Abstract
Study Objectives:
To investigate differences in brain gray matter concentrations or volumes in patients with obstructive sleep apnea syndrome (OSA) and healthy volunteers.
Designs:
Optimized voxel-based morphometry, an automated processing technique for MRI, was used to characterize structural differences in gray matter in newly diagnosed male patients.
Setting:
University hospital
Patients and Participants:
The study consisted of 36 male OSA and 31 non-apneic male healthy volunteers matched for age (mean age, 44.8 years).
Interventions:
Using the t-test, gray matter differences were identified. The statistical significance level was set to a false discovery rate P < 0.05 with an extent threshold of kE > 200 voxels.
Measurements and Results:
The mean apnea-hypopnea index (AHI) of patients was 52.5/ h. On visual inspection of MRI, no structural abnormalities were observed. Compared to healthy volunteers, the gray matter concentrations of OSA patients were significantly decreased in the left gyrus rectus, bilateral superior frontal gyri, left precentral gyrus, bilateral frontomarginal gyri, bilateral anterior cingulate gyri, right insular gyrus, bilateral caudate nuclei, bilateral thalami, bilateral amygdalo-hippocampi, bilateral inferior temporal gyri, and bilateral quadrangular and biventer lobules in the cerebellum (false discovery rate P < 0.05). Gray matter volume was not different between OSA patients and healthy volunteers.
Conclusions:
The brain gray matter deficits may suggest that memory impairment, affective and cardiovascular disturbances, executive dysfunctions, and dysregulation of autonomic and respiratory control frequently found in OSA patients might be related to morphological differences in the brain gray matter areas.
Citation:
Joo EY; Tae WS; Lee MJ; Kang JW; Park HS; Lee JY; Suh M; Hong SB. Reduced brain gray matter concentration in patients with obstructive sleep apnea syndrome. SLEEP 2010;33(2):235-241.
Keywords: Obstructive sleep apnea, Brain, Gray matter concentration, MRI, Voxel based morphometry
OBSTRUCTIVE SLEEP APNEA SYNDROME (OSA) IS A DISORDER CHARACTERIZED BY EXCESSIVE SNORING AND PERIODIC BREATHING WITH REPETITIVE apneas and arousals, which leads to fragmented sleep.1 OSA may cause daytime sleepiness, reduce work performance, increase the risk of a traffic accident, and diminish quality of life.2 Neurocognitive problems, such as deficits in memory, attention, and visuoconstructive abilities, frequently accompany OSA.3 The pathophysiology of these deficits remains controversial, although the main contributory factors are presumed to be sleep fragmentation and intermittent nocturnal hypoxemia during sleep apneas. Deterioration of cognitive performance has been significantly correlated with the degree of nocturnal hypoxemia4 and with the severity of nocturnal breathing irregularities.5 The structural changes seen on brain MRI imply a chronic state of disease rather than functional change of glucose metabolism or regional blood flow. Previous studies show that OSA patients have hippocampal atrophy,6 selective reduced gray matter volume in the hippocampus and parahippocampal gyrus,7,8 and frontoparietal cortices, temporal lobe, anterior cingulate, and cerebellum.9 This may suggest neuronal cell loss in untreated OSA brains and that significant morphologic changes in OSA brains may partly explain the incomplete modulation of upper airway motor function or cardiovascular and respiratory control during sleep,9 as well as cognitive decline during wakefulness.6 However, other investigators could not find any gray matter volume deficits or focal structural changes in patients with severe OSA compared to healthy volunteers.10 It has been presumed that inconsistent neuroimaging findings may reflect discrepancies in the procedure of data analysis or the characteristics of study subjects.11 Previous studies investigated gray matter volume (GMV) changes only.7,9,10 In this study, we tried to investigate not only GMV but also gray matter concentration (GMC) changes, using optimized VBM with modulated or unmodulated images. We hypothesized that severe patients with severe OSA, despite having visually normal brain MRIs, may show a decrease of GMV or GMC of brain compared to age-matched healthy volunteers.
METHODS
Patients and Healthy Volunteers
Forty-two male patients with severe OSA and 31 age-matched healthy volunteers were recruited. Patients were recruited from the sleep disorder clinic of the Samsung Medical Center located in South Korea. Inclusion criteria were as follows: (1) male; (2) older than 18 years but younger than 55 years old; and (3) an apnea-hypopnea index > 30. Thirty-one age-matched healthy volunteers were recruited using an advertisement in a local community. Each candidate had a detailed clinical interview, sleep questionnaire, and overnight polysomnography. Volunteer candidates with evidence of OSA (apnea-hypopnea index [AHI] > 5 measured by thermistor) or other sleep disorders were excluded. Exclusion criteria for patients and healthy volunteers were: (1) mean daily sleep time 6-7 h; (2) abnormal sleep-wake rhythm; (3) other sleep disorders, such as restless legs syndrome, periodic limb movement during sleep, sleep-related eating disorders, narcolepsy, and insomnia; (4) hypertension, diabetes, heart, and respiratory diseases; (5) history of cerebrovascular disease; (6) other neurological (neurodegenerative diseases, epilepsy, head injury) or psychiatric diseases (psychosis, current depression); (7) alcohol or illicit drug abuse or current intake of psychoactive medications; and (8) structural lesion on brain MRI. Six patients with diffuse brain atrophy on brain MRI were excluded from the patient group. Finally, 36 patients and 31 healthy volunteers were included in the present study.
