Abstract
Selective estrogen receptor modulators (SERMs) represent a class with a growing number of compounds that act as either estrogen receptor agonists or antagonists in a tissue-specific manner. This article reviews lasofoxifene, a new-generation SERM that has completed phase III development for the prevention and treatment of osteoporosis in postmenopausal women. Consistent with preclinical observations, this new SERM demonstrated improved skeletal efficacy over raloxifene and at an oral dose of 0.5 mg/day was effective in the prevention of both vertebral and nonvertebral fractures in postmenopausal women with osteoporosis. At the same dosage, lasofoxifene treatment also reduced estrogen receptor-positive breast cancer risk and the occurrence of vaginal atrophy, but, like the other SERMs, was associated with hot flushes and an increased risk of venous thromboembolic events. With its increased efficacy on the prevention of nonvertebral fractures than current available SERMs and its positive effects on the vagina, this new compound may represent an alternative and cost-effective therapy for osteoporosis in postmenopausal women.
Keywords: SERM, lasofoxifene, postmenopausal osteoporosis, fractures, bone density, menopause
Introduction
Osteoporosis is a skeletal disorder characterized by compromised bone strength and increased risk of fracture.1,2 It is one of the most common disorders in elderly subjects and represents a major public health problem, affecting up to 50% postmenopausal women and 20% of men older than 50 years.2 Worldwide, osteoporosis is estimated to be present in over 200 million individuals, with 75 million of these in Europe, Japan and the US.2,3 Its clinical significance lies in the occurrence of fractures, involving most commonly the forearm, the vertebral bodies and the hip, but fractures at other sites may be also associated with the disease. Each year more than 1.5 million people suffer hip, vertebral, and wrist fractures due to osteoporosis. The occurrence of osteoporotic fractures leads to considerable mortality, morbidity, reduced mobility and decreased quality of life.4 Up to 50% of women who sustain a hip fracture need assistance for activities of daily living, about 20% will die within 1 year, and about the same percentage will require long-term care.5,6 Moreover, future risk of osteoporotic fractures is greatly increased in patients with one or more vertebral fractures.4 The burden of osteoporosis and fractures will further increase in absolute terms over the next years because of the aging of the population.7 Given the magnitude of the problem, the prevention and treatment of osteoporosis is, therefore, of major importance for health organizations in all countries.
Osteoporosis occurs as the result of multiple mechanisms that together cause loss of bone mass and strength.8 Failure to acquire optimal bone mass and strength during growth and or an unbalance in bone remodeling leading to bone loss throughout life may all contribute to the development of the disease. In women, osteoporosis and fractures mainly occur as a consequence of estrogen deficiency after menopause9 and result from an imbalance between bone resorption by osteoclasts and bone formation by osteoblasts, leading to a net bone loss with each remodeling cycle. Additional age-related mechanisms such as vitamin D deficiency and secondary hyperparathyroidism or reduced mechanical loading may also increase bone loss in elderly subjects.
One of the major and easily measurable determinant of bone strength and osteoporotic fracture risk is bone mineral density (BMD), as assessed by dual energy X-ray absorptiometry (DXA). According to World Health Organization criteria, osteoporosis is defined to exist when BMD values fall more than 2.5 standard deviations below the young adult reference mean.1 Many studies indicate that the risk of fragility fractures increases progressively as BMD declines.10,11 It has been estimated that the risk of new vertebral fractures increases by a factor of 2.0 or more for each standard deviation decrease in BMD, irrespective of the site of bone density measurement.10 However, several other skeletal characteristics contribute to bone strength and interact with BMD in determining the risk of fracture. These include bone macroarchitecture (shape and geometry), bone microarchitecture (at the trabecular and cortical level), matrix and mineral composition, as well as the rate of bone turnover and the degree of mineralization or microdamage accumulation, affecting the structural and material properties of bone.2,12,13 The recognition and measurement of these parameters is becoming more important, and their incorporation into algorithms of fracture detection remains the subject of active research.
Estrogen and postmenopausal osteoporosis
Despite the remarkable progress made during the last two decades, the mechanism by which estrogen affects bone metabolism is complex and not fully elucidated.14–16 Estrogen acts through the binding and activation to two different estrogen receptors (ER), ERα and ERβ, which have been identified in osteoblasts, osteoclasts, osteocytes and bone marrow stromal cells.14 Positive effects of estrogen in bone include a decrease in the production and lifespan of osteoclasts (the bone resorbing cells), stimulation of osteoblast (the bone forming cells) activity and additional effects on calcium homeostasis.14,15 The antiresorptive action of estrogen is mediated via effects on the receptor activator of the NF-kappaB ligand (RANKL)/RANK/osteoprotegerin system (the major regulator of osteoclast activity), as well as by reducing the production of a number of proresorptive cytokines, along with direct effects on osteoclasts.15,16 Bone formation is also affected by estrogen deficiency with a reduction in the life span of osteoblast, thereby aggravating the negative bone balance. This combination of increased bone resorption and decreased bone formation accelerates bone loss and the structural decay of the skeleton. Importantly, the decrease in estrogen levels after menopause also leads to death of osteocytes by apoptosis, cells that forms a communicating network within the bone that is able to identify sites for remodeling when the prevailing physical loads are sensed.12,13 This effect on osteocyte apoptosis impairs the ability of bone to adapt to its loading circumstances and leads to a further loss of bone strength independently of BMD.12 Moreover, there is increasing evidence that, in addition to direct effects on bone cells, estrogen may be directly influencing calcium handling in the bowel and the kidney.14 ERs have been found in the gut and the kidney and some observations suggest that estrogen may positively affect calcium absorption, independently from other circulating calciotropic hormones.14 Thus, estrogen deficiency, in addition to directly increase bone resorption, may result in a negative calcium balance due to an impaired calcium absorption from the gut and to an increased renal calcium excretion.
