Abstract
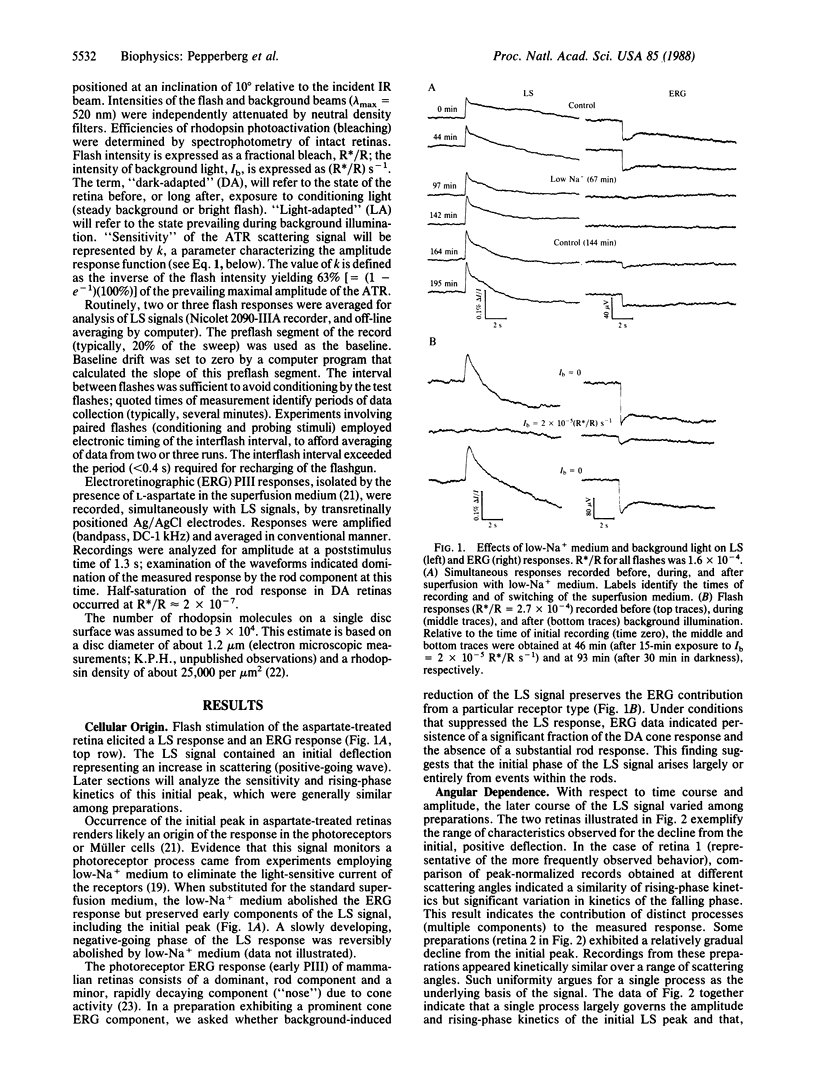
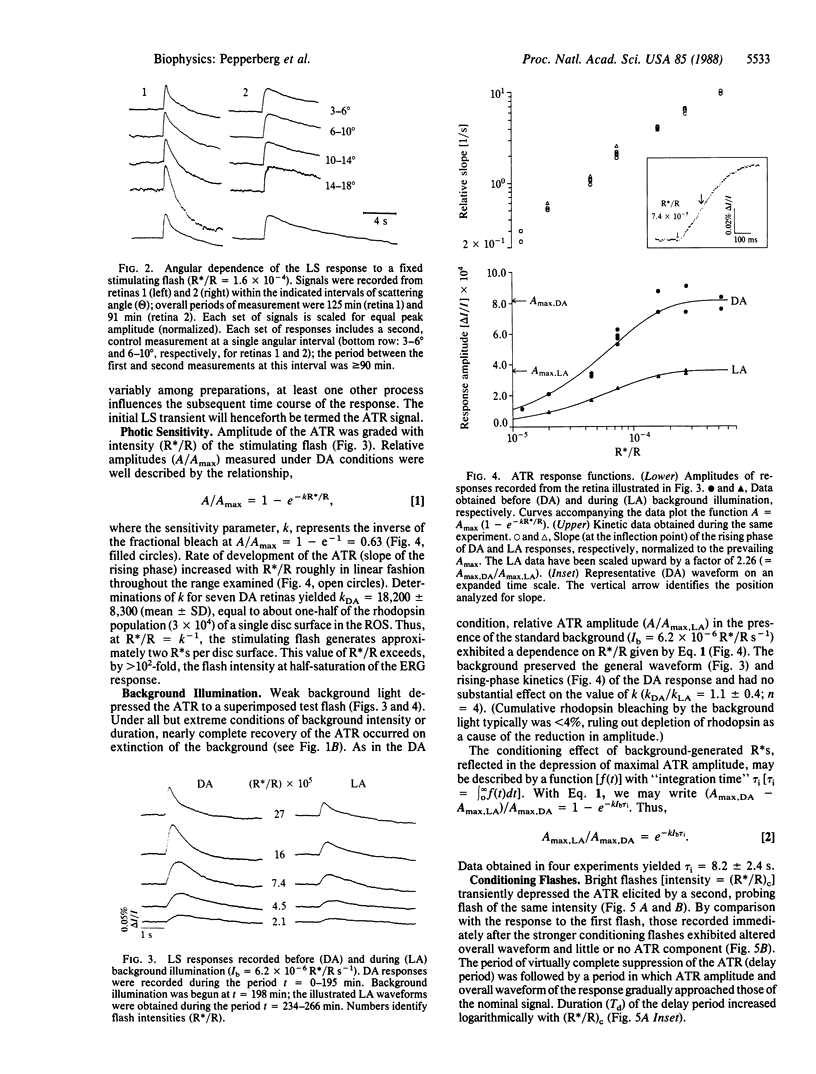
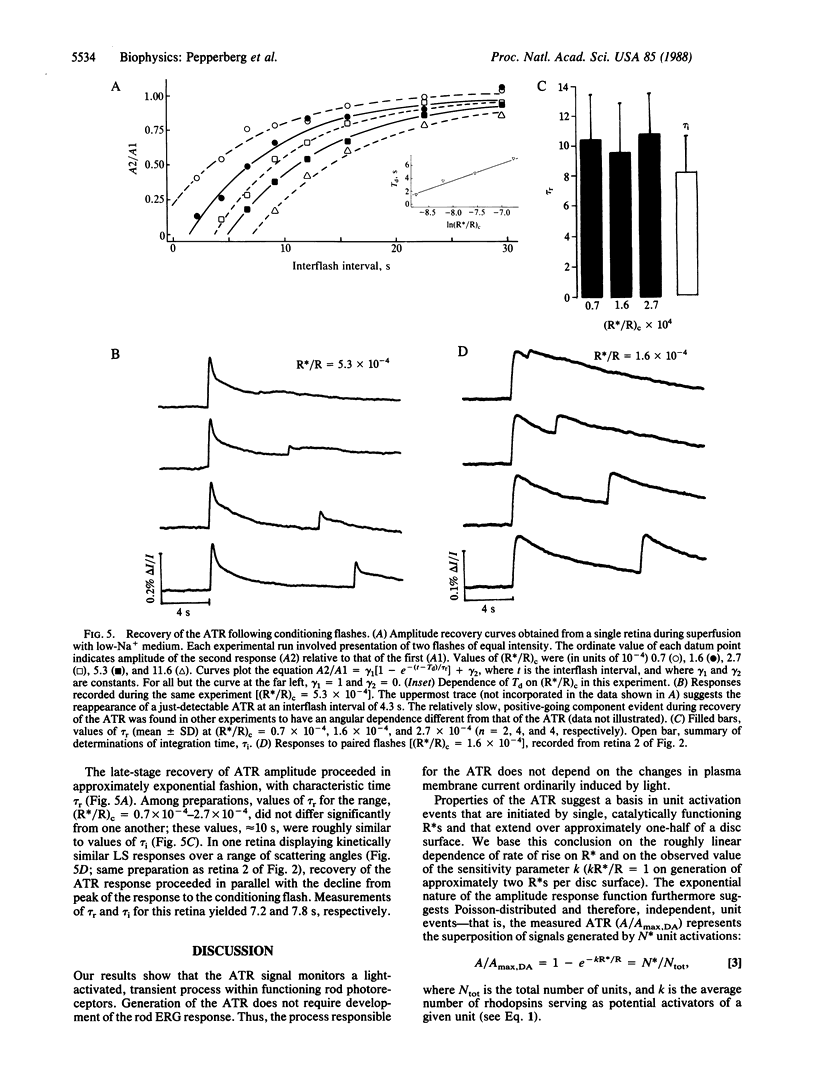
On stimulation by green flashes, the isolated, aspartate-treated bovine retina exhibits transient changes in the scattering of near-infrared (880 nm) light. A single component, termed the "ATR" (a flash-induced scattering signal, where ATR designates amplified transient-retina), dominates the amplitude and rising-phase kinetics of the initial peak of the light-scattering response. Superfusion with physiological solution containing low Na+ concentration reversibly abolishes the photoreceptor electroretinographic response but preserves the ATR signal, indicating a receptoral origin for the ATR. The increase of ATR amplitude (A/Amax) with flash intensity (R*/R, where R indicates rhodopsin) is described by A/Amax = (1- e-kR*/R), with R*/R = k-1 occurring on generation of approximately two photoactivated rhodopsins (R*s) per disc surface in the rod outer segment. Weak background light and bright flashes reversibly depress the ATR. Kinetic and sensitivity data suggest a basis of the ATR in stochastic, unit activation events, each initiated by a single R*. They further suggest an essential invariance of the unit event under differing conditions of illumination. A delay, apparently governed by the lifetime of a light-activated substance regulating ATR generation, precedes ATR recovery after a bright flash. The flash dependence of the delay period indicates an upper limit of 3 s for the lifetime of R* in the ATR-generating process. The unit event appears to be an R*-catalyzed and disc-localized reaction of phototransduction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Nunn B. J., Schnapf J. L. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984 Dec;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett N. A functional link between the dark Mg-ATPase activity and the light-induced enzymatic cascade in rod outer segments. Eur J Biochem. 1986 Jun 16;157(3):487–495. doi: 10.1111/j.1432-1033.1986.tb09693.x. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Pinto L. H. Ionic mechanism for the photoreceptor potential of the retina of Bufo marinus. J Physiol. 1974 Feb;236(3):575–591. doi: 10.1113/jphysiol.1974.sp010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clack J. W., Pepperberg D. R. Desensitization of skate photoreceptors by bleaching and background light. J Gen Physiol. 1982 Dec;80(6):863–883. doi: 10.1085/jgp.80.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deterre P., Bigay J., Robert M., Pfister C., Kühn H., Chabre M. Activation of retinal rod cyclic GMP-phosphodiesterase by transducin: characterization of the complex formed by phosphodiesterase inhibitor and transducin alpha-subunit. Proteins. 1986 Oct;1(2):188–193. doi: 10.1002/prot.340010210. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Ripps H. Adaptation in skate photoreceptors. J Gen Physiol. 1972 Dec;60(6):698–719. doi: 10.1085/jgp.60.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harary H. H., Brown J. E., Pinto L. H. Rapid light-induced changes in near infrared transmission of rods in Bufo marinus. Science. 1978 Dec 8;202(4372):1083–1085. doi: 10.1126/science.102035. [DOI] [PubMed] [Google Scholar]
- Hofmann K. P., Emeis D. Differential light detector. Rev Sci Instrum. 1979 Feb;50(2):249–249. doi: 10.1063/1.1135804. [DOI] [PubMed] [Google Scholar]
