Abstract
The chemokine CXCL10 is expressed in the central nervous system (CNS) during neuroinflammatory conditions. Neurons express CXCR3, the receptor for CXCL10, and neuronal function has been shown to be altered by acute exposure to CXCL10. Little is known about the effects of chronic exposure to CXCL10 on neuronal function. Results from our studies show that chronic exposure of cultured rat hippocampal neurons to CXCL10 results in altered levels of protein for GABA and glutamate receptors and altered synaptic network activity. These effects of CXCL10 may contribute to altered CNS function that occurs in some chronic neuroinflammatory conditions.
Keywords: chemokine, synapse, neuroinflammation, receptor, calcium
Introduction
Emerging research indicates that chemokines and their receptors are part of a signaling system within the CNS that is utilized for communication between cells of the CNS as well as between immune cells and cells of the CNS (Adler et al., 2005). Although a number of chemokines are expressed in the CNS, there is growing interest in the role of CXCL10, primarily driven by the higher expression of this chemokine in certain neuroinflammatory conditions and correlations between a high level of expression of CXCL10 and altered CNS function. For example, CXCL10 levels in the CSF are elevated in HIV infection, with higher levels in HIV-infected individuals with neurologic disorders than in HIV-infected individuals without neurological deficits (Cinque et al., 2005; Kolb et al., 1999). Moreover, a significant positive correlation between scores on standardized mental tests and CXCL10 concentrations in the CSF was observed in Alzheimer’s patients with mild cognitive dysfunction (Galimberti et al., 2006).
CNS cells, including neurons and glia, have been shown to express CXCR3, the receptor for CXCL10 (Bajetto et al., 2002; Biber et al., 2002; Flynn et al., 2003; Tran et al., 2007; Xia et al., 2000), and to produce CXCL10 under normal conditions or during pathological states, when chemokine production can be significantly elevated (Carter et al., 2007; Klein et al., 2005; Oh et al., 1999; Omari et al., 2005; Van Heteren et al., 2008; Wang et al., 1998). The expression of CXCR3 by CNS neurons suggests a role for neurons as a target for CXCL10 in the CNS. However, little is known about the neuronal effects of CXCL10. Recent studies of synaptic function in hippocampal slices from adult mice showed that acute exposure to CXCL10 reduced hippocampal long-term potentiation (LTP) at the Schaffer collateral to CA1 pyramidal neuron synapse (Vlkolinsky et al., 2004). LTP is a form of synaptic plasticity that is considered to be a cellular mechanism of learning and memory (Miyamoto, 2006). There was no effect of CXCL10 on basal synaptic responses (Vlkolinsky et al., 2004). Another study showed that acute exposure to CXCL10 altered spontaneous synaptic network activity, spike firing and intracellular Ca2+ levels associated with the synaptic network activity in the cultured hippocampal neurons (Nelson and Gruol, 2004). Taken together, these two studies showing that acute exposure to CXCL10 can alter neuronal activity support a potential role for CXCL10 signaling in normal CNS physiology or during neuroinflammatory conditions associated with CNS disease or injury.
During neuroinflammation, CNS levels of CXCL10 can be upregulated for a prolonged period. Our recent studies showed that prolonged exposure to CXCL10 produced alterations in the level of protein for signal transduction molecules that regulate neuronal function (e.g., ERK1/2) and transcription factors that regulate gene expression in rat hippocampal cultures (e.g., CREB) (Bajova et al., 2008). These results raise the possibility that the levels of other neuronal proteins that are important for neuronal function are also altered by prolonged CXCL10 exposure. To address this possibility, in the current study we examined the effect of chronic exposure to CXCL10 on the relative level of synaptic proteins and synaptic network activity in rat hippocampal cultures.
Materials and methods
The animal procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animal facilities and experimental protocols were in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care.
Cell cultures
Primary cultures were prepared from rat hippocampus (Sprague–Dawley; Charles River, Wilmington, MD, USA) and maintained in vitro as described previously (Nelson and Gruol, 2004). Briefly, hippocampi were isolated from the brain of embryonic day 20 rat pups, minced, and triturated in Ca2+ and Mg2+-free saline containing (in mM): 137 NaCl, 5.4 KCl, 0.17 Na2HPO4, 0.22 KH2PO4, 27.7 glucose, 43.8 sucrose, 10 HEPES–NaOH (pH 7.3 with NaOH). The resulting suspension of cells and small tissue pieces was plated on glass cover slips (MatTek, Ashland, MA, USA) coated with Matrigel (BD Biosciences, Bedford, MA, USA) or on 35-mm tissue culture dishes coated with Matrigel. During the culture period the small tissue pieces flatten as cells migrate out with astrocytes forming a substratum on which the neuronal population grows. The plating medium contained Minimal Essential Medium (MEM) with Earle’s salts and l-glutamine (Gibco-Invitrogen, Carlsbad, CA, USA) and was supplemented with 10% heat-inactivated horse serum (Gibco-Invitrogen), 10% heat-inactivated fetal calf serum (Gibco-Invitrogen), and D-glucose (5.0 g/l). Medium was replaced twice weekly with medium having a similar composition but without the calf serum. Cultures were incubated at 37 °C in a 5% CO2 humidified atmosphere. Brief treatment with the anti-mitotic agent 5-fluorodeoxyuridine (20 μg/ml, days 2–6 in vitro; Sigma, St. Louis, MO, USA) limited the number of non-neuronal cells in culture. Antibiotics were not used. Unless otherwise noted, chemicals were of reagent grade and were obtained from Sigma.
CXCL10 treatment
The treatment protocol was the same as used in our previous study (Bajova et al., 2008). Treatments were started on the second day in vitro (DIV) and lasted for 9 days. The CXCL10 treatment group was treated with human recombinant CXCL10 (BSA- and endotoxin-free; R&D Systems, Minneapolis, MN, USA), which is active at rat receptors (recombinant rat CXCL10 is not commercially available), by addition of a stock solution of CXCL10 to the cultures to give final concentrations of 100 nM or 250 nM CXCL10. To the control cultures, the same amount of 0.1% BSA (fatty acid-free) was added. CXCL10 or 0.1% BSA (control cultures) were reapplied every third day during normal media changes. The stock solution of CXCL10 was prepared by reconstitution in 0.1 % bovine serum albumin (BSA; fatty acid free) to a final concentration of 50 μM and stored at −20 °C.
Western blot assays
At 11 DIV the cultures were processed for Western blot assay following standard protocols reported previously (Vereyken et al., 2007). Tissue culture dishes were placed on ice, washed 3 times with ice cold phosphate-buffered saline (PBS) and the cells harvested and homogenized in the lysis buffer. The buffer contained 50 mM Tris/HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 0.5% NP-40, a Protease Inhibitor Cocktail Tablet (Boehringer Mannheim, Indianapolis, IN, USA), and a cocktail of phosphatase inhibitors (4.5 mM sodium pyrophosphate, 10 mM β-glycerophosphate, 1 mM sodium fluoride, 1 mM sodium orthovanadate). After incubation, the homogenates were centrifuged at 15,000 rpm (30 min), the supernatants collected and protein concentration in the samples determined using the Bio-Rad Protein Assay Kit (Bio-Rad, Hercules, CA, USA).
