Abstract
Study Objectives:
The International Classification of Sleep Disorders (ICSD-2) criteria for low CSF hypocretin-1 levels (CSF hcrt-1) still need validation as a diagnostic tool for narcolepsy in different populations because inter-assay variability and different definitions of hypocretin deficiency complicate direct comparisons of study results.
Design and Participants:
Interviews, polysomnography, multiple sleep latency test, HLA-typing, and CSF hcrt-1 measurements in Danish patients with narcolepsy with cataplexy (NC) and narcolepsy without cataplexy (NwC), CSF hcrt-1 measurements in other hypersomnias, neurological and normal controls. Comparisons of hypocretin deficiency and frequency of HLA-DQB1*0602-positivity in the Danish and eligible NC and NwC populations (included via MEDLINE search), by (re)calculation of studyusing the ICSD-2 criterion for low CSF hcrt-1 (<30% of normal mean).
Measurements and Results:
In Danes, low CSF hcrt-1 was present in 40/46 NC, 3/14 NwC and 0/106 controls (P < 0.0001). Thirty-nine of 41 NC and 4/13 NwC patients were HLA-DQB1*0602-positive (P < 0.01). Hypocretin-deficient NC patients had higher frequency of cataplexy, shorter mean sleep latency, more sleep onset REM periods (P < 0.05) and more awakenings (NS) than did NC patients with normal CSF hcrt-1. Across populations, low CSF hcrt-1 and HLA-DQB1*0602-positivity characterized the majority of NC (80% to 100%, P = 0.53; 80% to 100%, P = 0.11) but a minority of NwC patients (11% to 29%, P = 0.75; 29% to 89%, P = 0.043).
Conclusion:
The study provides evidence that hypocretin deficiency causes a more severe NC phenotype. The ICSD-2 criterion for low CSF hcrt-1 (<30% of normal mean) is valid for diagnosing NC, but not NwC. HLA-typing should precede CSF hcrt-1 measurements because hypocretin deficiency is rare in HLA-DQB1*0602-negative patients.
Citation:
Knudsen S; Jennum PJ; Alving J; Sheikh SP; Gammeltoft S. Validation of the ICSD-2 criteria for CSF hypocretin-1 measurements in the diagnosis of narcolepsy in the Danish population. SLEEP 2010;33(2):169-176.
Keywords: Hypocretin-1, HLA-DQB1*0602, narcolepsy with cataplexy, narcolepsy without cataplexy, ICSD-2
NARCOLEPSY WITH CATAPLEXY (NC) IS CAUSED BY ALMOST COMPLETE LOSS OF HYPOCRETIN (OREXIN) NEURONS IN THE HYPOTHALAMUS. THESE NEURONS produce the sleep-wake and REM sleep-regulating neuropeptides hypocretin-1 and hypocretin-2 (orexin-A and orexin-B).1, 2 Several studies have detected a low level of hypocretin-1 in the cerebrospinal fluid (CSF hcrt-1) in the majority of NC patients as well as in some patients with narcolepsy without cataplexy (NwC). Thus, determination of CSF hcrt-1 may be of diagnostic value for narcolepsy, and it has been included as a diagnostic tool in the current International Classification of Sleep Disorders (ICSD-2).3
However, the prevalence of low CSF hcrt-1 seems to vary greatly between studied populations: 77%4 to 94%5 in NC patients and 11%6 to 38%7 in NwC patients. This may reflect true between-population differences, but this is difficult to determine mainly because of different study definitions of low and normal CSF hcrt-1 levels and the great inter-assay variability of the CSF hcrt-1 radioimmunoassay. Hypocretin-deficient patients often carry the HLA-DQB1*0602-allele,8 so different HLA-DQB1*0602 frequencies in the study populations might also contribute to the variation observed.
Direct extrapolation of study results from different sleep laboratories has therefore not yet been possible. Hence, the ICSD-2 criterion for low CSF hcrt-1 still needs to be validated in different populations.
We aimed to (1) characterize the relationship between the clinical profile, the paraclinical profile (sleep investigations, HLA-type), and the ICSD-2 criterion for low CSF hcrt-1 (< 30% of normal mean) in a Danish population consisting of patients with narcolepsy with or without cataplexy, other hypersomnias, neurological disorders, and normal controls; and (2) recalculate the CSF hcrt-1 results of all eligible NC and NwC populations (identified by MEDLINE search) according to the ICSD-2 criterion for low CSF hcrt-1 (< 30% of normal mean), and then directly compare all study results.
METHODS
The Danish Population
After approval (KA03119) from the Danish Ethical Committee (Capital Region) and written informed consent, CSF was collected from Danish Caucasian patients with different hypersomnias, neurological disorders and normal controls seen at the Danish Center for Sleep Medicine and the Department of Neurology, Glostrup Hospital, Denmark.
Narcolepsy and Other Hypersomnias
Eighty-one consecutively diagnosed hypersomnia patients were included: NC (46) and NwC (14), idiopathic hypersomnia (13), Kleine-Levin Syndrome (1), possible narcolepsy (1), periodic limb movements (2) and post-anoxic hypersomnia (1). Four of the 46 NC patients were originally diagnosed at the Danish Epilepsy Centre, Dianalund, Denmark. Exclusion criteria were additional neurological or psychiatric disorders. All patients except one (who had severe cataplexy) were free of antidepressants and stimulants 7-14 days before inclusion. The patients were evaluated by neurological examination, polysomnography (PSG), multiple sleep latency test (MSLT), HLA-typing, determination of routine blood characteristics, and CSF hcrt-1 levels. Their hypersomnia history was obtained by a semi-structured interview based on the Stanford Sleep Questionnaire.9 The diagnoses of hypersomnia were based on the ICSD-2 criteria.3 A clear relation between emotional triggers and cataplexy was present in all patients diagnosed as NC. Narcolepsy with no or atypical cataplexy (absence of emotional triggers) was classified as NwC.
Neurological Disorders
Fifty-nine patients with neurological disorders were included. The diagnoses were conducted by neurology specialists. All patients were screened for sleep disorders by interview and for sleepiness by the Epworth Sleepiness Scale.10 Fifty-one patients had no history of sleep disorders; a PSG was conducted in 8 patients with possible additional sleep disorders. An MRI scan was part of the neurological examination in 50/59 patients; 49/50 had no pathological changes in the hypothalamus or brainstem, and one patient with MSA and REM sleep behavior disorder had pontine atrophy. Forty-five patients had been previously reported.11
Normal Controls
Twenty-nine normal controls without neurological or sleep disorders were included. We recruited 12/29 normal controls by advertising for normal volunteers (www.forsoegsperson.dk). The Epworth Sleepiness Scale10, neurological examination, and routine blood characteristics confirmed that they were healthy. The remaining 17/29 normal controls were originally referred for subjective neurological symptoms or subjective sleepiness, which were excluded by interview, neurological examination, PSG, and MSLT.