Informed consent was obtained from all subjects and the institutional review board of our hospital authorized the study protocol.
Overnight Polysomnography
The day before sleep studies, subjects were asked not to drink alcohol or caffeinated beverages. Sleep studies were recorded using an Alice-3 system (Healthdyne, USA) or a Somnologica system (Embla, USA). Overnight polysomnography was performed using a 4-channel electroencephalogram (EEG, C3/A2; C4/A1; O1/A2; O2/A1), 4-channel electrooculogram (EOG), electromyogram (EMG; submental, intercostal, and anterior tibialis muscles), and an electrocardiogram with surface electrodes. A thermistor (for monitoring nasal airflow), a nasal air pressure monitor, an oximeter (for measuring oxygen saturation), piezoelectric bands (for determining thoracic and abdominal wall motion), and a body position sensor were also attached to patients. Subjects were recorded on videotape using an infrared video camera and were continuously observed by a polysomnography technician. Subjects went to bed at 23:00 and were awakened at 07:00. Sleep architecture was scored in 30-sec epochs, and sleep staging was interpreted according to the standard criteria of Rechtschaffen and Kales.
Apneas and hypopneas were defined by criteria.12 An obstructive apnea was defined as a reduction in airflow > 90% lasting ≥ 10 s during which there was evidence of persistent respiratory effort. A hypopnea was defined as reduction in airflow by 50% with duration ≥ 10 s or reduction of airflow by 30% > 10 s, accompanied by EEG arousal and/or a 3% or greater oxygen desaturation.
According to the American Sleep Disorders Association Task Force criteria,13 arousals were classified as breathing-related arousals (occurring within 3 s following apnea, hypopnea, or snoring) and other type of arousals (spontaneous arousal, periodic limb movement-associated arousals).
Assessment of Excessive Daytime Sleepiness (EDS)
Subjective sleepiness was assessed on the Epworth Sleepiness Scale (ESS), a simple self-administered questionnaire with 8-item and 4-point scales that evaluate daytime somnolence among subjects suffering from sleep-wake disorders.14 Objective sleepiness was evaluated by Multiple Sleep Latency Test (MSLT).15 In brief, MSLT consists of a series of five 20-min naps at 2-h intervals in the morning and the afternoon. Patients are asked to try to sleep in a dark room with a montage of EEG, EOG, and EMG. The mean of the individual latencies to sleep onset from light-off was calculated in 5 nap trials. A final diagnosis of OSA was based on overnight polysomnography findings and associated clinical symptoms.
Magnetic Resonance Imaging (MRI)
MRI scanning was performed using a GE Signa 1.5 Tesla scanner (GE Medical Systems, Milwaukee, WI). All subjects underwent SPGR, T2-weighted, and fluid attenuated inversion recovery (FLAIR) imaging protocols. Coronal SPGR MR images were obtained using the following scanning variables; 1.6 mm thickness, no gap, 124 slices, repetition time/echo time (TR/TE) = 30/7 msec, flip angle (FA) = 45°, number of excitations (NEX) = 1, matrix = 256 × 192, and field of view (FOV) = 22 × 22 cm. The voxel dimension of SPGR MR images was 0.86 × 0.86 × 1.6 mm. Oblique coronal FLAIR MRI was performed using; a 4.0 mm slice thickness, 1.0 mm gap, 32 slices, TR/TE = 10002/127.5 msec, 1 NEX, matrix = 256 × 192, and FOV = 20 × 20 cm. Oblique coronal T2-weighted MR images were obtained with a 3.0 mm slice thickness, 0.3 mm gap, 56 slices, TR/TE = 5300/99 msec, FA = 90o, 3 NEX, matrix = 256 × 192, and FOV = 20 × 20 cm.
Voxel-Based Morphometry
Using SPM2 (Wellcome Department of Cognitive Neurology, Institute of Neurology, University College London) and MATLAB 7.0 (MathWorks, MA, USA), an optimized voxel-based morphometry (VBM) protocol16 was performed to determine the abnormality of GMC in patients with OSA.
To create customized templates and prior images of gray matter and white matter, all T1 MR images of the patients with OSA and healthy volunteers were spatially normalized to the MNI (ICBM 152) T1 template (the Montreal Neurological Institute standard T1 template). Spatial normalizations were applied using the following parameters: voxel size = 1 ×x 1 × 1 mm, cutoff spatial normalization 25 mm cutoff, nonlinear regularization = medium, and 16 nonlinear iterations. Normalized images were segmented into gray matter, white matter, CSF, and sub-sampled into a voxel size of 2 × 2 × 2 mm. To remove isolated voxels of one tissue class which were unlikely to be member of this tissue type, Hidden Markov Random Field model was applied in all segmentation processes. Spatially normalized and sub-sampled gray matter, white matter, and CSF images were averaged and saved into the customized T1 template, gray matter, white matter, or CSF prior images, respectively. Finally, 3 prior images and the customized T1 template were smoothed using an 8 mm full-width at half-maximum Isotropic Gaussian Kernel.
The raw T1 images of all subjects were automatically segmented into gray matter, white matter, and CSF partitions in naive space. Spatial normalization parameters were estimated by spatially normalizing individual gray matter to the study specific gray matter template of this study. Then, using the normalization parameter of the segmented gray matter image, raw T1 image was normalized to MNI space.
