Abstract
The association between the 22q11.2 deletion syndrome (22q11DS) and psychiatric disorders, particularly psychosis, suggests a causal relationship between 22q11DS genes and abnormal brain function. The genes catechol-O-methyl-transferase (COMT) and proline dehydrogenase both reside within the commonly deleted region of 22q11.2. COMT activity and proline levels may therefore be altered in 22q11DS individuals. Associations of both COMT158 genotype and elevated serum proline levels with abnormal brain function have been reported. Fifty-six 22q11DS children and 75 healthy controls were assessed on physiological measures of brain function, including prepulse inhibition (PPI) of startle, P50 auditory sensory gating and smooth pursuit eye movements (SPEM). COMT158 genotype and plasma proline levels were determined in the 22q11DS children. We hypothesized an interaction between the COMT158 genotype and proline, predicting the strongest negative effect of high proline on brain function to occur in 22q11DS children who are carriers of the COMTmet allele. Of the three physiological measures, only SPEM and PPI were abnormal in the patient sample. With regard to the SPEM performance, there was a significant interaction between the COMT158 genotype and proline level with significantly decreased SPEM performance in children with high plasma proline levels and the low activity COMTmet allele. A similar interaction effect was not observed with regard to PPI. These findings are consistent with a model in which elevated proline negatively affects brain function by an increase in dopamine in the prefrontal cortex. 22q11DS patients with low dopamine catabolic capacity are therefore especially vulnerable to this functional disruption.
Keywords: 22q11.2DS, endophenotype, proline, catechol-O-methyl transferase, smooth pursuit, prepulse inhibition
INTRODUCTION
The 22q11.2 deletion syndrome (22q11DS) is a congenital multisystem disorder caused by deletion of a small region on the long arm of chromosome 22 (Edelmann et al, 1999; Shaikh et al, 2000). Individuals with 22q11DS have specific cognitive deficits and are at increased risk for a variety of psychiatric illnesses. Among children and adolescents, attention deficit hyperactivity disorder, obsessive compulsive disorder, mood disorders, and autism spectrum disorders have all been reported (Baker and Skuse, 2005; Fine et al, 2005; Gothelf et al, 2004; Niklasson et al, 2001; Antshel et al, 2006; Vorstman et al, 2006). In adults, there is an increased prevalence of schizophrenia (Murphy et al, 1999).
In schizophrenia patients without concurrent 22q11DS, physiological measures considered to reflect genetic vulnerability are often abnormal. Among these endophenotypes, prepulse inhibition (PPI) of startle, P50 auditory sensory gating (P50), and smooth pursuit eye movements (SPEM) are among the most commonly studied (Turetsky et al, 2007). PPI refers to the reduction in magnitude of the startle response when a startle-inducing stimulus is preceded by a weaker subthreshold stimulus. P50 gating refers to a similar reduction of the P50 auditory evoked potential response to the second of two auditory stimuli presented in rapid succession. SPEM refers to the ability to smoothly follow and maintain a moving target in the central foveal area of the retina without using additional saccadic movements to recapture central fixation. Of these three measures, only PPI has been examined in 22q11DS subjects. Consistent with the increased occurrence of psychotic symptoms in the 22q11DS population, the 22q11DS subjects exhibited PPI deficits similar to those seen in schizophrenia (Sobin et al, 2005).
Given the co-occurrence of a cytogenetic deletion and an increased risk of psychopathology in 22q11DS, a causal relationship between genes in the deleted region of chromosome 22 and abnormal brain function is likely. This paper examines the effects of two specific genes located at 22q11.2, catechol-O-methyl-transferase (COMT) and proline dehydrogenase (PRODH). COMT is an extracellular enzyme involved in the breakdown of catecholamines. A common functional polymorphism of COMT at amino-acid position 158 (COMTMet) is associated with a significant decrease in enzyme activity relative to the other allelic form (COMTval; Chen et al, 2004; Lachman et al, 1996). In 22q11DS, a critical effect of this polymorphism can be expected because only one copy of the COMT gene is present. Several studies have now reported significant associations between cognition and the COMT158 genotype in 22q11DS (Shashi et al, 2006; Kates et al, 2006; Bearden et al, 2004; Gothelf et al, 2005; Baker et al, 2005), although not consistently (Glaser et al, 2006). PRODH catalyzes the first step in the degradation of the amino-acid proline. Increased plasma proline levels have been reported in 22q11DS patients (Goodman et al, 2000), caused by haploinsufficiency and/or functional mutations within the remaining PRODH allele or its promoters (Bender et al, 2005). Evidence supporting proline’s role in brain function include its modulation of glutamatergic neurotransmission in the murine hippocampus in vitro (Cohen and Nadler, 1997a, b) and the presence of high affinity proline transporter molecules in a subset of glutamatergic neurons in the rat brain (Fremeau Jr et al, 1992). Direct evidence that PRODH dysfunction increases proline and leads to altered brain function was provided by a study on PRODH knockout mice, who displayed both elevated levels of proline and reduced PPI (Gogos et al, 1999).