Collection and Measurements of CSF Hypocretin-1
CSF (10 mL) was collected between 07:00 and 10:00, cooled on ice water and stored within 15-30 min at −80°C until analysis. Hcrt-1 was analyzed in crude CSF by a radioimmunoassay (Phoenix Pharmaceuticals, Belmont, CA, USA).12 All samples were blindly measured as duplicates and the means of the results were calculated. The detection limit was 10 pg/mL. If the intra-assay variability was > 5%, the sample was reanalyzed. The standard curve range was 10-1280 pg/mL. The results were analyzed using the Analytical Data Sheet (ADS) for radioimmunoassay (www.bachem.com). Assay quality was controlled by the positive control sample included in the assay kit. In addition, 3 reference control samples of pooled CSF from patients or normal control individuals, representing low (approximately 150 pg/mL), intermediate (approximately 325 pg/mL), and normal (approximately 475 pg/mL) CSF hcrt-1 levels, were included to adjust for inter-assay variability. The low and intermediate reference control samples were kindly donated by Dr. Mignot's laboratory (Stanford, USA).
Stability of CSF Hypocretin-1
CSF hcrt-1 levels were measured in 84 samples (from 12 normal controls) kept at −80°C, room temperature (RT) or 4°C for 24, 48, and 96 hours before storage at −80°C. All samples were measured within a single assay.
Other Narcolepsy Populations (MEDLINE search)
All CSF hcrt-1 studies, consisting of narcolepsy populations with ICSD-2-defined cataplexy (emotional triggers present), ICSD-2-defined narcolepsy without cataplexy and a normal control group, were identified by a literature search on MEDLINE (1966-June 2008). Details of the laboratory protocol and study results (including CSF hcrt-1 normal mean and normal area) from the included CSF hcrt-1 studies were obtained by e-mail correspondence. It was ensured that all included studies corrected the inter-assay variability by the use of reference control samples. Subsequently, the CSF hcrt-1 results of each study were recalculated into “low” and “normal” groups based on the ICSD-2 criterion for low CSF hcrt-1 (< 30% of the normal mean).3 Intermediate CSF hcrt-1 levels very close to the ICSD-2 cutoff value were recategorized as “low” (see Table 3 and 4). The remaining CSF hcrt-1 values ≥ 30% of the normal mean were categorized as normal.
Table 3.
ICSD-2 Criterion for low CSF hcrt-1 (<30% of the normal mean) applied on populations with narcolepsy with cataplexy
| STUDY | No. of Subjects |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Narcolepsy cataplexy | Normal controls | Low CSF hcrt-1 | Recalculated ICSD-2 Cutoff Values | ||||||
| CSF hcrt-1 |
CSF hcrt-1 |
sens. | spec. | PPV | NPV | (low <30% of the normal mean) | |||
| Low | normal | low | normal | (%) | (%) | (%) | (%) | ||
| Knudsen et al (this study) | 40 (35/36 HLA+) | 6 (4/5HLA+) | 0 | 29 | 87 | 100 | 100 | 83 | normal mean CSF hcrt-1 = 430 pg/mL low CSF hcrt-1 cutoff < 129 pg/mL 5 HLA-types were unavailable |
| Bourgin et al (2008)8 | 86 (84/86HLA+) | 39 (14/39HLA+) | 0 | 64 | 69 | 100 | 100 | 62 | normal mean CSF hcrt-1 = 363 pg/mL low CSF hcrt-1 cutoff < 108 pg/mL |
| Serra et al (2008)16 | 12* (12/12HLA+) | 0 | 0 | 64§ | 100 | 100 | 100 | 100 | normal mean CSF hcrt-1 = 363 pg/mL low CSF hcrt-1 cutoff < 109 pg/mL *1/12 was intermediate value close to low cutoff. § CSF hcrt-1 measured at Stanford |
| Martinez-Rodriguez et al (2007)5 | 30 | 2 | 0 | 64* | 94 | 100 | 100 | 97 | normal mean CSF hcrt-1 = 381 pg/mL low CSF hcrt-1 cutoff < 114 pg/mL *linked to Stanford normal controls by reference CSF samples. 31/32 patients were HLA+ |
| Baumann et al (2006)14 | 15 | 3 | 0 | 20 | 83 | 100 | 100 | 87 | normal mean CSF hcrt-1 = 497 pg/mL low CSF hcrt-1 cutoff < 149 pg/mL 15/17 patients were HLA+ |
| Kanbayashi et al (2003)15+ Arii et al (2004)13 | 12* (12/12DR2+) | 3 (0/3DR2+) | 0 | 12 | 80 | 100 | 100 | 80 | normal mean CSF hcrt-1 = 293 pg/mL low CSF hcrt-1 cutoff < 88 pg/mL *2/12 are intermediate values close to low cutoff. Study populations are combined (same laboratory) |
| Dauvilliers et al (2003)6 | 23 (23/23HLA+) | 3 (2/3HLA+) | 0 | 64* | 88 | 100 | 100 | 96 | normal mean CSF hcrt-1=363 pg/mL low CSF hcrt-1 cutoff < 108 pg/mL *linked to Stanford normal controls by reference CSF samples |
CSF hcrt-1 (cerebrospinal fluid, hypocretin-1 level), sens. (sensitivity), spec. (specificity), PPV (positive predictive value), NPV (negative predictive value). ICSD-2 (International Classification of Sleep Disorders, 2nd edition, 2005), HLA+ (HLA-DQB1*0602-positive), DR2+ (DR2-positive)
Table 4.
