Abstract
Study Objectives:
To evaluate the use of sham-continuous positive airway pressure (CPAP) treatment as a placebo intervention.
Design and Setting:
Analysis of polysomnograms performed in fixed order without sham-CPAP and on the first night of the sham-CPAP intervention in participants in the CPAP Apnea Trial North American Program (CATNAP), a randomized, placebo controlled trial evaluating the effects of CPAP treatment on daytime function in adults with newly diagnosed mild to moderate obstructive sleep apnea (apnea hypopnea index (AHI) 5 - 30).
Participants:
The first 104 CATNAP participants randomized to the sham-CPAP intervention arm.
Measurements and Results:
Compared to the polysomnographic measures without sham-CPAP, the study on the first night with sham-CPAP had statistically significant differences that suggested a decrease in sleep quality: decreased sleep efficiency, increased arousal index, increased time in stage 1 NREM sleep, and prolonged latency to REM sleep. However, all of these differences had a relatively small effect size. Compared to the polysomnogram without sham-CPAP, the number of hypopneas on the sham-CPAP polysomnogram was significantly increased and the number of apneas significantly decreased. Relatively minor differences in AHI with and without sham-CPAP were present and were dependent on the criteria used to score hypopneas.
Conclusion:
Comparison of polysomnograms with and without sham-CPAP revealed differences that, although statistically significant, were small in magnitude and had relatively low effect sizes suggesting minimal clinical significance. The results support the use of sham-CPAP as a placebo intervention in trials evaluating the effects of CPAP treatment in patients with obstructive sleep apnea.
Clinical Trial Information:
This paper was a secondary analysis of clinical trial data. CATNAP: CPAP Apnea Trial North American Program, the trial from which the data were obtained, is registered with clinicaltrial.gov. Registration #NCT00089752.
Citation:
Rodway GW; Weaver TE; Mancini C; Cater J; Maislin G; Staley B; Ferguson KA; George CFP; Schulman DA; Greenberg H; Rapoport DM; Walsleben JA; Lee-Choing T; Kuna ST. Evaluation of sham-CPAP as a placebo in CPAP intervention studies. SLEEP 2010;33(2):260-266.
Keywords: CPAP, randomized controlled trial, placebo, sham-CPAP, polysomnogram
CONTINUOUS POSITIVE AIRWAY PRESSURE (CPAP) IS THE PRIMARY MEDICAL TREATMENT FOR OBSTRUCTIVE SLEEP APNEA (OSA).1,2 NUMEROUS RANDOMIZED controlled trials (RCTs) have assessed the effectiveness of this treatment in patients with OSA.3 initial trials used either an oral placebo tablet 4–8 or conventional care9,10 for patients randomized to non-CPAP treatment. as noted by farrée and colleagues, these studies failed to apply identical instrumental constraints to both treatment and non-treatment groups.11 in an effort to create a more appropriate placebo, farrée and colleagues modified the CPAP apparatus so that pressure levels at the mask interface were < 1 cm H2o.11 comparing polysomnograms (PSGs) in patients with OSA recorded with and without sham-CPAP, the modified CPAP circuit had no effect on apnea-hypopnea index (AHI), a measure of OSA severity.11 sham-CPAP is currently the placebo intervention of choice in RCTs evaluating the effectiveness of CPAP treatment.12–15 although these studies uniformly report no difference in AHI on PSGS with and without sham-CPAP in patients randomized to the placebo intervention arm, no systematic evaluation in a large number of subjects has assessed the effect of sham-CPAP on multiple other PSG measures that might influence clinical outcomes.
The primary purpose of this study was to perform a comprehensive evaluation of sham-CPAP effects on PSG outcomes in subjects with OSA. To make this comparison, we used data obtained from participants in the CPAP Apnea Trial North American Program (CATNAP), a multi-center, randomized, triple-blind study utilizing sham-CPAP as the placebo intervention. One of the secondary purposes of this study was to validate the placebo used in CATNAP.
METHODS
The primary purpose of the CATNAP study is to determine the impact of CPAP treatment on functional outcomes in 281 subjects with newly diagnosed mild to moderate OSA (AHI 5-30). Participants were enrolled at 5 clinical sites: University of Western Ontario, London, Ontario, Canada; Emory University, Atlanta GA; New York University School of Medicine, New York, NY; North Shore-Long Island Jewish Health System, Long Island, NY; and National Jewish Health, Denver, CO. The CATNAP protocol was approved by the institutional review board of each institution, as well as the University of Pennsylvania. Written informed consent was obtained from each subject prior to study participation.