Among the several therapeutic interventions in osteoporosis, hormone replacement therapy (HRT, estrogen plus progestins) or estrogen replacement therapy (ERT) have traditionally been seen as the gold standard method in postmenopausal women for many years, as well as for the management of menopausal symptoms. In fact, estrogen replacement (via HRT or ERT) returns bone turnover to premenopausal levels, increases BMD and, if instituted at the time of menopause, completely prevents postmenopausal bone loss and the development of fractures.9,17,18 The best prospective fracture data have been provided by the Women’s Health Initiative (WHI), showing that 0.625 mg/day of conjugated equine estrogen alone or in combination with 5 mg/day methoxyprogesterone significant reduces the risk of fractures at all skeletal sites compared to placebo in osteoporotic and nonosteoporotic women.18,19 However, despite biologically plausible mechanisms for cardiac protection by estrogen, and observational studies indicating that HRT or ERT confers cardiovascular benefit,20 WHI and other randomized, controlled trials have failed to confirm any potential benefit in reducing the risk of coronary artery disease and stroke.21,22 Indeed, early increases in cardiac event and stroke rate have been seen in women taking combination HRT, especially in those starting treatment when older than 70 years.23 Moreover, estrogen replacement, especially if long term, leads to an increased risk of breast cancer and, when unopposed by progestins, endometrial cancer.23 Thus, HRT and ERT remain an option only for short-term early use for menopausal symptom management, with treatment individualized for each woman. In contrast, estrogen therapy must be long term, possibly lifelong, to have any lasting impact on bone health. While lower doses of estrogen have been shown to reduce bone turnover and increase BMD, prospective evidence showing a reduction in risk for fracture with-low dose estrogen replacement is lacking.24 Alternative therapies for the prevention and the treatment of osteoporosis in postmenopausal women include bisphosphonates, calcitonin, vitamin D, strontium ranelate, parathyroid hormone, and selective estrogen receptor modulators (SERMs).
SERMs in clinical use and postmenopausal osteoporosis
SERMs are a class of compounds that interact with intracellular ERs in target organs as estrogen agonists and antagonists. 25 They include chemically diverse molecules that lack the steroid structure of estrogens, but possess a tertiary structure that allows them to bind to ERα and/or ERβ. For many years these compounds were classified simply by estrogen agonists or antagonists, but several experimental and clinical observations have led to rethink this classification with the development of the concept of “selective estrogen receptor modulation.”26–28
Over the past decade, different compounds that possess a SERM profile have been intensively studied and have proven to be a highly versatile group for the treatment of different conditions associated with aging, including hormone-responsive cancer and osteoporosis. Most of the unique pharmacology of SERMs as well as their agonistic and antagonistic activity on estrogen target tissues can be explained by three main interactive mechanisms:29 1) differential ERα and ERβ expression, 2) differential ER conformation on ligand binding, and 3) differential expression and binding to the ER of coregulator proteins (coactivators or corepressors). Notably, several studies clearly demonstrated that binding by estradiol, pure antiestrogen (ie, ICI 164,384) or different SERMs compounds results in a unique ER conformation for each ligand.30–32
Currently there are two main chemical classes of SERMs approved for clinical use: the triphenylethylene derivatives tamoxifen and toremifene that are used to treat breast cancer, and raloxifene, a benzothiopene derivative indicated for the treatment and prevention of osteoporosis and in the US for the prevention of breast cancer.25,29,33–35 All three also have beneficial effects on serum lipids, but are associated with venous thromboembolism and hot flushes. The effects of raloxifene on bone are well established. Clinical trials demonstrated that at a daily dose of 60 mg is effective in the prevention and treatment of postmenopausal osteoporosis and vertebral fractures. 36–38 Although tamoxifen has a positive effect on bone as well, the increased risk of endometrial cancer eliminates it as a possible therapy for postmenopausal osteoporosis. Moreover, results from clinical trials and observational studies suggest that both raloxifene and tamoxifen are less potent on the skeleton than estrogen,34,39–42 and their effect in the prevention of hip and other nonvertebral fractures remains uncertain. Thus, the benefits of these compounds in reducing the risks of fracture and invasive breast cancer should be weighed against the increased risks of venous thromboembolism, fatal stroke, and in case of tamoxifen, uterine cancer. Moreover, a consistent number of women taking available SERMs for different indications reported moderate or severe vasomotor or gynecologic symptoms (especially vaginal dryness and hot flashes) that could hinder compliance. Among the different SERM compounds actually under investigation, ospemifene, lasofoxifene, bazedoxifene, and arzoxifene, have shown to be effective in animal models of osteoporosis to a comparable extent to that observed during conventional HRT and are now in clinical phase III studies or have already completed the phase III development program.34,43,44 In this article we revise the clinical evidence for the use of lasofoxifene in women after menopause and to discuss how it will fit into the treatment of postmenopausal osteoporosis.
Chemistry, pharmacokinetics and metabolism of lasofoxifene
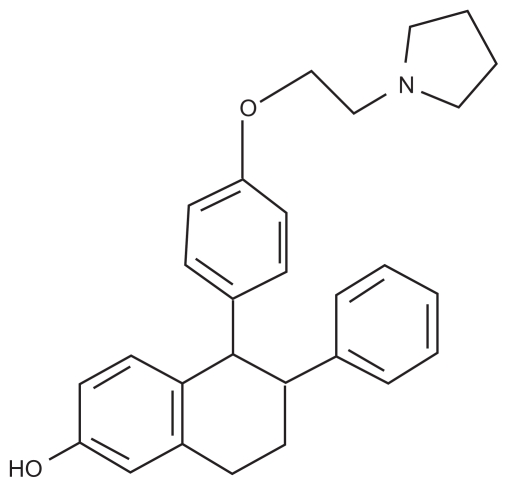
Lasofoxifene (Figure 1) is a naphthalene derivative, third-generation SERM that was discovered through a synthetic program aimed to isolate novel molecules with a good oral bioavailability and higher in vivo potency.45 This compound is structurally distinct from the first- and second-generation SERMs raloxifene (a benzothiopene derivative), tamoxifen and toremifene (both triphenylethylene derivatives). Lasofoxifene selectively binds to both ERα and ERβ with high affinity.46,47 In particular, its half-inhibition concentration for ERα (1.5 nM) is similar to that seen with estradiol (4.8 nM) and at least 10-fold higher than those reported for raloxifene and tamoxifen.47–49
Figure 1.
Chemical structure for lasofoxifene.