ICSD-2 criterion for low CSF hcrt-1 (<30% of the normal mean) applied on populations with narcolepsy without cataplexy
| STUDY | No. of Subjects |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Narcolepsy without cataplexy | Normal controls | Low CSF hcrt-1 | Recalculated ICSD-2 Cutoff Values | ||||||
| CSF hcrt-1 |
CSF hcrt-1 |
sens. | spec. | PPV | NPV | (low <30% of the normal mean) | |||
| low | normal | low | normal | (%) | (%) | (%) | (%) | ||
| Knudsen et al (this study) | 3 (3/3HLA+) | 11 (1/10HLA+)* | 0 | 27 | 19 | 100 | 100 | 73 | normal mean CSF hcrt-1 = 430 pg/mL low CSF hcrt-1 cutoff < 129 pg/mL *1/11 HLA-type was unavailable |
| Bourgin et al (2008)8 | 29* (24/29HLA+) | 133 (46/133HLA+) | 0 | 64 | 18 | 100 | 100 | 33 | normal mean CSF hcrt-1 = 363 pg/mL low CSF hcrt-1 cutoff < 108 pg/mL *5/29 are intermediate values close to low cutoff |
| Martinez-Rodriguez et al (2007)5 | 3 | 8 | 0 | 64* | 27 | 100 | 100 | 89 | normal mean CSF hcrt-1 = 381 pg/mL low CSF hcrt-1 cutoff < 114 pg/mL *linked to Stanford normal controls by reference CSF samples. 6/11 patients were HLA+ |
| Kanbayashi et al (2003)15, Oka et al (2006)17 | 5 (5/5HLA+) | 12 (4/12HLA+) | 0 | 12 | 29 | 100 | 100 | 50 | normal mean CSF hcrt-1 = 293 pg/mL low CSF hcrt-1 cutoff < 88 pg/mL Study populations are combined (same laboratory) |
| Dauvilliers et al (2003)6 | 1 (1/1HLA+) | 8 (7/8HLA+) | 0 | 64* | 11 | 100 | 100 | 89 | normal mean CSF hcrt-1 = 363 pg/mL low CSF hcrt-1 cutoff <108 pg/mL *linked to Stanford normal controls by reference CSF samples |
CSF hcrt-1 (cerebrospinal fluid, hypocretin-1 level), sens. (sensitivity), spec. (specificity), PPV (positive predictive value), NPV (negative predictive value). ICSD-2 (International Classification of Sleep Disorders, 2nd edition, 2005), HLA+ (HLA-DQB1*0602-positive)
Statistical Analysis
GraphPad Prism version 4.01 and SAS version 9.1 were used for statistical analyses. Results are reported as the mean ± SEM unless otherwise stated. Values of P < 0.05 were considered statistically significant. Data presented in Tables 1, 3, and 4 and data from the included MEDLINE articles were analyzed by nonparametric Kruskal-Wallis, χ2, and Fisher exact tests. Due to selection bias, the 2008 data of Bourgin et al8 (Tables 3 and 4) were not included in the statistical analyses. Data presented in Table 2 and Figure 2 were analyzed by nonparametric Kruskal-Wallis and Dunn multiple comparison tests. CSF hcrt-1 levels of neurological patients with or without sleep disorders were not significantly different (P = 0.16) and so were analyzed as a single group. Data presented in Figure 3 were analyzed by the Friedman nonparametric paired test, and those in Figure 1 by Spearman nonparametric correlation test.
Table 1.
Demographic, clinical, and paraclinical data of the Danish narcolepsy population by presence of cataplexy and hypocretin deficiency
| Narcolepsy with cataplexy | Narcolepsy without cataplexy | P-value | Low CSF hcrt-1 | Normal CSF hcrt-1 | P-value | |
|---|---|---|---|---|---|---|
| N = 46 | N = 14 | N = 43 | N = 17 | |||
| Demography | ||||||
| Gender (male), n (%) | 20 (43.5%) | 7 (50.0%) | 0.76 | 19 (44.2%) | 8 (47.1%) | 1.00 |
| Age (y), mean ± SEM | 36.18 ± 2.37 | 29.57 ± 2.96 | 0.18 | 36.2 ± 2.56 | 30.71 ± 2.32 | 0.28 |
| Age at disease onset (y), mean ± SEM | 18.80 ± 1.17 | 15.96 ± 1.73 | 0.19 | 18.17 ± 1.21 | 18.06 ± 1.76 | 0.99 |
| Disease duration (y), mean ± SEM | 17.37 ± 2.19 | 13.61 ± 2.76 | 0.63 | 18.01 ± 2.29 | 12.65 ± 2.42 | 0.32 |
| HLA-DQB1*0602-positivity,n (%) | 39 (95.12%) | 4 (30.77%) | < 0.01 | 38 (97.4%) | 5 (33.3%) | < 0.01 |
| N = 41 | N = 13 | N = 39 | N = 15 | |||
| CSF hcrt-1 < 129 pg/mL, n (%) | 40 (87.0%) | 3 (21.4%) | < 0.01 | - | - | |
| N = 46 | N = 14 | |||||
| Sleepiness | ||||||
| Daytime sleepiness, n (%) | 46 (100%) | 14 (100%) | 1.00 | 43 (100%) | 17 (100%) | 1.00 |
| Epworth Sleepiness Scale, (sum), mean ± SEM | 18.71 ± 0.50 | 16.50 ± 0.86 | 0.03 | 18.42 ± 0.55 | 17.56 ± 0.72 | 0.29 |
| N = 45 | N = 14 | N = 43 | N = 16 | |||
| Age at onset (y), mean ± SEM | 19.32 ± 1.34 | 15.96 ± 1.73 | 0.23 | 18.49 ± 1.43 | 18.65 ± 1.58 | 0.67 |
| N = 46 | N = 14 | N = 43 | N = 17 | |||
| Cataplexy characteristics* | ||||||
| Frequency** | ||||||
| Grade 1, n (%) | 1 (2.2%) | 0 (0%) | - | 0 (0%) | 1 (16.7%) | |
| Grade 2, n (%) | 4 (8.7%) | 0 (0%) | 3 (7.5%) | 1 (16.7%) | 0.036 | |
| Grade 3, n (%) | 4 (8.7%) | 0 (0%) | 3 (7.5%) | 1 (16.7%) | ||
