Abstract
Objectives:
To assess the influence of total or selective REM sleep deprivation on the dopamine transporter (DAT) densities and sleep patterns of healthy volunteers.
Design:
Prospective study.
Setting:
Evaluation of polysomnography recordings and DAT density after 4 nights of selective REM sleep deprivation followed by 3 nights of sleep recovery compared to a control group and a group that was subjected to 2 nights of total sleep deprivation. Single positron emission computed tomography and [99mTc]TRODAT-1 were used to assess the cerebral DAT density in the striatum at baseline, after REM sleep deprivation and total sleep deprivation as well as after sleep recovery. Blood was collected daily to examine prolactin and estradiol levels, which were correlated with dopaminergic activity.
Patients or Participants:
Thirty healthy male volunteers ranging from 19 to 29 years of age were randomly assigned to one of three experimental groups after giving written informed consent (10 non-sleep deprived, 10 total sleep deprived, and 10 REM sleep deprived).
Measurements and Results:
Four nights of REM sleep deprivation and 2 nights of total sleep deprivation induced distinct and heterogeneous patterns of sleep recovery. No significant modulation of DAT availability was observed within groups. In the recovery nights, changes in cortisol, prolactin and estradiol concentrations were significantly correlated with specific sleep stages in the total and REM sleep deprived groups. In addition, DAT density was positively correlated with estradiol concentration and inversely associated with SWS latency only after total sleep deprivation.
Conclusion:
Our study demonstrates that although sleep deprivation did not promote significant alterations in DAT density within the striatum, there were significant correlations among transporter availability, hormonal concentrations and sleep parameters.
Citation:
Martins, RCS; Andersen ML; Garbuio SA; Bittencourt LR: Guindalini C; Shih MC; Hoexter MQ; Bressan RA; Castiglioni MLV; Tufik S. Dopamine transporter regulation during four nights of REM sleep deprivation followed by recovery – an in vivo molecular imaging study in humans. SLEEP 2010;33(2):243-251.
Keywords: Sleep deprivation, recovery, SPECT, dopamine, prolactin, estradiol, rebound sleep, TRODAT
CHRONIC SLEEP LOSS IS INCREASINGLY COMMON IN INDUSTRIALIZED SOCIETIES, AFFECTING ABOUT 45% OF ADULTS.1 SLEEP DEPRIVATION INDUCES behavioral, hormonal, and neurochemical alterations.2 The stress inherent in sleep deprivation causes changes in the concentration of hormones such as cortisol3 as well as in prolactin and estradiol, which are known to influence dopaminergic transmission.4
Studies have suggested that dopamine (DA) is responsible for the behavioral changes observed after sleep deprivation. Since the 1970s, Tufik and colleagues demonstrated an enhancement in DA receptor sensitivity after rapid eye movement (REM) sleep deprivation in rats.2,5 Specifically, REM sleep deprivation has been shown to be related to changes in D2 post-synaptic receptor sensitivity in the rat striatum (STR).6,7 Recently, a study using microdialysis coupled with electrophysiological recordings reported an increase in DA concentrations during wakefulness and REM sleep compared to the concentration associated with slow wave sleep (SWS) in rats.8 Profound DA depletion in mice promotes the complete suppression of REM sleep,9 and DA transporter (DAT) knockout mice exhibit increased wakefulness and less SWS.10 An autoradiographic study in REM sleep deprived rats showed an increase in DA post-synaptic receptor sensitivity,6 however there was no observable increase in DAT density.11 A recent imaging study in humans indicated that DA release increases after 24 hours of total sleep deprivation, as reflected by DA cell firing and/or release, rather than reduced DA reuptake.12 Although the recovery of sleep deprivation is marked by several neurochemical alterations13 and studies have suggested that REM sleep is the main modulator of the catecholaminergic system,2,5–7 the effect of longer periods of selective REM sleep deprivation on DAT availability, associated with electroencephalography, has not yet been evaluated. In addition, neuroimaging studies have not been performed in individuals subjected to both total and REM sleep deprivation, followed by sleep recovery.
Therefore, we set out to assess DAT density in the human brain after 4 nights of REM sleep deprivation and 3 nights of sleep recovery using single positron emission computed tomography (SPECT) and [99mTc]TRODAT-1, a selective DAT imaging agent. The specific sleep deprivation and recovery periods were chosen based on the fact that REM sleep is concentrated in the second half of sleep. Thus, individuals who experience daily sleep deprivation as a consequence of bedtime restriction lose a considerable percentage of this stage of sleep. Frequently, this sleep debt is compensated during the weekends by obtaining extra sleep over and above the daily requirement, a scenario that was replicated in this investigation by the REM sleep-deprived group. Reproducibility of the DAT density was ensured by a control group that slept regularly and had SPECT imaging conducted at three time-points. Based on another common type of individual who spends long periods of time without sleeping as a consequence of professional or personal burden, volunteers in a third experimental group were sleep deprived for 2 consecutive nights and subjected to the same evaluations. We monitored sleep architecture and measured cortisol, prolactin, and estradiol levels during the course of entire investigation. The working hypotheses were (1) REM sleep would induce increases in dopaminergic activity after sleep deprivation and (2) selective REM sleep deprivation for a prolonged period would result in down-regulation of DAT, enhancing dopaminergic neurotransmission.
METHODS
Participants
The study was conducted at the Sleep Laboratory of the Department of Psychobiology at the Universidade Federal de São Paulo (UNIFESP) with the approval of the Ethics Committee of the University as well as the Radiation Protection Center (#1676/06). Thirty healthy male volunteers ranging from 19 to 29 years of age were randomly assigned to one of three experimental groups after giving written informed consent (10 non-sleep deprived, 10 total sleep deprivaved, and 10 REM sleep deprived). The 10-year age range and the gender of volunteers were chosen based on neuroimaging studies that have documented an age-related decline in striatal DAT and the influence of sexual hormones in DAT density.4,14 Volunteers were carefully screened by obtaining a detailed medical history and performing physical and neurological examinations, routine blood tests, and urine toxicology for psychotropic drugs to ensure they fulfilled inclusion and exclusion criteria. Exclusion criteria included the following: shift work, sleep disorders, extreme morningness-eveningness, a history of neurological or psychiatric diseases and medical conditions, smoking, and alcohol or substance abuse. Volunteers underwent urine analysis to detect amphetamine, methamphetamine, cocaine, tetrahydrocannabinol, barbiturates, opiates, and benzodiazepines. All participants had normal results on validated questionnaires, including the Pittsburgh Sleep Quality Index, the Epworth Sleepiness Scale, and the Beck Depression Inventory. Normal sleep-wake rhythms and average sleep durations (between 7 and 9 h of sleep per night, with a morning wake time between 06:00 and 09:00) were verified by a sleep diary and actigraphy for a period of one week before participation in the study. Volunteers underwent a polysomnography recording to verify that they had no kind of sleep disturbances. This screening night also helped the volunteers to adapt to the recording equipment and the study environment.