Spatially normalized T1 images were segmented into gray matter, white matter, and CSF partitions in MNI space using HMRF operation and prior images (gray matter, white matter, and CSF partitions), and the spatially normalized, segmented gray matter images were modulated by the Jacobian determinants derived from spatial normalization for regional volume change analysis. Finally unmodulated gray matter images smoothed using a 12-mm FWHM IGK, and modulated gray matter images were smoothed using an 8 mm FWHM IGK to apply same smoothing effect compared to unmodulated image. The final voxel sizes of all unmodulated or modulated gray matter images were 1 × 1 × 1 mm.
Statistics
A one-way ANCOVA with covariate of age was used for the VBM of unmodulated images for studying the GMC differences, and one-way ANCOVA with covariates of age and intracranial volume was used for the VBM of modulated images for investigating the GMV differences.17 To correct for multiple comparisons, the results were corrected using a false discovery rate (FDR) at a corrected P < 0.05 level, and voxel clusters were corrected with extent threshold of kE > 200 voxels. Multiple regression analyses for age, apnea-hypopnea indices, arousal index, ESS, and gray matter concentrations or volumes were performed with SPM2 using a whole brain mask. Coordinates were defined as by the Montreal Neurological Institute (MNI) coordinate system and cluster regions were named as described in Henri M. Duvernoy's atlas.18
RESULTS
Clinical Characteristics
All patients and healthy volunteers were right-handed and mostly middle-aged (mean 44.7 years). We inspected T1, T2, and FLAIR images to exclude patients or healthy volunteers with gross structural abnormality of brain MRI. All subjects underwent overnight polysomnography. Detailed overnight polysomnographic findings in OSA patients and healthy volunteers were summarized in Table 1. With the exception of sleep latency, all sleep related parameters in PSG were worse in patients than in healthy volunteers. OSA patients had more sleep disordered breathing (higher apnea-hypopnea indices) and more awakening from sleep (higher arousal indices). Sleep structures were more fragmented in patients (more stage 1 and 2 sleep, less REM sleep and slow wave sleep). Two of 36 patients had periodic limb movements during sleep (PLMS; total PLMX index, 47.6/h and 74.1/h; movement arousal index, 5.2/h and 9.0/h); this was not seen in any healthy volunteers.
Table 1.
Patients characteristics
| OSA patients (n = 36) | Healthy volunteers (n = 31) | P | |
|---|---|---|---|
| Mean age, y | 44.7 ± 6.7 (31-56) | 44.8 ± 5.4 (30-54) | 0.473 |
| Body mass index, kg/m2 | 26.0 ± 2.7 (22-35) | 25.8 ± 3.3 (22-30) | 0.254 |
| Overnight Polysomnography | |||
| Mean sleep latency, min | 28.1 ± 52.6 (1-60) | 20.5 ± 20.4 (4.5- 48) | 0.068 |
| Mean AHI, per h | 52.5 ± 21.7 (31.6-105.9) | 2.8 ± 0.9 (1.5 - 3.8) | < 0.001* |
| Mean AI, per h | 57.1 ± 14.3 (19.7-105.4) | 11.4 ± 6.9 (5.2-17.9) | < 0.001* |
| Stage 1, % | 49.7 ± 20.1 (11.5-75.5) | 7.8 ± 13.5 (4.0-15.3) | < 0.001* |
| Stage 2, % | 39.6 ± 17.0 (10.5-73.0) | 55.5 ± 21.5 (35.5-68.4) | 0.032* |
| Stage 3+4, % | 0.9 ± 5.9 (0-17.2) | 11.3 ± 4.5 (7.9-15.3) | < 0.001* |
| REM, % | 9.8 ± 5.9 (0-28.8) | 24.5 ± 6.8 (18.3-30.1) | < 0.001* |
| % TST 90% | 13.2 ± 16.9 (0-58.9) | 0.15 ± 0.18 (0-0.39) | < 0.001 |
| Mean ESS | 10.4 ± 3.7 (2-22) | 3.3 ± 2.4 (0-8) | < 0.001* |
| Mean SSS | 2.5 ± 1.7 (1-6) | 1.7 ± 1.3 (0 -4) | 0.015* |
REM, rapid eye movement; AHI, apnea-hypopnea index; AI, arousal index; % TST < 90%, percentage of total sleep time spent at oxygen saturations less than 90% ESS, Epworth sleepiness scale; SSS, Stanford sleepiness scale.
All values are expressed as mean ± SD, and the range of values is parenthesized.
Independent t-test, P < 0.05
Eight patients underwent MSLT on the day following overnight polysomnography because they complained of severe daytime sleepiness (> 16 in ESS). Mean sleep latency for MSLT was 9.5 ± 4.1 min (5.9–15.5). No sleep onset REM periods were observed.
Regional Difference of Gray Matter Concentrations between OSA Patients and Healthy Volunteers (Table 2)
Table 2.