| Grade 4, n (%) | 17 (37.0%) | 0 (0%) | 17 (42.5%) | 0 (0%) | ||
| Grade 5, n (%) | 20 (43.5%) | 0 (0%) | 17 (42.5%) | 3 (50.0%) | ||
| Injurious cataplexy, n (%) | 21 (47.7%) | 0 (0%) | - | 18 (46.2%) | 3 (60.0%) | 0.66 |
| N = 44 | N = 39 | N = 5 | ||||
| Age at onset (y), mean ± SEM | 22.16 ± 1.45 | - | - | 22.56 ± 1.56 | 19.50 ± 4.02 | 0.61 |
| N = 46 | N = 0 | N = 40 | N = 6 | |||
| Hypnagogic Hallucinations | ||||||
| Hypnagogic hallucinations, n (%) | 41 (89.1%) | 7 (50.0%) | < 0.01 | 37 (86.0%) | 11 (64.7%) | 0.08 |
| Age at onset (y), mean ± SEM | 21.70 ± 1.45 | 16.71 ± 1.87 | 0.14 | 21.94 ± 1.53 | 17.72 ± 2.10 | 0.23 |
| N = 40 | N = 7 | N = 36 | N = 11 | |||
| Sleep paralysis | ||||||
| Sleep paralysis, n (%) | 35 (76.1%) | 4 (28.6%) | < 0.01 | 31 (72.1%) | 8 (47.1%) | 0.08 |
| Age at onset (y), mean ± SEM | 22.91 ± 1.60 | 12.33 ± 3.48 | 0.05 | 23.45 ± 1.69 | 16.0 ± 3.37 | 0.10 |
| N = 35 | N = 3 | N = 31 | N = 7 | |||
| Automatic Behaviour | ||||||
| Automatic behaviour, n (%) | 25 (61.0%) | 7 (53.9%) | 0.64 | 22 (55.0%) | 10 (71.4%) | 0.35 |
| N = 41 | N = 13 | N = 40 | N = 14 | |||
| Disrupted night sleep*** | ||||||
| Awakenings/ night, mean ± SEM | 5.60 ± 0.60 | 2.93 ± 0.94 | 0.01 | 5.78 ± 0.61 | 3.00 ± 0.89 | < 0.01 |
| N = 43 | N = 14 | N = 40 | N = 17 | |||
| MSLT (multiple sleep latency test) | ||||||
| Sleep latency (seconds) mean ± SEM | 228.82 ± 23.97 | 267.14 ± 38.20 | 0.20 | 214.10 ± 22.29 | 297.59 ± 42 | 0.048 |
| N = 46 | N = 14 | N = 43 | N = 17 | |||
| SOREMPs (number) mean ± SEM | 3.04 ± 0.18 | 3.29 ± 0.24 | 0.74 | 3.33 ± 0.16 | 2.53 ± 0.31 | 0.02 |
| N = 46 | N = 14 | N = 43 | N = 17 |
Comparisons within the cataplexy group.
1-5 indicates cataplexy attacks 1/year or less, 1/month, 1/week, several times/week, or 1 or several/day.
Number of awakenings on polysomnography. SOREMPs (sleep onset REM periods), CSF hcrt-1 (hypocretin-1 levels in the cerebrospinal fluid).
Table 2.
Demographic and CSF hcrt-1 data of Danish patients with narcolepsy, other hypersomnias, neurological disorders and normal controls
| Diagnosis | No. of subjects | Gender | Age (years) | CSF hcrt-1 (pg/mL) |
|---|---|---|---|---|
| mean ± SEM (range) | mean ± SEM (range) | |||
| Narcolepsy | ||||
| Narcolepsy with cataplexy | 46 | 20M, 26F | 36.2 ± 2.37 (10-75) | 74.9 ± 21.0 (10-543)* |
| Narcolepsy without cataplexy | 14 | 7M, 7F | 29.6 ± 2.96 (9-48) | 346.9 ± 49.7 (35-563) |
| Other hypersomnias | ||||
| Idiopathic hypersomnia | 13 | 3M,10F | 36.4 ± 4.6 (21-63) | 438.4 ± 18.9 (267-524)** |
| Kleine-Levin (during a hypersomnia period) | 1 | 1F | 15 | 396 |
| Possible narcolepsy without cataplexy | 1† | 1M | 45 | 317 |
| Periodic limb movements (PLMs) | 2 | 2M | 53.0 ± 8.0 (45-61) | 450.0 ± 28.0 (422-478) |
| Post-anoxic hypersomnia | 1‡ | 1M | 34 | 367 |
| Neurological disorders without hypersomnia | ||||
| Multiple sclerosis | 22 | 8M,14F | 39.2 ± 1.8 (27-57) | 454.9 ± 8.7 (385-535) |
| Optic Neuritis | 23 | 8M,15F | 32.3 ± 1.7 (18-48) | 450.1 ± 8.9 (332-518) |
| ADHD | 1 | 1M | 13 | 414 |
| Stroke (sequelae) | 1 | 1F | 61 | 551 |
| Chronic headache | 1 | 1F | 39 | 478 |
| Cluster headache (and previous SAH) | 1 | 1M | 24 | 389 |
| Polyneuropathy | 2 | 2M | 53.0 ± 4.0 (49-57) | 449.5 ± 36.5 (413-486) |
| Neurological disorders with hypersomnia | ||||
| Multiple system atrophy with RBD | 1 | 1M | 59 | 446 |
| Parkinson disease with RBD | 1 | 1M | 61 | 401 |
| Huntington chorea with OSAS and PLMs | 1 | 1M | 64 | 502 |
| Machado-Joseph with PLMs | 2 | 2M | 52.5 ± 4.5 (48-57) | 385.0 ± 20.0 (365-405) |
| Myotonic dystrophy with RBD | 1 | 1M | 41 | 422 |
| Sarcoidosis | 2 | 1M, 1F | 33.0 ± 9.0 (24-42) | 468.5 ± 19.5 (449-488) |
| Normal controls | 29 | 15M, 14F | 34.5 ± 2.7 (9-77) | 429.7 ± 8.8 (343-534) |
| 429.7 ± 95 (mean ± 2SD) |
CSF hcrt-1 (hypocretin-1 value in cerebrospinal fluid), ADHD (attention deficit hyperactivity disorder), SAH (subarachnoidal hemorrhage), RBD (REM sleep behaviour disorder), OSAS (obstructive sleep apnea syndrome),
mean CSF hcrt-1: P < 0.0001 compared to all other diagnoses and normal controls,
(sleepy, no cataplexy, MSLT with short sleep latency and 5 SOREMPs, but loss of PSG data (father of patient with narcolepsy with cataplexy)),
(hypersomnia onset after cardiac revival, normal brain MRI),
7 HLA-types were available; 1/7 was HLA-DQB1*0602-positive (CSF hcrt-1 level 267 pg/mL)
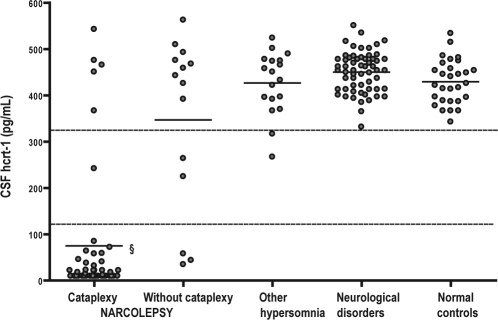
Figure 2.