There is evidence to support a functional association between COMT activity and proline. Interference with glutamatergic neurotransmission through antagonism of the NMDA receptor induces dopamine (DA) release in the prefrontal cortex (PFC; Moghaddam, 2002). Similarly, the putative modulatory effect of proline on glutamatergic transmission could induce DA release in the PFC. The efficiency with which this increased DA can be catabolyzed is dependent on COMT activity, which in turn can be predicted by the COMT158 genotype. Evidence supporting this mechanism comes from a recent study in which brain function was found to be most profoundly disrupted in mice having both increased levels of proline and decreased COMT activity (Paterlini et al, 2005). Given this, we hypothesized a negative effect of elevated proline levels on brain function in children with 22q11DS, with the strongest effect to be expected in the subgroup of children with the low activity COMTmet allele. To test this hypothesis, we measured PPI, P50, and SPEM performance in children with 22q11DS and in typically developing children. 22q11DS subjects were genotyped for the COMT158 allele and assessed for plasma proline levels.
PATIENTS AND METHODS
Recruitment
Children with 22q11DS (n = 56) were recruited from the child psychiatry clinic at the University Medical Center Utrecht, the Netherlands. Control subjects (n = 75) were recruited from high schools in and around Utrecht. Inclusion criteria for the control group included age between 12 and 18 years, no known history of closed head injury, neurological illness or endocrine dysfunction, no drug or alcohol abuse over the preceding 12 months, and no use of psychoactive medications. Absence of psychiatric illness was assessed using the Mini International Neuropsychiatric Interview (Sheehan et al, 2007; van Vliet et al, 2000). This study was approved by Dutch Central Committee on Research Involving Human Subjects (CCMO) and written informed consent was obtained from participants and their parents or guardians.
Cognitive Assessment
A detailed account of the assessment protocol used for the 22q11DS sample has been published previously (Vorstman et al, 2006). Intelligence level (FSIQ) was assessed using the Dutch version of the Wechsler Intelligence Scales: WISC-III (Wechsler et al, 2002). In three cases the WISC-R and in three cases the adult scale (WAIS-III) was used instead. In one case data from a Wechsler assessment for preschool children, assessed at an earlier age, (WPSSI-R) were available. In the control group, intelligence level was assessed using the WISC-III in children younger than 16 years and the WAIS-III in children 16 years of age or older.
Proline Measurement
Plasma proline levels were assessed by automated ion exchange chromatography with post-column ninhydrin derivatization. Plasma amino-acid analyses were performed on a JEOL AminoTac (JEOL AminoTac JLC-500/V, Tokyo, Japan) following AM blood draw. Overnight fast was confirmed in 25 children; in 27 children overnight fasting status was uncertain. One outlier (proline of 580 μM) was identified in the confirmed fasting group, but not removed because abnormally high proline levels can be seen in 22q11DS. Mean proline levels did not differ between the uncertain fasting (278±70 μM) and confirmed fasting (280±110 μM) groups (p = 0.94). Exclusion of the outlier did not alter these results.
COMT158 Genotyping
COMT genotyping was carried out using allele-specific TaqMan probes. Genomic DNA (20 ng) were mixed with the assays and TaqMan® mastermix (Applied Biosystems, Foster City, CA) in a final volume of 5 μl. Four replicates were used for each sample. Samples were treated with the following profile: 95°C for 10 min pretreatment to activate the Taq Gold and then 40 cycles of 95°C for 15 s and 60°C for 1 min. Data were collected during amplification using the Sequence Detection System software (version 2.2) and a postread run was performed for allelic discrimination.
P50 Data Acquisition and Processing
EEG activity was recorded from 12 tin electrodes (Electro-Cap International, Eaton, OH) located at 10–20 system scalp sites referenced to left mastoid, using Psylab hardware and software (Contact Precision Instruments, London, UK). Horizontal electrooculography (EOG) was recorded from electrodes adjacent to the outer canthus of each eye. Vertical EOG was measured from left infra and supraorbital electrodes. Impedances: below <5kΩ, bandpass filter settings: 0.01–100 Hz, digital sampling rate: 500 Hz.
Auditory clicks (86 dB 1.5 ms duration white noise) were presented binaurally through stereo insert earphones (Eartone ABR). Software settings were calibrated by an artificial ear (Brüel and Kjær, type 4152) to ensure adequate stimulus intensities. Before the actual experiment, two click pairs were presented to the subjects, who were instructed to count the click pairs. A block of 36 click pairs, with an interstimulus interval of 500 ms and an intertrial interval of 10 s, was then presented, after which the subjects reported the number of stimulus pairs.
Continuous EEG data were digitally filtered with a 10–100 Hz zero phase-shift Butterworth filter (24 dB/octave). An automated artifact rejection algorithm excluded EEG intervals with voltages beyond the range of ±75 μV. The data were then segmented into artifact-free intervals beginning 50 ms before each click and ending 125 ms poststimulus and single-trial segments were averaged. P50 amplitude was measured as the maximum voltage response at the vertex (Cz), between 50 and 80 ms poststimulus, and P50 gating was computed as the click2/click1 ratio.
PPI Data Acquisition and Processing
The electromyographic (EMG) activity of the right orbicularis oculi muscle was recorded from bipolar electrodes, one placed over the medial aspect of the muscle and one displaced 0.5 cm laterally. The EMG was recorded with analog filter settings of 30–200 Hz. Digital sampling rate was 500Hz.
The prepulse and startle stimuli were bursts of white noise (duration 22.5 and 32.5ms, intensity 87 and 106.5 dB, respectively, rise/fall 0.1ms), with a fixed interstimulus interval of 120 ms. The stimuli were presented binaurally through stereo insert earphones (Eartone ABR) without any background noise. Before testing, four startle stimuli of rising intensity were presented, with two preceded by a prepulse, to accustom the subjects to the loud noise. The actual experiment consisted of 24 randomized trials: 12 startle stimuli preceded by a prepulse and 12 startle stimuli with no prepulse. The intertrial interval varied between 12 and 23 s.