The samples (10–20 μg of protein in duplicate) were diluted with 2 × Laemmli sample buffer, separated by SDS-PAGE gel electrophoresis on 4–12% Bis-Tris NuPAGE® gels (Invitrogen, Carlsbad, CA, USA) and transferred onto Immobilon P membranes (Millipore, Billerica, MA, USA). Membranes were stained with Ponceau S to confirm uniform transfer. After destaining, the membranes were blocked with casein (Pierce Biotechnology, Rockford, IL, USA) and then incubated in primary antibody followed by the secondary antibody coupled to HRP (SuperSignal West Pico Chemiluminescent Substrate, Thermo Scientific, Rockford, IL). The immunoreactive bands were detected using chemiluminescence. The membranes were exposed to Kodak Biomax MR film (Kodak, Rochester, New York) and the density of the immunoreactive bands quantified using the NIH Image software program (http://rsb.info.nih.gov/nih-image). All membranes were stripped (Pierce Restore Stripping buffer, Pierce Biotechnology) and reblotted for β-actin. To minimize the potential effects of any loading errors, density measurements for each treatment group were first normalized to density measurements for β-actin in the same lane of the gel and then the normalized values for the different treatment groups were normalized to values for the control treatment group. This method of normalization adjusted for differences in total protein levels, which could vary across culture sets, and was applied to all studies. All treatment groups from the same culture set were run on the same gel to facilitate normalization of data. Summarized results are the normalized data. Statistical calculations were performed using one group t-test with statistical significance set at p < 0.05.
Immunocytochemistry
Immunocytochemical staining of the hippocampal cultures was performed according to previously published methods (Nelson and Gruol, 2004). In brief, cultures were rinsed with serum-free MEM, fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS, 100 mM, pH 7.3) and permeablized with 0.05% Triton X-100 in PBS for 30 min. The cultures were incubated overnight (4 °C) in PBS containing the primary antibodies in dilutions of 1:1000 and 0.05% BSA as a blocking agent. Immunoreactivity was detected with the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA, USA). No immunostaining was observed when the antibody was first blocked by pre-incubation with the respective antigenic peptide (1:10 dilution) used for production of the primary antibodies.
Antibodies
The following primary antibodies were used for immunohistochemistry or Western blot: an affinity purified goat polyclonal antibody raised against a peptide corresponding to an amino acid sequence at the C-terminus of the precursor form of the rat GABABR1 (Sc7338; 1:1000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA); a rabbit polyclonal antibody raised against a synthetic peptide from the rat GABAA receptor alpha-2 subunit from the C-terminus (AB5948; 1:1000; Chemicon International, Inc., Temecula, CA); a rabbit polyclonal antibody raised against a synthetic peptide to the C-terminus of rat GAD 65/67 (AB1511; 1:1000; Chemicon International, Inc.); an affinity purified goat polyclonal antibody raised against a peptide corresponding to an amino acid sequence at the C-terminus of the precursor form of the NR1 subunit of the human NMDA receptor (NMDAR1)(sc-1467, 1:1000; Santa Cruz); a purified rabbit polyclonal antibody that recognizes both GluR2 and GluR3 subunits (GluR2/3) of the AMPA receptor (AB1506; 1–1000; Chemicon International, Inc); a purified rabbit polyclonal antibody raised against a carboxy terminus peptide of the rat mGluR2/3 conjugated to BSA with glutaraldehyde (AB1553; 1–1000; Chemicon International, Inc.); monoclonal antibody to glial fibrillary acidic protein (GFAP; 1:5000, MAB360, Chemicon International, Inc); a purified mouse antibody produced against GST fusion protein corresponding to residues 77–299 of human-PSD-95 (clone K28.42; 1:1000; IC Davis/NINDA/NIH NeuroMab Facility, Davis, CA); an affinity purified rabbit polyclonal antibody raised against synapsin 1 (A-6442; 1:5000; Molecular Probes/Invitrogen); a monoclonal antibody raised against beta-actin (AC-15; 1–5000; Sigma). Secondary antibodies were rabbit anti-goat, goat anti-rabbit or goat anti-mouse IgG (H+L) (1:10000, Southern Biotech, Birmingham, AL, USA).
Ca2+ imaging
Standard microscopic digital imaging techniques were used as described previously (Nelson & Gruol, 2004) and based on the methods of Grynkiewicz et al. (1985). The hippocampal cultures were loaded with the Ca2+ sensitive dye fura-2/AM (Molecular Probes) at 3 μM in physiological saline containing 0.02% pluronic F-127 (Molecular Probes) for 30 minutes at room temperature. The composition of the physiological saline was (in mM): 140 NaCl, 3.5 KCl, 0.4 KH2PO4, 1.25 Na2HPO4, 2.2 CaCl2, 2 MgSO4, 10 glucose and 10 HEPES. After removal of the fura-2, the cultures were incubated in dye-free saline for at least 45 minutes to allow for the de-esterification of the fura-2AM.
Live fluorescence images of the selected field of neurons were acquired at excitation wavelengths of 340 and 380 nm using a SIT-66 video camera (DAGE-MTI, Dage, Michigan City, IN) and digitized for real-time display using the MCID imaging software (Imaging Research, St. Catharines, Ontario, Canada). Data were collected at 0.6 to 1.5 s intervals (4 frames per wavelength were averaged for each time point) depending on the experiment. Measurements of fluorescence levels were made in individual neurons and intracellular Ca2+ levels were calculated by converting the fluorescence ratios (340/380 nm) to intracellular Ca2+ concentrations. The following formula was used for this conversion: [Ca2+]i = Kd(R−Rmin)/(Rmax − R)*Fo/Fs, where R is the ratio value, Rmin is the ratio for a Ca2+ free solution, Rmax is the ratio for a saturated Ca2+ solution, Kd is 225 (the dissociation constant for fura-2), Fo is the intensity of a Ca2+ free solution at 380 nm and Fs is the intensity of a saturated Ca2+ solution at 380 nm. The low level of background fluorescence and adjustment of the black level of the SIT camera eliminated the need for background subtraction methods. Calibration was done using fura-2 salt (100 μM) in solutions of known Ca2+ concentration (Molecular Probes kit #C-3009). Typical Rmax, Rmin, and Fo/Fs values were 0.61, 2.85 and 2.5, respectively. Recordings were made at room temperature.
Ca2+ signals generated by spontaneous synaptic activity were quantified by measurement of total Ca2+ load (area under of the curve) during a standardized recording period (typically 3 min) using AxoGraph software (Axon Instruments, Foster City, CA). For data analysis, on each experimental day the mean Ca2+ level was calculated for the population of control neurons studied and the measured Ca2+ level for each neuron studied on that day (control and CXCL10-treated) was normalized to the mean control value. This normalization adjusted for potential differences in synaptic activity (which generates the Ca2+ signals) across culture sets. Normalized values from all experiments were then combined according to treatment group and experimental condition and analyzed statistically for differences using the unpaired t-test. Statistical significance was set at p < 0.05.