Experimental Protocol
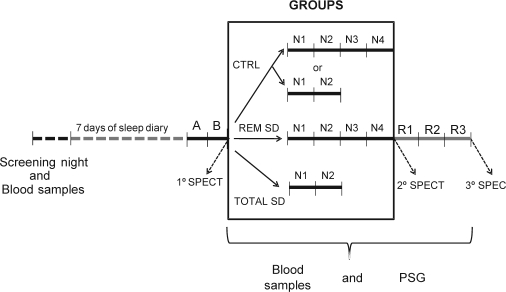
During the week preceding the study as well as during the study period, participants were asked to abstain from alcohol, chocolate, and caffeinated beverages and to maintain a standardized bedtime schedule in accordance with their regular habits. Subjects received 4 meals per day (08:30, 12:00, 16:00, and 20:00) plus a late evening snack at 00:00 for the total sleep deprivation group during the sleep deprivation period. The volunteers wore a wrist activity monitor (Actiwatch, MiniMitter, USA) to verify the compliance of the subjects with scheduled bedtimes for one week before and during the entire experimental protocol. All subjects underwent an adaptation night followed by a baseline polysomnography at the Sleep Institute. In the laboratory, subjects were instructed to be in bed between 23:00 and 08:00, and sleep was recorded each night. The control subjects had regular nights of sleep monitored by polysomnography during the entire experimental protocol, and naps were not permitted. All subjects had their first [99mTc]-TRODAT-1 injection after the baseline night between 08:00 and 13:00 under identical conditions and were scanned 4 hours after the injection. The control group underwent three trials of SPECT at intervals of 2, 3, or 4 days. The 20 sleep deprived volunteers were subjected to SPECT prior to the sleep deprivation, and a second scan after 2 nights of total sleep deprivation (N1 and N2) or 4 nights of REM sleep deprivation (N1, N2, N3, and N4). The last scan was performed after 3 nights of sleep recovery (R1, R2, and R3). Fasting blood samples were collected once each morning to measure cortisol, prolactin, and estradiol levels. The study flow chart for the various groups and nights of study is depicted in Figure 1.
Figure 1.
Schematic representation of the experimental protocol of the study.
Polysomnography
Sleep recordings were performed using a digital electroencephalogram (EEG) acquisition system with an Embla Digital A10 recording/amplifier and Somnologica software (Flaga, Reykjavik, Iceland). Surface electrodes were used to record EEG signals—2 central electrodes (C3-A2 and C4-A1), and 2 occipital electrodes (O1-A2 and O2-A1)—along with bilateral electroculograms (EOG), and submental electromyograms (EMG). Recordings included the oronasal airflow signal as measured by thermocouple and by nasal cannula with pressure transducer, the respiratory effort signal by thoracic and abdominal piezoelectric belts, leg movements by tibial EMG, and arterial oxygen saturation levels as assessed by pulse oximetry. Sleep recordings were visually scored at 30-s intervals as wakefulness, REM, or stages 1, 2, and SWS.15 Respiratory events,16 periodic limb movements,17 and arousals18 were scored according to established criteria. During the acquisition, EEG signals were filtered (0.3-70 Hz) and sampled at 200 Hz with a 16-bit resolution.
Sleep deprivation and rested wakefulness procedures
REM sleep deprived subjects were awakened by having their names called over an intercom at the first sign of REM sleep. If there was no response, the experimenter entered the room and gently shook the subject until receiving a response. This stage was determined by visual polysomnographic inspection in an on-line recording interval. The criteria for intervention were a desynchronized EEG without spindles or K-complexes and a concomitant reduction of the tonic EMG amplitude and occurrence of REM. The volunteers were kept awake for sufficient time to avoid an immediate relapse into REM sleep while keeping the waking episodes short enough to allow for frequent interventions. Throughout the protocol, sleep deprived volunteers could read, play games, watch television, or ambulate within the building to help them stay awake. Vigorous or stressful levels of exercise were avoided. To ensure that subjects would not fall asleep during the study, investigators were continuously present to monitor wakefulness.
SPECT Imaging
All subjects received an intravenous injection of 2 mL containing among 22 to 25 mCi of [99mTc]-TRODAT-1 in an antecubital peripheral vein. Images were acquired 4 hours after the injection. TRODAT-1 kits were produced by the Institute of Nuclear Energy Research (INER-Taiwan R.O.C.) and labeled according to methods published previously by Shih et al.19 At the start of the scan, individuals were placed at rest on the scanning bed in the supine position. All images were acquired on a double-headed gamma camera equipped with fan beam collimators (General Electric Medical System, Hawkeye USA). The energy window was 140 ± 14 keV, and a matrix of 128 × 128 in circular orbit with step and shoot movements of 64 steps was used for each head, with a diameter and degree of rotation of 30 cm and 360°, respectively. Acquisition time for the projection was 30 seconds, with a zoom of 1.45 and a slice thickness of 1.95 mm. During the 35-min period of SPECT data acquisition, all subjects were requested to stay awake and keep their eyes open. Sleep-deprived subjects were monitored by staff members throughout the recording time; when subjects closed their eyes for a longer period than usual, they were instructed not to sleep and to open their eyes again.