CATNAP participants were recruited from consecutive patients referred to the sleep centers at the participating clinical sites who were newly diagnosed with mild to moderate OSA on a routine full-night diagnostic PSG. The subjects selected for this sub-study were the first 104 individuals randomized to the placebo arm of CATNAP. CATNAP inclusion criteria included: age > 18 y; subjective sleepiness operationally defined as an Epworth Sleepiness Scale score ≥ 1116; absence of another recognized sleep disorder; stability of any chronic medical problems; no change in medications in the last 3 months; no regular use (> 3 times/week) of sedative, hypnotic, or alerting medications in the last 3 months; not pregnant; and no history of a sleepiness-related driving accident or current sleepiness-sensitive occupation. Exclusion criteria included any previous treatment for OSA, inability to return for instructions or follow-up testing, chronic nasal congestion over the prior 3 months that would prevent the use of a nasal mask, recent or recurring history of substance or alcohol abuse (determined by the CAGE questionnaire17) leading to tolerance or dependence, residing with an individual currently using CPAP treatment, and inability to perform written and verbal tests.
Following enrollment, participants were assigned randomly by computer to one of 2 treatment groups: (1) active CPAP; or (2) sham-CPAP (placebo). Subjects randomized to the placebo intervention were fitted with one of the following nasal mask interfaces: Comfort Gel, Comfort Classic, Comfort Select, and Profile Lite (Philips Respironics, Murrysville, PA), and scheduled for a full night PSG using sham-CPAP. The mean time span between the diagnostic and sham-CPAP titration studies was 57 + 22.7 (SD) days, range 10–125 days.
To standardize data collection across sites, the following PSG signals were recorded at each site during both the diagnostic and sham-CPAP PSGs: electroencephalograms (C3M2, C4M1, O2M1), bilateral electroculograms, electromyograms of the chin muscles and right and left anterior tibialis, movement of the rib cage and abdomen (piezoelectric crystal), oxygen saturation (Spo2) by pulse oximetry, electrocardiogram (Lead 1), and body position. Nasal pressure (ProTech PTAF2) was the surrogate airflow signal recorded on the diagnostic PSGs, and mask pressure (ProTech PTAF2) was used as the airflow signal on the sham-CPAP studies. The airflow signal from the CPAP machine could not be used since the large expiratory leak and orifice restrictor in the sham-CPAP circuit (see below) prevented the signal from being received by the machine’s sensors. The only equipment that was standardized across all sites was the amplifier for the nasal pressure signal (Pro-Tech Services, Inc., Mukilteo, WA). To further standardize data collection, each site adhered to uniform criteria for signal processing, e.g., digitization rates and AC filters.
Similar to the sham-CPAP design of Farrée and co-workers,11 the sham-CPAP apparatus (RemStar Pro, Philips Respironics) used in the CATNAP study consisted of an enlarged air leak incorporated into the exhalation valve (Philips Respironics, Whisper Swivel) positioned between the mask and the CPAP tubing (Figure 1) and an orifice restrictor in the CPAP circuit (Figure 2). The modification in the exhalation valve was not visually noticeable when the valve was fully assembled. During the sham-CPAP PSG, the technologists used the sleep centers' remotely controlled CPAP machines as the sham-CPAP device to avoid the possibility of unblinding participants if they recognized that they were receiving a different type of intervention than other patients being studied in the laboratory. The laboratory CPAP machine was converted into a sham device by inserting the orifice restrictor into the circuit at the point where the CPAP tubing connected to the machine and attaching the sham expiratory valve to the mask interface. With the machine set at 10 cm H2O throughout the night, the pressure at the mask interface was < 1 cm H2O. Prior to the participant's arrival in the sleep center, the PSG technologist set up the sham circuit and confirmed with a portable manometer that, when the CPAP machine was turned on, the pressure in the occluded mask was less than 1 cm H2O. During the sham-CPAP PSGs, the tubing from the pressure sensor was attached to a port on the mask interface. The sham-CPAP apparatus (Philips Respironics) distributed to the participants for home use had the same circuit as that used during the PSG with sham-CPAP with the exception that the orifice restrictor was incorporated in the CPAP machine so that it was not visible and the machine looked identical to that used by participants randomized to active CPAP treatment.
Figure 1.
Exhalation connectors inserted between the mask interface and tubing in the active and sham-CPAP circuits. Figure 1a shows the inner part of the exhalation connector used in the active CPAP (left) and sham-CPAP (right) circuits. The inner part of the exhalation connector used in the sham-CPAP circuit had an enlarged port for expiratory airflow. This modification was not noticeable when the connector was fully assembled (Fig. 1b, active connector on left, sham-CPAP connector on right).
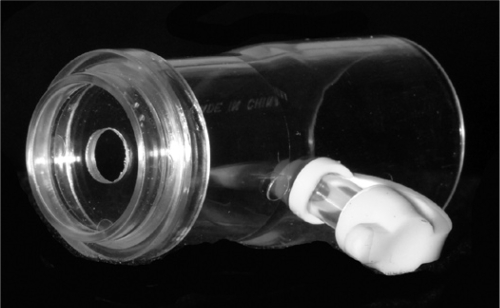
Figure 2.
Orifice restrictor used in the sham-CPAP circuit. During the sham-CPAP polysomnograms, the orifice restrictor was inserted between the laboratory's remotely controlled CPAP machine and tubing. A port on the side of the orifice restrictor was occluded by a white plastic cap. For the device that the participants used at home, the orifice restrictor was located within the CPAP machine. Placement of the orifice restrictor and exhalation connector with enlarged air leak into the CPAP circuit, with the machine set at 10 cm H2O, resulted in a pressure at the mask interface less than 1 cm H2O.