Lasofoxifene is well absorbed orally, and circulates highly bound to plasma proteins.47,49 Food does not significantly affect its bioavailability relative to the fasting state,50 and its metabolism occurs almost exclusively in the liver, through both oxidative and conjugative pathways. In different studies this SERM demonstrated linear pharmacokinetics over a wide dose range (from 0.01 to 100 mg), with a mean time until maximum concentration (Tmax) of approximately 6 hours, and an estimated terminal elimination half-life (t1/2) of approximately 6 days (150 hours), that is more than 2-fold longer than for raloxifene (16 to 87 hours).48,49,51–53 Moreover, lasofoxifene has a remarkably improved oral bioavailability with respect to raloxifene. In fact, while raloxifene and other benzothiopene derivatives undergo extensive first pass glucuronidation, due to the presence of phenolic groups, lasofoxifene has a nonpolar tetrahydronaphthalene structure that confers an increased resistance to intestinal wall glucuronidation. 45,49,50,54 Both lasofoxifene and its metabolites are recovered primarily in the feces and secondarily in urine.55 Hence, there is little change in the pharmacokinetics of the drug in patients with impaired renal function. In addition, age, mild to moderate hepatic impairment or use of concomitant medications (ie, ketaconazole, digoxin or warfarin) have not been associated with substantial differences in lasofoxifene pharmacokinetics.56–59
Preclinical studies with lasofoxifene
The effects of lasofoxifene in both skeletal and extraskeletal tissues have been tested in different in vitro and in vivo experimental models.34,47,49 Initial studies in bone cells showed an estrogen-like activity of lasofoxifene, with a proapoptotic effect on osteoclast precursors.46 Different short-term and long-term in vivo studies in ovariectomyzed (OVX) rats confirmed this in vitro evidence and demonstrated that lasofoxifene treatment (at doses of 10 to 1000 μg/kg/day) reduces bone turnover and is effective in protecting from OVX-induced bone loss without any major adverse finding.45,46,60,61 In a bone histomorphometry study lasofoxifene (at doses of 10 to 1,000 μg/kg/day) completely blocked the OVX-induced decrease in trabecular number and thickness as well as the increase in bone resorption indices (osteoclast number, percent osteoclast perimeter, percent eroded perimeter) and bone formation indices (labeling perimeter, BFR/BV).46 Long-term studies in the same models showed that lasofoxifene maintains its efficacy on bone over time without any major adverse finding.61 Moreover, peripheral quantitative computerized tomography analysis of proximal tibial metaphysis and biomechanical testing of the fourth lumbar vertebra clearly indicated that lasofoxifene treatment maintained bone quality and preserved bone strength in treated animals.61 Interestingly, lasofoxifene was also effective in the prevention of bone loss induced by aging or orchidectomy (ORX) in male models of osteoporosis, without significant effects on the prostate,62,63 suggesting a potential application of this compound for the treatment of osteoporosis not only in postmenopausal women but also in elderly men. A higher dose (10 to 100 vs 0.01 to 0.1 μg/kg/day) was required to prevent ORX-induced than age-related decrease in bone mass. Importantly, at these dosages lasofoxifene did not significantly affect the prostate.
The preclinical studies demonstrated extraskeletal benefits of lasofoxifene on serum lipids45,46,60–64 as well as chemopreventive and therapeutic effects on breast cancer.65,66 No uterine hypertrophic effects were observed at doses of 1 to 1000 mcg/kg/day in OVX rats,45,61 and at doses of 0.1 to 100 mcg/kg/day in immature (3 weeks old) or aged (17 months old) intact female rats.46 No toxicity was reported in the preclinical literature in either female and male rats of different ages.47,49
Clinical evidence with lasoxoxifene in osteoporosis
An extensive development clinical program has been conducted with lasofoxifene, including 23 clinical pharmacology studies and 17 phase II/III clinical trials.67 This development program included more than 10,000 women and was designed to support the use of lasofoxifene for the prevention or treatment of postmenopausal osteoporosis and for the treatment of vulvar-vaginal atrophy.
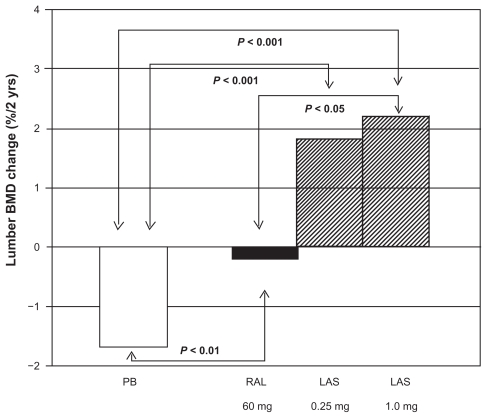
Initial, phase I/II studies demonstrated safety and efficacy of lasofoxifene given over a 600-fold dose range (from 0.017 mg/day to 10 mg/day) after an overnight fast.51,52,68,69 The overall results from the dose selection analyses suggested that the lowest lasofoxifene dose necessary to achieve a fully efficacious response on BMD and low-density lipoprotein cholesterol (LDL-C) levels is 0.25 mg/day.69–71 All lasofoxifene treatment regimens were also associated with improvements in vaginal atrophy measures, namely maturation index and vaginal pH, compared with placebo. In a different phase II study lasofoxifene increased BMD as effectively as conjugate estrogen (Prempro® [premarin conjugated estrogen with medroxyprogesterone acetate]; Wyeth Pharmeceuticals, Inc.).68,72,73 A subsequent, 2-year, phase II study was specifically designed to compare the skeletal effects of lasofoxifene (0.25 and 1.0 mg/day) to raloxifene (60 mg/day) or placebo in 410 postmenopausal women (average T-score –1.0).74,75 All women also received daily calcium (1000 mg) and vitamin D (250 IU) supplementation. At the lumbar spine, both doses of lasofoxifene significantly increased BMD compared with raloxifene or placebo (+1.8% and +2.2% for 0.25 and 1.0 mg lasofoxifene respectively; −0.1% and −1.7% for raloxifene and placebo, respectively) (Figure 2). Conversely, lasofoxifene and raloxifene were equally effective at increasing total hip BMD (+1.9 and +1.3% for 0.25 and 1.0 mg lasofoxifene respectively; +1.5 and –0.1% for raloxifene and placebo respectively). Consistent with the effects on BMD, biochemical markers of bone turnover significantly decreased after 2 years of lasofoxifene treatment with respect to placebo, at a similar or even greater extent than with raloxifene. Moreover, at 2 years, both doses of lasofoxifene resulted in greater reductions in LDL-C and total cholesterol levels when compared with raloxifene and placebo.
Figure 2.
Effects of lasofoxifene (LAS) treatment (0.25 and 1.0 mg/day) on lumbar bone mineral density (BMD) (% change at 2 years) in the phase II comparative trial vs raloxifene (RAL) (60 mg/day) or placebo. Drawn from data of McClung et al.75
The key evidence with lasofoxifene for the treatment of osteoporosis and vaginal atrophy in postmenopausal women comes from 2 phase III trials: the Osteoporosis Prevention and Lipid Lowering (OPAL) studies, and the Postmenopausal Evaluation and Risk-Reduction with Lasofoxifene (PEARL) study. Both trials assessed the effects of lasofoxifene on BMD and bone turnover markers as well as different vaginal endpoints, while only PEARL evaluated the outcome of fractures. Results from this trial have been only released in abstract form. Additional information has been derived from two clinical documents about lasofoxifene released on the web [http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4381b1-01-FDA.pdf and http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4381b1-02-Pfizer.pdf].