Ca2+ signals produced by brief application of NMDA were quantified by measurement of the peak amplitude. For data analysis, on each experimental day the mean value for the peak amplitude of the Ca2+ signal produced by NMDA was calculated for the population of control neurons studied. The peak amplitude measure for each neuron studied on that experimental day (control and CXCL10-treated) was normalized to the mean control value. Normalized values from all experiments were then combined according to treatment group and experimental condition and analyzed statistically for differences using the unpaired t-test. Statistical significance was set at p < 0.05
Drug application
Experiments were performed either in normal physiological saline or saline with reduced Mg2+ (final concentration, 30 μM) plus 5 μM glycine. In some experiments, transmitter receptor antagonists were added to the recording saline. These antagonists included 5 μM NBQX (2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulphonamide disodium; Tocris Cookson, Ltd., Langford, Bristol, UK), 50 μM APV (D(−)-2-amino-5-phosphonopentanoic acid; Tocris Cookson), 100 μM picrotoxin (Sigma), and 1 μM CGP55854A (Novartis, Basel, Switzerland) to block AMPA, NMDA, GABAA, and GABAB receptors, respectively. In some studies the bath also contained 0.2 μM tetrodotoxin (TTX, Sigma) to block Na+ based action potentials.
In some studies, receptor ligands were applied by brief micropressure application (Picospritzer II; Parker Hannifin Corporation, Fairfield, NJ, USA) from a glass micropipette (1–2 μm tip) positioned to expose the target neurons. Fast green (0.03%; Sigma) was included in drug solutions applied by microperfusion to visualize cell exposure to drugs. Fast green by itself had no effect on the measured fluorescence or excitability of the neurons. The ligands tested were NMDA (10 μM), to activate NMDA receptors, baclofen, to activate GABAB receptors, and CGP55854A (CGP), to block responses mediated by GABAB receptors.
Analyses
Statistical significance (P < 0.05) was determined using the one group t-test, unpaired t-test or ANOVA. Values are reported as the mean ± SEM. n = the number of cells, microscopic fields or cultures studied. For each study, results were obtained from at least three different culture sets.
Results
CXCL10 alters the levels of proteins involved in synaptic transmission
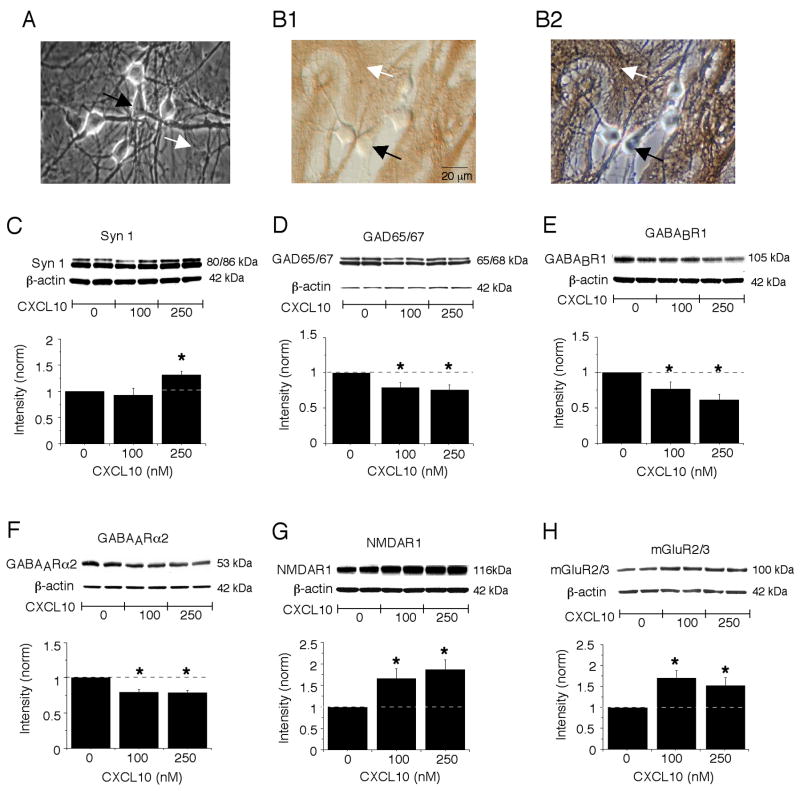
The effect of chronic exposure to CXCL10 on the level of several representative neuronal proteins involved in synaptic transmission was determined by Western blot analyses of control and CXCL10-treated hippocampal cultures. The hippocampal cultures contain both neuron and glial cells, primarily astrocytes (Fig. 1A,B). The cultures were treated chronically with 0, 100 or 250 nM CXCL10 for 9 days starting at 2 DIV.
Figure 1.
Chronic CXCL10 alters the level of synaptic proteins in cultured hippocampal neurons. A. Digitized phase contrast images showing cell characteristics of the hippocampal cultures. The primary cell types in the hippocampal cultures are the hippocampal neurons and astrocytes, which can be identified based on morphological criteria developed in immunohistochemical studies using cell specific antibodies. The neurons (black arrow) are rounder or oval cells with processes that grow on top of the astrocytes (white arrow), which are large flat cells that form the substratum upon which the neurons grow. B. Digitized images of hippocampal cultures immunostained with an antibody to the astrocyte specific protein GFAP. The unstained neurons (black arrow) and immunostained astrocytes (white arrow) are shown under Hoffman optics (B1), which reveals the immunostaining, and phase contrast optics (B2), which reveals all cells in the field. C-H. Graphs showing mean (±SEM) values for the level of synapsin 1(C), GAD 65/67 (D), GABABR1 (E), GABAARα2 (F), NMDAR1 (G) and mGluR2/3 (H) protein in the CXCL10-treated cultures relative to the level in control cultures (control values =1; represented by the dotted line; see methods for normalization protocol). Representative Western blots are shown above the graphs. * = significantly different from control group (one group t-test, P<0.05). n=5 to 8 different culture sets.
Western blot analysis showed that the level of synapsin I, a presynaptic protein that plays a central role in neurotransmitter release, was significantly increased in cultures chronically treated with 250 nM CXCL10 compared with the level in control cultures. However, this effect was not observed in cultures chronically treated with 100 nM CXCL10 (Fig. 1C). The increase in synapsin I at the 250 nM dose may reflect a growth promoting action of CXCL10. A growth promoting action of CXCL10 was identified in our previous studies as evidenced by an increase in the relative level of both neuron specific enolase and beta-actin in cultures treated with 250 nM CXCL10 compared with control cultures but not in cultures treated with 100 nM CXCL10 (Bajova et al., 2008).
The hippocampal cultures contain both excitatory neurons that use glutamate as a transmitter and inhibitory neurons that use GABA as a transmitter and both neuronal types express neuron specific enolase and beta-actin. However, the GABAergic neurons uniquely express the protein GAD 65/67, the synthetic enzyme for the transmitter GABA. A growth promoting effect of CXCL10 on this protein could affect the level of GABA in the inhibitory neurons and consequently inhibitory synaptic transmission. Therefore, we determined if the level of GAD 65/67 protein in control and CXCL10-treated cultures differed using Western blot. Interestingly, the relative level of GAD 65/67 protein was significantly reduced in the chronic CXCL10-treated cultures compared with levels in control cultures at both the 100 and 250 nM CXCL10 dose (Fig. 1D). The reduced level of GAD 65/67 protein could reflect a loss of inhibitory neurons in the CXCL10-treated cultures or a reduction in the GAD 65/67 level in the inhibitory neurons. In either case, this result raises the possibility that GABAergic inhibition could be reduced in hippocampal neurons by prolonged exposure to CXCL10.