Significant reduction of gray matter concentration in patients with severe obstructive sleep apnea syndrome
| Location | Side | MNI Coordinates (mm) |
T | *Corrected P | uncorrected P | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Gyrus rectus | L | −8 | 27 | −23 | 5.03 | 0.001 | 0.000002 |
| Superior frontal gyrus | L | −9 | −4 | 67 | 5.84 | < 0.001 | < 0.000001 |
| R | 22 | −3 | 65 | 3.92 | 0.006 | 0.00011 | |
| Precentral gyrus | L | −7 | −37 | 72 | 4.55 | 0.002 | 0.000012 |
| Cingulate gyrus | L | −6 | 36 | 23 | 4.93 | 0.002 | 0.000003 |
| R | 8 | 36 | 19 | 5.21 | < 0.001 | 0.0000011 | |
| Frontomarginal gyrus | L | −9 | 60 | −15 | 4.25 | 0.003 | 0.000035 |
| R | 9 | 62 | −14 | 5.63 | < 0.001 | < 0.000001 | |
| Insular gyrus | R | 34 | −10 | 10 | 3.97 | 0.006 | 0.00009 |
| Caudate nucleus | L | −17 | −3 | 20 | 3.40 | 0.014 | 0.00058 |
| R | 15 | 15 | 11 | 3.12 | 0.024 | 0.0014 | |
| Thalamus | L | −12 | −23 | 9 | 4.32 | 0.003 | 0.000028 |
| R | 14 | −23 | 8 | 4.66 | 0.002 | 0.0000083 | |
| Amygdala | L | −24 | −2 | −23 | 4.90 | 0.002 | 0.0000034 |
| R | 23 | −2 | −24 | 4.72 | 0.002 | 0.0000066 | |
| Hippocampus | L | −25 | −9 | −27 | 5.31 | < 0.001 | < 0.000001 |
| R | 21 | −8 | −24 | 5.92 | < 0.001 | < 0.000001 | |
| Inferior temporal gyrus | L | −28 | 3 | −42 | 5.03 | < 0.001 | 0.000002 |
| L | −50 | −3 | −38 | 4.54 | 0.002 | 0.000013 | |
| L | −61 | −23 | −21 | 3.83 | 0.007 | 0.00015 | |
| R | 27 | 4 | −42 | 5.76 | < 0.001 | < 0.000001 | |
| R | 52 | −4 | −35 | 5.73 | < 0.001 | < 0.000001 | |
| R | 64 | −26 | −18 | 4.58 | 0.002 | 0.000011 | |
| Quadrangular lobule (cerebellum) | L | −23 | −39 | −28 | 4.62 | 0.002 | 0.0000095 |
| R | 12 | −37 | −26 | 4.51 | 0.002 | 0.000014 | |
| Biventer lobule (cerebellum) | L | −32 | −46 | −53 | 4.80 | 0.001 | 0.000005 |
| R | 26 | −50 | −54 | 6.89 | < 0.001 | < 0.000001 | |
MNI: Montreal Neurologic Institute, B: bilateral, L: left, R: right
Significant at the false discovery rate (FDR) corrected P, Extent threshold Ke > 200
In patients with severe OSA, gray matter concentrations were significantly reduced in the ventromedial frontal cortex (bilateral superior frontal gyri, left gyrus rectus, and bilateral frontomarginal gyri), bilateral anterior cingulate gyri, right anterior insular gyrus, bilateral caudate nuclei, bilateral thalami, bilateral amygdalo-hippocampal gyri, bilateral inferior temporal gyri, and bilateral cerebellar cortices (quadrangular and biventer lobules) at FDR corrected P < 0.05 (Figure 1). Gray matter concentrations were not increased in any brain region of OSA patients compared to healthy volunteers.
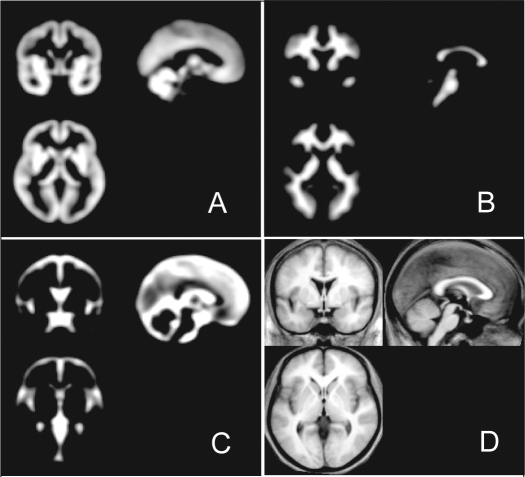
Figure 1.
The prior images of brain tissue partitions and averaged T1 template in voxel-based morphometry
(A) Gray matter, (B) white matter, and (C) CSF prior maps. Averaged T1 template was created from all OSA patients and healthy volunteers
Regional Difference of Gray Matter Volume between OSA Patients and Healthy Volunteers
There were no regional differences between OSA patients and healthy volunteers regarding GMV.
Multiple regression analyses of gray matter concentrations or volumes with age, apnea-hypopnea indices, arousal index, and Epworth sleepiness scale did not show statistically significant results in OSA patients and healthy volunteers.
DISCUSSION
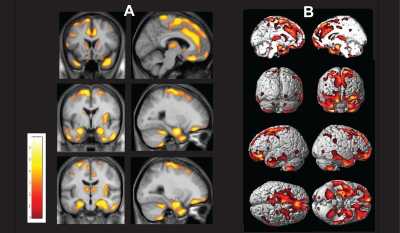
In the present study, we found decrease of gray matter concentration in multiple brain regions of OSA patients compared to healthy volunteers (Figure 2).
Figure 2.
A statistical brain map showing a decrease in gray matter concentration (GMC) in patients with severe obstructive sleep apnea syndrome (OSA).