Polysomnographic files were electronically transmitted to the University of Pennsylvania by means of the CATNAP web portal or File Transfer Protocol for centralized, manual, computer software assisted scoring (Sandman NT software; Covidien, Mansfield, MA). Three of the clinical sites recorded the PSGs using software different than that used by the scoring lab. In order for these recordings to be analyzed, the files were converted into European Data Format prior to being transmitted to the scoring lab.18,19 In these instances, the technologists used a standardized PSG event log to record events during the studies since electronic tags on the files were lost when the files were converted to European Data Format.
Sleep stages were characterized by Rechtschaffen and Kales criteria.20 Stages 3 and 4 NREM sleep were scored as stage 3-4 NREM sleep. Arousals were characterized by the ASDA criteria.21 An arousal was associated with a respiratory event if it began within 3 sec of the termination of the event. Apneas were identified if the airflow signal was flat or nearly flat, i.e., below at least 10% of baseline, and the decrease lasted for > 10 s. Apneas associated with respiratory effort were scored as obstructive apneas. Apneas that were not associated with respiratory effort were scored as central apneas. Mixed apneas were scored as obstructive apneas. Hypopneas were scored using several different criteria: (1) a greater than 50% decrease in amplitude of any respiratory signal below baseline ≥ 10 sec, and (2) decrease in amplitude of a respiratory signal below baseline ≥ 10 sec associated with a > 3% transient fall in Spo2 (Hypopneas with > 3% desaturation) or an arousal (hypopneas with arousal). The total number of hypopneas (Hypopnea Total) reported in Results include the events scored using all of the above criteria. Apnea-hypopnea index (AHI)was calculated as the mean number of apneas and hypopneas per hour of sleep. AHIs were calculated using the different hypopnea determinations: AHI that included all scored hypopneas (Total AHI), AHI that included only those hypopneas associated with arousals (AHI with arousals), and AHI that included only those hypopneas associated with a greater than 3% oxygen desaturation (AHI with > 3% desaturation). The latter AHI was used to determine participant eligibility in CATNAP. The oximetry recording was used to determine: oxygen desaturation index (mean number of transient oxygen desaturation events > 3% per hour of sleep), minimum Spo2, mean Spo2, and sleep time spent below 95%, 90%, and 85% Spo2.
The PSG outcome measures related to sleep staging included: sleep efficiency (total sleep time divided by total recording time), sleep latency (time from “lights out” to the first epoch of any stage of sleep), arousal index (mean number of arousals per hour of sleep), latency to stage REM and stage 1, 2, and 3/4 NREM sleep (time from sleep onset to the first epoch of REM and a particular stage of NREM sleep), wake after sleep onset (minutes awake following sleep onset), episodes of wakefulness following sleep onset, total sleep time, and amount of time in each sleep stage. In addition, we measured the sleep time spent in the supine and non-supine body position.
Scorers in the centralized scoring laboratory, as well as PSG technicians recording the studies, were not blinded to study condition. However, study participants, site investigators, study coordinators, Project Manager, statistician, and Principal Investigator were blinded. The scored diagnostic and sham-CPAP PSG data were electronically exported to Excel spreadsheets by customizing software options in the Sandman software. Intra-scorer reliability was determined by having the scorer rescore 32 randomly chosen deidentified PSGs. The intraclass correlation coefficient for AHI was 0.89. A value > 0.80 is considered excellent.22
Analysis
A paired samples t-test was used to assess differences between the diagnostic and sham-CPAP PSG variables. For this analysis, the change in PSG variables from diagnostic to sham-CPAP PSG was computed for each subject. To test the hypothesis, the mean difference divided by the standard error of the difference was compared to the expected value of zero (for the null hypothesis of no difference between the diagnostic and sham-CPAP PSG). Statistical significance was established at P < 0.05 a priori. The application of paired t-tests to multiple variables increases the risk of a type I error. This issue is frequently addressed with a Bonferroni correction. However, we decided to take a more conservative approach and elected not to apply a Bonferroni correction to the results, thereby making it easier to reject the null hypothesis. Effect size (Cohen's “d”) was calculated as the mean difference between diagnostic PSG and sham-CPAP PSG values divided by the standard deviation of this difference. Although advice differs regarding how to interpret effect size, perhaps the most accepted opinion is that of Cohen,23 where 0.2 is indicative of a small effect, 0.5 a medium effect, and 0.8 a large effect size. In inferential statistics, effect size helps to determine whether a statistically significant difference is a difference of practical concern. A value of 0.5 or greater is generally considered to be clinically meaningful.
RESULTS
Description of sample
Of the 104 CATNAP participants (42% female) included in this analysis, mean age was 51.8 + 11.5 (SD) y, height 170.8 + 11.0 cm, weight 98.7 + 22.9 kg, and BMI 33.2 + 6.8 kg/m2. The sample consisted of 12.5% African Americans, 81.7% Caucasians, and 6.7% Hispanics.