The OPAL trial was composed by two identical phase III, 24-month, prospective, multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase III studies, in which 1907 nonosteoporotic women aged 40 to 75 years (3 to 20 years postmenopausal) were randomized to lasofoxifene 0.025, 0.25 or 0.5 mg/day, or placebo for 2 years.76,77 All patients received calcium or vitamin D supplementation. Exclusions criteria were any disease associated with altered bone metabolism, malignancy within the previous 5 years, ovarian or uterine pathology, spinal deformities that would affect lumbar densitometry, hip prosthesis, or a history of nontraumatic vertebral or hip fractures. Overall, lasofoxifene treatment significantly increased BMD and decreased bone turnover compared to placebo, with beneficial changes observed as early as 6 months.77 A significant increase in lumbar and femoral BMD at 6, 12, and 24 months was observed with all the three lasofoxifene doses compared with a decrease observed in calcium and vitamin D supplemented placebo group. A significant decrease in bone turnover markers (osteocalcin, C-terminal telopeptide of type 1 collagen and N-terminal propeptide of type 1 procollagen) was also observed at 6 and 24 months in lasofoxifene treatment groups with respect to placebo. Importantly, bone biopsies in lasofoxifene treated subjects showed bone of normal quality. Moreover, neither breast density (assessed by mammography in 351 women) nor breast pain increased with lasofoxifene treatment. Changes in signs and self-assessed symptoms of vaginal atrophy or cognitive function and variations in lipid levels were also periodically analyzed over the 24 months of the study.77 Of interest, there was a significant improvement in vaginal pH at 1 and 2 years for all doses of lasofoxifene versus placebo. The assessment of the degree of vaginal maturation indicated significantly lower percentages of parabasal cells and significantly higher proportions of intermediate and superficial cells in lasofoxifene treated women with respect to placebo. Moreover, at all time points lasofoxifene treatment was associated with significant reductions in total and LDL cholesterol relative to placebo in all treatment groups. Conversely, small but significant increases in triglycerides were observed at all time points with both lasofoxifene doses with respect to placebo.
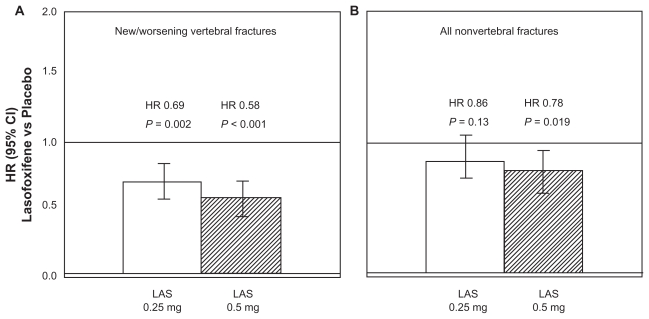
The PEARL trial was a randomized, double-blind, placebo-controlled, parallel assignment study specifically designed to determine the safety and effectiveness of 2 doses of lasofoxifene (0.25 and 0.5 mg/day) in reducing the risk of new/worsening radiographic spinal fractures at 3 years (primary endpoint) in women with osteoporosis.68 [http://www.clinicaltrials.gov/ct]. Secondary outcomes included nonvertebral fractures, BMD, bone markers, breast cancer, cardiovascular events, and gynecological safety events. The study was originally designed as a 3-year study, but was extended by 2 additional years via a protocol amendment, in order to provide long-term exposure data. All patients received calcium or vitamin D supplementation. Women were also excluded if they had other metabolic bone disease, if they were taking medications approved for osteoporosis, if they have had a recent fracture (within 1 year), or more than 3 prevalent vertebral fractures on baseline X-ray and/or a BMD < −4.5 SD at the lumbar spine or the femoral neck. At 3 years, lasofoxifene significantly reduced bone turnover markers and improved BMD at the spine (3.3% for both doses, P < 0.001) and femoral neck (2.7% for 0.25 mg and 3.3% for 0.5 mg, P < 0.001), compared with placebo.78–80 A signif icant reduction in vertebral fracture risk was demonstrated with both treatment arms (31% for 0.25 mg, P = 0.002 and 42% for 0.5 mg, P < 0.001)80 (Figure 3A). This effect was observed as early as 1 year for both doses of lasofoxifene and was sustained through 5 years. A similar risk reduction was observed with both doses in women with or without prevalent fracture at baseline. Conversely, the risk of nonvertebral fractures at 3 years was significantly reduced with lasofoxifene 0.5 mg/day (22%, P = 0.02), but not with lasofoxifene 0.25 mg/day (Figure 3B). The antifracture efficacy at nonvertebral sites of the 0.5 mg dose was observed as early as 1 year and was maintained through 5 years. Based on these results lasofoxifene 0.5 mg/day was selected as the relevant dosage for the treatment of postmenopausal osteoporosis.
Figure 3.
Reduction in the risk (hazard ratio, HR) for new/worsening radiographic vertebral fractures A) and nonvertebral fractures B) after 3 years of lasofoxifene (LAS) (0.25 or 0.50 mg/day) treatment, compared with placebo. Drawn from data of results of the PEARL trial, http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4381b1-02-Pfizer.pdf.
The analysis of extraskeletal outcomes of PEARL study confirmed preclinical evidence and indicated that lasofoxifene 0.25 mg and 0.5 mg also reduces the risk of ER positive breast cancer (by 84% and 67%, respectively). This effect was also evident for all breast cancers (a composite endpoint consisting of ER+, ER–, invasive, and ductal cancer in situ) with lasofoxifene 0.5 mg dose (65% and 79% risk reduction compared to placebo through 3 and 5 years, respectively).67,80 Efficacy of lasofoxifene treatment on vulvar and vaginal atrophy endpoints (symptoms, vaginal pH and maturation index, percentage of parabasal cells and of superficial cells) was also demonstrated with both lasofoxifene doses.67
Based on the positive results of the PEARL trial and of the 2-year extension on safety concerns, a FDA application (NDA 22-242) was submitted to request approval of Fablyn® (lasofoxifene tartrate, 0.5 mg, film-coated oral tablets; Pfizer) for the treatment of osteoporosis in postmenopausal women at increased risk of fracture. On September 2008, an FDA scientific advisory panel voted 9 to 3 that there is a population of postmenopausal women with osteoporosis in which the benefits of lasofoxifene likely outweigh the risks; however, in a response letter FDA asked for additional information on the compound. On 18 December 2008 the Committee for Medicinal Products for Human Use (CHMP) of the European Medicine Agency (EMEA) adopted a positive opinion, recommending to grant a marketing authorization for Fablyn 0.5 mg intended for treatment of osteoporosis in postmenopausal women at increased risk of fracture.81 The CHMP, on the basis of the submitted quality, safety and efficacy data, considered that there is a favorable benefit to risk balance for this compound.