(A) Gray matter concentrations were reduced in patients with OSA compared with healthy volunteers (at the false discovery rate corrected P < 0.05, independent t test) in the following brain structures: bilateral superior frontal gyri, left gyrus rectus, and bilateral frontomarginal gyri, bilateral anterior cingulate gyri, right anterior insular gyrus, bilateral caudate nuclei, bilateral thalami, bilateral amygdala and hippocampi, bilateral inferior temporal gyri, and bilateral cerebellar cortices. The results were superimposed on the 2-dimensional planes of averaged T1 template of all subjects. Scales in color bar are t scores. Left-hand sides of images represent the left hemisphere of the brain.
(B) The overall areas with reduced gray matter concentrations are shown in a three-dimensional brain rendering view.
Reduced Gray Matter Concentrations in the Limbic Areas (Amygdalo-Hippocampal Formation, Insular, Ventral Anterior Thalamus) and Cingulate Gyri
The hippocampal area of the brain is metabolically active and is highly susceptible to hypoxic insult. A deficit in unilateral hippocampal gray matter was previously reported in patients with OSA.7 These findings have been suggested to be relevant to the memory disturbances observed in OSA patients.3,20,21 The amygdala and insular cortex are known to have a role in mediating affective perceptions.22
Insula and cingulate gyri are recognized to be involved in cardiovascular control. The insular cortex receives nociceptive and viscerosensory input and exerts significant influences on sympathetic and parasympathetic nervous system activity.23–26 Insular functional MRI signals were disturbed to Valsalva and cold pressor challenges in OSA patients,27,28 who showed increased sympathetic tone and higher heart rates.26 The cingulate cortex forms the circuit of Papez and is involved in functions of the gut and emotions. This structure is also related to the control of certain autonomic functions, including respiration, blood pressure, and salivary secretion. During respiration, some cingulate neurons discharged at pace with respiration.29 Moreover, cingulate areas are activated by dyspnea,30 breathlessness,31 and emotion related to the need for air,32 which suggested that the cingulate cortex has a complex and indirect relationship to central networks that control respiration.29,33 Recent studies confirmed higher cardiac events over a 10-year period in patients with untreated severe OSA.34,35
Therefore, decreased gray matter concentrations in the limbic areas, as well as the cingulate cortex in our results, may explain clinical manifestations such as memory impairment and affective and cardiovascular disturbances in patients with severe OSA.
Reduced Gray Matter Concentrations in Prefrontal Cortices and Caudate Nuclei
The present study showed a significant GMC decrease in dorsolateral (bilateral superior frontal gyri) and ventromedial frontal cortices (left gyrus rectus and bilateral frontomarginal gyri), as well as bilateral caudate nuclei. The head of the caudate nucleus is supposed to be involved in the “prefrontal” circuit. Sleep apneas lead to sleep fragmentation and a chronic sleep deprived state. Patients with OSA frequently report neurocognitive problems as well as excessive daytime sleepiness.1–5 Recently it was reported that sleep disturbances preferentially lead to dysfunction in the prefrontal cortex, a region of the brain that controls various executive functions, such as behavioral inhibition, set shifting, and self-regulation of affect and arousal.36 These OSA-induced executive function deficits cannot be accounted for by sleepiness itself37 and may represent neuronal damage.38 Studies of the metabolic and cognitive consequences of sleep deprivation support a role for sleep in the restoration of function in the prefrontal cortex.39 OSA is associated with a loss of slow wave sleep in addition to the behavioral deficits reflecting altered prefrontal cortex function.38
Reduced Gray Matter Concentrations in the Cerebellum
Cerebellar structures have been associated with roles in regulation of autonomic and respiratory patterns.40–42 The anatomic arrangement of climbing fibers from the inferior olive to cerebellar hemispheric neurons leads to an extraordinary sensitivity to excitotoxic processes from ischemia or toxins.43,44 Significant gray matter loss was reported bilaterally in the caudal quadrangular lobule of the cerebellar cortex in patients with heart failure.45 The cerebellum is known to be vulnerable to anoxia or ischemia. Exposure to repetitive intermittent hypoxia of sleep disordered breathing may be related to the abnormal gray matter concentrations in the bilateral cerebellar hemispheres in our results.
Optimized Voxel-Based Morphometry findings in OSA
Optimized voxel-based morphometry (VBM) was developed to increase the sensitivity of standard VBM in detecting neuroanatomical differences in vivo using structural MRIs.16,46 Generally, the most important advantage of VBM is its ability to examine the entire brain, rather than a particular structure, in an unbiased and objective manner. In spatial normalization, there are two options—unmodulation process for brain tissue concentration, and modulation process for brain tissue volume change. To preserve the total amount of signal in the images, areas expanded during warping are correspondingly reduced in intensity, and areas contracted during warping are increased in intensity to the contrary. This process is modulation, which multiplies tissue voxel values by the Jacobian determinants, the determinant of the deformation parameters obtained from spatial normalization. Thus, the use of modulation process could reflect regional brain volume change. The unmodulated images preserve their own signal intensities regardless the expansion or contraction during warping. Therefore, the VBM results using unmodulated images could reflect the gray or white matter concentrations.16,46
A previous VBM study by Australian researchers demonstrated scattered areas of GMV reduction in posterior and mesial temporal lobe bilaterally and in the left insular region in OSA patients compared with control subjects (uncorrected P < 0.001); but no areas of GMV reduction were still significant after correction for multiple comparisons.10 Similarly our study showed no significant GMV differences by optimized VBM of modulated images. However, optimized VBM of unmodulated images showed multiple areas of GMC decrease, including bilateral mesial and inferior temporal gyri. Our patients had larger sample size, lower BMI, and lower mean AHI than Australian patients (24 male, mean BMI 33.2/h, mean AHI 71.7/h in O'Donoghue et al vs. 36 male, mean BMI 26.0/h, mean AHI 52.5/h in our patients).10
Good et al. reported differences in VBM between analyses of GMC and GMV, suggesting that analyses of GMC and GMV detect different aspects of MR assessable neuroanatomy.16 It should be clearly noted that previous optimized VBM studies had used modulated images in OSA. Some found significant results,9 but some did not.10 In our study, GMC reduction was observed in multiple brain areas, whereas GMV reduction was not observed in any areas.