The continuously recorded EMG activity was bandpass filtered between 1 and 1000Hz (24 dB/octave slope), using a zero phase-shift Butterworth filter. Data were segmented into intervals beginning 50 ms before each startle stimulus and ending 250 poststimulus. Voltage polarity was rectified to yield all positive amplitudes. Individual startle segments were visually inspected for excessive electrical noise and/or voluntary eye blinks. Acceptable segments were averaged separately for the ‘startle alone’ and ‘startle with prepulse’ conditions. Startle magnitude was defined as the most positive peak occurring 20–100 ms after stimulus presentation. PPI of startle was computed as 1 minus the ‘startle with prepulse’/‘startle alone’ ratio (expressed as a percentage). Individual PPI results exceeding 100% were relabeled as ‘100%’, negative PPI results were relabeled as ‘0%’.
SPEM Data Acquisition and Processing
Stimuli were displayed on a 21-inch computer screen (42×32 cm) positioned 1m in front of the subject. Display resolution was 800×600 pixels. Eye movements were recorded using EOG with Psylab hardware and software (Contact Precision Instruments). Tin electrodes were placed above and below the left eye and adjacent to the outer canthi of both eyes, with a ground electrode at AFz. EOG recordings were filtered online with a bandpass of 0.1–100 Hz and digitally sampled at 250 Hz.
The visual target was a white dot of 1 pixel on the 21 inch monitor, which was clearly visible against a black background. This dot moved horizontally in a harmonic (sinusoidal) motion described by X(t) = A sin(2πft) (A = 300 pixels). The subject was seated at 100 cm from the monitor, so that the amplitude A of 300 pixels (spanning nearly 18 cm) was 10° of visual angle. The eyes moved from full left to full right position over a total arc of 20°. Seven trials were presented, each consisting of 10 s of sinusoidal motion. Each trial began with an additional 2 s, during which the dot began to move slowly in the middle of the screen and speeded up to the desired speed for the trial. The average trial speeds were, respectively: 8, 13, 16, 20, 24, 29, and 35 degrees per second. For training purposes, subjects were shown two trials with the slowest velocities and asked to follow the dot carefully.
There was a large amount of data dropout in the 22q11DS sample for the three fastest target frequencies (28.7% of data). Many 22q11DS children appeared to have trouble following the target at these higher speed levels. In contrast, missing data for the three fastest frequencies in the control sample was only 4.5%. At the four slower target frequencies (8, 13, 16, and 20 degrees per second) only 5.6% of 22q11DS data were missing. Therefore, only the data from these 4 frequencies were included in the analysis. The proportion of smooth pursuit movements relative to the occurrence of saccadic movements, termed ‘velocity gain’, was computed for each target frequency and averaged across the four frequencies (see Supplementary Figure 1).
Statistical Analysis
All statistical analyses were conducted with SPSS version 11.5 statistical analysis software. The three dependent measures were the auditory P50 gating ratio, the percent PPI of startle, and the mean SPEM velocity gain. To obtain optimal matching for age and gender distribution with the 22q11DS group, matched subgroups were composed out of the original n = 75 controls. Normal distributions of data in the different subgroups were assessed with Kolmogorov–Smirnov tests (significance level 0.05). For each dependent measure, outliers were detected and removed using the boxplot function (see online Supplementary Figure 2). This resulted in the exclusion of two subjects for P50 (one case, one control) and two subjects for SPEM (both controls). For six cases and one control the PPI results were outside the 0–100% range, and therefore relabeled to either 0 or 100%. Comparison of demographic characteristics between the 22q11DS subjects and controls was performed using Student’s t-test for FSIQ and age, and the χ2-test for gender distribution (p<0.05, two-tailed). Plasma proline levels in the 22q11DS sample were compared to published normative values using a one sample t-test. For the analyses of the effect of proline on the outcome measures, the 22q11DS sample was divided in two subgroups: ‘high plasma proline’ (levels above the group median value of 262 μM) and ‘low plasma proline’ (below 262 μM).
Analysis of the three physiological measures proceeded in three stages. Stage 1 examined differences between 22q11DS subjects and controls using analysis of variance. Stage 2 examined the effects of proline and the COMT158 allele, independently, on those physiological measures that were significantly altered in the 22q11DS group. Stage 3 addressed the central hypothesis of this study, that the effect of proline would be evident principally in the COMTmet subgroup, by examining COMT158 genotype×plasma proline level interaction on the outcome measure. As it is difficult to exclude a relevant effect of age, gender, and IQ on the physiological measures, these variables were included as covariates in the analysis. Before these tests, normality of the data distribution in all subgroups was ascertained, and mean age and gender distributions of the COMT158 allele and high/low proline subgroups were compared.
Several allelic variants of both PRODH (Bender et al, 2005) and COMT (Oosterhuis et al, 2008) have been reported. However, given the modest size of this sample and the loss of statistical power associated with multiple comparisons, we limited the analysis to the biological effect of PRODH variation (ie variation in plasma proline level) and the COMT158 polymorphism, the allelic variant most clearly associated with altered COMT enzyme activity.
Given the previously reported effect of plasma proline levels and the COMT158 genotype on FSIQ (Shashi et al, 2006; Gothelf et al, 2005; Raux et al, 2006), we examined these associations post hoc in the current sample utilizing the Student’s t-test and ANCOVA.