Safety and tolerability of lasofoxifene
In all of the clinical trials performed to date, lasofoxifene appeared to be well tolerated. In general, adverse events were mild or moderate and usually resolved within a few days, without requiring treatment discontinuation. Moreover, there were no major changes in adverse-event frequency or intensity with increasing dose. In the comparative phase II study, the adverse event profile of lasofoxifene was similar to that of raloxifene.74,75 In phase III studies adverse events reported by more than 5% of lasofoxifene treated subjects included hot flashes, leg cramps and increased vaginal moisture.67,76,77,80
Major general safety events of special interest for any SERM have been comprehensively addressed in phase III clinical trials, and particularly in the PEARL study, as secondary endpoints [http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4381b1-02-Pfizer.pdf].67 Overall, there were three safety findings of note associated with lasofoxifene treatment: an increased incidence of uterine diagnostic procedures, an increase in venous thromboembolic events, and a slight but significant increase in all-cause mortality with lasofoxifene 0.25 mg (but not 0.5 mg) compared with placebo The gynecologic adverse events that occurred more frequently than placebo (leading to an increase in diagnostic procedures during the PEARL trial) included uterine polyps and endometrial hypertrophy, both considered benign findings. These effects have been attributed to increased vascular permeability by lasofoxifene, which results in uterine imbibition and accumulation of fluid in both the glands and stroma of the endometrium. This is consistent with the cystic echotexture and increased endometrial thickness observed on ultrasound, together with the benign cystic atrophy observed on biopsy. Vaginal bleeding was reported with low frequency, but was more common in lasofoxifene-treated patients compared to placebo. Importantly, the 5-year extensions of the clinical safety database from the PEARL trial did not show any evidence of an increased risk of endometrial cancer or hyperplasia associated with the use of lasofoxifene. However, during the 5 years of study follow up, lasofoxifene was associated with an approximate 2-fold increased risk of venous thromboembolism, mainly driven by an increased risk of deep vein thrombosis. Pulmonary embolism occurred less frequently but was also significantly increased in lasofoxifene-treated patients compared to placebo. Conversely, lasofoxifene was not associated with an increased risk of stroke. The slightly increased percentage of all-cause mortality was observed with lasofoxifene 0.25 mg on both the 3-year and 5-year safety data. The percentage of subjects who died in the 0.25 mg lasofoxifene group exceeded that in the 0.5 mg group and was significantly greater than that in the placebo-treated subjects based on 5-year data (90 vs 65, respectively, P = 0.049). The excess numbers of deaths were found primarily in the noncoronary vascular and cancer categories. The latter did not appear to be focused in any specific organ system. Slightly more cancer deaths occurred in the brain, lung, and gastrointestinal system in the lasofoxifene-treated subjects.
Markers of cardiovascular risk (total cholesterol, LDL-cholesterol, and high sensitivity C-reactive protein) were measured at 3 years and showed a significant reduction in lasofoxifene-treated subjects compared to placebo. Moreover, lasofoxifene 0.5 mg was associated with a significant 32% reduction in major coronary events (including coronary death, nonfatal myocardial infarctions, coronary revascularization procedures, documented new ischemic heart disease, and hospitalizations for unstable angina) through 5 years.
Patient-focused perspectives and cost-effectiveness
Available data from randomized trials indicate that lasofoxifene is a very well tolerated and versatile compound. It can be taken orally with or without food with few side effects. Adherence could be impaired in some women because of the occurrence of hot flushes or vaginal bleeding.
Pharmacoeconomic evaluation on the use of lasofoxifene in osteoporosis prevention or treatment has been not released. Due to favorable extraskeletal effects and based on current data on fracture prevention, the cost-effectiveness of this compound could be improved compared with other antiresorptive agents. In this context, it is important to differentiate between the effect on quality of life impact caused by the antifracture efficacy of an intervention and any general quality of life effect that it may have independently of that antifracture efficacy. In a UK meta-analysis considering postmenopausal women unselected for low BMD and modeled for additional conditions such as breast cancer and cardiovascular health, only raloxifene proved cost-effective for the prevention of vertebral fractures at 60 years of age, with a cost per quality adjusted life-year (QALY) of £26,000 (assuming no effect on hip fractures) that was below the estimated threshold of £35,000 corresponding to no treatment (with the assumption that all women had sufficient intakes of calcium and vitamin D).82 Bisphosphonates such as alendronate, etidronate and risedronate had a cost per QALY above that threshold. Moreover, none of the considered interventions was shown to cost-effectively reduce the risk of nonvertebral fractures in women unselected for low BMD. These net costs were markedly different by age, with some treatment regimens becoming cost-saving at higher age ranges in patients with a prior fracture. Importantly, the released results from the phase II comparative study and the phase III trials indicate that lasofoxifene has an improved efficacy on bone parameters (ie, markers of bone resorption and BMD) with significant improvement in fracture prevention (particularly in nonvertebral fractures) over the current leading SERM, raloxifene. This new SERM also retains a similar positive effect in breast cancer prevention to raloxifene, with potentially improved cardiovascular benefits. Thus if lasofoxifene will be marketed at a similar cost to current products, its cost-effectiveness should be improved with respect to raloxifene.
Conclusions
During the past 10 years, much effort has been devoted to understanding the skeletal effects of estrogen and the developing compounds that interact with intracellular ERs in target organs, such as estrogen agonist and antagonists. The search for a SERM molecule with an ideal pharmacologic profile, which has estrogen-like activity on the bone and the lipid profile, antiestrogenic activity on the breast and neutral activity on the uterus, represented for many years the goal to be achieved by pharmaceutical companies. This need has been further emphasized by the recent negative results from WHI and other randomized controlled trials on HRT, particularly for long-term treatment regimens. However, it is now clear from several observations that the ideal SERM profile for one patient may be far from the ideal profile for another. Thus, it is likely that different groups of SERM compounds will be available in the future, each with a somewhat different profile that may be rationally applied to various patients with a spectrum of needs.
While retaining some of the adverse effects of other available SERMs (ie, hot flushes and an increased risk of venous thromboembolic events) lasofoxifene shows an improved skeletal efficacy with a demonstrated efficacy in the prevention of vertebral and nonvertebral fractures. The latter endpoint has not been addressed by raloxifene or other SERMs in clinical development such as ospemifene or arzoxifene. Potential additive beneficial effects of treatment include vaginal atrophy, breast cancer prevention and heart disease. Confirmation of such positive results in these areas would make the drug very attractive to patients at risk for those conditions and postmenopausal bone loss, vastly extending the drug’s patient potential. Some concerns on the overall risk/benefit profile of lasofoxifene are related to the slight increase in the number of deaths observed at 5 years in the PEARL study in subjects treated with lasofoxifene 0.25 mg/day versus placebo. Thus, specific studies on different outcomes (ie, the prevention of breast cancer or coronary events in high risk populations) and longer-term analysis in larger samples for clinically relevant adverse events will be needed to obtain a reasonable view of the future and cost-effectiveness of this compound in the management of women’s health following menopause.