We examined further potential effects of chronic CXCL10 exposure on proteins involved in inhibitory synaptic transmission by comparing the levels of protein for GABA receptors in chronic CXCL10-treated and control cultures. Western blot analysis showed that the level of protein for GABABR1, a metabotropic GABA receptor, was significantly reduced in cultures exposed to chronic CXCL10 treatment at both the 100 nM and 250 nM dose compared with control cultures (Fig. 1E). GABABR1 is localized to postsynaptic sites where it mediates GABAergic inhibition involving K+ channels and on presynaptic terminals of GABAergic neurons where it is involved in feedback inhibition of transmitter release (Bettler et al., 2004). The relative level of the alpha-2 subunit of the ionotropic GABAA receptor (GABAARα2), a subunit that is expressed in high abundance in hippocampal neurons (Prenosil et al., 2006), was also significantly reduced by chronic CXCL10 treatment at both the 100 nM and 250 nM dose (Fig. 1F). GABAARα2 is localized to postsynaptic sites where it mediates fast time-course GABAergic inhibition involving Cl− channels. Taken together, these results suggest that chronic CXCL10 exposure targets several mechanisms involved in GABAergic inhibition in CXCL10-treated culture, with the effects likely to result in reduced inhibitory synaptic transmission depending on the relative contribution of the opposing actions of pre- vs. postsynaptic GABABR1s.
In contrast to the effects of chronic CXCL10 on the level of proteins associated with inhibitory GABAergic synaptic transmission, the relative level of protein for two glutamate receptors that mediate excitatory synaptic transmission was increased in the hippocampal cultures. The relative level of the NR1 subunit of the NMDA receptor (NMDAR1), the receptor subunit essential for ion flux, was significantly increased by chronic CXCL10 treatment at both the 100 nM and 250 nM dose compared with control cultures (Fig. 1G). The relative level of the alpha subunit of group II metabotropic glutamate receptors (mGluR2/3; the antibody recognizes both mGluR2 and mGluR3), the primary subunit of mGluR2/3, was also significantly increased in hippocampal cultures exposed to chronic CXCL10 treatment at both the 100 nM and 250 nM dose compared with levels in control cultures (Fig. 1H). However, there was no significant effect of CXCL10 treatment on the level of GluR2/3 subunits (the antibody recognizes both subunits) of the AMPA subtype of glutamate receptor (AMPAR) (normalized data, control =1, 100 nM CXCL10 = 0.91 ±0.03; 250 nM CXCL10 = 0.96 ± 0.07; n=4 for all, p>0.05). The relative level of PSD 95 protein, an essential scaffolding component of the postsynaptic specialization at glutamatergic synapses (Kennedy, 2000), was also not altered by chronic CXCL10 treatment (normalized data; control =1, 100 nM CXCL10 = 1.14 ±0.12; 250 nM CXCL10 = 0.99 ± 0.13; n=6–7l, p>0.05). NMDAR1 and GluR2/3 are localized primarily postsynaptically where they mediate fast glutamatergic excitatory synaptic transmission. mGluR2/3 is localized primarily at presynaptic glutamatergic synapses where it is involved in feedback inhibition of transmitter (i.e., glutamate) release (Shigemoto et al., 1997; Tamaru et al., 2001). Some hippocampal astrocytes also express mGluR2/3 (Mudo et al., 2007; Schools and Kimelberg, 1999). Taken together, these results indicate glutamate receptors involved in excitatory synaptic transmission are a target of chronic CXCL10, with the net effects likely to result in enhanced excitatory synaptic transmission, depending on the relative contribution of the opposing actions of mGluR2/3 vs. NMDAR.
Chronic CXCL10 alters neuronal activity in hippocampal cultures
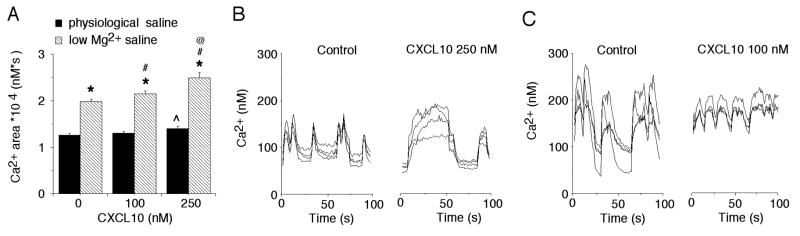
The above results from our Western blot studies show that prolonged exposure to CXCL10 can have significant effects on the levels of hippocampal neuronal proteins involved in excitatory and inhibitory synaptic transmission. In the hippocampal cultures, interactions between excitatory and inhibitory neurons results in spontaneous synaptic network activity, which is comprised of excitatory and inhibitory synaptic potentials and action potentials. Thus, effects of CXCL10 on synaptic proteins could result in altered spontaneous synaptic network activity. To address this possibility, we used Ca2+ imaging techniques to measure intracellular Ca2+ oscillations that are generated by the synaptic network activity. The Ca2+ oscillations result from Ca2+ influx through NMDARs and voltage-gated Ca2+ channels that are activated by the synaptic responses and action potentials that comprise the network activity (Przewlocki et al., 1999). By using Ca2+ imaging techniques, we were able to simultaneously monitor network activity in several neurons in a microscopic field that were part of a neuronal network. Intracellular Ca2+ oscillations were observed in both control and chronic CXCL10-treated neurons under baseline conditions, indicating that synaptic network activity was maintained in the chronic CXCL10-treated cultures. The Ca2+ oscillations were quantified by measuring the total Ca2+ load (area under the curve) during a standardized recording period (typically 3 min). Measurements were made under physiological recording conditions (physiological saline in the bath) and after replacing the physiological saline with reduced Mg2+ saline. The reduced Mg2+ saline induces conditions reflective of strong synaptic drive by removing Mg2+ block of NMDARs.
With physiological saline in the bath, intracellular Ca2+ levels (i.e., total Ca2+ load during a standardized recording period) generated by the network activity were similar in control neurons and neurons treated with 100 nM CXCL10. A small but significant increase in intracellular Ca2+ levels was observed in neurons treated with 250 nM CXCL10 compared with control neurons (Fig. 2). Replacement of physiological saline with low Mg2+ saline significantly increased intracellular Ca2+ oscillations in all treatment groups resulting in higher Ca2+ levels (Fig. 2). Ca2+ levels were significantly higher in the 100 nM and 250 nM CXCL10-treated neurons compared with control neurons, suggesting that alterations produced by chronic CXCL10 treatment resulted in enhanced synaptic network activity in the hippocampal cultures.
Figure 2.