A previous study showed that whole brain volume reduced by about 4% after nasal CPAP treatment in OSA patients.10 Increased intracranial pressure and papilledema were reported in OSA patients, and they improved after nasal CPAP treatment.47,48 Frequent episodes of nocturnal hypoxemia and hypercarbia induce vasodilation and disturbances in autoregulation of brains of OSA patients. Thus the changes of brain volume in OSA patients may be obscured by increased cerebral blood volume or whole brain water content from OSA-induced changes in autoregulation.49
This may be a reason why gray matter concentration was decreased without significant changes of gray matter volume in OSA patients. The present study is to our knowledge, the first study to assess the results with both modulated and unmodulated images in OSA patients.
The registration processes are the same on modulated images and unmodulated images in optimized VBM analysis, which provides evidence that our findings of unmodulated images are not related with the errors from registration. There was no significant difference in intracranial volume between our patients and normal subjects (mean 1675525.3 ± 94223.1 mm3 vs. 1676637.1 ± 102387.9 mm3, independent t-test, P = 0.963). Therefore, there does not seem to be any systematic difference in region size and modulation maps between the groups. Our GMC results suggest it may be of great value to investigate the brain in OSA, supposing that there may be methodological limitations with unmodulated data.
Macey and colleagues suggested that bilateral reductions may result from ischemic or other physiologic changes accompanying obstruction and that unilateral volume changes may originate from an initial brain insult or ischemic event, which leads to a cascade of neural damage resulting in ineffective capabilities to respond to minor respiratory challenges within sleep.9 In our study, decreased gray matter concentrations appeared bilaterally in most portions of cingulate gyri, caudate nuclei, thalami, and frontal and temporal cortices including amygdalo-hippocampal areas, and cerebellar hemispheres in patients with OSA. Unilateral reductions were only found in the gyrus rectus and precentral and insular gyri. It could be assumed that our morphological findings also support Macey's two theories; (1) gray matter loss may be a consequence of apnea, or (2) preexisting abnormalities may contribute to the genesis or maintenance of the disorder.9
We applied strict inclusion and exclusion criteria to enroll the patients and healthy volunteers to exclude possible other factors may affect gray matter concentrations. We excluded 6 patients who showed diffuse brain atrophy on brain MRI from the patient group to avoid bias during the analysis. Their mean age was 53.5 y (51-55) and AHI was 45.4/h. Two of them seemed to have hypertension (untreated) and one had a history of meningoencephalitis in childhood. Another 3 patients did not have any neurological diseases or history of other problems which change brain volume. It would be suggested that older age (mean 44.7 y) was related to brain atrophy.
Our findings had a strong statistical significance; a threshold of P < 0.05 was set with a multiple comparison adjustment for a false discovery rate. It has been recognized that the adjustment for a false discovery rate (the estimated fraction of false positive voxels) provides adequate control for multiple comparisons.47,50–52 O'Donoghue and colleagues also chose to use a threshold of P < 0.05 with a multiple comparison adjustment for false discovery rate; however, none of the comparisons showed a significant effect at this level.10
In summary the present study revealed significant GMC deficits in multiple brain regions of patients with severe OSA, whereas their brain MRIs were visually normal. Disturbances of these brain areas may result in memory impairment, affective and cardiovascular disturbances, executive dysfunctions, and the problems of autonomic and respiratory control.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was supported by a Grant (2009K001257) from Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science, by a grant (no. A090579) of the Good Health R&D Project, Ministry of Health – Welfare, Republic of Korea, and by the Samsung Medical Center Clinical Research Development Program Grant, #CRS-108-11-1.