CSF hcrt-1 values in the Danish patients with narcolepsy with cataplexy, narcolepsy without cataplexy, other hypersomnias, neurological disorders, and normal controls.
Every circle represents the CSF hcrt-1 level of an individual patient or control. The upper horizontal line marks the lower limit of the normal area (2 SD below the mean value of the normal controls). The lower horizontal line marks the cutoff limit for a low CSF hcrt-1 value (30% of the mean value of the normal controls [ICSD-2 criterion]).
§ Significantly lower mean CSF hcrt-1 value compared to all other groups (P < 0.0001).
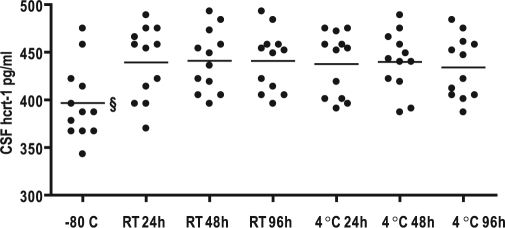
Figure 3.
Temperature and time stability of CSF hcrt-1 levels in normal controls.
Comparison of hcrt-1 levels in CSF immediately frozen (−80°C) or stored for respectively 24, 48, and 96 hours at room temperature (RT) or 4°C before frozen (−80°C). § indicates significantly lower mean value (P < 0.0001)
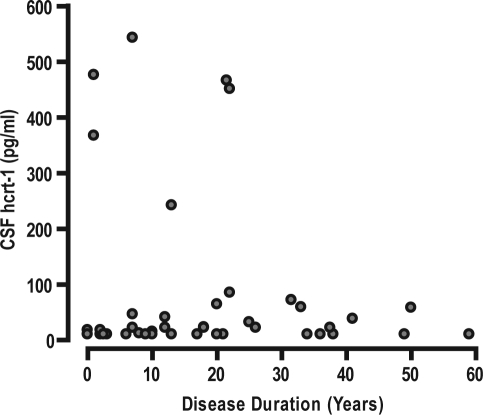
Figure 1.
No correlation between CSF hcrt-1 levels and disease duration of Danish patients with narcolepsy with cataplexy (r = 0.12, P = 0.44). Every circle represents the CSF hcrt-1 level and the disease duration of an individual patient.
RESULTS
The Danish Population
Table 1 shows the demographic data of the narcolepsy population. NC patients had a higher prevalence of hypocretin deficiency (40/46, 87%) and HLA-DQB1*0602-positivity (39/41, 95%) than NwC patients (3/14, 21% low CSF hcrt-1; 4/13, 31% HLA-DQB1*0602-positivity) (P < 0.01). NC patients had higher Epworth Sleepiness Scores (ESS), greater prevalence of hypnagogic hallucination, sleep paralysis, and more nightly awakenings than NwC patients (P < 0.01). There were no significant differences between the NC and NwC groups regarding mean age, percentage of males, mean age at disease onset, disease duration, prevalence of automatic behavior, MSLT mean sleep latency, and number of sleep onset REM periods (SOREMPs).
Within the NC group, 6/46 patients had normal CSF hcrt-1; 4/5 (80%) of these were HLA-DQB1*0602-positive (one HLA-type was unavailable), hence not significantly different from the hypocretin-deficient NC patients (35/36 HLA-DQB1*0602-positive, 97% [4 HLA-types were unavailable]) (P = 0.23) (Table 3). Within the NwC group, hypocretin-deficient patients had a greater prevalence of HLA-DQB1*0602-positivity (3/3, 100%) than patients with normal CSF hcrt-1 (1/10, 10% one HLA-type was unavailable) (P = 0.014) (Table 4).
Within the NC group, but not the NwC group, hypocretin deficiency significantly predicted lower sleep latency (199.6 ± 21 sec; 377.1 ± 96 sec, P = 0.027), more SOREMPs (3.23 ± 0.16; 1.50 ± 0.56, P = 0.005), and a trend towards more nightly awakenings (5.95 ± 0.65; 3.50 ± 1.43, P = 0.13). Both hypocretin deficiency and HLA-DQB1*0602-positivity independently predicted a higher cataplexy frequency (P = 0.03; P = 0.04), but not the location of cataplexy (knee, arms/hands, jaw, head/shoulder, speech) or the prevalence of injurious cataplexy, hypnagogic hallucinations, and sleep paralysis (data not shown). CSF hcrt-1 levels were not correlated with disease duration in NC patients (P = 0.44) (Figure 1), regardless of HLA or cataplexy status (data not shown).
Table 2 shows the demographic and CSF hcrt-1 data of patients and controls. The CSF hcrt-1 data are futher illustrated by Figure 2. Normal controls had a mean CSF hcrt-1 value of 429.7 ± 95 pg/mL (mean ± 2 SD). The Danish low CSF hcrt-1 cutoff value was 129 pg/mL (< 30% of the normal mean). The Danish cutoff value for a normal CSF hcrt-1 value was 336 pg/mL (2 SD below the normal mean). Intermediate CSF hcrt-1 levels (129-335 pg/mL) were found in 1/46 NC patient, 2/14 NwC patients, and 2/18 with other hypersomnias. The remaining patients with other hypersomnias, neurological disorders, and normal controls had normal CSF hcrt-1 levels. The mean CSF hcrt-1 level in NC patients was significantly lower than in all other groups (P < 0.0001). There was no significant difference between the mean CSF hcrt-1 levels of NwC patients, other hypersomnias, neurological disorders, and normal controls.
Stability of CSF Hypocretin-1
CSF hcrt-1 levels were significantly lower (11%) in samples kept at −80°C than those stored at RT or at 4°C (P < 0.0001) (Figure 3). There was no difference in CSF hcrt-1 levels between samples kept at 4°C or RT, regardless of storage time (P = 0.32).