Footnotes
Disclosures
The authors declare no conflicts of interest.
References
- 1.Osteoporosis Prevention, Diagnosis, and Therapy Consensus Statement 2000 JAMA 2001285785–795.11176917 [Google Scholar]
- 2.Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367:2010–2018. doi: 10.1016/S0140-6736(06)68891-0. [DOI] [PubMed] [Google Scholar]
- 3.Reginster JY, Burlet N. Osteoporosis: a still increasing prevalence. Bone. 2006;38:S4–S9. doi: 10.1016/j.bone.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 4.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 5.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 6.Ross PD. Osteoporosis. Frequency, consequences, and risk factors. Arch Intern Med. 1996;156:1399–1411. doi: 10.1001/archinte.156.13.1399. [DOI] [PubMed] [Google Scholar]
- 7.Dennison E, Mohamed MA, Cooper C. Epidemiology of osteoporosis. Rheum Dis Clin North Am. 2006;32:617–629. doi: 10.1016/j.rdc.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts and prospects. J Clin Invest. 2005;115:3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindsay R, Hart DM, Forrest C, Baird C. Prevention of spinal osteoporosis in oophorectomised women. Lancet. 1980;2(8205):1151–1154. doi: 10.1016/s0140-6736(80)92592-1. [DOI] [PubMed] [Google Scholar]
- 10.Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fractures. Lancet. 1993;341:72–75. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 11.Hui SL, Slemenda CW, Carey MA, Johnston CC. Choosing between predictors of fractures. J Bone Miner Res. 1995;10:1816–1822. doi: 10.1002/jbmr.5650101126. [DOI] [PubMed] [Google Scholar]
- 12.Seeman E, Delmas PD. Bone quality-the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 13.Martin TJ, Seeman E. Bone remodeling: its local regulation and the emergence of bone fragility. Best Pract Res Clin Endo Endocrinol Metab. 2008;22:701–722. doi: 10.1016/j.beem.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Riggs BL, Khosla S, Melton LJ., III Sex steroids and the construction and conservation of the adult skeleton. Endo Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 15.Syed F, Khosla S. Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun. 2005;328:688–696. doi: 10.1016/j.bbrc.2004.11.097. [DOI] [PubMed] [Google Scholar]
- 16.Pacifici R. Mechanisms of estrogen action in bone. In: Bilezikian JP, Raisz LG, Martin TJ, editors. Principles of Bone Biology. 3rd ed. USA: Elsevier Inc; 2008. pp. 921–933. [Google Scholar]
- 17.Bush TL, Wells HB, James MK, et al. Effects of hormone therapy on bone mineral density–results from the postmenopausal estrogen/progestin interventions (PEPI) trial. JAMA. 1996;276:1389–1396. [PubMed] [Google Scholar]
- 18.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 19.Jackson RD, Shidham S. The role of hormone therapy and calcium plus vitamin D for reduction of bone loss and risk for fractures: lessons learned from the Women’s Health Initiative. Current Osteoporosis Reports. 2007;5(4):153–159. doi: 10.1007/s11914-007-0010-4. [DOI] [PubMed] [Google Scholar]
- 20.Grodstein F, Manson JE, Colditz GA, et al. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933–941. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- 21.Manson JE, Hsia J, Johnson KC, et al. Women’s Health Initiative Investigators. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 22.The Writing Group for the PEPI Trial. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women: the Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1995;273:199–208. [PubMed] [Google Scholar]
- 23.Vassilopoulou-Sellin R. Breast cancer and hormonal replacement therapy. Ann N Y Acad Sci. 2003;997:341–350. doi: 10.1196/annals.1290.037. [DOI] [PubMed] [Google Scholar]
- 24.The North American Menopause Society. Estrogen and progestogen use in postmenopausal women: July 2008 position statement of The North American menopause society. Menopause. 2008;15(4):584–602. doi: 10.1097/gme.0b013e31817b076a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho CH, Nuttall ME. Therapeutic potential of oestrogen receptor ligands in development for osteoporosis. Expert Opin Emerg Drugs. 2001;6:137–154. doi: 10.1517/14728214.6.1.137. [DOI] [PubMed] [Google Scholar]
- 26.Jordan VC. Chemosuppression of breast cancer with tamoxifen-laboratory evidence and future clinical investigations. Cancer Investigation. 1988;6:589–595. doi: 10.3109/07357908809082124. [DOI] [PubMed] [Google Scholar]
- 27.Lerner LJ, Jordan VC. Development of antiestrogens and their use in breast cancer (Eighth Cain Memorial award lecture) Cancer Res. 1990;50:4177–4189. [PubMed] [Google Scholar]
- 28.Avioli LV. SERM Drugs for the prevention of osteoporosis. Trends Endocrinol Metab. 1999;10:317–319. doi: 10.1016/s1043-2760(99)00176-9. [DOI] [PubMed] [Google Scholar]
- 29.Riggs L, Hartmann LC. Selective Estrogen-Receptor Modulators- Mechanisms of Action and Application to Clinical Practice. N Engl J Med. 2003;348:618–629. doi: 10.1056/NEJMra022219. [DOI] [PubMed] [Google Scholar]
- 30.Brzozowski AM, Pike AC, Dauter Z, et al. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- 31.Shiau AK, Barstad D, Loria PM, et al. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95:927–937. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- 32.Paige LA, Christensen DJ, Grøn H, et al. Estrogen receptor (ER) modulators each induce distinct conformational changes in ERa and ERb. Proc Natl Acad Sci U S A. 1999;96:3999–4004. doi: 10.1073/pnas.96.7.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nilsson S, Koehler KF. Oestrogen receptors and selective oestrogen receptor modulators: molecular and cellular pharmacology. Basic Clin Pharmacol Toxicol. 2005;96:15–25. doi: 10.1111/j.1742-7843.2005.pto960103.x. [DOI] [PubMed] [Google Scholar]