Sleep Staging
No significant differences between the PSGs without and with sham-CPAP were noted for the following measures: sleep latency, latency to stages 1, 2, and 3-4 NREM sleep, wake time after sleep onset, minutes in stage 2 NREM sleep, minutes in REM, minutes in NREM sleep, and total sleep time (Table 1). Compared to the PSG without sham-CPAP, the sham-CPAP PSG was associated with the following statistically significant differences (P < 0.05): decreased sleep efficiency, increased arousal index, increased latency to REM sleep, increased episodes of wakefulness following sleep onset, increased minutes in stage 1 NREM sleep, and decreased minutes in stage 3-4 NREM sleep (Table 1). The effect size of all of these differences was ≤ 0.40 and therefore unlikely to be of clinical significance.
Table 1.
Sleep-related variables measured during the polysomnograms with and without sham-CPAP
| Variable | PSG without sham-CPAP mean (SD) | PSG with sham-CPAP mean (SD) | P-value* | Effect size (d) |
|---|---|---|---|---|
| Sleep efficiency (%) | 81.4 (10.4) | 78.3 (12.9) | 0.015 | 0.24 |
| Arousal index (events/h) | 29.7 (11.0) | 35.2 (16.1) | < 0.001 | 0.40 |
| Sleep latency (min) | 17.4 (23.0) | 13.9 (18.1) | 0.156 | 0.14 |
| Latency to stage 1 NREM (min) | 3.6 (19.2) | 0.8 (6.5) | 0.161 | 0.14 |
| Latency to stage 2 NREM (min) | 4.8 (8.7) | 6.4 (15.6) | 0.338 | 0.09 |
| Latency to stage 3/4 NREM† (min) | 41.1 (43.8) | 50.3 (57.1) | 0.322 | 0.14 |
| Latency to REM sleep (min) | 140.6 (93.2) | 177.1 (112.3) | 0.004 | 0.29 |
| Wake after sleep onset (min) | 67.5 (42.2) | 72.6 (57.7) | 0.391 | 0.08 |
| Episodes of wakefulness | 33.2 (15.2) | 39.1 (18.7) | 0.002 | 0.30 |
| Minutes in stage 1 NREM | 35.7 (22.1) | 42.1 (29.1) | 0.028 | 0.22 |
| Minutes in stage 2 NREM | 255.0 (50.5) | 250.1 (56.2) | 0.417 | 0.08 |
| Minutes in stage 3-4 NREM | 21.0 (26.9) | 16.1 (24.2) | 0.039 | 0.21 |
| Minutes in REM sleep | 56.9 (25.5) | 51.5 (31.5) | 0.071 | 0.18 |
| Minutes in NREM sleep | 311.6 (52.8) | 308.3 (55.1) | 0.570 | 0.06 |
| Total sleep time (min) | 368.5 (55.1) | 360.2 (66.1) | 0.206 | 0.12 |
| Total recording time (min) | 453.7 (42.0) | 460.7 (41.8) | 0.147 | 0.14 |
paired t-test P-values representing comparison of PSGs with and without sham-CPAP
PSG = polysomnogram; NREM = non-rapid eye movement; REM = rapid eye movement;
n = 50; all other variables n = 104
Respiratory Variables
As required by the inclusion criteria, all participants had objectively documented OSA. On the PSG without sham-CPAP, the mean AHI based on scoring hypopneas associated with > 3% oxygen desaturations was 12.1 + 6.3 events/h (Table 2). No statistically significant differences were noted with and without sham-CPAP in total AHI, minimum Spo2, total number of central apneas, and percent time the Spo2was below 90% and 85% (Table 2). Statistically significant differences (P < 0.05) were observed for some respiratory variables (Table 2); however, the clinical significance of these changes is doubtful due to the relatively small magnitude of the changes and the modest effect sizes (d ≤ 0.59). For example, the increases in oxygen desaturation index, AHI with desaturations > 3%, and AHI with arousals on the sham-CPAP study were < 3 events/h compared to their respective values on the PSG without sham-CPAP. Perhaps most importantly, when differences in AHI were present, the value was higher during the sham-CPAP PSG.
Table 2.