Other Narcolepsy Populations Compared with the Danish Population
It was possible to include 7 studies of NC patients5,6,8,13–16 and 5 studies of NwC patients5,6,8,15,17 through the MEDLINE search. Sixteen eligible studies were excluded because they overlapped with included study populations (marked in bold): Martinez-Rodriguez et al., 200718 included in Martinez-Rodriguez et al., 20075; Kanbayashi et al., 200219, Kubota et al., 200320 and Tsukamoto et al., 200221 included in Arii et al., 200413 and Kanbayashi et al., 200315; Hong et al., 200222, Jeong et al., 20077 included in Hong et al., 200623 (later included in Bourgin et al., 2008)8; Arnulf et al., 200624, Nishino et al., 200025 and 200126, Overeem et al. 200427 and 200628, and Ripley et al., 200129 included in Mignot et al., 200212 (later included in Bourgin et al., 2008)8; Lin et al., 200730, and Bassetti et al., 200331 included in Bourgin et al., 2008.8
Tables 3 and 4 show the (re)calculated ICSD-2 CSF hcrt-1 results of the Danish and the other included NC and NwC populations.
Hypocretin deficiency and HLA-DQB1*0602-positivity were found in the majority of NC patients (80% to 100%) with no significant differences between the populations (P = 0.53; P = 0.11). Detailed HLA-data available from 5/7 studies 6,8,13,15,16 were compared with the Danish data. Only 3/186 (1.6%) hypocretin-deficient NC patients were HLA-DQB1*0602-negative. Among the NC patients with normal CSF hcrt-1 levels, 20% to 100% were HLA-DQB1*0602-negative (Table 3).
Hypocretin deficiency and HLA-DQB1*0602-positivity were found in the minority of NwC patients (11% to 29% 29% to 89%) with no significant differences between the populations regarding hypocretin deficiency (P = 0.75) but a marginally higher HLA-DQB1*0602-positivity in the French population (P = 0.043). A total of only 5/41 hypocretin-deficient NwC patients were HLA-DQB1*0602-negative (Table 4).
DISCUSSION
The present study shows that the ICSD-2 criterion for low CSF hcrt-1 (< 30% of the normal mean) is valid for diagnosing NC, but not NwC, in Danes. The association between hypocretin deficiency and HLA-DQB1*0602-positivity is well established and is confirmed in the present study, since only 1/43 hypocretin-deficient patients was HLA-DQB1*0602-negative. Moreover, hypocretin-deficient NC patients seem to present a more severe phenotype than NC patients with normal CSF hcrt-1 levels.
The hypocretin neuropeptides are believed to stabilize the inhibition of REM sleep onset and REM sleep atonia mainly through excitation of REM-off neurons,32 and to consolidate wakefulness through excitation of wake-promoting neurons.33 It is plausible that the almost total loss of hypocretin neurons found in most NC patients1,2 causes instability of sleep-wake, REM sleep onset, and REM sleep atonia regulation. The corresponding clinical manifestation could be the severe hypocretin-deficient NC phenotype that we observed, characterized by more frequent cataplectic attacks (intrusions of REM sleep atonia), increased sleepiness (lower sleep latency), more intrusions of REM sleep (SOREMPs), and more fragmented sleep (more awakenings). The prevalence of hypnagogic hallucinations and sleep paralysis was similar in the two NC subgroups, as indicated previously.4 This suggests that these phenomena may involve neuronal systems other than the hypocretin system.
The study results also indicate that this NC phenotype emerges when the hypocretin neurons are already largely lost, because disease duration and CSF hcrt-1 levels were not correlated, as also indicated previously.4,8 Moreover, two Danish NC children were already hypocretin deficient (CSF hcrt-1, 20 pg/mL and 24 pg/mL) 3 and 6 months after disease onset, respectively, in accordance with case reports from North America,12 Asia,13,22 and Central Europe.16,34,35
Our finding of a milder phenotype in the subgroup of NC patients with normal CSF hcrt-1 is supported by less daytime sleepiness,14 longer sleep latency,14 and fewer SOREMPs14,19 in other NC patients with normal CSF hcrt-1. Moreover, less extensive cataplexy was found in Norwegian NC patients with normal CSF hcrt-1,4 and less extensive and less frequent cataplexy were reported in HLA-negative NC patients (of unknown CSF hcrt-1 status).36 The pathogenesis in NC or NwC with normal CSF hcrt-1 levels is unclear. Dysfunctions downstream in the hypocretin system or a partial destruction of hypocretin neurons without resulting in low CSF hcrt-1 levels2 could be possible explanations. As hypocretin cell destruction is believed to be caused by an autoimmune attack, the preserved high prevalence of HLA-DQB1*0602-positivity in the milder NC phenotype in the Danish (80%) and Norwegian population (75%)4 favors the latter hypothesis in this subgroup.
Regarding the CSF hcrt-1 measurements, we found that it was essential to adjust for inter-assay variability by reference control CSF samples, in accordance with the current recommadations.8 Moreover, a small but significant difference in CSF hcrt-1 levels depending on the storage time and temperature was observed. This would need to be taken into account to ensure the correct diagnosis of cases whose levels lie close to the cutoff limits. Our finding is not at first sight consistent with the stable CSF hcrt-1 levels after repeated CSF freezing/thawing found by another laboratory,12 although this may be due to differences in that laboratory's protocols (it is not known whether CSF had been frozen before first thawing).
Extrapolation of CSF hcrt-1 results have previously been hampered by the inter-assay variability of the available radioimmunoassay and different definitions of hypocretin deficiency (i.e., all values under the local normal area,15 detectable versus undetectable levels14 or < 110 pg/mL as a fixed standard3). Considering the variable normal mean values and consequently variable low cutoff limits of individual laboratories (88-149 pg/mL, Tables 3 and 4), a fixed standard value for hypocretin deficiency (which is proposed as a possibility by ICSD-2) is obviously currently problematic. However, as CSF hcrt-1 levels of individual patient samples were found to be highly correlated when determined in different laboratories (in spite of different CSF hcrt-1 level ranges),37 this suggests that it would be possible to establish an international fixed standard value for hypocretin deficiency if all laboratories agreed to use reference samples from the same pooled CSF batches.
To our knowledge, the ICSD-2 criterion for low CSF hcrt-1 (< 30% of the normal mean) has not previously been used to compare study results from different laboratories directly. We found that this criterion is valid as a sensitive and specific diagnostic tool for NC patients but not NwC patients, across all included nationalities and ethnic groups. In all included NC and NwC populations, the CSF hcrt-1 levels clustered in two groups; below the local ICSD-2 low cutoff limit, or within the normal area of the individual sleep laboratory (mean ± 2 SD). A total of only 8/158 hypocretin-deficient NC and NwC patients were HLA-DQB1*0602-negative (Table 3 and 4).8 Intermediate values were rare and most often occurred in the proximity of the cutoff limits. In all included studies, only one patient with low CSF hcrt-1 levels (idiopathic hypersomnia)5 and three patients with intermediate CSF hcrt-1 levels (Kleine-Levin Syndrome [2], obstructive sleep apnea [1])8 were detected in any of the control groups.