- 34.Gennari L, Merlotti D, Valleggi F, Martini G, Nuti R. Selective estrogen receptor modulators for postmenopausal osteoporosis: current state of development. Drugs Aging. 2007;24:361–379. doi: 10.2165/00002512-200724050-00002. [DOI] [PubMed] [Google Scholar]
- 35.Gennari L, Merlotti D, De Paola V, Martini G, Nuti R. Bazedoxifene for the prevention of postmenopausal osteoporosis. Ther Clin Risk Manag. 2008a;4(6):1229–1242. doi: 10.2147/tcrm.s3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delmas PD, Bjarnason NH, Mitlak BH, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997;337:1641–1647. doi: 10.1056/NEJM199712043372301. [DOI] [PubMed] [Google Scholar]
- 37.Lufkin EG, Whitaker MD, Nickelsen T, et al. Treatment of established postmenopausal osteoporosis with raloxifene: a randomized trial. J Bone Miner Res. 1998;13:1747–1754. doi: 10.1359/jbmr.1998.13.11.1747. [DOI] [PubMed] [Google Scholar]
- 38.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282(7):637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 39.Jordan VC, Phelps E, Lindgren JU. Effects of anti-estrogens on bone in castrated and intact female rats. Breast Cancer Res Treat. 1987;10:31–35. doi: 10.1007/BF01806132. [DOI] [PubMed] [Google Scholar]
- 40.Love RR, Barden HS, Mazess RB, et al. Effect of tamoxifen on lumbar spine bone mineral density in postmenopausal women after 5 years. Arch Intern Med. 1994;154:2585–2588. [PubMed] [Google Scholar]
- 41.Prestwood KM, Gunness M, Muchmore DB, et al. A comparison of the effects of raloxifene and estrogen on bone in postmenopausal women. J Clin Endocrinol Metab. 2000;85:2197–2202. doi: 10.1210/jcem.85.6.6654. [DOI] [PubMed] [Google Scholar]
- 42.Weinstein RS, Parfitt AM, Marcus R, et al. Effects of raloxifene, hormone replacement therapy, and placebo on bone turnover in postmenopausal women. Osteoporos Int. 2003;14:814–822. doi: 10.1007/s00198-003-1434-z. [DOI] [PubMed] [Google Scholar]
- 43.Gennari L, Merlotti D, De Paola V, Nuti R. Raloxifene in breast cancer prevention. Expert Opin Drug Saf. 2008b;7:259–270. doi: 10.1517/14740338.7.3.259. [DOI] [PubMed] [Google Scholar]
- 44.Gennari L, Merlotti D, Valleggi F, Nuti R. Ospemifene use in postmenopausal women. Expert Opin Investig Drugs. 2009;18(6):839–849. doi: 10.1517/13543780902953715. [DOI] [PubMed] [Google Scholar]
- 45.Rosati RL, Da Silva Jardine P, Cameron KO. Discovery and preclinical pharmacology of a novel, potent, nonsteroidal estrogen receptor agonist/antagonist, CP-336156, a diaryltetrahydronaphthalene. J Med Chem. 1998;41:2928–2931. doi: 10.1021/jm980048b. [DOI] [PubMed] [Google Scholar]
- 46.Ke HZ, Paralkar VM, Grasser WA. Effects of CP-336,156, a new, nonsteroidal estrogen agonist/antagonist, on bone, serum cholesterol, uterus and body composition in rat models. Endocrinology. 1998;139:2068–2076. doi: 10.1210/endo.139.4.5902. [DOI] [PubMed] [Google Scholar]
- 47.Gennari L, Merlotti D, Martini G, Nuti R. Lasofoxifene: a thirdgeneration selective estrogen receptor modulator for the prevention and treatment of osteoporosis. Expert Opin Investig Drugs. 2006a;15:1091–1103. doi: 10.1517/13543784.15.9.1091. [DOI] [PubMed] [Google Scholar]
- 48.Morello KC, Wurz GT, DeGregorio MW. Pharmacokinetics of selective estrogen receptor modulators. Clin Pharmacokinet. 2003;42:361–372. doi: 10.2165/00003088-200342040-00004. [DOI] [PubMed] [Google Scholar]
- 49.Gennari L. Lasofoxifene: a new type of selective estrogen receptor modulator for the treatment of osteoporosis. Drugs Today (Barc) 2006b;42:355–367. doi: 10.1358/dot.2006.42.6.973583. [DOI] [PubMed] [Google Scholar]
- 50.Moller R, Fisher J, Taylor A, et al. Effect of Food on the Pharmacokinetics of Lasofoxifene, in Healthy Postmenopausal Women. J Bone Miner Res. 2004;19 Abstract SU487. [Google Scholar]
- 51.Gardner M, Nishizawa Y, Wei G, et al. A Single-Dose Pharmacokinetic Study of Lasofoxifene in Japanese and Caucasian Postmenopausal Women. J Bone Miner Res. 2004;19 Abstract M469. [Google Scholar]
- 52.Gardner M, Taylor A, Wei G, et al. Clinical pharmacology of multiple doses of lasofoxifene in postmenopausal women. J Clin Pharmacol. 2006;46:52–58. doi: 10.1177/0091270005283280. [DOI] [PubMed] [Google Scholar]
- 53.Fountaine RJ, Nishizawa Y, Wei G, Dogolo L, Calcagni A, Gardner MJ. Clinical pharmacology of lasofoxifene in Japanese and white postmenopausal women. J Clin Pharmacol. 2006;46:693–699. doi: 10.1177/0091270006288213. [DOI] [PubMed] [Google Scholar]
- 54.Katzenellenbogen JA. D-1,2-Dyaril-3,4-dihydronaphthalenes: photofluorogenic ligands for the estrogen receptor. J Steroid Biochem. 1985;23:929–937. doi: 10.1016/0022-4731(85)90049-4. [DOI] [PubMed] [Google Scholar]
- 55.Jhonson KA, Gardner MJ, Prakash C. In vivo and in vitro metabolites of a next-generation selective estrogen receptor modulator, lasofoxifene, in humans. Drug Metab Rev. 2004;36:246. [Google Scholar]
- 56.Roman D, Bramson C, Ouellet D, et al. Effect of lasofoxifene on the pharmacokinetics of digoxin in healthy postmenopausal women. J Clin Pharmacol. 2005;45:1407–1412. doi: 10.1177/0091270005282627. [DOI] [PubMed] [Google Scholar]
- 57.Moller RA, Fisher JM, Taylor AE, et al. Effects of Steady-State Lasofoxifene on CYP2D6- and CYP2E1-Mediated Metabolism. Ann Pharmacother. 2006;40:32–37. doi: 10.1345/aph.1G347. [DOI] [PubMed] [Google Scholar]
- 58.Bramson C, Ouellet D, Roman D, et al. A single-dose pharmacokinetic study of lasofoxifene in healthy volunteers and subjects with mild and moderate hepatic impairment. J Clin Pharmacol. 2006;46:29–36. doi: 10.1177/0091270005283278. [DOI] [PubMed] [Google Scholar]