Image Analysis
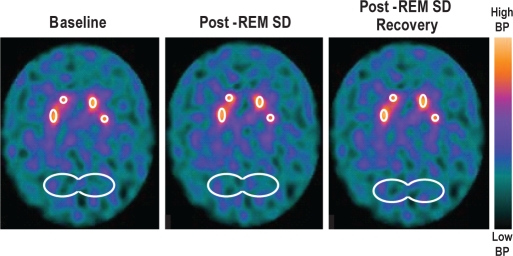
The SPECT images were reconstructed using an algorithm of filtered backprojection and a Butterworth filter of 0.4 cut-off with pixels of the 10th order. Photon attenuation correction was performed using Chang's first-order correction method with an attenuation coefficient of 0.11/cm.20 The SPECT studies with [99mTc]TRODAT-1 were evaluated through visual inspection and quantitative evaluation of the regions of interest (ROI). The ROIs were different shapes (some more round and some more oblong, ranging from 7 to 14 pixels) to accommodate the different structures, including the STR and occipital region (OCC). Average STR images based on 3 consecutive slice ROIs were used to estimate the concentration of DAT in the striatal body (right and left). An elliptical ROI was placed on three consecutive slices in the occipital cortex, an area used for reference of non-specific DAT binding (Figure 2). To quantitatively evaluate DAT, the binding potential (BP) was calculated, where BP = [STR-OCC]/OCC.21
Figure 2.
Averaged brain images based on three consecutive slice regions of interest (ROIs) for [99mTc]TRODAT-1 were used to estimate the concentration of DAT in the striatum (right and left at baseline) post-SD, and post-sleep recovery. An elliptical ROI was placed on three consecutive slices in the occipital cortex, an area used for reference of non-specific DAT binding.
Blood Sampling
Blood samples were collected every morning from each volunteer during the experimental protocol to evaluate if the sleep deprivation promoted changes in cortisol, prolactin, and estradiol levels (hormones related to dopaminergic activity and stress). Blood samples were centrifuged immediately at 4°C, and serum and plasma were stored at −80°C until the end of the study. Serum cortisol (coefficient of variation (CV): 5.3%; Immulite 2000, DPC Corporation, USA), plasma prolactin and estradiol concentrations were measured by the chemiluminescence method (prolactin CV: 5.7% and estradiol CV: 6.7%; Advia Centaur, Bayer Corporation, USA).
Statistical Analysis
Average values are given as means ± SDs. Statistical significance was assessed by a repeated-measures analysis of variance (ANOVA). If the day-by-group interaction showed a significant effect (P < 0.05) a post hoc Tukey test was used for comparison. Pearson correlation analyses were performed to examine the relationships between sleep parameters, DAT density and hormonal profile.
RESULTS
Effects of Sleep Deprivation on Sleep Pattern
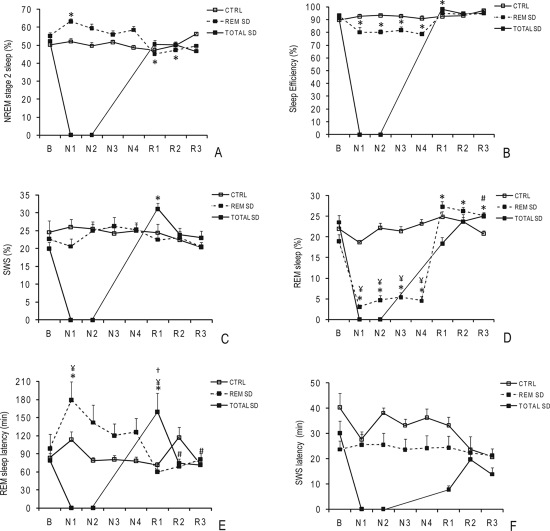
Sleep analyses in the control, total, and REM sleep deprived groups examined REM and SWS latency (the time from sleep onset to the first of ≥ 3 consecutive epochs of REM or SWS), sleep efficiency (the proportion of sleep in the period potentially filled by sleep; e.g., the ratio of total sleep time by total sleep recording), and sleep stage percentages (Figure 3). At baseline, no significant differences were observed between groups (Figure 3).
Figure 3.
Sleep architecture during the experimental protocol in control and sleep-deprived (SD) groups at baseline (B), during nights of SD (N1, N2, N3, N4), and during nights of sleep recovery (R1, R2, R3).
The data are expressed as the mean ± SEM (n = 10 volunteers per group). *, significant differences vs. baseline; #, significant differences vs. R1; ¥, significant differences vs. the control group in each respective time-point (ANOVA for repeated measures, followed by a Tukey-Kramer test, see text for p-values). Percentage of NREM stage 2 sleep (A); percentage of sleep efficiency (B); percentage of slow wave sleep (SWS) (C); percentage of REM sleep (D); REM sleep latency in minutes(E); and SWS latency in minutes (F).
Sleep Patterns in the Control Group
The control group exhibited no statistical differences in any polysomnographic variables analyzed across all days of the experimental protocol, as shown in Figure 3.
Sleep Patterns in the REM Sleep Deprived Group
An increase in the amount of NREM stage 2 sleep was evident during the first night of REM sleep deprivation (P < 0.03), as depicted in Figure 3A. The duration of this stage decreased in the recovery period and only reached baseline levels during the third night of recovery (R3) (P < 0.03). A significant decrease in sleep efficiency was observed during the sleep deprivation nights compared to baseline (93.1% ± 1.4% vs. 81.7% ± 1.8%, P < 0.01) (Figure 3B). No significant differences in the percentage of sleep time spent in SWS were induced by 4 nights of REM sleep deprivation, as depicted in Figure 3C. Relative to the baseline, 4 nights of REM sleep deprivation promoted a pronounced decrease (∼70%) in the amount of REM sleep during the 4 experimental sleep deprivation nights (P < 0.0001). On average, the percentage of REM sleep diminished from 19 ± 1.6% at baseline to 5 ± 1.2% during the sleep deprivation nights (Figure 3D). During the recovery nights, the amount of REM sleep increased by only 50% (18.4% ± 1.6% vs. 26.5% ± 0.7%, P < 0.05) and did not return to baseline values even after 3 nights of sleep recovery (Figure 3D). The percentage of REM sleep during 4 nights of REM sleep deprivation was significantly lower than that in the control group at the corresponding time-points (P < 0.0001) (Figure 3D). During the first night of REM sleep deprivation, volunteers showed an increase in REM sleep latency relative to baseline values (P < 0.001) and to the control group (P < 0.02) (Figure 3E). No significant changes in SWS latency were promoted by four nights of REM sleep deprivation during the protocol in the REM sleep deprived group (Figure 3F).