Respiratory-related variables measured during the polysomnograms with and without sham-CPAP
| Variable | PSG without sham-CPAP mean (SD) | PSG with sham-CPAP mean (SD) | P-value* | Effect size (d) |
|---|---|---|---|---|
| AHI w/ desats > 3% (events/h)§ | 12.1 (6.3) | 14.5 (12.0) | 0.024 | 0.22 |
| Total AHI (events/h) ‡ | 33.8 (13.8) | 34.0 (20.1) | 0.921 | 0.01 |
| AHI during REM (events/h) | 48.0 (22.6) | 39.8 (25.9) | 0.004 | 0.29 |
| AHI w/ arousals (events/h)** | 18.4 (9.4) | 21.1 (15.1) | 0.026 | 0.22 |
| Oxygen desaturation index (events/h) | 13.6 (6.8) | 16.4 (13.5) | 0.021 | 0.23 |
| Minimum Spo2 (%) | 81.7 (7.6) | 80.6 (11.5) | 0.318 | 0.10 |
| Mean Spo2 (%) | 94.3 (1.6) | 95.1 (1.5) | < 0.001 | 0.59 |
| Number of obstructive apneas † | 67.7 (63.7) | 39.3 (51.0) | < 0.001 | 0.50 |
| Number of central apneas† | 1.6 (5.0) | 3.8 (16.2) | 0.112 | 0.16 |
| Number of Hypopneas total†‡ | 140.7 (68.5) | 158.7 (92.0) | 0.029 | 0.22 |
| % time Spo2 below 95%†† | 61.0 (31.7) | 53.3 (32.0) | 0.008 | 0.27 |
| % time Spo2 below 90%†† | 3.8 (6.4) | 2.6 (3.8) | 0.063 | 0.19 |
| % time Spo2 below 85%†† | 0.6 (1.8) | 0.4 (1.0) | 0.085 | 0.17 |
paired t-test P-values representing baseline vs. sham-CPAP PSG comparison
PSG = polysomnogram; AHI = apnea-hypopnea index; Spo2 = arterial oxygen saturation; REM = rapid eye movement
Data missing on apnea/hypopnea totals for 2 subjects
Total AHI and Hypopnea total = AHI and number of hypopneas based on scoring criteria detailed in the 1999 American Academy of Sleep Medicine Task Force publication.35
AHI w/desats > 3% = AHI based on the requirement that hypopneas be associated with greater than 3% oxygen desaturation; this determination of AHI was used for study entry criteria;
AHI w/ arousals = AHI based on requirement that hypopneas be associated with an arousal;
% time Spo2 below 95%, 90%, and 85% = percent of total sleep time that oxygen saturation was below the specified level.
Body Position-Related Variables
No significant differences in body position were noted between the PSGs with and without sham-CPAP.
DISCUSSION
The primary purpose of this study was to perform a comprehensive evaluation of sham-CPAP effects on PSG measurements in subjects with OSA. The changes in sleep staging variables are indicative of decreased sleep quality during the subjects' first night using sham-CPAP compared to the preceding PSG without sham-CPAP. With regard to AHI, the primary PSG outcome measure in most studies of patients with OSA, the presence or absence of a difference in AHI between the PSGs with and without sham-CPAP was dependent on the method used to score hypopneas. When a statistically significant difference in an AHI determination with and without sham-CPAP was present, the difference was relatively small and the AHI was slightly greater on the sham-CPAP PSG. Comparison of sleep stage and body position variables across the two conditions offered no clear explanation for the observed changes in AHI.
We recognize that a Bonferroni correction is often applied when paired t-tests are used to analyze multiple variables in order to decrease the risk of type I statistical error. In this study, we desired the null hypothesis and therefore elected the more conservative approach of not applying a Bonferroni correction. This reduced the possibility of type II statistical errors and made it easier to reject the null hypothesis.24 With this approach, we did find statistically significant changes in some PSG variables, but none of the comparisons had a large effect size (≥ 0.8) and only a few were in the medium effect size range. The relatively low effect sizes suggest that the observed statistical differences in PSG variables are of doubtful clinical importance.
Perhaps the most interesting finding related to the respiratory parameters was the decrease in the number of obstructive apneas and the increase in the number of hypopneas on the sham-CPAP PSG compared to the PSG without sham-CPAP. Despite these changes in the type of respiratory event, the AHI determinations on the sham-CPAP PSG were either similar or minimally increased compared to those on the study without sham-CPAP. The increase in hypopneas (and decrease in apneas) during the sham-CPAP PSG may at least partially explain the observed improvement seen in Spo2 measures. The shift from apneas to hypopneas was not explained by differences in sleep staging or time spent in the supine position. One can speculate that the shift in the type of respiratory event may have been due to increased inhaled carbon dioxide on the sham-CPAP study because of mask dead space, or the application of the small positive airway pressure delivered by the sham-CPAP device (< 1 cm H2O). In addition, the increase in arousal index and wakefulness after sleep onset episodes on the sham-CPAP PSG may have resulted in the shift in the type of respiratory event between the two conditions.
The change in the proportion of apneas and hypopneas between the PSGs with and without sham-CPAP may also have been due to the different methods used to record airflow. During the diagnostic PSG, nasal cannula were used to detect pressure swings inside the nostril and the nares opening served to provide a low resistance that caused pressure to oscillate in proportion to airflow. Using this technique under conditions of low airflow, however, these pressure oscillations may have been greatly attenuated making it more likely that reductions in airflow would be scored as apneas rather than hypopneas. In contrast, during the sham CPAP PSGs, we recorded pressure oscillations within the mask with a small bias flow going through a large leakport. This arrangement produces a pressure swing that may be more linearly related to airflow at the lowest airflows, making it more likely that reductions in airflow would be recorded by the pressure sensor and therefore scored as hypopneas rather than apneas.