REFERENCES
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Bennett LS, Barbour C, Langford B, Stradling JR, Davies RJ. Health status in obstructive sleep apnea: relationship with sleep fragmentation and daytime sleepiness, and effects of continuous positive airway pressure treatment. Am J Respir Crit Care Med. 1999;159:1884–90. doi: 10.1164/ajrccm.159.6.9808107. [DOI] [PubMed] [Google Scholar]
- 3.Decary A, Rouleau I, Montplaisir J. Cognitive deficits associated with sleep apnea syndrome: a proposed neuropsychological test battery. Sleep. 2000;23:369–81. [PubMed] [Google Scholar]
- 4.Montplaisir J, Bedard MA, Richer F, Rouleau I. Neurobehavioral manifestations in obstructive sleep apnea syndrome before and after treatment with continuous positive airway pressure. Sleep. 1992;15:S17–9. doi: 10.1093/sleep/15.suppl_6.s17. [DOI] [PubMed] [Google Scholar]
- 5.Valencia-Flores M, Bliwise DL, Guilleminault C, Cilveti R, Clerk A. Cognitive function in patients with sleep apnea after acute nocturnal nasal continuous positive airway pressure (CPAP) treatment: sleepiness and hypoxemia effects. J Clin Exp Neuropsychol. 1996;18:197–210. doi: 10.1080/01688639608408275. [DOI] [PubMed] [Google Scholar]
- 6.Gale SD, Hopkins RO. Effects of hypoxia on the brain: neuroimaging and neuropsychological findings following carbon monoxide poisoning and obstructive sleep apnea. J Int Neuropsychol Soc. 2004;10:60–71. doi: 10.1017/S1355617704101082. [DOI] [PubMed] [Google Scholar]
- 7.Morrell MJ, McRobbie DW, Quest RA, Cummin AR, Ghiassi R, Corfield DR. Changes in brain morphology associated with obstructive sleep apnea. Sleep Med. 2003;4:451–4. doi: 10.1016/s1389-9457(03)00159-x. [DOI] [PubMed] [Google Scholar]
- 8.Morrell MJ, Twigg G. Neural consequences of sleep disordered breathing: the role of intermittent hypoxia. Adv Exp Med Biol. 2006;588:75–88. doi: 10.1007/978-0-387-34817-9_8. [DOI] [PubMed] [Google Scholar]
- 9.Macey PM, Henderson LA, Macey KE, et al. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166:1382–7. doi: 10.1164/rccm.200201-050OC. [DOI] [PubMed] [Google Scholar]
- 10.O'Donoghue FJ, Briellmann RS, Rochford PD, et al. Cerebral structural changes in severe obstructive sleep apnea. Am J Respir Crit Care Med. 2005;171:1185–90. doi: 10.1164/rccm.200406-738OC. [DOI] [PubMed] [Google Scholar]
- 11.Macey PM, Harper RM. OSA brain morphology differences: magnitude of loss approximates age-related effects. Am J Respir Crit Care Med. 2005;172:1056–7. doi: 10.1164/ajrccm.172.8.954. author reply 1057-8. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Medicare and Medicaid Services. National Coverage Determination for Continuous Positive Airway Pressure (CPAP) Therapy for Obstructive Sleep Apnea (OSA) NCD #240.4. 2005. [cited 2008:22;September]. Available from: http://www.cms.hhs.gov.
- 13.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 14.Johns MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 15.Carskadon M, Dement W. Effects of total sleep loss on sleep tendency. Percept Mot Skills. 1979;48:495–506. doi: 10.2466/pms.1979.48.2.495. [DOI] [PubMed] [Google Scholar]
- 16.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 17.Chey J, Na DG, Tae WS, Ryoo JW, Hong SB. Medial temporal lobe volume of nondemented elderly individuals with poor cognitive functions. Neurobiol Aging. 2006;27:1269–79. doi: 10.1016/j.neurobiolaging.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Duvernoy HM. The human brain: surface, three-dimensional sectional anatomy with MRI and blood supply. New York: Springer-Verlag; 1991. [Google Scholar]
- 19.Kamba M, Suto Y, Ohta Y, Inoue Y, Matsuda E. Cerebral metabolism in sleep apnea. Evaluation by magnetic resonance spectroscopy. Am J Respir Crit Care Med. 1997;156:296–8. doi: 10.1164/ajrccm.156.1.9611063. [DOI] [PubMed] [Google Scholar]
- 20.Engleman HM, Kingshott RN, Martin SE, Douglas NJ. Cognitive function in the sleep apnea/hypopnea syndrome (SAHS) Sleep. 2000;23(Suppl 4):S102–8. [PubMed] [Google Scholar]
- 21.Fulda S, Schulz H. Cognitive dysfunction in sleep-related breathing disorders: a meta-analysis. Sleep Res Online. 2003;5:19–51. [Google Scholar]
- 22.Amaral DG. The amygdala, social behavior, and danger detection. Ann N Y Acad Sci. 2003;1000:337–47. doi: 10.1196/annals.1280.015. [DOI] [PubMed] [Google Scholar]
- 23.Cechetto DF, Chen SJ. Subcortical sites mediating sympathetic responses from insular cortex in rats. Am J Physiol. 1990;258:R245–55. doi: 10.1152/ajpregu.1990.258.1.R245. [DOI] [PubMed] [Google Scholar]
- 24.Kim JE, Song H, Jeong JH, Choi KG, Na DL. Bilateral ageusia in a patient with a left ventroposteromedial thalamic infarct: Cortical localization of taste sensation by statistical parametric mapping analysis of PET images. J Clin Neurol. 2007;3:161–4. doi: 10.3988/jcn.2007.3.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saper CB. Convergence of autonomic and limbic connections in the insular cortex of the rat. J Comp Neurol. 1982;210:163–73. doi: 10.1002/cne.902100207. [DOI] [PubMed] [Google Scholar]