Sleep Patterns of the Total Sleep Deprived Group
No significant differences in NREM stage 2 sleep were evident in the sleep deprivation group during the experimental protocol (Figure 3A). The total sleep deprived group was the only group to show statistical significance in NREM stage 2 sleep compared to baseline (R1, P < 0.01) (Figure 3B). The SWS stage strongly recovered during R1 (20% ± 1.8% vs. 31.1% ± 1.6%, P < 0.01) (Figure 3C). During R3, there was a significant decrease in the percentage of REM sleep in relation to R1 (P < 0.01), as shown in Figure 3D. Indeed, this group showed an 80% increase in REM sleep latency during R1 when compared to the baseline (79% ± 11.1% vs. 160.5% ± 30.9%, P < 0.01), to the respective time-points in the control group (P < 0.01) and to R2 and R3 (P < 0.01) (Figure 3E). The difference in SWS latency between R1 and baseline did not reach statistical significance (P < 0.09).
Effects of Sleep Deprivation on Hormonal Profile
The results show that after either 2 nights of total sleep deprivation or 4 nights of selective suppression of REM sleep, there were no statistical differences in prolactin, estradiol, and cortisol levels, as assessed by intra-group analysis (Table 1).Correlation analyses were performed between sleep parameters and hormonal profiles to further investigate the effect of total and selective sleep deprivation in each recovery day. After total sleep deprivation, estradiol levels were positively correlated with sleep latency and the percentage of SWS (r = 0.74; P = 0.02; r = 0.77; P = 0.01, respectively) in R1, while prolactin levels were positively correlated with REM sleep latency (r = 0.65; P = 0.04) in R2. Cortisol levels were negatively correlated with the percentage of REM sleep in R2 (r = −0.75; P = 0.01). Additionally, we observed a tendency towards a positive correlation between cortisol and the percentage of SWS (r = 0.6; P = 0.07) in R2. During sleep recovery in the REM sleep deprived group, estradiol levels were positively correlated with REM sleep latency in R3 (r = 0.85; P = 0.002). In addition, prolactin levels were positively correlated with SWS latency in R1 (r = 0.68; P = 0.046) and negatively correlated with REM sleep latency in R2 (r = −0.74; P = 0.01) (Table 2).
Table 1.
Hormonal concentrations of prolactin, estradiol, and cortisol during the protocol in control and sleep deprived groups at baseline during the nights of sleep deprivation and during nights of sleep recovery.
| Prolactin (ng/mL) | B | N1 | N2 | N3 | N4 | R1 | R2 | R3 |
| Control | 12 ± 3 | 13.6 ± 5 | 13 ± 4 | 14.7 ± 5 | 13.9 ± 5 | 14.6 ± 6 | 12 ± 4 | 16.3 ± 10 |
| REM Sleep Deprivation | 13 ± 4 | 10.1 ± 4 | 10.8 ± 4 | 10.1 ± 3 | 9.6 ± 3 | 14.5 ± 5 | 13.2 ± 5 | 11.7 ± 2 |
| Total Sleep Deprivation | 8.8 ± 4 | 7.7 ± 5 | 8.2 ± 4 | 12 ± 6 | 11.3 ± 6 | 11.8 ± 6 | ||
| Estradiol (pg/mL) | ||||||||
| Control | 22.9 ± 7 | 23.5 ± 9 | 18.4 ± 7 | 24.5 ± 11 | 26.3 ± 11 | 20.9 ± 10 | 30.4 ± 15 | 25.1 ± 14 |
| REM Sleep Deprivation | 22.3 ± 8 | 21 ± 9 | 23.3 ± 14 | 19.7 ± 8 | 24.8 ± 10 | 20.8 ± 11 | 20.2 ± 11 | 19.9 ± 11 |
| Total Sleep Deprivation | 26.8 ± 11 | 23.1 ± 8 | 22.7 ± 9 | 27.9 ± 11 | 29.2 ± 12 | 29.7 ± 13 | ||
| Cortisol (μg/dL) | ||||||||
| Control | 16.2 ± 3 | 17.7 ± 6 | 16.2 ± 3 | 17.2 ± 4 | 15.5 ± 3 | 15.2 ± 5 | 14.9 ± 5 | 14.1 ± 5 |
| REM Sleep Deprivation | 18.7 ± 3 | 14.8 ± 3 | 14.9 ± 4 | 14.7 ± 4 | 14.1 ± 2 | 15.7 ± 4 | 15.3 ± 5 | 15.2 ± 4 |
| Total Sleep Deprivation | 16.9 ± 6 | 16.8 ± 5 | 16.1 ± 5 | 18 ± 6 | 17.8 ± 5 | 17.8 ± 5 |
The data are means ± SD (n = 10 volunteers per group). No significant differences were detected. B, baseline night; N1, first night of sleep deprivation; N2, second night of sleep deprivation; N3, third night of sleep deprivation; N4, fourth night of sleep deprivation; R1, first night of sleep recovery; R2, second night of sleep recovery; R3, third night of sleep recovery.
Table 2.
Estimated Pearson correlation coefficients between sleep parameters, hormonal concentrations and dopamine transporter density in the correspondent recovery night after REM and total sleep deprivation.
| REM Sleep Deprivation |
TOTAL Sleep Deprivation |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estradiol | Prolactin | Cortisol | DAT density R Striatum | DAT density L Striatum | Estradiol | Prolactin | Cortisol | DAT density R Striatum | DAT density L Striatum | |
| SWS Latency R1 | −0.24 | 0.68* | 0.02 | 0.74* | 0.49 | 0.20 | ||||
| SWS (%) R1 | −0.23 | 0.53 | −0.34 | 0.77* | 0.44 | 0.20 | ||||
| REM sleep latency R1 | 0.52 | −0.62 | 0.52 | −0.19 | −0.09 | 0.27 | ||||
| REM sleep (%) R1 | −0.27 | 0.25 | 0.29 | 0.10 | 0.27 | −0.52 | ||||
| SWS latency R2 | 0.28 | 0.40 | −0.41 | −0.37 | 0.46 | −0.11 | ||||
| SWS (%) R2 | −0.24 | −0.33 | −0.53 | 0.31 | −0.47 | 0.60 | ||||
| REM sleep latency R2 | 0.00 | −0.74* | 0.03 | −0.40 | 0.65* | −0.16 | ||||
| REM sleep (%) R2 | 0.28 | −0.10 | −0.23 | −0.09 | 0.54 | −0.75* | ||||
| SWS latency R3 | −0.16 | 0.51 | −0.14 | 0.40 | −0.21 | 0.25 | −0.32 | 0.28 | −0.77* | −0.35 |
| SWS (%) R3 | −0.45 | −0.58 | −0.39 | −0.30 | 0.09 | −0.32 | 0.57 | 0.06 | −0.17 | 0.37 |
| REM sleep latency R3 | 0.85* | −0.22 | 0.42 | 0.10 | −0.29 | −0.24 | 0.09 | −0.06 | 0.13 | −0.49 |
| REM sleep (%) R3 | −0.07 | 0.45 | 0.15 | 0.26 | 0.34 | 0.40 | 0.00 | 0.18 | 0.15 | 0.33 |
P < 0.05; R1, first night of sleep recovery; R2, second night of sleep recovery; R3, third night of sleep recovery; SWS, slow wave sleep; DAT, dopamine transporter; R Striatum, Right Striatum; L Striatum, Left Striatum.