Using a fixed order study design similar to that in the current study, Henke and colleagues25 compared the outcome measures from PSGs without and with sham-CPAP in 18 subjects with OSA (mean AHI on the PSG without sham-CPAP 68.1 + 25.2 events/h) and found that the placebo intervention in this relatively small sample did not significantly affect any of the measured sleep parameters (total sleep time, sleep efficiency, sleep maintenance efficiency, sleep latency, time in REM and NREM stages, AHI, apnea index, desaturation index, minimum Spo2, and average minimum oxygen desaturation during apneas and hypopneas). No previous studies, however, have examined the effect of sham-CPAP on other potentially important PSG measures such as arousal index, AHI during REM, and AHI events associated with oxygen desaturation > 3%. The effect of sham-CPAP on such parameters could conceivably influence treatment outcomes in a randomized, placebo controlled trial such as CATNAP, and thus were analyzed in the current study. In addition to our larger sample size than the study of Henke et al.,25 the subjects studied by Henke and coworkers had more severe OSA than the subjects in the present investigation. It is conceivable that limiting our comparison to patients with mild to moderate OSA may have contributed to the more notable PSG changes in our study.
When taken together, the changes in sleep-staging related measures are consistent with decreased sleep quality during the sham-CPAP PSG. Sleep efficiency and minutes in slow wave sleep decreased, while arousal index, latency to REM sleep, episodes of wakefulness, and minutes in stage 1 NREM sleep increased during the sham-CPAP PSG. We suggest that the probable explanation for these findings is a “mask effect” of wearing the CPAP apparatus for the first time. The novel experience of wearing this device may have accounted for the participants' decrease in sleep quality on the sham-CPAP study. It is likely that similar effects of the initial mask intervention on sleep would be present on a PSG with “active” CPAP. Such a possible device-driven alteration in sleep may in some respects be analogous to the so-called “first night effect” on diagnostic PSG results.30–32
Previous studies comparing two or more nights of diagnostic PSGs have reported a “first night effect” on sleep stage variables similar to those found in our study on the sham-CPAP PSG: decreased total sleep time, less REM sleep, increased wakefulness after sleep onset, lower sleep efficiency, and a prolonged REM sleep latency during the first night's study.30,33 It is consequently plausible that the sleep stage differences seen on the first night using sham-CPAP compared to the diagnostic PSG would not have been present if the sham-CPAP PSG had been performed following several nights of use. Unfortunately, a limitation of our study was the inability to test this possible explanation by performing repeat PSGs on sham-CPAP during the 8-week intervention period of the CATNAP protocol.
Some of the differences we observed between the PSGs with and without sham-CPAP may be explained by the known night-to-night variability in PSG outcome measures in normal subjects and patients with OSA.26–29 In studies comparing multiple study nights in the same subjects without any intervention, there is evidence of broad inter-PSG variation in AHI between study nights.28,34 This known night-to-night variability in AHI has led to the contention that a negative first-night study is insufficient to exclude OSA in patients with clinical markers of the disease,27,29 although not all investigators agree with this appraisal.28
Although the differences in PSG measures that we observed were relatively small, we cannot rule out the possibility that some of the observed differences in PSG measures on sham-CPAP might become clinically significant if they were to persist. Indeed, previous RCTs using sham-CPAP devices similar to ours report significant subjective improvements in daytime sleepiness and quality of life in participants randomized to the sham-CPAP intervention.14,25 These improvements have been attributed to a placebo effect but could conceivably have been due to some undetected therapeutic benefit of sham-CPAP, such as our observed shift from apneas to hypopneas. To account for the placebo and possible therapeutic effects on sham-CPAP, determination of clinically-related outcomes on CPAP treatment in RCTs should be based on comparison of the change in the selected outcome measures between participants randomized to the active and sham-CPAP intervention arms.
In summary, the sham-CPAP apparatus utilized in CATNAP appears to be an appropriate placebo even if the observed differences between the PSGs with and without sham-CPAP are long lasting. The changes in sleep staging variables between the two PSG conditions were suggestive of decreased sleep quality during the sham-CPAP PSG. To further evaluate this possibility, future RCTs using sham-CPAP might consider assessing the effect of this intervention on PSG measures following a week or more of participant use. Despite the significant increase in the number of hypopneas and significant decrease in the number of apneas on sham-CPAP, relatively minor differences in AHI with and without sham-CPAP were present and were dependent on how AHI was calculated. These changes are unlikely to be of clinical significance and may be explained by the different methods used to measure airflow during the baseline and sham-CPAP studies. Taken in their entirety, the results of this study support the use of sham-CPAP as a placebo in RCTs evaluating the effects of CPAP treatment in patients with OSA.
