google Scholar]
- 33.Martín MC, Fueyo JM, González-Hevia MA, Mendoza MC. Genetic procedures for identification of enterotoxigenic strains of Staphylococcus aureus from three food poisoning outbreaks. International Journal of Food Microbiology. 2004;94(3):279–286. doi: 10.1016/j.ijfoodmicro.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Pinto B, Chenoll E, Aznar R. Identification and typing of food-borne Staphylococcus aureus by PCR-based techniques. Systematic and Applied Microbiology. 2005;28(4):340–352. doi: 10.1016/j.syapm.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Nema V, Agrawal R, Kamboj DV, Goel AK, Singh L. Isolation and characterization of heat resistant enterotoxigenic Staphylococcus aureus from a food poisoning outbreak in Indian subcontinent. International Journal of Food Microbiology. 2007;117(1):29–35. doi: 10.1016/j.ijfoodmicro.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Grundmann H, Hori S, Enright MC, et al. Determining the genetic structure of the natural population of Staphylococcus aureus: a comparison of multilocus sequence typing with pulsed-field gel electrophoresis, randomly amplified polymorphic DNA analysis, and phage typing. Journal of Clinical Microbiology. 2002;40(12):4544–4546. doi: 10.1128/JCM.40.12.4544-4546.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pereira MSV, Leal NC, Leal TCA, et al. Typing of human and bovine Staphylococcus aureus by RAPD-PCR and ribotyping-PCR. Letters in Applied Microbiology. 2002;35(1):32–36. doi: 10.1046/j.1472-765x.2002.01127.x. [DOI] [PubMed] [Google Scholar]
- 38.Lee JH. Methicillin (oxacillin)-resistant Staphylococcus aureus strains isolated from major food animals and their potential transmission to humans. Applied and Environmental Microbiology. 2003;69(11):6489–6494. doi: 10.1128/AEM.69.11.6489-6494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reinoso E, Bettera S, Frigerio C, DiRenzo M, Calzolari A, Bogni C. RAPD-PCR analysis of Staphylococcus aureus strains isolated from bovine and human hosts. Microbiological Research. 2004;159(3):245–255. doi: 10.1016/j.micres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Neela V, Mariana NS, Radu S, Zamberi S, Raha AR, Rosli R. Use of RAPD to investigate the epidemiology of Staphylococcus aureus infection in Malaysian hospitals. World Journal of Microbiology and Biotechnology. 2005;21(3):245–251. [Google Scholar]
- 41.Vautor E, Jay C, Chevalier N, Visomblin N, Vernet G, Pépin M. Characterization of 26 isolates of Staphylococcus aureus, predominantly from dairy sheep, using four different techniques of molecular epidemiology. Journal of Veterinary Diagnostic Investigation. 2005;17(4):363–368. doi: 10.1177/104063870501700411. [DOI] [PubMed] [Google Scholar]
- 42.Araki M, Kariyama R, Monden K, Tsugawa M, Kumon H. Molecular epidemiological studies of Staphylococcus aureus in urinary tract infection. Journal of Infection and Chemotherapy. 2002;8(2):168–174. doi: 10.1007/s101560200029. [DOI] [PubMed] [Google Scholar]
- 43.Normanno G, Corrente M, La Salandra G, et al. Methicillin-resistant Staphylococcus aureus (MRSA) in foods of animal origin product in Italy. International Journal of Food Microbiology. 2007;117(2):219–222. doi: 10.1016/j.ijfoodmicro.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Jurkovič D, Križková L, Dušinský R, et al. Identification and characterization of enterococci from Bryndza cheese. Letters in Applied Microbiology. 2006;42(6):553–559. doi: 10.1111/j.1472-765X.2006.01918.x. [DOI] [PubMed] [Google Scholar]
- 45.Morandi S, Brasca M, Andrighetto C, Lombardi A, Lodi R. Technological and molecular characterization of enterococci isolated from north-west Italian dairy products. International Dairy Journal. 2006;16:867–875. [Google Scholar]
- 46.Psoni L, Kotzamanides C, Andrighetto C, Lombardi A, Tzanetakis N, Litopoulou-Tzanetaki E. Genotypic and phenotypic heterogeneity in Enterococcus isolates from Batzos, a raw goat milk cheese. International Journal of Food Microbiology. 2006;109:109–120. doi: 10.1016/j.ijfoodmicro.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 47.Vimercati C, Cremonesi P, Castiglioni B, et al. Molecular typing of Staphylococcus aureus isolated from cows, goats and sheep with intramammary infections on the basis of gene polymorphisms and toxins genes. Journal of Veterinary Medicine Series B. 2006;53(9):423–428. doi: 10.1111/j.1439-0450.2006.00980.x. [DOI] [PubMed] [Google Scholar]