Chronic CXCL10 enhances synaptic network activity. A. Graph showing mean (±SEM) Ca2+ levels in neurons in control and chronic CXCL10-treated cultures. For all studies in this figure, Ca2+ levels were measured as total Ca2+ load (area under the curve) during a standardized recording period (180 s). The neurons were recorded in physiological saline or reduced Mg2+ saline. Data are from 6 different culture sets; n>50 for each condition. B,C. Representative recordings of Ca2+ levels in four neurons of a microscopic field in cultures from different treatment groups. Recordings in B were made under physiological conditions. Recordings in C were made in reduced Mg2+ saline. The oscillations in Ca2+ levels in the recordings are produced by spontaneous synaptic network activity. ^significantly different from control (0 CXCL10) recordings in physiological saline; *significantly different from recordings made in physiological saline for the same treatment group; # significantly different from control (0 CXCL10) for recordings made in low Mg2+ saline; @ significantly different from 100 nM CXCL10 treatment group for recordings made in low Mg2+ saline.
In addition to an effect on network synaptic activity, CXCL10 could alter resting Ca2+ levels. Because we measured Ca2+ oscillations associated with synaptic network activity relative to 0 Ca2+ (i.e., area under the curve), effects of CXCL10 on resting Ca2+ levels would have been incorporated into the measures of Ca2+ levels. To determine if CXCL10 altered resting Ca2+ levels, Ca2+ levels were measured after the addition of TTX and GABA and glutamate receptor antagonists to the bath to block action potentials and synaptic transmission. Results showed that resting Ca2+ levels were similar in control neurons and neurons treated with 100 nM CXCL10, whereas resting levels were slightly but significantly higher in neurons treated with 250 nM CXCL10 (control, 91±2 nM, n=665; 100 nM CXCL10, 87±2 nM, n= 353; 250 nM CXCL10, 98±2 nM, n=300, p<0.05 vs. control, ANOVA). These results indicate that a small increase in resting Ca2+ contributes to the increased Ca2+ levels in the CXCL10-treated neurons under conditions of low Mg2+ saline, but the primary mechanism mediating the increased Ca2+ levels involves network synaptic activity.
CXCL10 treatment alter sensitivity to GABABR1 and NMDAR1 ligands
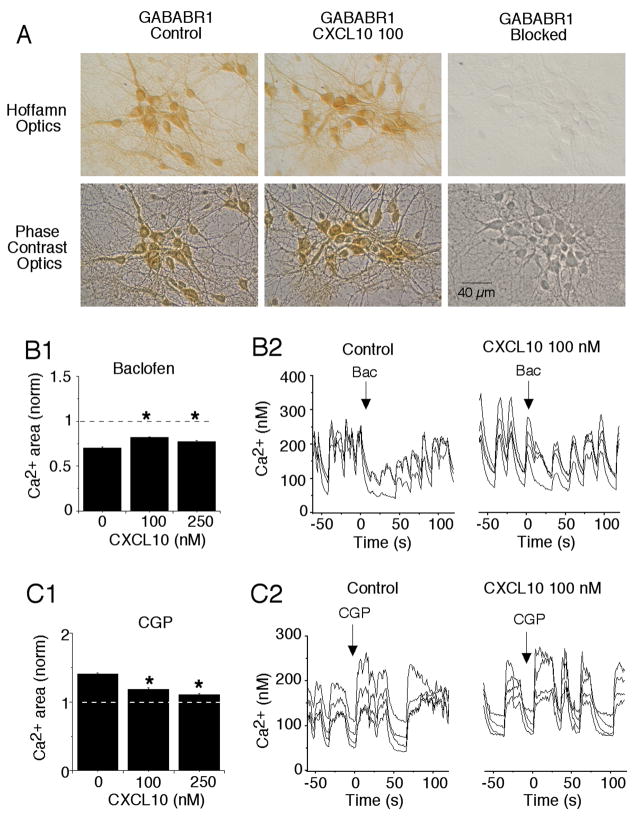
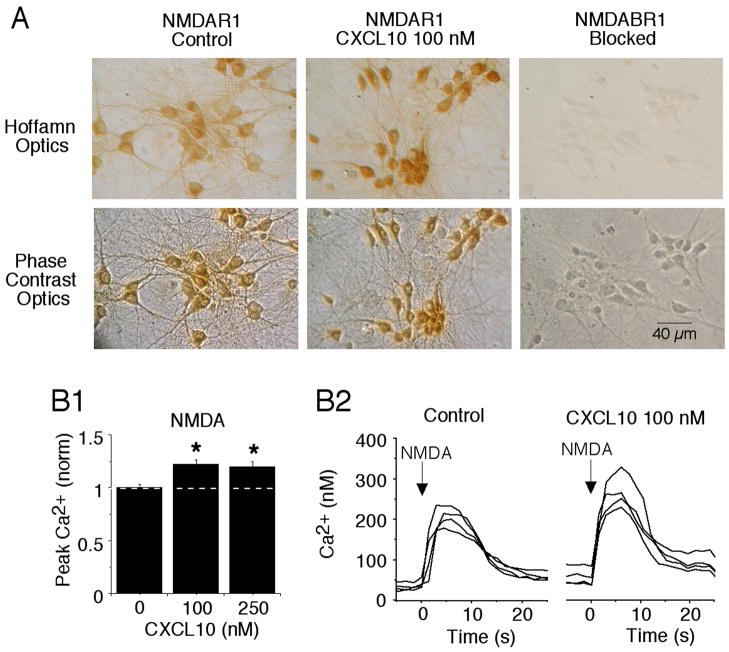
The spontaneous network activity in the hippocampal cultures involves interplay between excitatory (glutamate-mediated) and inhibitory (GABA-mediated) synaptic input. Thus, changes in the level of receptor proteins observed in our Western blot studies could contribute to the CXCL10-induced changes in synaptic network activity noted above. Immunostaining of the cultures showed that GABABR1 and NMDAR1 were expressed by most if not all of the cultured hippocampal neurons and were absent from glial cells (Fig. 3A,4A).
Figure 3.
Chronic CXCL10 decreases the effectiveness of GABAB receptor ligands on spontaneous synaptic activity in the hippocampal cultures. A. Digitized images showing GABABR1 immunostaining in control and CXCL10 treated cultures (100 nM). Hoffman images show the immunostained neurons; phase contrast images show all neurons in the field. Most if not all the cultured hippocampal neurons express GABABR1. Glial cells that form the substratum upon which the neurons grow do not express GABABR1 and are unstained. Immunostaining is blocked by the antigenic peptide used to produce the antibody. B. Effect of the GABABR1 agonist baclofen on spontaneous Ca2+ signals recorded in hippocampal neurons in low Mg2+ saline. Mean values are shown in B1 and sample recordings in B2. Baclofen reduced the Ca2+ signals in both control and CXCL10-treated cultures, consistent with an inhibition of synaptic activity. The extent of inhibition by baclofen was smaller in CXCL10-treated cultures. The data are normalized within each treatment group to baseline values prior to the addition of baclofen. Control levels equal 1 (dotted line). Ca2+ levels less than 1 indicate an inhibition by baclofen. C. Effect of the GABABR1 antagonist CGP on spontaneous Ca2+ signals in hippocampal neurons recorded in low Mg2+ saline. Mean values are shown in C1. The data are normalized within each treatment group to baseline values prior to the addition of CGP. Baseline values equal 1 (dotted line). CGP increased the Ca2+ levels consistent with an inhibition of inhibitory synaptic activity. The effects of CGP were smaller in CXCL10-treated cultures. Sample recordings are shown in C2. Data are from at least 3 culture sets (> 150 neurons measured for each condition) *significantly different from control treatment group (0 CXCL10).