RESULTS
Demographic Characteristics
Table 1 displays the sample characteristics of the 22q11DS and typically developing children with available data for each dependent measure. Between the 22q11DS group and controls mean FSIQ was significantly different in all subsamples (P50, PPI, and SPEM), whereas mean age and gender distribution were not significantly different.
Table 1.
Demographics of the 22q11DS and Control Samples
| 22q11DS |
Normal controls |
Statisticsa |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| nb | Mean age±SD | Mean FSIQ±SD | M: F | nb | Mean age±SD | Mean FSIQ±SD | M: F | Agec | IQc | M:F | |
| P50 | 49 | 13 yr, 8 m±2 yr, 7 m | 66±14 | 0.58 | 59 | 14 yr,1 m±2 yr, 5 m | 108±15 | 0.74 | p = 0.40 | p<0.01 | p = 0.55 |
| PPI | 40 | 14 yr, 1 m±2 yr, 6 m | 66±13 | 0.54 | 33 | 14 yr, 6 m±1 yr, 1 m | 106±13 | 0.57 | p = 0.39 | p<0.01 | p = 0.90 |
| SPEM | 56 | 13 yr, 7 m±2 yr, 6 m | 65±14 | 0.56 | 54 | 13 yr, 10m±2 yr, 11 m | 111±15 | 0.74 | p = 0.68 | p<0.01 | p = 0.46 |
SD; standard deviation, yr; year, m; month, M: F; male to female ratio.
Comparison of means cases vs controls. For age and FSIQ: Student’s t-test, for M: F ratio: χ2-test.
Sample size after removal of outliers.
Age and IQ normally distributed in all subgroups of 22q11DS and controls (p>0.05, Kolmogorov–Smirnov test).
In the 22q11DS sample (proline data available from n = 52 subjects), mean plasma proline levels were significantly higher than normal population values (p<0.01, see online Supplementary Figure 3).
Psychophysiological Measures
Data from P50 gating, PPI of startle, and SPEM were normally distributed in all subgroups. SPEM and PPI were significantly different (decreased) in 22q11DS compared to controls (see Table 2). Inclusion of outliers did not alter these findings. The SPEM results were not changed when velocity gain data from all target speed velocities were included. Group differences remained significant when the analyses were repeated without FSIQ as a covariate.
Table 2.
Comparison of Psychophysiology Results between 22q11DS and Controls
| P50a |
PPI |
SPEMa,b |
||||
|---|---|---|---|---|---|---|
| F-statistic | P | F-statistic | P | F-statistic | P | |
| 22q11DS vs controls | 0.257 | 0.613 | 8.785 | 0.004 | 7.259 | 0.008 |
| FSIQc | 0.719 | 0.398 | 0.002 | 0.968 | 0.595 | 0.442 |
Results of the univariate analysis of variance with the brain function measure (respectively, P50, SPEM, and PPI) as dependent variable and FSIQ as covariate.
Results of SPEM and P50 were not essentially changed when including outliers.
Results of SPEM were not essentially changed when using data from all target speed velocities.
Results of statistical comparisons without FSIQ as a covariate (Student’s t-test): P50: p = 0.033, PPI: p<0.001, SPEM: p<0.001.
Effects of Proline Level and COMT158 Genotype on Identified Brain Measures
Based upon these findings we proceeded to analyze the effects of proline and COMT158 genotype on SPEM and PPI, the measures that differentiated the 22q11DS group from controls. For both SPEM and PPI, there was no main effect of COMT158 allele status or proline level on any of the demographic variables (age, FSIQ, gender).
There was a trend towards a significant PPI difference between the COMTmet (mean PPI±SD: 40.1±32.5) and the COMTval subgroups (59.4±26.4, p = 0.065), but no difference was found between the high/low proline subgroups (p = 0.567). There was no difference in SPEM performance between the COMT158 subgroups (p = 0.421), but a difference was found between those with high proline (0.284±0.130) and those with low proline (0.361±0.164, p = 0.050). However, this finding did not remain significant after applying Bonferroni correction for the number of hypotheses tested.
Analysis revealed a significant effect of COMT158 allele status×proline level on SPEM, with covariation for age, FSIQ, and gender (F = 13.825, p = 0.003, see Table 3). This effect remained significant after applying Bonferroni correction (p = 0.036). A high proline level was associated with a significantly decreased SPEM performance only in the COMTmet group (p = 0.028) but not in the COMTval group (p = 0.827, see Figure 1). There was no interactive effect of COMT158 allele status×proline level for PPI; in both COMT158 allele subgroups the mean PPI performance was not significantly different between those with high proline levels and those with low proline levels (COMTmet: p = 0.748, COMTval: p = 0.296). Scatter plots of individual PPI and SPEM values are available online (supplemental figures 4 and 5)
Table 3.
Univariate Analysis of Covariance on SPEM
| Variable | F | Sig. |
|---|---|---|
| COMT158 allele status | 0.040 | 0.874 |
| Proline high/low subgroup | 6.445 | 0.492 |
| FSIQ | 0.000 | 0.996 |
| Age | 0.092 | 0.764 |
| Gender | 0.649 | 0.524 |
| COMT158×proline | 13.825 | 0.003 |
Univariate analysis of covariance with SPEM as the dependent measure, COMT158 allele status and high/low proline groups as fixed factors and FSIQ, age and gender as covariates.
Figure 1.