Effects on DAT density
The DAT density in the STR exhibited no statistically significant intra-group differences between baseline, total or REM sleep deprivation and recovery period samples in representative ROIs (Table 3). Additional correlation analyses showed that after the third recovery night, DAT density in the right STR was inversely correlated with SWS latency in the total sleep deprived group (r = −0.77; P = 0.01) (Table 2). Further exploratory analyses showed that in the total sleep deprived group, higher estradiol levels at baseline were associated with higher DAT densities in the right and left STR after sleep deprivation (r = 0.71; P = 0.02 and r = 0.82; P = 0.004, respectively) and in the right STR after recovery (r = 0.68; P = 0.03) (Table 4). In the REM sleep deprived group, concentration of prolactin after the fourth night of sleep deprivation were positively correlated with DAT densities in the left STR after recovery sleep (r = 0.63; P = 0.049) (Table 4).
Table 3.
The average dopamine transporter density proportional to the counts per pixel in the right (R) and left (L) striatum of control and sleep deprived groups. The scans were performed at baseline, after the respective total or REM sleep deprivation protocol, and after three nights of sleep recovery.
| R Striatum | Baseline | Sleep Deprivation | Recovery |
| Control | 2.3 ± 0.2 | 2.3 ± 0.2 | 2.1 ± 0.2 |
| REM Sleep Deprivation | 2.2 ± 0.3 | 2.1 ± 0.3 | 2.1 ± 0.3 |
| Total Sleep Deprivation | 2.4 ± 0.2 | 2.3 ± 0.3 | 2.3 ± 0.3 |
| L Striatum | |||
| Control | 2.2 ± 0.3 | 2.3 ± 0.2 | 2.1 ± 0.3 |
| REM Sleep Deprivation | 2.2 ± 0.3 | 2.1 ± 0.2 | 2.2 ± 0.3 |
| Total Sleep Deprivation | 2.5 ± 0.3 | 2.4 ± 0.4 | 2.3 ± 0.4 |
The data are shown as mean ± SD (n = 10 volunteers per group). R Striatum, Right Striatum; L Striatum, Left Striatum.
Table 4.
Estimated Pearson correlation coefficients between hormonal concentrations and dopamine transporter density in the right and left striatum for the REM and total sleep deprived groups.
| REM Sleep Deprivation (DAT density) |
Total Sleep Deprivation (DAT density) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Basal |
Sleep Deprivation |
Recovery |
Basal |
Sleep Deprivation |
Recovery |
|||||||
| R Striatum | L Striatum | R Striatum | L Striatum | R Striatum | L Striatum | R Striatum | L Striatum | R Striatum | L Striatum | R Striatum | L Striatum | |
| Estradiol B | 0.05 | 0.05 | −0.53 | 0.23 | −0.37 | −0.44 | 0.42 | 0.33 | 0.71* | 0.82* | 0.68* | 0.18 |
| Prolactin B | 0.09 | 0.36 | 0.28 | 0.56 | −0.06 | 0.17 | 0.37 | −0.04 | −0.06 | −0.24 | −0.03 | 0.14 |
| Cortisol B | 0.38 | −0.03 | −0.58 | −0.43 | −0.41 | −0.21 | −0.50 | -0.37 | −0.18 | 0.12 | −0.39 | −0.48 |
| Estradiol N4/N2 | −0.18 | -0.20 | 0.12 | 0.28 | 0.07 | -0.19 | −0.27 | 0.33 | 0.05 | 0.58 | 0.08 | 0.01 |
| Prolactin N4/N2 | 0.34 | 0.51 | 0.08 | 0.55 | 0.19 | 0.63* | 0.62 | 0.06 | −0.05 | −0.05 | 0.25 | 0.29 |
| Cortisol N4/N2 | 0.32 | 0.36 | −0.36 | −0.08 | 0.09 | 0.57 | −0.47 | 0.29 | −0.13 | 0.23 | 0.21 | 0.15 |
| Estradiol R3 | −0.47 | −0.69* | −0.02 | −0.62 | −0.08 | −0.28 | −0.32 | −0.24 | −0.17 | 0.17 | −0.17 | −0.36 |
| Prolactin R3 | 0.50 | 0.21 | 0.28 | 0.42 | 0.47 | 0.37 | 0.25 | 0.32 | −0.10 | −0.18 | 0.02 | 0.52 |
| Cortisol R3 | −0.20 | −0.42 | −0.41 | −0.68* | −0.09 | −0.32 | −0.60 | −0.12 | −0.34 | 0.14 | −0.37 | −0.13 |
P < 0.05; N2, second night of sleep deprivation in the total sleep deprived group; N4, fourth night of sleep deprivation in the REM sleep deprived group; R3, third recovery night; SWS, slow wave sleep; DAT, dopamine transporter; R Striatum, Right Striatum; L Striatum, Left Striatum.
DISCUSSION
The major finding reported here is that four nights of REM sleep deprivation and two nights of total sleep deprivation do not directly influence DAT availability in the STR. However, they do promote distinct and heterogeneous patterns of sleep recovery as well as significant cross-talk between DAT expression, the endocrine system and sleep parameters.