Figure 4.
Chronic CXCL10 enhances NMDAR-mediated Ca2+ responses in hippocampal cultures. A. Digitized images shown NMDAR1 immunostaining in control and CXCL10 (100 nM) treated cultures. Hoffman images show the immunostained cells, which are all neurons; phase contrast images show all cells in the field. Most if not all of the cultured hippocampal neurons express NMDAR1. Glial cells that form the substratum do not express NMDAR1 and are unstained. Immunostaining is blocked by the antigenic peptide used to produce the antibody. B. Ca2+ signals evoked by brief 1 s application of NMDA (at the arrow) to cultured hippocampal neurons. Ca2+ signals were recorded in hippocampal neurons in low Mg2+ saline containing antagonists for AMPAR, GABABR and GABAAR. Mean values are shown in B1. All data are normalized to values for the control treatment group. Control levels equal 1 (dotted line). *significantly different from control treatment group (0 CXCL10). Sample recordings are shown in B2. Data are from 6 culture sets (~ 300 neurons measured for each treatment group)
To gain information on the relative contribution of GABABR1 to the synaptic network activity we determined the effect of receptor specific ligands applied by micropressure on intracellular Ca2+ levels associated with spontaneous network activity. Baclofen, a GABABR1 agonist, significantly decreased Ca2+ levels in both control and CXCL10-treated neurons (Fig. 3B,C). This effect is likely to result from the activation of a slow membrane hyperpolarization involving activation of K+ channels known to be linked to GABABR activation in hippocampal neurons (Dutar and Nicoll, 1988). The slow hyperpolarization would depress synaptic network activity and associated Ca2+ levels. In contrast CGP, a GABABR1 antagonist, significantly increased Ca2+ levels in both control and CXCL10-treated neurons (Fig. 3D,E), indicative of an increase in neuronal activity. The increased activity presumably involves CGP blockade of postsynaptic GABABRs that mediate the slow membrane hyperpolarization mediated by K+ channels. The effects of baclofen and CGP on Ca2+ levels were significantly smaller in the CXCL10-treated neurons compared with control neurons (Fig. 3B,D), a result consistent with the lower levels of GABABR1 measured by Western blot in the CXCL10-treated cultures (Fig. 1E).
To assess NMDAR-mediated function we measured the magnitude of NMDA-evoked Ca2+ responses in control and chronic CXCL10-treated hippocampal neurons. Exogenous micropressure application of NMDA (10 μM; 1 s duration) produced a Ca2+ response in both control and CXCL10-treated neurons. The Ca2+ responses produced by NMDA in control and chronic CXCL10-treated were similar in time course (duration at half max amplitude = 18.6±11 s for control, n=232 and 18.5±10 s for all CXCL10, n= 239), but the peak amplitude was significantly larger in the CXCL10-treated neurons compared with control neurons (Fig. 4), These results are consistent with the higher level of protein for NMDAR1 observed in our Western blot studies. Thus, chronic CXCL10-induced alterations in NMDAR1 levels were accompanied by corresponding changes in the Ca2+ signal evoked by NMDA.
Discussion
Chemokines are expressed within the CNS and are thought to regulate neuronal and glial functions both under physiological and pathological conditions (Adler et al., 2005; Ambrosini and Aloisi, 2004; Cartier et al., 2005). Our previous studies showed that acute exposure to the chemokine CXCL10 results in altered physiological function of cultured rat hippocampal neurons. The physiological changes induced by CXCL10 included an enhancement of spontaneous synaptic network activity, spike activity and activity dependent Ca2+ signaling (Nelson and Gruol, 2004). These changes are consistent with a neuroregulatory role for CXCL10 in the CNS. In the current study, we examined the effects of chronic exposure to CXCL10 in cultured rat hippocampal neurons to identify neuronal effects produced by prolonged exposure to CXCL10, such as occurs in a chronic neuroinflammatory condition when levels of CXCL10 are persistently elevated. Results from Western blot analysis identified several changes in the level of synaptic proteins produced by chronic CXCL10 including alterations in the level of glutamate and GABA receptors such as NMDAR1, mGluR2/3, GABABR1 and GABAAR-α2. Results from parallel Ca2+ imaging studies of Ca2+ oscillations generated by spontaneous synaptic network activity that occurs in the hippocampal cultures showed that the network activity was increased by the chronic exposure to CXCL10. Because glutamate and GABA receptors play a central role in generating the spontaneous synaptic activity, altered levels of the synaptic proteins could be an important factor in the effects of CXCL10 on synaptic network activity. CXCL10 was not present in the bath saline during the Ca2+ imaging studies to measure synaptic network activity, indicating that chronic CXCL10 exposure produced neuroadaptive changes that remained after removal of the chemokine.
The effects of chronic exposure to CXCL10 on the level of neuronal proteins observed in the current study would predict a net outcome of reduced synaptic inhibition in the hippocampal cultures. Thus, chronic CXCL10 exposure resulted in a reduction in the level of GAD65/67, the synthetic enzyme responsible for the production of the inhibitory transmitter GABA, GABABR1, a receptor that mediates a slow inhibitory postsynaptic potential, and GABAAR-α2, a receptor that mediates a fast inhibitory postsynaptic potential. All of these effects are consistent with a net reduction in inhibitory synaptic drive. In addition, chronic CXCL10 increased the excitatory influences by increasing the level of NMDAR1, the primary subunit of NMDAR, a receptor that mediates excitatory synaptic transmission in the hippocampus. These effects of chronic CXCL10 on GABA and NMDAR-mediated synaptic transmission are consistent with the enhanced synaptic network activity observed in the Ca2+ imaging studies. Chronic CXCL10 also increased the level of mGluR2/3, a glutamate receptor that provides feedback inhibition of transmitter release at excitatory synapses and mediates a slow inhibitory postsynaptic potential. Because both excitatory input to glutamatergic neurons and GABAergic neurons could be affected by the changes in mGluR2/3 levels, the net effect of this change would depend on the relative contribution of the excitatory and inhibitory synaptic drive to the network activity.
Ca2+ imaging studies also showed that the GABABR agonist baclofen reduced Ca 2+ oscillations associated with synaptic network activity, whereas the GABABR antagonist CGP increased the Ca2+ oscillations. The effects of these GABABR ligands were smaller in the chronic CXCL10-treated neurons than in the control neurons. The Ca2+ response produced by exogenous application of NMDA to the chronic CXCL10-treated neurons was larger than the Ca2+ response produced by exogenous application of NMDA to control neurons. These results are consistent with the observed changes in protein levels for GABABR and NMDAR1 in the Western blot studies of chronic CXCL10-treated and control cultures. However, measurement of Ca2+ signals is an indirect method for assessing synaptic network activity and further direct studies of synaptic transmission using electrophysiological recordings will be necessary to confirm that the changes in the level of synaptic proteins identified in our Western blot studies correlates with changes in the magnitude of synaptic responses.