DISCLOSURE STATEMENT
This work was supported by NIH grant R01 HL076101 (T. Weaver) and the Respironics Sleep and Respiratory Foundation. Philips Respironics, Inc. provided the mask interfaces, whisper swivels, restrictor valves, and sham CPAP devices; ProTech Services, Inc. donated the pressure transducer units (PTAF2); and Coviden loaned the Sandman NT software used for PSG scoring. Dr. Weaver has received research support from Respironics Respiratory and Sleep Research Foundation and Cephalon, Inc.; has consulted for Apnex Medical, Inc. and Cephalon, Inc.; has received the use of equipment for research from Respironics, Inc. and Protech; and has FOSQ License Agreements with Sanofi-Aventis, Merck, Sleep Solutions, N.V. Organon, Apnex Medical, Inc., Ventus Medical, GlaxoSmithKline and Respironics, Inc. Dr. Ferguson is a director of Critical Outcome Technologies (COTI) and has participated in speaking engagements for Pfizer. Dr. Schulman has participated in speaking engagements for GlaxoSmithKline. Dr. Rapoport has received research support from Advance Brain Monitoring, Fisher & Paykel Healthcare, Genzyme, Guidant, Restore Medical (Medtronics) and Ventis Medical; has consulted for Apnex Medical, Inc. and Mannkind (DSMB); and holds multiple US and foreign patents covering techniques and analysis algorithms for the diagnosis of OSAHS and techniques for administering CPAP. Several of these have been licensed to Biologics, Fisher & Paykel Healthcare, Covidien, and Advance Brain Monitoring. Dr. Lee-Chiong has received research support from Takeda and has consulted for Covidien. Dr. Kuna has received research support from Respironics, Inc. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We wish to thank Mary Jones-Parker, RPSGT, and Haideliza Soto-Calderon of the CATNAP PSG Reading Center at the University of Pennsylvania. We also thank Shawn Ballard and Bridget Small of the Clinical Research Computing Unit of the Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania School of Medicine that served as the Data Coordinating Center and the Site Research Coordinators and PSG Technologists: Mala Ramu, Leila MacBean, Pat Clemens and Nina Marinkovic, University of Western Ontario; Laura Beth Straight, Kara Barrett and Jasmine Konn, Emory University; Bien Pagan-Lee and Ming Chen, New York University; Kristen Cruz, Asha John, Ana Hallinan and Rod Massop, North Shore Long Island Jewish Health System; Jen Goldschmeid and Allison Rankin, National Jewish Health, Philips Respironics, Inc., ProTech Services, Inc., and Coviden.
REFERENCES
- 1.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–5. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 2.Basner RC. Continuous positive airway pressure for obstructive sleep apnea. N Engl J Med. 2007;356:1751–8. doi: 10.1056/NEJMct066953. [DOI] [PubMed] [Google Scholar]
- 3.Gay P, Weaver T, Loube D, Iber C. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29:381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 4.Barnes M, Houston D, Worsnop CJ, et al. A randomized controlled trial of continuous positive airway pressure in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:773–80. doi: 10.1164/ajrccm.165.6.2003166. [DOI] [PubMed] [Google Scholar]
- 5.Engleman HM, Kingshott RN, Wraith PK, Mackay TW, Deary IJ, Douglas NJ. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:461–7. doi: 10.1164/ajrccm.159.2.9803121. [DOI] [PubMed] [Google Scholar]
- 6.Engleman HM, Martin SE, Deary IJ, Douglas NJ. Effect of CPAP therapy on daytime function in patients with mild sleep apnoea/hypopnoea syndrome. Thorax. 1997;52:114–9. doi: 10.1136/thx.52.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engleman HM, Martin SE, Deary IJ, Douglas NJ. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet. 1994;343:572–5. doi: 10.1016/s0140-6736(94)91522-9. [DOI] [PubMed] [Google Scholar]
- 8.Engleman HM, Martin SE, Kingshott RN, Mackay TW, Deary IJ, Douglas NJ. Randomised placebo controlled trial of daytime function after continuous positive airway pressure (CPAP) therapy for the sleep apnoea/hypopnoea syndrome. Thorax. 1998;53:341–5. doi: 10.1136/thx.53.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballester E, Badia JR, Hernéandez L, et al. Evidence of the effectiveness of continuous positive airway pressure in the treatment of sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:495–501. doi: 10.1164/ajrccm.159.2.9804061. [DOI] [PubMed] [Google Scholar]
- 10.Redline S, Adams N, Strauss ME, Roebuck T, Winters M, Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care Med. 1998;157:858–65. doi: 10.1164/ajrccm.157.3.9709042. [DOI] [PubMed] [Google Scholar]
- 11.Farrée R, Hernréandez L, Montserrat JM, Rotger M, Ballester E, Navajas D. Sham continuous positive airway pressure for placebo-controlled studies in sleep apnoea. Lancet. 1999;353:1154. doi: 10.1016/S0140-6736(99)01056-9. [DOI] [PubMed] [Google Scholar]