So, even if the ICSD-2 criterion for low CSF hcrt-1 was a very sensitive and specific diagnostic tool across different populations, it could be argued that its clinical use would be limited: low CSF hcrt-1 mainly detects clear-cut NC patients who are already relatively easy to recognize and diagnose with the conventional diagnostic tools (ICSD-2-defined cataplexy, PSG, and MSLT).
However, CSF hcrt-1 measurements can be the determinant diagnostic tool in cases of doubtful cataplexy, intolerance to sleep investigations (e.g., children), and inconclusive sleep investigations caused by additional sleep disorders, psychiatric disorders, or ongoing medication (e.g., anti-cataplexy drugs, stimulant treatment/abuse). As HLA-DQB1*0602-negativity is extremely rare in hypocretin-deficient patients, CSF hcrt-1 measurements are not diagnostically relevant in this patient category. For diagnostic purposes, it is therefore recommended that HLA-typing precede CSF hcrt-1 measurements.
There are some limitations of our study that should be borne in mind when interpreting the results. The Danish neurological controls did not include disorders in which hypocretin deficiency had been (casuistically) reported (e.g., Guillain Barr<940>e Syndrome [in Asian38 but not Caucasian39 patients], hypothalamic lesions)8 which could have led to overestimation of the specificity of the test. All NwC patients in Table 4 were diagnosed solely by ICSD-2 PSG and MSLT criteria, which is a matter for broader discussion since 1% to 6% of the general population fulfilled these criteria in a recent study.40 As hypocretin deficiency is associated with cataplexy, atypical cataplexy and cataplexy-like phenomena were excluded from the cataplexy group by a validated cataplexy questionnaire9 and interview in the Danish study. It is therefore less likely that the observed milder NC phenotype with normal CSF hcrt-1 was caused by cataplexy misclassification, as is further supported by the HLA-DQB1*0602-positivity of the group. Likewise, ICSD-2-defined cataplexy was an inclusion criterion of the NC populations identified by the MEDLINE search. Finally, the limited sample size of NC patients with normal CSF hcrt-1, hypocretin-deficient NwC patients, and normal controls in the smaller studies of Tables 3 and 4 imply a risk of producing type II errors.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We thank the corresponding researchers from the collaborating sleep laboratories for their e-mail correspondence, Jens Hallgren Romlund and Birte Kofoed for practical help with hcrt-1 analyses, and Svend Kreiner (Biostatistic Institute, University of Copenhagen) for statistical assistance. The research was supported by the Copenhagen County Research Foundation and the Eilif Trier Hansen and Wife Ane Trier Hansen Foundation.
REFERENCES
- 1.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–7. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 2.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–74. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Academy of Sleep Medicine. International classification of sleep disorders, 2nd ed.: diagnostic and coding manual. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 4.Heier MS, Evsiukova T, Vilming S, Gjerstad MD, Schrader H, Gautvik K. CSF hypocretin-1 levels and clinical profiles in narcolepsy and idiopathic CNS hypersomnia in Norway. Sleep. 2007;30:969–73. doi: 10.1093/sleep/30.8.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez-Rodriguez JE, Iranzo A, Casamitjana R, Graus F, Santamaria J. [Comparative analysis of patients with narcolepsy-cataplexy, narcolepsy without cataplexy and idiopathic hypersomnia] Med Clin (Barc) 2007;128:361–4. doi: 10.1157/13099970. [DOI] [PubMed] [Google Scholar]
- 6.Dauvilliers Y, Baumann CR, Carlander B, et al. CSF hypocretin-1 levels in narcolepsy, Kleine-Levin syndrome, and other hypersomnias and neurological conditions. J Neurol Neurosurg Psychiatry. 2003;74:1667–73. doi: 10.1136/jnnp.74.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong JH, Hong SC, Shin YK, Han JH, Lee SP. HLA-DQB1 allele and hypocretin in Korean narcoleptics with cataplexy. J Korean Med Sci. 2007;22:127–31. doi: 10.3346/jkms.2007.22.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourgin P, Zeitzer JM, Mignot E. CSF hypocretin-1 assessment in sleep and neurological disorders. Lancet Neurol. 2008;7:649–62. doi: 10.1016/S1474-4422(08)70140-6. [DOI] [PubMed] [Google Scholar]
- 9.Anic-Labat S, Guilleminault C, Kraemer HC, Meehan J, Arrigoni J, Mignot E. Validation of a cataplexy questionnaire in 983 sleep-disorders patients. Sleep. 1999;22:77–87. [PubMed] [Google Scholar]
- 10.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 11.Knudsen S, Jennum PJ, Korsholm K, Sheikh SP, Gammeltoft S, Frederiksen JL. Normal levels of cerebrospinal fluid hypocretin-1 and daytime sleepiness during attacks of relapsing-remitting multiple sclerosis and monosymptomatic optic neuritis. Mult Scler. 2008;14:734–8. doi: 10.1177/1352458508088939. [DOI] [PubMed] [Google Scholar]
- 12.Mignot E, Lammers GJ, Ripley B, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–62. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 13.Arii J, Kanbayashi T, Tanabe Y, et al. CSF hypocretin-1 (orexin-A) levels in childhood narcolepsy and neurologic disorders. Neurology. 2004;63:2440–2. doi: 10.1212/01.wnl.0000147328.15956.b4. [DOI] [PubMed] [Google Scholar]