Sleep is regulated through both homeostatic and circadian mechanisms.22 The importance of observed alterations in sleep architecture caused by sleep deprivation is not fully known, but improved SWS and sleep efficiency could mean better sleep quality.23 The current polysomnographic findings show that the SWS stage displayed both the earliest and most dramatic recovery after a prolonged period of wakefulness. Increases in REM latency that were observed during the first recovery night in the total sleep deprived group (Figure 2E) may partly be a consequence of the inhibitory action of the enhanced SWS propensity.24 The significant increase in SWS during the first night of the sleep recovery period might reflect the homeostatic process after extended wakefulness.22
The pattern of sleep recovery in REM sleep deprived volunteers was marked by a selective increase in REM sleep that persisted throughout the three recovery nights, though the last recovery day exhibited a smaller percentage of this stage than the first day of recovery, indicating a gradual recuperation. Notably, the pressure for this recovery was not enough to decrease REM latency or decrease the percentage of SWS; instead, a concomitant decrease in NREM stage 2 during R1 and R2 was observed. These findings are in accordance with the hypothesis that SWS is a priority for sleep.25 In line with this idea, the polysomnographic results show that this group did not present any decreases in the percentage of SWS, even during the REM sleep deprivation period.
Deprivation of sleep is a stressful situation that has multiple effects on neuroendocrine function. In rats, REM sleep deprivation leads to increasing levels of corticosterone,26 and in humans, studies often show that sleep deprivation and sleep restriction are accompanied by increased cortisol levels in the afternoon and evening.27 On the other hand, a recent study that suppressed SWS for three nights did not show this pattern.3 Corroborating the results of Schussler et al.28 and Voderholzer et al.,29 we did not find changes in cortisol secretion during the recovery within groups. However, correlation analyses revealed that, during R2, volunteers subjected to total sleep deprivation showed cortisol levels that were negatively correlated to the percentage of REM sleep but that had a tendency towards a positive correlation to the percentage of SWS, supporting previous studies in animals and humans.26–28 In addition, stress conditions are known to stimulate prolactin release30 and a number of studies suggest that, in humans, the concentration of prolactin is related to REM sleep occurrence.31,32 In the REM sleep deprived group, a negative correlation was observed between the levels of prolactin and REM sleep latency, which is in accordance with the hypothesis that reduction in the percentage of REM sleep and the consequent increases in prolactin levels are related to stress.26 Taken together, these results highlight the fact that, even for young and healthy individuals, acute sleep deprivation is capable of inducing significant alterations not only in the sleep physiology, but also in stress related hormone release.
Adaptive hormonal responses, including changes in estradiol levels, have also been associated with sleep deprivation.33 Our data show that in the first night of recovery after total sleep deprivation, estradiol levels were positively correlated with the SWS percentage. Moreover, in the REM sleep deprived group, estradiol levels were positively correlated with REM sleep latency in the third day of recovery. Interestingly, since this hormone is present in women in a much higher concentration than in men, these results corroborate recent data from our group showing that women spend significantly more time in SWS and have a higher REM latency than men.34 Our results suggested that the levels of physiologic estradiol in women are related to their sleep pattern. In addition, a growing body of research has examined the modulation of brain dopaminergic systems by estrogen.4 Specifically, striatal DAT activity in rats has been correlated with estradiol concentrations.35 In support of this result, our examination of the total sleep deprived group revealed that higher estradiol levels were associated with a higher DAT density in the right STR after total sleep deprivation and recovery.
Clinical and experimental evidence indicates that increases in DA release occur after sleep deprivation periods.12,36 In animal models, sleep deprivation is responsible for a number of alterations similar to the effects caused by psychostimulant drugs, especially those affecting dopaminergic pathways.5,11 Thus, experimental manipulations of the sleep-awake cycle have proven to be efficient in intensifying stimulant behaviors, such as aggressiveness, stereotypy, locomotion, erection, and ejaculation.2,5,11,37 Moreover, wake-promoting substances that act on the dopaminergic system, such as psychostimulant drugs, have acute and chronic effects on sleep architecture.38 A recent study39 suggests that both sleepiness and slow waves in the EEG observed after injection of psychostimulants40 might be explained by the close interaction between the DA activity and the retinoic acid receptors.41 Retinoic acid receptors are highly expressed in the brain,42 where they are involved in the regulation of neural functions such as the control of locomotion41 and possibly in the neurobiology of Parkinson disease, and addiction,39 through their effect on DA neurotransmission.41 Delta oscillations, characteristic of the EEG of SWS, estimate sleep depth and need and are thought to be closely linked to the recovery function of sleep. Maret and colleagues demonstrate in the mouse that the gene encoding the retinoic acid receptor β determines the contribution of delta oscillations to the sleep EEG.39 Thus, retinoic acid signaling, which is involved in the patterning of the brain and DA pathways, regulates cortical synchrony in the adult.
Psychostimulant drug abusers often have an increased propensity for REM sleep during periods of acute and subacute withdrawal, which has been attributed to a state of relative functional DA depletion in the brain during abstinence.43 In animal models, profound DA depletion promotes the complete suppression of REM sleep,9 and DAT knockout mice exhibit increased wakefulness and less SWS.10 Our results did not provide evidence for correlations between DAT availability and the percentages of REM and SW sleep. However, DAT density in the right STR was inversely associated with SWS latency in the total sleep deprived group. This finding warrants further investigation to elucidate its implications in the neurobiology of sleep; however, it can be speculated that reduced SWS latency might be related to augmentation of SWS activity in the recovery nights.
Although DAT is the key regulator of DA levels in the synaptic cleft and the density of this neurotransmitter reflects the homeostatic tonus of the dopaminergic system,44,45 a recent imaging study showed that D2/D3 post-synaptic receptors, but not DAT, are affected by one night of total sleep deprivation in humans.12 In this study, Volkow and colleagues demonstrated that the D2/D3 receptor radioligand binding potential was reduced in the STR after total sleep deprivation.12 The authors consider that this reduction could be a result of a DA increase in the synaptic cleft, as a consequence of sleep deprivation. However, the possibility of a reduction in either the number of D2/D3 receptors or in their affinity cannot be excluded. Based on these results, the authors suggest that the increase in DA release after 24 hours of total sleep deprivation is a reflection of DA cell firing and/or release rather than reduced DA reuptake.12 Earlier studies had shown that the influence of DA on sleep is modulated by mesencephalic DA cells.46 This finding agrees with our results, showing that longer total sleep deprivation periods as well as selective REM sleep deprivation periods were not able to change the DAT density in the STR of healthy humans.