- 59.Ouellet D, Bramson C, Carvajal-Gonzalez S, Roman D, Randinitis E, Remmers A, et al. Effects of lasofoxifene on the pharmacokinetics and pharmacodynamics of single-dose warfarin. Br J Clin Pharmacol. 2006;61:741–745. doi: 10.1111/j.1365-2125.2006.02589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ke HZ, Qi H, Chidsey-Frink KL, et al. Effects of different dose regimens of lasofoxifene (CP-336,156) in preventing bone loss in ovariectomized rats. J Bone Miner Res. 2000a;15:S1–S310. [Google Scholar]
- 61.Ke HZ, Foley GL, Simmons HA, Shen V, Thompson DD. Long-term treatment of lasofoxifene preserves bone mass and bone strength and does not adversely affect the uterus in ovariectomized rats. Endocrinology. 2004;145:1996–2005. doi: 10.1210/en.2003-1481. [DOI] [PubMed] [Google Scholar]
- 62.Ke HZ, Qi H, Crawford DT, et al. Lasofoxifene (CP-336-156), a selective estrogen receptor modulator, prevents bone loss induced by aging and orchidectomy in the adult rat. Endocrinology. 2000b;141:1338–1344. doi: 10.1210/endo.141.4.7408. [DOI] [PubMed] [Google Scholar]
- 63.Ke HZ, Oi H, Chidsey-Frink KL, et al. Lasofoxifene (CP-336-156) protects against the age-related changes in bone mass, bone strength, and total serum cholesterol in intact aged male rats. J Bone Miner Res. 2001;16:765–773. doi: 10.1359/jbmr.2001.16.4.765. [DOI] [PubMed] [Google Scholar]
- 64.Kharode YP, Green PD, Marzolf JT, et al. Comparison of the effects of bazedoxifene, raloxifene, lasofoxifene and risedronate, co-treatment on h-PTH-induced reversal of established osteopenia in ovariectomized rats. J Bone Miner Res. 2003;18:S273. [Google Scholar]
- 65.Cohen LA, Pittman B, Wang CX, et al. LAS, a novel selective estrogen receptor modulator with chemopreventive and therapeutic activity in the N-nitroso-N-methylurea-induced rat mammary tumor model. Cancer Res. 2001;61:8683–8688. [PubMed] [Google Scholar]
- 66.Gennari L, Merlotti D, De Paola V, Nuti R. Lasofoxifene: the evidence of its therapeutic value in osteoporosis. Core Evidence. 2009;4:113–129. doi: 10.2147/ce.s6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chesworth R, Zawistoski MP, Lefker BA, et al. Tetrahydroisoquinolines as subtype selective estrogen agonists/antagonists. Bioorganic and Medicinal Chemistry Letters. 2004;14:2729–2733. doi: 10.1016/j.bmcl.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 68.Lee A, Radecki D, Wolter K, et al. Lasofoxifene Phase 2 and Phase 3 Clinical Trial Design and Strategy. J Bone Miner Res. 2005;20 Abstract M384. [Google Scholar]
- 69.Day W, Martel J, Lee A. Lasofoxifene phase 2 dose response analysis in postmenopausal women. J Bone Miner Res. 2005;20 Abstract M385. [Google Scholar]
- 70.Moffett AH, Ettinger M, Bolognese M, et al. Lasofoxifene, a Next Generation SERM, is Effective in Preventing Loss of BMD and Reducing LDL-C in Postmenopausal Women. J Bone Miner Res. 2004;19 Abstract SA426. [Google Scholar]
- 71.Portman DJ, Moffett AH, Bachman GA, West C, Symons J. Lasofoxifene, a selective estrogen receptor modulator, improves objective measures of vaginal atrophy. Obstetrics and Gynecology. 2004;103:25S–26S. [Google Scholar]
- 72.Ettinger M, Schwartz E, Emkey R, et al. Lasofoxifene: a next generation selective estrogen receptor modulator (SERM) in the prevention of bone loss in postmenopausal women. 86th Annual Meeting of the Endocrine Society; 2004 June 16–19; New Orleans, LA. [Google Scholar]
- 73.Lingand pharmaceutical Internetional Inc. Annual Report 2000. Available from http//investors.ligand.com.
- 74.McClung M, Portman D, Emkey R, et al. Comparison of the extraskeletal effects of lasofoxifene and raloxifene. J Bone Miner Res. 2004;19 Abstract SA423. [Google Scholar]
- 75.McClung MR, Siris E, Cummings S, et al. Prevention of bone loss in postmenopausal women treated with lasofoxifene compared with raloxifene. Menopause. 2006;13:377–386. doi: 10.1097/01.gme.0000188736.69617.4f. [DOI] [PubMed] [Google Scholar]
- 76.McClung M, Siris E, Cummings S, et al. Lasofoxifene Increased BMD of the Spine and Hip and Decreased Bone Turnover Markers in Postmenopausal Women with Low or Normal BMD. J Bone Miner Res. 2005;20 Abstract F429. [Google Scholar]
- 77.Davidson M, Moffett A, Welty F, et al. Extraskeletal Effects of Lasofoxifene on Postmenopausal Women. J Bone Miner Res. 2005;20 Abstract SA428. [Google Scholar]
- 78.Eastell R, Reid DM, Vukicevic S, et al. The Effects of Lasofoxifene on Bone Turnover Markers: the PEARL Trial. J Bone Miner Res. 2008;23 Abstract 1287. [Google Scholar]
- 79.Glover SJ, Rogers A, Gossiel F, Eastell R. A randomized double blinded controlled trial of individual response in biochemical markers of bone turnover to lasofoxifene therapy. J Bone Miner Res. 2008;23 doi: 10.1016/j.bone.2009.07.089. Abstract M507. [DOI] [PubMed] [Google Scholar]
- 80.Cummings SR, Eastell R, Ensrud K, et al. The effects of lasofoxifene on fractures and breast cancer: 3-year results from the PEARL Trial. J Bone Miner Res. 2008;23 Abstract 1288. [Google Scholar]
- 81.European Medicines Agency, Pre-Authorisation Evaluation of Medicines for Human Use. Committee for Medicinal Products for Human Use summary of positive opinion for Fablyn; London. 2008 December 18; Doc Ref EMEA/CHMP/609979/2008. [Google Scholar]
- 82.Stevenson N, Jones ML, De Nigris E, Brewer N, Davis S, Oakley J. A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis. Health Technol Assess. 2005;9:1–160. doi: 10.3310/hta9220. [DOI] [PubMed] [Google Scholar]