- 26.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harper RM, Macey PM, Henderson LA, et al. fMRI responses to cold pressor challenges in control and obstructive sleep apnea subjects. J Appl Physiol. 2003;94:1583–95. doi: 10.1152/japplphysiol.00881.2002. [DOI] [PubMed] [Google Scholar]
- 28.Henderson LA, Woo MA, Macey PM, et al. Neural responses during Valsalva maneuvers in obstructive sleep apnea syndrome. J Appl Physiol. 2003;94:1063–74. doi: 10.1152/japplphysiol.00702.2002. [DOI] [PubMed] [Google Scholar]
- 29.Frysinger RC, Harper RM. Cardiac and respiratory relationships with neural discharge in the anterior cingulate cortex during sleep-walking states. Exp Neurol. 1986;94:247–63. doi: 10.1016/0014-4886(86)90100-7. [DOI] [PubMed] [Google Scholar]
- 30.Peiffer C, Poline JB, Thivard L, Aubier M, Samson Y. Neural substrates for the perception of acutely induced dyspnea. Am J Respir Crit Care Med. 2001;163:951–7. doi: 10.1164/ajrccm.163.4.2005057. [DOI] [PubMed] [Google Scholar]
- 31.Banzett RB, Mulnier HE, Murphy K, Rosen SD, Wise RJ, Adams L. Breathlessness in humans activates insular cortex. Neuroreport. 2000;11:2117–20. doi: 10.1097/00001756-200007140-00012. [DOI] [PubMed] [Google Scholar]
- 32.Evans KC, Banzett RB, Adams L, McKay L, Frackowiak RS, Corfield DR. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J Neurophysiol. 2002;88:1500–11. doi: 10.1152/jn.2002.88.3.1500. [DOI] [PubMed] [Google Scholar]
- 33.Brannan S, Liotti M, Egan G, et al. Neuroimaging of cerebral activations and deactivations associated with hypercapnia and hunger for air. Proc Natl Acad Sci U S A. 2001;98:2029–34. doi: 10.1073/pnas.98.4.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doherty LS, Kiely JL, Swan V, McNicholas WT. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest. 2005;127:2076–84. doi: 10.1378/chest.127.6.2076. [DOI] [PubMed] [Google Scholar]
- 35.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 36.Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11:1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- 37.Harrison Y, Horne JA. The impact of sleep deprivation on decision making: a review. J Exp Psychol Appl. 2000;6:236–49. doi: 10.1037//1076-898x.6.3.236. [DOI] [PubMed] [Google Scholar]
- 38.Nofzinger EA. Neuroimaging and sleep medicine. Sleep Med Rev. 2005;9:157–72. doi: 10.1016/j.smrv.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–52. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 40.Lutherer LO, Williams JL. Stimulating fastigial nucleus pressor region elicits patterned respiratory responses. Am J Physiol. 1986;250:R418–26. doi: 10.1152/ajpregu.1986.250.3.R418. [DOI] [PubMed] [Google Scholar]
- 41.Xu F, Frazier DT. Respiratory-related neurons of the fastigial nucleus in response to chemical and mechanical challenges. J Appl Physiol. 1997;82:1177–84. doi: 10.1152/jappl.1997.82.4.1177. [DOI] [PubMed] [Google Scholar]
- 42.Yates BJ. Vestibular influences on the autonomic nervous system. Ann N Y Acad Sci. 1996;781:458–73. doi: 10.1111/j.1749-6632.1996.tb15720.x. [DOI] [PubMed] [Google Scholar]
- 43.O'Hearn E, Molliver ME. The olivocerebellar projection mediates ibogaine-induced degeneration of Purkinje cells: a model of indirect, trans-synaptic excitotoxicity. J Neurosci. 1997;17:8828–41. doi: 10.1523/JNEUROSCI.17-22-08828.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welsh JP, Yuen G, Placantonakis DG, et al. Why do Purkinje cells die so easily after global brain ischemia? Aldolase C, EAAT4, and the cerebellar contribution to posthypoxic myoclonus. Adv Neurol. 2002;89:331–59. [PubMed] [Google Scholar]
- 45.Woo MA, Macey PM, Fonarow GC, Hamilton MA, Harper RM. Regional brain gray matter loss in heart failure. J Appl Physiol. 2003;95:677–84. doi: 10.1152/japplphysiol.00101.2003. [DOI] [PubMed] [Google Scholar]
- 46.Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–66. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Purvin VA, Kawasaki A, Yee RD. Papilledema and obstructive sleep apnea syndrome. Arch Ophthalmol. 2000;118:1626–30. doi: 10.1001/archopht.118.12.1626. [DOI] [PubMed] [Google Scholar]
- 48.Lee AG, Golnik K, Kardon R, Wall M, Eggenberger E, Yedavally S. Sleep apnea and intracranial hypertension in men. Ophthalmology. 2002;109:482–5. doi: 10.1016/s0161-6420(01)00987-3. [DOI] [PubMed] [Google Scholar]
- 49.Karl A. Franklin. Cerebral haemodynamics in obstructive sleep apnoea and Cheyne–Stokes respiration. Sleep Med Rev. 2002;6:429–41. doi: 10.1053/smrv.2001.0206. [DOI] [PubMed] [Google Scholar]
- 50.Joo EY, Hong SB, Tae WS, et al. Cerebral perfusion abnormality in narcolepsy with cataplexy. Neuroimage. 2005;28:410–6. doi: 10.1016/j.neuroimage.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 51.Joo EY, Tae WS, Han SJ, Cho JW, Hong SB. Reduced cerebral blood flow during wakefulness in obstructive sleep apnea-hypopnea syndrome. Sleep. 2007;30:1515–20. doi: 10.1093/sleep/30.11.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joo EY, Tae WS, Kim ST, Hong SB. Gray matter concentration abnormality in brains of narcolepsy patients. Korean J Radiol. 2009;10:552–8. doi: 10.3348/kjr.2009.10.6.552. [DOI] [PMC free article] [PubMed] [Google Scholar]