The data presented here are consistent with recent studies in humans and animals linking the impacts of sleep deprivation on cortisol, prolactin and estradiol release and DA activity. In addition, our results show that, although sleep deprivation did not promote significant changes in DAT availability in the STR, DAT density showed a positive correlation with the estradiol concentration and a negative correlation with SWS latency in the recovery nights after total sleep deprivation. Taken together, the current evidence suggests that strategies to improve sleep quality, as well as, to avoid periods of sleep deprivation should be considered as a potential intervention to prevent or delay physiologic and mental impacts associated with reduced sleep periods.
STUDY LIMITATIONS
The fact that sleep nights were limited to 8 hours may have obscured a recovery that would have appeared during extended sleep. However, permitting unlimited sleep during the first recovery night would have led to large individual variations in sleep duration, which would have made it difficult to assess the changes during the second and third night. Additionally, unlimited sleep could have possibly disrupted the volunteers' sleep cycles.
The restricted spatial resolution of SPECT did not allow us to measure small regions of [99mTc]TRODAT-1 labeling.
Blood samples were collected once a day at 08:00. We cannot exclude that the hormones assessed did not lose their periodic pattern of excretion during sleep loss. Since the volunteers were injected with a radioactive isotope to perform the SPECT, additional blood samples to evaluate hormone levels were not recommended after this intervention.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Bressan has received research support from and has participated in speaking engagements for Eli Lilly, Astra-Zeneca, and Janssen. The other authors have indicated no financial conflicts of interest.
ACKNOWLEGMENTS
The authors would like to express their sincere thanks to Centro de Estudos em Psicobiologia e Exercício (CEPE), Associação Fundo de Incentivo à Psicofarmacologia (AFIP), and Instituto do Sono staff. We are very appreciative of the help from the experimenters and research staff, as well as Andrew Newberg. This TRODAT-1&SPECT study was made possible through collaboration between the Institute of Nuclear Energy Research Council (INER-Taiwan, R.O. China) and the Federal University of São Paulo (UNIFESP). We would like to thank all volunteers who participated in the experimental protocol. ST and MLA are recipients of CNPq fellowships. This work was supported by grants from AFIP and FAPESP (CEPID #98/14303-3 to ST and 06/58276-8 to RCMS).
REFERENCES
- 1.Bonnet MH, Arand DL. We are chronically sleep deprived. Sleep. 1995;18:908–11. doi: 10.1093/sleep/18.10.908. [DOI] [PubMed] [Google Scholar]
- 2.Tufik S, Lindsey CJ, Carlini EA. Does REM sleep deprivation induce a supersensitivity of dopaminergic receptors in the rat brain? Pharmacology. 1978;16:95–108. doi: 10.1159/000136753. [DOI] [PubMed] [Google Scholar]
- 3.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105:1044–9. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lammers CH, D'Souza U, Qin ZH, Lee SH, Yajima S, Mouradian MM. Regulation of striatal dopamine receptors by estrogen. Synapse. 1999;34:222–7. doi: 10.1002/(SICI)1098-2396(19991201)34:3<222::AID-SYN6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 5.Tufik S. Changes of response to dopaminergic drugs in rats submitted to REM-sleep deprivation. Psychopharmacology. 1981;72:257–60. doi: 10.1007/BF00431826. [DOI] [PubMed] [Google Scholar]
- 6.Nunes GP, Tufik S, Nobrega JN. Autoradiographic analysis of D1 and D2 dopaminergic receptors in rat brain after paradoxical sleep deprivation. Brain Res Bull. 1994;34:435–56. doi: 10.1016/0361-9230(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 7.Lima MM, Andersen ML, Reksidler AB, et al. Blockage of dopaminergic D(2) receptors produces decrease of REM but not of slow wave sleep in rats after REM sleep deprivation. Behav Brain Res. 2008;188:406–11. doi: 10.1016/j.bbr.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 8.Lena I, Parrot S, Deschaux O, et al. Variations in extracellular levels of dopamine, noradrenaline, glutamate, and aspartate across the sleep-wake cycle in the medial prefrontal cortex and nucleus accumbens of freely moving rats. J Neurosci Res. 2005;81:891–9. doi: 10.1002/jnr.20602. [DOI] [PubMed] [Google Scholar]
- 9.Dzirasa K, Ribeiro S, Costa R, et al. Dopaminergic control of sleep-wake states. J Neurosci. 2006;26:10577–89. doi: 10.1523/JNEUROSCI.1767-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21:1787–94. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen ML, Papale LA, Hipolide DC, Nobrega JN, Tufik S. Involvement of dopamine receptors in cocaine-induced genital reflexes after paradoxical sleep deprivation. Behav Brain Res. 2005;160:44–50. doi: 10.1016/j.bbr.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Volkow ND, Wang GJ, Telang F, et al. Sleep deprivation decreases binding of [11C]raclopride to dopamine D2/D3 receptors in the human brain. J Neurosci. 2008;28:8454–61. doi: 10.1523/JNEUROSCI.1443-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benington JH. Debating how REM sleep is regulated (and by what) J Sleep Res. 2002;11:29–31. doi: 10.1046/j.1365-2869.2002.0282a.x. [DOI] [PubMed] [Google Scholar]
- 14.van Dyck CH, Seibyl JP, Malison RT, et al. Age-related decline in striatal dopamine transporter binding with iodine-123-beta-CITSPECT. J Nucl Med. 1995;36:1175–81. [PubMed] [Google Scholar]
- 15.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 16.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 17.American Sleep Disorders Association. The Atlas Task Force. Recording and scoring leg movements. Sleep. 1993;16:748–59. [PubMed] [Google Scholar]