- Hofmann K. P., Uhl R., Hoffmann W., Kreutz W. Measurements on fast light-induced light-scattering and -absorption changes in outer segments of vertebrate light sensitive rod cells. Biophys Struct Mech. 1976 Apr 15;2(1):61–77. doi: 10.1007/BF00535653. [DOI] [PubMed] [Google Scholar]
- Kamps K. M., Hofmann K. P. ATP can promote activation and deactivation of the rod cGMP-phosphodiesterase. Kinetic light scattering on intact rod outer segments. FEBS Lett. 1986 Nov 24;208(2):241–247. doi: 10.1016/0014-5793(86)81025-0. [DOI] [PubMed] [Google Scholar]
- Kamps K. M., Reichert J., Hofmann K. P. Light-induced activation of the rod phosphodiesterase leads to a rapid transient increase of near-infrared light scattering. FEBS Lett. 1985 Aug 19;188(1):15–20. doi: 10.1016/0014-5793(85)80866-8. [DOI] [PubMed] [Google Scholar]
- Kohl B., Hofmann K. P. Temperature dependence of G-protein activation in photoreceptor membranes. Transient extra metarhodopsin II on bovine disk membranes. Biophys J. 1987 Aug;52(2):271–277. doi: 10.1016/S0006-3495(87)83214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn H., Bennett N., Michel-Villaz M., Chabre M. Interactions between photoexcited rhodopsin and GTP-binding protein: kinetic and stoichiometric analyses from light-scattering changes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6873–6877. doi: 10.1073/pnas.78.11.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovic K. N., Dowling J. E., Kim Y. Y. Background and bleaching equivalence in steady-state adaptation of vertebrate rods. J Neurosci. 1987 Apr;7(4):1056–1063. doi: 10.1523/JNEUROSCI.07-04-01056.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A., Parker K. R., Dratz E. A. The molecular mechanism of visual excitation and its relation to the structure and composition of the rod outer segment. Annu Rev Physiol. 