The mechanisms responsible for the altered protein levels in the CXCL10-treated neurons have yet to be identified. Our previous studies showed that chronic exposure to CXCL10 increases the level of signal transduction proteins (e.g., ERK1/2) and transcription factors (pCREB, NFkB) that regulate gene expression (Bajova et al., 2008). These alterations could be important contributors to the mechanisms mediating the changes in neuronal proteins involved in synaptic network activity in the CXCL10-treated cultures. Moreover, the CXCL10-induced increases in ERK1/2 could contribute to the altered synaptic network activity by additional actions on other neuronal proteins and/or signal transduction molecules involved in network activity. Future studies will be needed to address these issues.
The effects of CXCL10 did not reflect a general alteration in protein levels, because the changes observed differed for the various proteins studied. Moreover, the levels of some proteins (e.g., levels of the GluR2/3, subunits of the AMPA receptor) were not changed by the prolonged exposure to CXCL10. Although the effects produced by chronic CXCL10 in our studies involved neuronal properties and neurons are known to express CXCR3, the receptor for CXCL10, the cellular site of CXCL10 action cannot be unequivocally determined by our studies. Both neurons and glial cells (microglia and astrocytes, the primary glial cell types in our cultures) are known to express CXCR3. However, our previous immunohistochemical studies showed that the cultured hippocampal neurons are strongly immunoreactive for CXCR3, whereas the glial cells in the hippocampal cultures were only weakly immunoreactive (Nelson and Gruol, 2004). Therefore, the neuroadaptive effects observed in our studies could have occurred through direct effects on the neuronal population. Further studies in which neurons are studied in isolation from glia will be required to identify the cell type that induces the effects of CXCL10 in neurons. Moreover, although we assume CXCL10 produced neuronal effects by acting at CXCR3, our studies did not determine the receptor involved in CXCL10 actions. Future studies are needed to establish this point.
In the current study, we tested a CXCL10 concentration range of 100–250 nM, which is likely to reflect high physiological to pathophysiological levels for CNS neurons. The ED50 for physiological actions of the CXCL10 used in our studies was reported to range from 15–75 ng/ml (~1–5 nM) in transfected immune cells (R&D systems; measured by the ability to chemoattract mouse BaF/3 cells transfected with CXCR3). However, our previous studies showed that in rat hippocampal neurons higher concentrations of CXCL10 (100 nM) were required to elicit neurophysiological actions (Nelson and Gruol, 2004), concentrations similar to those reported for biological actions of other chemokines on neurons and glia (Biber et al., 2001; Liu et al., 2003; Meucci et al., 1998; Oh et al., 2002). A number of factors can affect the ED50 of a ligand for a G protein-coupled receptor including the endogenous complement of G proteins and other cell specific factors. This situation may explain the higher concentrations of CXCL10 that are reported for chemokine actions in CNS cells. Moreover, our studies used recombinant human CXCL10, which may have different affinity towards rat cells.
Although levels of CXCL10 in CNS parenchyma are not commonly measured, elevated levels of CXCL10 in the CSF have been reported for a number of chronic conditions that involve altered CNS function including Aicardi-Goutières syndrome (Van Heteren et al., 2008), HIV infection (Cinque et al., 1998), Alzheimer’s disease (Galimberti et al., 2006), neuropsychiatric lupus (Okamoto et al., 2006) and multiple sclerosis (Sorensen et al., 2002). Our results showing that prolonged exposure to CXCL10 can alter neuronal function suggest that CXCL10 may play a role in the altered CNS function observed in these diseases, even in the absence of frank neuronal degeneration or before such neurodegeneration has commenced. The effect of chronic CXCL10 exposure on network synaptic activity (Fig. 2A) was most pronounced under conditions of low Mg2+ in the recording saline to simulate strong synaptic drive. This result suggests that the effects of chronic CXCL10 would be most evident under conditions that place higher demand on CNS neuronal circuits.
Acknowledgments
This research was supported by NIH grants MH63712 and P30 MH62261, a grant (KRF-2006-531-E00015) from Korea Research Foundation, and a grant (PF06216-00) from the Plant Diversity Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology of Korean government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler MW, Geller EB, Chen X, Rogers TJ. Viewing chemokines as a third major system of communication in the brain. Aaps J. 2005;7:E865–870. doi: 10.1208/aapsj070484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosini E, Aloisi F. Chemokines and glial cells: a complex network in the central nervous system. Neurochem Res. 2004;29:1017–1038. doi: 10.1023/b:nere.0000021246.96864.89. [DOI] [PubMed] [Google Scholar]
- Bajetto A, Bonavia R, Barbero S, Schettini G. Characterization of chemokines and their receptors in the central nervous system: physiopathological implications. J Neurochem. 2002;82:1311–1329. doi: 10.1046/j.1471-4159.2002.01091.x. [DOI] [PubMed] [Google Scholar]
- Bajova H, Nelson TE, Gruol DL. Chronic CXCL10 alters the level of activated ERK1/2 and transcriptional factors CREB and NF-kB in hippocampal neuronal cell culture. J Neuroimmunology. 2008;195:36–46. doi: 10.1016/j.jneuroim.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- Biber K, Dijkstra I, Trebst C, De Groot CJ, Ransohoff RM, Boddeke HW. Functional expression of CXCR3 in cultured mouse and human astrocytes and microglia. Neuroscience. 2002;112:487–497. doi: 10.1016/s0306-4522(02)00114-8. [DOI] [PubMed] [Google Scholar]
- Biber K, Sauter A, Brouwer N, Copray SC, Boddeke HW. Ischemia-induced neuronal expression of the microglia attracting chemokine Secondary Lymphoid-tissue Chemokine (SLC) Glia. 2001;34:121–133. doi: 10.1002/glia.1047. [DOI] [PubMed] [Google Scholar]
- Carter SL, Muller M, Manders PM, Campbell IL. Induction of the genes for Cxcl9 and Cxcl10 is dependent on IFN-gamma but shows differential cellular expression in experimental autoimmune encephalomyelitis and by astrocytes and microglia in vitro. Glia. 2007;55:1728–1739. doi: 10.1002/glia.20587. [DOI] [PubMed] [Google Scholar]
- Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev. 2005;48:16–42. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Cinque P, Bestetti A, Marenzi R, Sala S, Gisslen M, Hagberg L, Price RW. Cerebrospinal fluid interferon-gamma-inducible protein 10 (IP-10, CXCL10) in HIV-1 infection. J Neuroimmunol. 2005;168:154–163. doi: 10.1016/j.jneuroim.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Cinque P, Vago L, Mengozzi M, Torri V, Ceresa D, Vicenzi E, Transidico P, Vagani A, Sozzani S, Mantovani A, Lazzarin A, Poli G. Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. Aids. 1998;12:1327–1332. doi: 10.1097/00002030-199811000-00014. [DOI] [PubMed] [Google Scholar]
- Dutar P, Nicoll RA. A physiological role for GABAB receptors in the central nervous system. Nature. 1988;332:156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- Flynn G, Maru S, Loughlin J, Romero IA, Male D. Regulation of chemokine receptor expression in human microglia and astrocytes. J Neuroimmunol. 2003;136:84–93. doi: 10.1016/s0165-5728(03)00009-2. [DOI] [PubMed] [Google Scholar]
- Galimberti D, Schoonenboom N, Scheltens P, Fenoglio C, Bouwman F, Venturelli E, Guidi I, Blankenstein MA, Bresolin N, Scarpini E. Intrathecal chemokine synthesis in mild cognitive impairment and Alzheimer disease. Arch Neurol. 2006;63:538–543. doi: 10.1001/archneur.63.4.538. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Kennedy MB. Signal-processing machines at the postsynaptic density. Science. 2000;290:750–754. doi: 10.1126/science.290.5492.750. [DOI] [PubMed] [Google Scholar]
- Klein RS, Lin E, Zhang B, Luster AD, Tollett J, Samuel MA, Engle M, Diamond MS. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J Virol. 2005;79:11457–11466. doi: 10.1128/JVI.79.17.11457-11466.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb SA, Sporer B, Lahrtz F, Koedel U, Pfister HW, Fontana A. Identification of a T cell chemotactic factor in the cerebrospinal fluid of HIV-1-infected individuals as interferon-gamma inducible protein 10. J Neuroimmunol. 1999;93:172–181. doi: 10.1016/s0165-5728(98)00223-9. [DOI] [PubMed] [Google Scholar]
- Liu Z, Geng L, Li R, He X, Zheng JQ, Xie Z. Frequency modulation of synchronized Ca2+ spikes in cultured hippocampal networks through G-protein-coupled receptors. J Neurosci. 2003;23:4156–4163. doi: 10.1523/JNEUROSCI.23-10-04156.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto E. Molecular mechanism of neuronal plasticity: induction and maintenance of long-term potentiation in the hippocampus. J Pharmacol Sci. 2006;100:433–442. doi: 10.1254/jphs.cpj06007x. [DOI] [PubMed] [Google Scholar]
- Mudo G, Trovato-Salinaro A, Caniglia G, Cheng Q, Condorelli DF. Cellular localization of mGluR3 and mGluR5 mRNAs in normal and injured rat brain. Brain Res. 2007;1149:1–13. doi: 10.1016/j.brainres.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Nelson TE, Gruol DL. The chemokine CXCL10 modulates excitatory activity and intracellular calcium signaling in cultured hippocampal neurons. J Neuroimmunol. 2004;156:74–87. doi: 10.1016/j.jneuroim.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Oh JW, Schwiebert LM, Benveniste EN. Cytokine regulation of CC and CXC chemokine expression by human astrocytes. J Neurovirol. 1999;5:82–94. doi: 10.3109/13550289909029749. [DOI] [PubMed] [Google Scholar]
- Oh SB, Endoh T, Simen AA, Ren D, Miller RJ. Regulation of calcium currents by chemokines and their receptors. J Neuroimmunol. 2002;123:66–75. doi: 10.1016/s0165-5728(01)00485-4. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Iikuni N, Kamitsuji S, Yoshio T, Minota S, Kamatani N. IP-10/MCP-1 ratio in CSF is an useful diagnostic marker of neuropsychiatric lupus patients. Rheumatology (Oxford) 2006;45:232–234. doi: 10.1093/rheumatology/kei233. [DOI] [PubMed] [Google Scholar]
- Omari KM, John GR, Sealfon SC, Raine CS. CXC chemokine receptors on human oligodendrocytes: implications for multiple sclerosis. Brain. 2005;128:1003–1015. doi: 10.1093/brain/awh479. [DOI] [PubMed] [Google Scholar]
- Prenosil GA, Schneider Gasser EM, Rudolph U, Keist R, Fritschy JM, Vogt KE. Specific subtypes of GABAA receptors mediate phasic and tonic forms of inhibition in hippocampal pyramidal neurons. J Neurophysiol. 2006;96:846–857. doi: 10.1152/jn.01199.2005. [DOI] [PubMed] [Google Scholar]
- Przewlocki R, Parsons KL, Sweeney DD, Trotter C, Netzeband JG, Siggins GR, Gruol DL. Opioid enhancement of calcium oscillations and burst events involving NMDA receptors and L-type calcium channels in cultured hippocampal neurons. J Neurosci. 1999;19:9705–9715. doi: 10.1523/JNEUROSCI.19-22-09705.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schools GP, Kimelberg HK. mGluR3 and mGluR5 are the predominant metabotropic glutamate receptor mRNAs expressed in hippocampal astrocytes acutely isolated from young rats. J Neurosci Res. 1999;58:533–543. doi: 10.1002/(sici)1097-4547(19991115)58:4<533::aid-jnr6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen TL, Trebst C, Kivisakk P, Klaege KL, Majmudar A, Ravid R, Lassmann H, Olsen DB, Strieter RM, Ransohoff RM, Sellebjerg F. Multiple sclerosis: a study of CXCL10 and CXCR3 co-localization in the inflamed central nervous system. J Neuroimmunol. 2002;127:59–68. doi: 10.1016/s0165-5728(02)00097-8. [DOI] [PubMed] [Google Scholar]
- Tamaru Y, Nomura S, Mizuno N, Shigemoto R. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience. 2001;106:481–503. doi: 10.1016/s0306-4522(01)00305-0. [DOI] [PubMed] [Google Scholar]
- Tran PB, Banisadr G, Ren D, Chenn A, Miller RJ. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J Comp Neurol. 2007;500:1007–1033. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heteren JT, Rozenberg F, Aronica E, Troost D, Lebon P, Kuijpers TW. Astrocytes produce interferon-alpha and CXCL10, but not IL-6 or CXCL8, in aicardi-Goutieres syndrome. Glia. 2008;56:568–578. doi: 10.1002/glia.20639. [DOI] [PubMed] [Google Scholar]
- Vereyken EJ, Bajova H, Chow S, de Graan PN, Gruol DL. Chronic interleukin-6 alters the level of synaptic proteins in hippocampus in culture and in vivo. Eur J Neurosci. 2007;25:3605–3616. doi: 10.1111/j.1460-9568.2007.05615.x. [DOI] [PubMed] [Google Scholar]
- Vlkolinsky R, Siggins GR, Campbell IL, Krucker T. Acute exposure to CXC chemokine ligand 10, but not its chronic astroglial production, alters synaptic plasticity in mouse hippocampal slices. J Neuroimmunol. 2004;150:37–47. doi: 10.1016/j.jneuroim.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Wang X, Ellison JA, Siren AL, Lysko PG, Yue TL, Barone FC, Shatzman A, Feuerstein GZ. Prolonged expression of interferon-inducible protein-10 in ischemic cortex after permanent occlusion of the middle cerebral artery in rat. J Neurochem. 1998;71:1194–1204. doi: 10.1046/j.1471-4159.1998.71031194.x. [DOI] [PubMed] [Google Scholar]
- Xia MQ, Bacskai BJ, Knowles RB, Qin SX, Hyman BT. Expression of the chemokine receptor CXCR3 on neurons and the elevated expression of its ligand IP-10 in reactive astrocytes: in vitro ERK1/2 activation and role in Alzheimer’s disease. J Neuroimmunol. 2000;108:227–235. doi: 10.1016/s0165-5728(00)00285-x. [DOI] [PubMed] [Google Scholar]