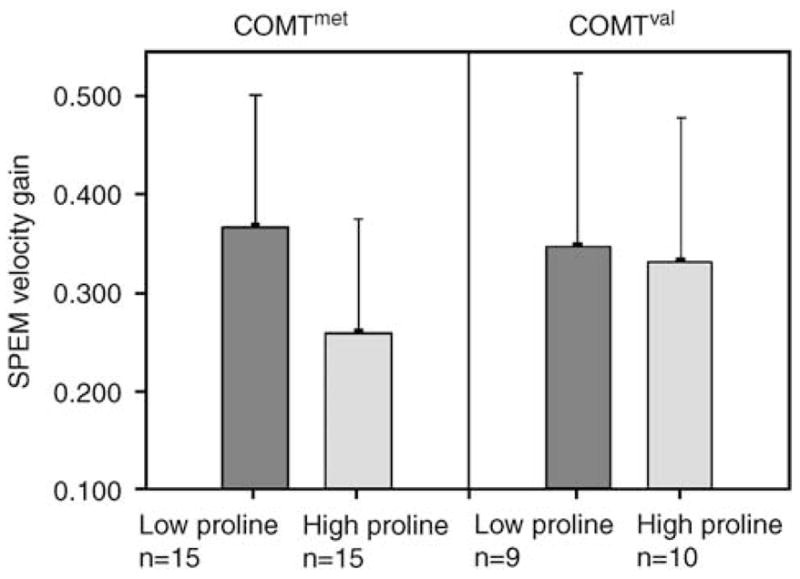
The association between proline and SPEM is moderated by the COMT158 genotype. Only in the COMTmet subgroup a decreased SPEM performance was associated with high plasma proline levels in 22q11DS individuals (p = 0.028), whereas in the COMTval group no significant difference was revealed between the high/low proline subgroups (p = 0.827). Proline levels were not available in seven subjects; therefore the sample size in this analysis is 49.
Post Hoc Analyses
Mean FSIQ was not significantly different between carriers of the COMTmet (64±14) and the COMTval allele (66±14, p = 0.575). Similarly, mean FSIQ was equal in those with low proline (64±13) and those with high proline levels (65±14, p = 0.817). Further analyses did not reveal an interactive effect of COMT158 allele status×proline level on FSIQ.
DISCUSSION
In this study we report decreased SPEM velocity gain and PPI of startle in children with 22q11DS compared to typically developing children of the same age. Decreased SPEM in 22q11DS is a novel finding because to date no studies have reported on SPEM performance in 22q11DS children. This study replicates the previously reported finding of decreased PPI in 22q11DS subjects (Sobin et al, 2005).
We further demonstrate a significant interactive effect of COMT158 allele status×proline level on SPEM; proline negatively affects SPEM in 22q11DS subjects who are hemizygous for the COMTmet allele. With regard to PPI such interactive effect of proline and COMT158 was not found; however a trend towards a main effect of the COMT158 genotype was demonstrated; individuals with the COMTmet allele showed decreased PPI performance.
Individuals with the 22q11DS carry one, instead of two copies of the genes that reside in the deleted region. This abnormal situation may have two consequences: first, for some genes one copy may be insufficient to generate adequate amounts of gene product. Second, any functional variant in the remaining allele of these affected genes can be expected to have a critical effect, as there is no compensating normal allele. Thus, in 22q11DS, the enzyme COMT may be generated in lesser amounts and in addition, the common functional variant COMT158 has a dominant impact on the enzyme’s effectiveness.
Consistent with both the central hypothesis of this study and previous findings in mice (Paterlini et al, 2005), we found that the effect of proline on SPEM was contingent upon the COMT158 genotype. These findings are also partly consistent with recently reported results (Raux et al, 2006), although the main outcome measure in that study was psychiatric diagnosis rather than SPEM. Findings of both studies indicate a negative effect of high proline on brain function in 22q11DS subjects with the COMTmet genotype. The consistency of these results is not surprising given the reported association between SPEM abnormalities and psychosis in numerous studies (Thaker, 2008). We did not analyze psychosis as a phenotypic outcome because the young age of the study sample excluded a reliable partition of subjects in this respect. Raux et al, also reported that increased proline levels were significantly associated with lower FSIQ, independent of the COMT158 genotype. Our post hoc analyses did not replicate this correlation.
Although P50 and PPI are both thought to reflect the brain’s capacity to filter information, they tend to be uncorrelated within individuals and, therefore, are likely to be mediated by different neurobiological mechanisms (Schwarzkopf et al, 1993; Braff et al, 2007). For PPI, a significant regulatory influence of the striatum is suggested by the findings of impaired PPI in Huntington’s disease patients (Swerdlow et al, 1995) and in animals with striatal lesions (Kodsi and Swerdlow, 1997). In contrast, several studies indicate a more critical (though not exclusive) role for the PFC (Grunwald et al, 2003; Knight et al, 1999; Nagel et al, 2008; Kurthen et al, 2007) and the hippocampus (Grunwald et al, 2003; Tanabe et al, 2006; Tregellas et al, 2004) in the regulation of P50 gating and SPEM. Although the anatomical loci of control for P50 gating and SPEM may overlap, the regulatory neurotransmitter systems are thought to be different. For P50, there are strong indications that cholinergic transmission is an essential part of its regulation (Adler et al, 1992, 1993). In SPEM, a regulatory role for DA is strongly suggested by the fact that the COMT158 allele affects SPEM performance in both healthy subjects and schizophrenia patients (Thaker et al, 2004). Similarly, DA signaling is likely involved in the regulation of PPI, as the administration of DA agonists attenuates PPI (Hutchison and Swift, 1999; Abduljawad et al, 1998) and a common functional variant of the DA D3 receptor significantly affects PPI in humans (Roussos et al, 2008).