- 18.American Sleep Disorders Association. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 19.Shih MC, Amaro JrE, Ferraz HB, et al. Neuroimaging of the dopamine transporter in Parkinson's disease – First study using [99mTc]-TRODAT-1 and SPECT in Brazil. Arq Neuropsiquiatr. 2006;64:628–34. doi: 10.1590/s0004-282x2006000400021. [DOI] [PubMed] [Google Scholar]
- 20.Chang LT. A method for attenuation correction in radionuclide computed tomography. IEEE Trans Nucl Sci. 1978;25:638–43. [Google Scholar]
- 21.Chou KL, Hurtig HI, Stern MB, et al. Diagnostic accuracy of [99mTc]TRODAT-1 SPECT imaging in early Parkinson's disease. Parkinsonism Relat Disord. 2004;10:375–9. doi: 10.1016/j.parkreldis.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 23.Kecklund G, Akerstedt T. Objective components of individual differences in subjective sleep quality. J Sleep Res. 1997;6:217–20. doi: 10.1111/j.1365-2869.1997.00217.x. [DOI] [PubMed] [Google Scholar]
- 24.Endo T, Roth C, Landolt HP, et al. Selective REM sleep deprivation in humans: effects on sleep and sleep EEG. Am J Physiol. 1998;274:R1186–94. doi: 10.1152/ajpregu.1998.274.4.R1186. [DOI] [PubMed] [Google Scholar]
- 25.Dement W, Greenberg S. Changes in total amount of stage four sleep as a function of partial sleep deprivation. Electroencephalogr Clin Neurophysiol. 1966;20:523–6. doi: 10.1016/0013-4694(66)90110-6. [DOI] [PubMed] [Google Scholar]
- 26.Andersen ML, Martins PJ, D'Almeida V, Bignotto M, Tufik S. Endocrinological and catecholaminergic alterations during sleep deprivation and recovery in male rats. J Sleep Res. 2005;14:83–90. doi: 10.1111/j.1365-2869.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- 27.Copinschi G. Metabolic and endocrine effects of sleep deprivation. Essent Psychopharmacol. 2005;6:341–7. [PubMed] [Google Scholar]
- 28.Schussler P, Yassouridis A, Uhr M, et al. Growth hormone-releasing hormone and corticotropin-releasing hormone enhance non-rapid-eye-movement sleep after sleep deprivation. Am J Physiol Endocrinol Metab. 2006;291:E549–56. doi: 10.1152/ajpendo.00641.2005. [DOI] [PubMed] [Google Scholar]
- 29.Voderholzer U, Hohagen F, Klein T, et al. Impact of sleep deprivation and subsequent recovery sleep on cortisol in unmedicated depressed patients. Am J Psychiatry. 2004;161:1404–10. doi: 10.1176/appi.ajp.161.8.1404. [DOI] [PubMed] [Google Scholar]
- 30.Henry JP. Biological basis of the stress response. Integr Physiol Behav Sci. 1992;27:66–83. doi: 10.1007/BF02691093. [DOI] [PubMed] [Google Scholar]
- 31.Roky R, Obál F, Jr, Valatx JL, et al. Prolactin and rapid eye movement sleep regulation. Sleep. 1995;18:536–42. [PubMed] [Google Scholar]
- 32.Obál F, Jr, Krueger JM, Hormones . cytokines and sleep. In: McEwen BS, editor. Coping with the environment neural and endocrine mechanisms: handbook of physiology. New York: Oxford University Press; 2000. pp. 331–49. Chap 16. [Google Scholar]
- 33.González-Santos MR, Gajá-Rodríguez OV, Alonso-Uriarte R, Sojo-Aranda I, Cortés-Gallegos V. Sleep deprivation and adaptive hormonal responses of healthy men. Arch Androl. 1989;22:203–7. doi: 10.3109/01485018908986773. [DOI] [PubMed] [Google Scholar]
- 34.Silva A, Andersen ML, De Mello MT, Bittencourt LR, Peruzzo D, Tufik S. Gender and age differences in polysomnography findings and sleep complaints of patients referred to a sleep laboratory. Braz J Med Biol Res. 2008;41:1067–75. doi: 10.1590/s0100-879x2008001200005. [DOI] [PubMed] [Google Scholar]
- 35.Morissette M, Di Paolo T. Sex and estrous cycle variations of rat striatal dopamine uptake sites. Neuroendocrinology. 1993;58:16–22. doi: 10.1159/000126507. [DOI] [PubMed] [Google Scholar]
- 36.Davies B, Shapiro CM, Daggett A, Gatt JA, Jakeman P. Physiological changes and sleep responses during and following a world record continuous walking record. Br J Sports Med. 1984;18:173–80. doi: 10.1136/bjsm.18.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gessa GL, Pani L, Fadda P, Fratta W. Sleep deprivation in the rat: an animal model of mania. Eur Neuropsychopharmacol. 1995;5(Suppl):89–93. doi: 10.1016/0924-977x(95)00023-i. [DOI] [PubMed] [Google Scholar]
- 38.Martins RC, Andersen ML, Shih MC, Tufik S. The effects of cocaine, methamphetamine and modafinil challenge on sleep rebound after paradoxical sleep deprivation in rats. Braz J Med Biol Res. 2008;41:68–77. doi: 10.1590/s0100-879x2008000100011. [DOI] [PubMed] [Google Scholar]
- 39.Maret S, Franken P, Dauvilliers Y, et al. Retinoic acid signaling affects cortical synchrony during sleep. Science. 2005;310:111–3. doi: 10.1126/science.1117623. [DOI] [PubMed] [Google Scholar]
- 40.Bo P, Ongini E, Giorgetti A, Savoldi F. Synchronization of the EEG and sedation induced by neuroleptics depend upon blockade of both D1 and D2 dopamine receptors. Neuropharmacology. 1988;27:799–805. doi: 10.1016/0028-3908(88)90094-9. [DOI] [PubMed] [Google Scholar]
- 41.Krezel W, Ghyselinck N, Samad TA, et al. Impaired locomotion and dopamine signaling in retinoid receptor mutant mice. Science. 1998;279:863–7. doi: 10.1126/science.279.5352.863. [DOI] [PubMed] [Google Scholar]
- 42.Krezel W, Kastner P, Chambon P. Differential expression of retinoid receptors in the adult mouse central nervous system. Neuroscience. 1999;89:1291–300. doi: 10.1016/s0306-4522(98)00342-x. [DOI] [PubMed] [Google Scholar]
- 43.Gillin JC, Pulvirenti L, Withers N, Golshan S, Koob G. The effects of lisuride on mood and sleep during acute withdrawal in stimulant abusers: a preliminary report. Biol Psychiatry. 1994;35:843–9. doi: 10.1016/0006-3223(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 44.Jaber M, Jones S, Giros B, Caron MG. The dopamine transporter: a crucial component regulating dopamine transmission. Mov Disord. 1997;12:629–33. doi: 10.1002/mds.870120502. [DOI] [PubMed] [Google Scholar]
- 45.Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci U S A. 1998;95:4029–34. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bagetta G, De Sarro G, Priolo E, Nistico G. Ventral tegmental area: site through which dopamine D2-receptor agonists evoke behavioural and electrocortical sleep in rats. Br J Pharmacol. 1988;95:860–6. doi: 10.1111/j.1476-5381.1988.tb11715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]