- 12.Campos-Rodriguez F, Grilo-Reina A, Perez-Ronchel J, et al. Effect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo-controlled trial. Chest. 2006;129:1459–67. doi: 10.1378/chest.129.6.1459. [DOI] [PubMed] [Google Scholar]
- 13.Loredo JS, Ancoli-Israel S, Dimsdale JE. Effect of continuous positive airway pressure vs placebo continuous positive airway pressure on sleep quality in obstructive sleep apnea. Chest. 1999;116:1545–9. doi: 10.1378/chest.116.6.1545. [DOI] [PubMed] [Google Scholar]
- 14.Profant J, Ancoli-Israel S, Dimsdale JE. A randomized, controlled trial of 1 week of continuous positive airway pressure treatment on quality of life. Heart Lung. 2003;32:52–8. doi: 10.1067/mhl.2003.8. [DOI] [PubMed] [Google Scholar]
- 15.Ziegler MG, Mills PJ, Loredo JS, Ancoli-Israel S, Dimsdale JE. Effect of continuous positive airway pressure and placebo treatment on sympathetic nervous activity in patients with obstructive sleep apnea. Chest. 2001;120:887–93. doi: 10.1378/chest.120.3.887. [DOI] [PubMed] [Google Scholar]
- 16.Gottlieb DJ, Whitney CW, Bonekat WH, et al. Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. Am J Respir Crit Care Med. 1999;159:502–7. doi: 10.1164/ajrccm.159.2.9804051. [DOI] [PubMed] [Google Scholar]
- 17.Mayfield D, McLeod G, Hall P. The CAGE questionnaire: Validation of a new alcoholism screening instrument. Am J Psychiatry. 1974;131:1121–3. doi: 10.1176/ajp.131.10.1121. [DOI] [PubMed] [Google Scholar]
- 18.Kemp B. European data format (EDF): Current availability and additional applications. J Sleep Res. 2002;11(Suppl. 1):120. [Google Scholar]
- 19.Kemp B, Varri A, Rosa AC, Nielsen KD, Gade J. A simple format for exchange of digitized polygraphic recordings. Electroencephalogr Clin Neurophysiol. 1992;82:391–3. doi: 10.1016/0013-4694(92)90009-7. [DOI] [PubMed] [Google Scholar]
- 20.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington, DC: Public Health Service, U.S. Government Printing Office; 1968. [Google Scholar]
- 21.American Sleep Disorders Association Atlas Task Force. EEG arousal: scoring rules and examples. Sleep. 1992;15:174–8. [PubMed] [Google Scholar]
- 22.Landis JKG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics. 1977;33:363–74. [PubMed] [Google Scholar]
- 23.Cohen J. A power primer. Psychol Bull. 1992;112:155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 24.Nakagawa S. A farewell to Bonferroni: The problems of low statistical power and publication bias. Behav Ecol. 2004;15:1044–5. [Google Scholar]
- 25.Henke KG, Grady JJ, Kuna ST. Effect of nasal continuous positive airway pressure on neuropsychological function in sleep apnea-hypopnea syndrome: A randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2001;163:911–7. doi: 10.1164/ajrccm.163.4.9910025. [DOI] [PubMed] [Google Scholar]
- 26.Bliwise DL, Benkert RE, Ingham RH. Factors associated with nightly variability in sleep-disordered breathing in the elderly. Chest. 1991;100:973–6. doi: 10.1378/chest.100.4.973. [DOI] [PubMed] [Google Scholar]
- 27.Le Bon O, Hoffmann G, Tecco J, et al. Mild to moderate sleep respiratory events: one negative night may not be enough. Chest. 2000;118:353–9. doi: 10.1378/chest.118.2.353. [DOI] [PubMed] [Google Scholar]
- 28.Mendelson WB. Use of the sleep laboratory in suspected sleep apnea syndrome: is one night enough? Cleve Clin J Med. 1994;61:299–303. doi: 10.3949/ccjm.61.4.299. [DOI] [PubMed] [Google Scholar]
- 29.Meyer TJ, Eveloff SE, Kline LR, Millman RP. One negative polysomnogram does not exclude obstructive sleep apnea. Chest. 1993;103:756–60. doi: 10.1378/chest.103.3.756. [DOI] [PubMed] [Google Scholar]
- 30.Agnew HWJ, Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiology. 1966;2:263–6. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 31.Le Bon O, Staner L, Hoffmann G, et al. The first-night effect may last more than one night. J Psychiatr Res. 2001;35:165–72. doi: 10.1016/s0022-3956(01)00019-x. [DOI] [PubMed] [Google Scholar]
- 32.Lorenzo JL, Barbanoj MJ. Variability of sleep parameters across multiple laboratory sessions in healthy young subjects: the “very first night effect”. Psychophysiology. 2002;39:409–13. doi: 10.1017.S0048577202394010. [DOI] [PubMed] [Google Scholar]
- 33.Verhulst SL, Schrauwen N, De Backer WA, Desager KN. First night effect for polysomnographic data in children and adolescents with suspected sleep disordered breathing. Arch Dis Child. 2006;91:233–7. doi: 10.1136/adc.2005.085365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mosko SS, Dickel MJ, Ashurst J. Night-to-night variability in sleep apnea and sleep-related periodic leg movements in the elderly. Sleep. 1988;11:340–8. [PubMed] [Google Scholar]
- 35.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: Recommendations for syndrome definitions and measurement techniques in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]