Given the putative roles of the hippocampus and PFC in regulating SPEM, it is notable that evidence now supports an influence of proline on excitatory neurotransmission in these regions. A high affinity proline transporter has been identified on a subset of glutamatergic neurons (Crump et al, 1999; Renick et al, 1999; Fremeau Jr et al, 1992) and proline-mediated modulation of glutamatergic neuron terminals has been demonstrated (Cohen and Nadler, 1997a, b; Martin et al, 1992). Regions with the highest levels of proline transporter expression include hippocampal (Schaffer collateral commissural and lateral perforant pathway) and corticostriatal pathways (Renick et al, 1999). Importantly, in a PRODH knockdown study, it has been shown that PRODH deficiency not only alters hippocampal glutamatergic transmission, but also significantly potentiates DA release (Paterlini et al, 2005).
In summary, the hypothesized model holds that the action of proline on specific glutamatergic neurons in the hippocampus induces two events: interference with glutamate transmission and secondary potentiation of the DA response in the PFC (Figure 2). Under this model, one can anticipate that the effect of proline will be accentuated in individuals with the low activity COMT enzyme, given their decreased capacity to effectively catabolize the augmented DA response in the forebrain. Our finding that proline significantly affects SPEM in the low activity COMTmet allele, but not in the high activity COMTval allele subgroup is both consistent with and supportive of this model.
Figure 2.
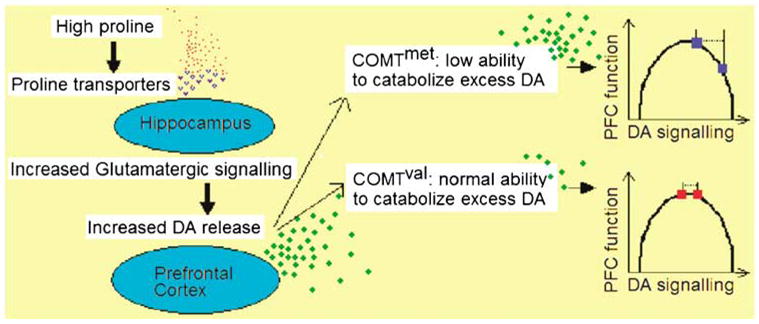
Schematic representation of the hypothesized model. High proline levels induce glutamatergic signaling in the hippocampus. Increased glutamatergic tone causes a release of DA in the PFC. In 22q11DS subjects hemizygous for COMTmet (in blue), the inefficiency in catabolizing DA leads to a large shift to the right (dotted line). This, in combination with a starting position somewhat right of the curve’s optimum, leads to a decrease in PFC function. In those hemizygous for COMTval (in red), excess DA can be more adequately catabolized; the resulting shift, if any, on the hypothetical model of the inverted U-shaped curve is more moderate and does not result in a substantial change in PFC function (inverted U-shape curve adapted from Mattay et al, 2003).
The effect reported in this study accords with the hypothetical model of an inverted U-shape relationship between DA signaling and prefrontal function (Mattay et al, 2003; Figure 2). In this model, optimum prefrontal function occurs within a restricted range of DA signaling, with decreased function in conditions of too high or too low DA availability.
Paterlini et al (2005), reported that PRODH deficient mice with elevated proline levels demonstrated significantly increased expression levels of COMT in the frontal cortex. Similarly, high proline levels may have led to an upregulation of COMT expression in the 22q11DS subjects of this study. However, given the haploidy of COMT in individuals with 22q11DS, the capacity for upregulation may be diminished, thereby restricting the effect of this compensatory mechanism. In addition, in those with the low activity COMTmet allele, a further reduction of the net effect of this compensatory mechanism can be expected.
Finally, the attenuating effect of the COMTmet allele, but not of proline, on PPI in this study is likely the result of haploinsufficiency, with the low activity COMT allele increasing DA availability in the striatum. Apparently, this effect occurs despite COMT expression being lower in the striatum than the PFC (Matsumoto et al, 2003). The absence of any influence of proline on PPI is consistent with the fact that proline appears to potentiate DA transmission in the murine cortex, but not in the striatum (Paterlini et al, 2005).
A limitation of the current study is the absence of plasma proline values from age-matched controls. This makes it difficult to delineate, which 22q11DS subjects have elevated plasma proline levels and to draw firm conclusions regarding the effects of increased proline. If all 22q11DS subjects have abnormally elevated proline levels, then an effect of proline on either of the physiological measures cannot be entirely ruled out. However, it is unlikely that this is the case for two reasons: (1) Abnormally high levels are not reported in all, but in approximately 50% of 22q11DS subjects (Goodman et al, 2000). (2) The variance of proline values in 22q11DS cases and controls shows considerable overlap (see Supplementary Figure 3), suggesting that many 22q11DS children in this study have plasma proline levels within the normal range.
Another limitation is that it is unknown to what extent changes in peripheral plasma concentrations of proline correlate with similar changes in proline concentration in the brain. This is an issue that needs to be addressed in future studies.
Findings of this study may contribute to our understanding of the pathophysiological mechanisms that lead to the increased vulnerability for psychosis in 22q11DS subjects. This finding not only is relevant to our understanding of 22q11DS-related psychopathology, but also contributes to our understanding of how factors such as proline influence DA metabolism and transmission in the brain.