1987;49:765–791. doi: 10.1146/annurev.ph.49.030187.004001. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Pugh E. N., Jr Gain, speed and sensitivity of GTP binding vs PDE activation in visual excitation. Vision Res. 1982;22(12):1475–1480. doi: 10.1016/0042-6989(82)90212-7. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Pugh E. N., Jr The control of phosphodiesterase in rod disk membranes: kinetics, possible mechanisms and significance for vision. Vision Res. 1979;19(4):375–380. doi: 10.1016/0042-6989(79)90097-x. [DOI] [PubMed] [Google Scholar]
- Miki N., Baraban J. M., Keirns J. J., Boyce J. J., Bitensky M. W. Purification and properties of the light-activated cyclic nucleotide phosphodiesterase of rod outer segments. J Biol Chem. 1975 Aug 25;250(16):6320–6327. [PubMed] [Google Scholar]
- Sillman A. J., Ito H., Tomita T. Studies on the mass receptor potential of the isolated frog retina. I. General properties of the response. Vision Res. 1969 Dec;9(12):1435–1442. doi: 10.1016/0042-6989(69)90059-5. [DOI] [PubMed] [Google Scholar]
- Vuong T. M., Chabre M., Stryer L. Millisecond activation of transducin in the cyclic nucleotide cascade of vision. Nature. 1984 Oct 18;311(5987):659–661. doi: 10.1038/311659a0. [DOI] [PubMed] [Google Scholar]
- Wensel T. G., Stryer L. Reciprocal control of retinal rod cyclic GMP phosphodiesterase by its gamma subunit and transducin. Proteins. 1986 Sep;1(1):90–99. doi: 10.1002/prot.340010114. [DOI] [PubMed] [Google Scholar]
- Whitten D. N., Brown K. T. The time courses of late receptor potentials from monkey cones and rods. Vision Res. 1973 Jan;13(1):107–135. doi: 10.1016/0042-6989(73)90168-5. [DOI] [PubMed] [Google Scholar]