- 14.Baumann CR, Khatami R, Werth E, Bassetti CL. Hypocretin (orexin) deficiency predicts severe objective excessive daytime sleepiness in narcolepsy with cataplexy. J Neurol Neurosurg Psychiatry. 2006;77:402–4. doi: 10.1136/jnnp.2005.067207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanbayashi T, Inoue Y, Kawanishi K, et al. CSF hypocretin measures in patients with obstructive sleep apnea. J Sleep Res. 2003;12:339–41. doi: 10.1046/j.0962-1105.2003.00373.x. [DOI] [PubMed] [Google Scholar]
- 16.Serra L, Montagna P, Mignot E, Lugaresi E, Plazzi G. Cataplexy features in childhood narcolepsy. Mov Disord. 2008;23:858–65. doi: 10.1002/mds.21965. [DOI] [PubMed] [Google Scholar]
- 17.Oka Y, Inoue Y, Kanbayashi T, et al. Narcolepsy without cataplexy: 2 subtypes based on CSF hypocretin-1/orexin-A findings. Sleep. 2006;29:1439–43. doi: 10.1093/sleep/29.11.1439. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Rodriguez JE, Sabater L, Graus F, Iranzo A, Santamaria J. Evaluation of hypothalamic-specific autoimmunity in patients with narcolepsy. Sleep. 2007;30:27–8. doi: 10.1093/sleep/30.1.27. [DOI] [PubMed] [Google Scholar]
- 19.Kanbayashi T, Inoue Y, Chiba S, et al. CSF hypocretin-1 (orexin-A) concentrations in narcolepsy with and without cataplexy and idiopathic hypersomnia. J Sleep Res. 2002;11:91–3. doi: 10.1046/j.1365-2869.2002.00284.x. [DOI] [PubMed] [Google Scholar]
- 20.Kubota H, Kanbayashi T, Tanabe Y, et al. Decreased cerebrospinal fluid hypocretin-1 levels near the onset of narcolepsy in 2 prepubertal children. Sleep. 2003;26:555–7. doi: 10.1093/sleep/26.5.555. [DOI] [PubMed] [Google Scholar]
- 21.Tsukamoto H, Ishikawa T, Fujii Y, Fukumizu M, Sugai K, Kanbayashi T. Undetectable levels of CSF hypocretin-1 (orexin-A) in two prepubertal boys with narcolepsy. Neuropediatrics. 2002;33:51–2. doi: 10.1055/s-2002-23601. [DOI] [PubMed] [Google Scholar]
- 22.Hong SC, Leen K, Park SA, et al. HLA and hypocretin studies in Korean patients with narcolepsy. Sleep. 2002;25:440–4. [PubMed] [Google Scholar]
- 23.Hong SC, Lin L, Jeong JH, et al. A study of the diagnostic utility of HLA typing, CSF hypocretin-1 measurements, and MSLT testing for the diagnosis of narcolepsy in 163 Korean patients with unexplained excessive daytime sleepiness. Sleep. 2006;29:1429–38. doi: 10.1093/sleep/29.11.1429. [DOI] [PubMed] [Google Scholar]
- 24.Arnulf I, Lin L, Zhang J, et al. CSF versus serum leptin in narcolepsy: is there an effect of hypocretin deficiency? Sleep. 2006;29:1017–24. doi: 10.1093/sleep/29.8.1017. [DOI] [PubMed] [Google Scholar]
- 25.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 26.Nishino S, Ripley B, Overeem S, et al. Low cerebrospinal fluid hypocretin (Orexin) and altered energy homeostasis in human narcolepsy. Ann Neurol. 2001;50:381–8. doi: 10.1002/ana.1130. [DOI] [PubMed] [Google Scholar]
- 27.Overeem S, Dalmau J, Bataller L, et al. Hypocretin-1 CSF levels in anti-Ma2 associated encephalitis. Neurology. 2004;62:138–40. doi: 10.1212/01.wnl.0000101718.92619.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Overeem S, Verschuuren JJ, Fronczek R, et al. Immunohistochemical screening for autoantibodies against lateral hypothalamic neurons in human narcolepsy. J Neuroimmunol. 2006;174:187–91. doi: 10.1016/j.jneuroim.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Ripley B, Overeem S, Fujiki N, et al. CSF hypocretin/orexin levels in narcolepsy and other neurological conditions. Neurology. 2001;57:2253–8. doi: 10.1212/wnl.57.12.2253. [DOI] [PubMed] [Google Scholar]
- 30.Lin L, Mignot E. Human leukocyte antigen and narcolepsy: present status and relationship with familial history and hypocretin deficiency. In: Bassetti C, Billiard M, Mignot E, editors. Narcolepsy and hypersomnia. New York: Informa Health Care; 2007. pp. 411–26. [Google Scholar]
- 31.Bassetti C, Gugger M, Bischof M, et al. The narcoleptic borderland: a multimodal diagnostic approach including cerebrospinal fluid levels of hypocretin-1 (orexin A) Sleep Med. 2003;4:7–12. doi: 10.1016/s1389-9457(02)00191-0. [DOI] [PubMed] [Google Scholar]
- 32.Boeve BF, Silber MH, Saper CB, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130(Pt 11):2770–8. doi: 10.1093/brain/awm056. [DOI] [PubMed] [Google Scholar]
- 33.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 34.Dauvilliers Y. Follow-up of four narcolepsy patients treated with intravenous immunoglobulins. Ann Neurol. 2006;60:153. doi: 10.1002/ana.20892. [DOI] [PubMed] [Google Scholar]
- 35.Lecendreux M, Maret S, Bassetti C, Mouren MC, Tafti M. Clinical efficacy of high-dose intravenous immunoglobulins near the onset of narcolepsy in a 10-year-old boy. J Sleep Res. 2003;12:347–8. doi: 10.1046/j.1365-2869.2003.00380.x. [DOI] [PubMed] [Google Scholar]
- 36.Mignot E, Hayduk R, Black J, Grumet FC, Guilleminault C. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients. Sleep. 1997;20:1012–20. [PubMed] [Google Scholar]
- 37.Baumann CR, Dauvilliers Y, Mignot E, Bassetti CL. Normal CSF hypocretin-1 (orexin A) levels in dementia with Lewy bodies associated with excessive daytime sleepiness. Eur Neurol. 2004;52:73–6. doi: 10.1159/000079749. [DOI] [PubMed] [Google Scholar]
- 38.Nishino S, Kanbayashi T, Fujiki N, et al. CSF hypocretin levels in Guillain-Barre syndrome and other inflammatory neuropathies. Neurology. 2003;61:823–5. doi: 10.1212/01.wnl.0000081049.14098.50. [DOI] [PubMed] [Google Scholar]
- 39.Baumann CR, Bassetti CL. CSF hypocretin levels in Guillain-Barre syndrome and other inflammatory neuropathies. Neurology. 2004;62:2337. doi: 10.1212/wnl.62.12.2337. [DOI] [PubMed] [Google Scholar]
- 40.Mignot E, Lin L, Finn L, et al. Correlates of sleep-onset REM periods during the multiple sleep latency test in community adults. Brain. 2006;129(Pt 6):1609–23. doi: 10.1093/brain/awl079. [DOI] [PubMed] [Google Scholar]