Supplementary Material
Acknowledgments
JAS Vorstman M.D., Ph. D. was supported by a 2006 NARSAD Young Investigator Award, funded by Stephen and Constance Lieber.
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
None.
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
References
- Abduljawad KA, Langley RW, Bradshaw CM, Szabadi E. Effects of bromocriptine and haloperidol on prepulse inhibition of the acoustic startle response in man. J Psychopharmacol. 1998;12:239–245. doi: 10.1177/026988119801200302. [DOI] [PubMed] [Google Scholar]
- Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry. 1993;150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- Adler LE, Hoffer LJ, Griffith J, Waldo MC, Freedman R. Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biol Psychiatry. 1992;32:607–616. doi: 10.1016/0006-3223(92)90073-9. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Fremont W, Roizen NJ, Shprintzen R, Higgins AM, Dhamoon A, et al. ADHD, major depressive disorder, and simple phobias are prevalent psychiatric conditions in youth with velocardiofacial syndrome. J Am Acad Child Adolesc Psychiatry. 2006;45:596–603. doi: 10.1097/01.chi.0000205703.25453.5a. [DOI] [PubMed] [Google Scholar]
- Baker K, Baldeweg T, Sivagnanasundaram S, Scambler P, Skuse D. COMT Val108/158 Met modifies mismatch negativity and cognitive function in 22q11 deletion syndrome. Biol Psychiatry. 2005;58:23–31. doi: 10.1016/j.biopsych.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Baker KD, Skuse DH. Adolescents and young adults with 22q11 deletion syndrome: psychopathology in an at-risk group. Br J Psychiatry. 2005;186:115–120. doi: 10.1192/bjp.186.2.115. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Jawad AF, Lynch DR, Sokol S, Kanes SJ, McDonald-McGinn DM, et al. Effects of a functional COMT polymorphism on prefrontal cognitive function in patients with 22q11.2 deletion syndrome. Am J Psychiatry. 2004;161:1700–1702. doi: 10.1176/appi.ajp.161.9.1700. [DOI] [PubMed] [Google Scholar]
- Bender HU, Almashanu S, Steel G, Hu CA, Lin WW, Willis A, et al. Functional consequences of PRODH missense mutations. Am J Hum Genet. 2005;76:409–420. doi: 10.1086/428142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Light GA, Swerdlow NR. Prepulse inhibition and P50 suppression are both deficient but not correlated in schizophrenia patients. Biol Psychiatry. 2007;61:1204–1207. doi: 10.1016/j.biopsych.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SM, Nadler JV. Proline-induced potentiation of glutamate transmission. Brain Res. 1997a;761:271–282. doi: 10.1016/s0006-8993(97)00352-1. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Nadler JV. Proline-induced inhibition of glutamate release in hippocampal area CA1. Brain Res. 1997b;769:333–339. doi: 10.1016/s0006-8993(97)00721-x. [DOI] [PubMed] [Google Scholar]
- Crump FT, Fremeau RT, Craig AM. Localization of the brain-specific high-affinity L-proline transporter in cultured hippocampal neurons: molecular heterogeneity of synaptic terminals. Mol Cell Neurosci. 1999;13:25–39. doi: 10.1006/mcne.1998.0727. [DOI] [PubMed] [Google Scholar]
- Edelmann L, Pandita RK, Spiteri E, Funke B, Goldberg R, Palanisamy N, et al. A common molecular basis for rearrangement disorders on chromosome 22q11. Hum Mol Genet. 1999;8:1157–1167. doi: 10.1093/hmg/8.7.1157. [DOI] [PubMed] [Google Scholar]
- Fine SE, Weissman A, Gerdes M, Pinto-Martin J, Zackai EH, McDonald-McGinn DM, et al. Autism spectrum disorders and symptoms in children with molecularly confirmed 22q11.2 deletion syndrome. J Autism Dev Disord. 2005;35:461–470. doi: 10.1007/s10803-005-5036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Caron MG, Blakely RD. Molecular cloning and expression of a high affinity L-proline transporter expressed in putative glutamatergic pathways of rat brain. Neuron. 1992;8:915–926. doi: 10.1016/0896-6273(92)90206-s. [DOI] [PubMed] [Google Scholar]
- Glaser B, Debbane M, Hinard C, Morris MA, Dahoun SP, Antonarakis SE, et al. No evidence for an effect of COMT Val158Met genotype on executive function in patients with 22q11 deletion syndrome. Am J Psychiatry. 2006;163:537–539. doi: 10.1176/appi.ajp.163.3.537. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Santha M, Takacs Z, Beck KD, Luine V, Lucas LR, et al. The gene encoding proline dehydrogenase modulates sensorimotor gating in mice. Nat Genet. 1999;21:434–439. doi: 10.1038/7777. [DOI] [PubMed] [Google Scholar]
- Goodman BK, Rutberg J, Lin WW, Pulver AE, Thomas GH. Hyperprolinaemia in patients with deletion (22)(q11.2) syndrome. J Inherit Metab Dis. 2000;23:847–848. doi: 10.1023/a:1026773005303. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Eliez S, Thompson T, Hinard C, Penniman L, Feinstein C, et al. COMT genotype predicts longitudinal cognitive decline and psychosis in 22q11.2 deletion syndrome. Nat Neurosci. 2005;8:1500–1502. doi: 10.1038/nn1572. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Presburger G, Zohar AH, Burg M, Nahmani A, Frydman M, et al. Obsessive-compulsive disorder in patients with velocardiofacial (22q11 deletion) syndrome. Am J Med Genet. 2004;126B:99–105. doi: 10.1002/ajmg.b.20124. [DOI] [PubMed] [Google Scholar]
- Grunwald T, Boutros NN, Pezer N, von Oertzen J, Fernandez G, Schaller C, et al. Neuronal substrates of sensory gating within the human brain. Biol Psychiatry. 2003;53:511–519. doi: 10.1016/s0006-3223(02)01673-6. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Swift R. Effect of D-amphetamine on prepulse inhibition of the startle reflex in humans. Psychopharmacology (Berl) 1999;143:394–400. doi: 10.1007/s002130050964. [DOI] [PubMed] [Google Scholar]
- Kates WR, Antshel KM, Abdulsabur N, Colgan D, Funke B, Fremont W, et al. A gender-moderated effect of a functional COMT polymorphism on prefrontal brain morphology and function in velo-cardio-facial syndrome (22q11.2 deletion syndrome) Am J Med Genet B Neuropsychiatr Genet. 2006;141:274–280. doi: 10.1002/ajmg.b.30284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight RT, Staines WR, Swick D, Chao LL. Prefrontal cortex regulates inhibition and excitation in distributed neural networks. Acta Psychol (Amst) 1999;101:159–178. doi: 10.1016/s0001-6918(99)00004-9. [DOI] [PubMed] [Google Scholar]
- Kodsi MH, Swerdlow NR. Mitochondrial toxin 3-nitropropionic acid produces startle reflex abnormalities and striatal damage in rats that model some features of Huntington’s disease. Neurosci Lett. 1997;231:103–107. doi: 10.1016/s0304-3940(97)00482-5. [DOI] [PubMed] [Google Scholar]
- Kurthen M, Trautner P, Rosburg T, Grunwald T, Dietl T, Kuhn KU, et al. Towards a functional topography of sensory gating areas: invasive P50 recording and electrical stimulation mapping in epilepsy surgery candidates. Psychiatry Res. 2007;155:121–133. doi: 10.1016/j.pscychresns.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Martin D, Ault B, Nadler JV. NMDA receptor-mediated depolarizing action of proline on CA1 pyramidal cells. Eur J Pharmacol. 1992;219:59–66. doi: 10.1016/0014-2999(92)90580-w. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Weickert CS, Akil M, Lipska BK, Hyde TM, Herman MM, et al. Catechol-O-methyltransferase mRNA expression in human and rat brain: evidence for a role in cortical neuronal function. Neuroscience. 2003;116:127–137. doi: 10.1016/s0306-4522(02)00556-0. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, et al. Catechol-O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol Psychiatry. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56:940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- Nagel M, Sprenger A, Hohagen F, Binkofski F, Lencer R. Cortical mechanisms of retinal and extraretinal smooth pursuit eye movements to different target velocities. Neuroimage. 2008;41:483–492. doi: 10.1016/j.neuroimage.2008.02.058. [DOI] [PubMed] [Google Scholar]
- Niklasson L, Rasmussen P, Oskarsdottir S, Gillberg C. Neuropsychiatric disorders in the 22q11 deletion syndrome. Genet Med. 2001;3:79–84. doi: 10.1097/00125817-200101000-00017. [DOI] [PubMed] [Google Scholar]
- Oosterhuis BE, Laforge KS, Proudnikov D, Ho A, Nielsen DA, Gianotti R, et al. Catechol-O-methyltransferase (COMT) gene variants: Possible association of the Val158Met variant with opiate addiction in hispanic women. Am J Med Genet B Neuropsychiatr Genet. 2008 Feb 12; doi: 10.1002/ajmg.b.30716. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterlini M, Zakharenko SS, Lai WS, Qin J, Zhang H, Mukai J, et al. Transcriptional and behavioral interaction between 22q11.2 orthologs modulates schizophrenia-related phenotypes in mice. Nat Neurosci. 2005;8:1586–1594. doi: 10.1038/nn1562. [DOI] [PubMed] [Google Scholar]
- Raux G, Bumsel E, Hecketsweiler B, van Amelsvoort T, Zinkstok J, Manouvrier-Hanu S, et al. Involvement of hyperprolinemia in cognitive and psychiatric features of the 22q11 deletion syndrome. Hum Mol Genet. 2006;16:83–91. doi: 10.1093/hmg/ddl443. [DOI] [PubMed] [Google Scholar]
- Renick SE, Kleven DT, Chan J, Stenius K, Milner TA, Pickel VM, et al. The mammalian brain high-affinity L-proline transporter is enriched preferentially in synaptic vesicles in a subpopulation of excitatory nerve terminals in rat forebrain. J Neurosci. 1999;19:21–33. doi: 10.1523/JNEUROSCI.19-01-00021.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussos P, Giakoumaki SG, Bitsios P. The dopamine D(3) receptor Ser9Gly polymorphism modulates prepulse inhibition of the acoustic startle reflex. Biol Psychiatry. 2008;64:235–240. doi: 10.1016/j.biopsych.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Schwarzkopf SB, Lamberti JS, Smith DA. Concurrent assessment of acoustic startle and auditory P50 evoked potential measures of sensory inhibition. Biol Psychiatry. 1993;33:815–828. doi: 10.1016/0006-3223(93)90023-7. [DOI] [PubMed] [Google Scholar]
- Shaikh TH, Kurahashi H, Saitta SC, O’Hare AM, Hu P, Roe BA, et al. Chromosome 22-specific low copy repeats and the 22q11.2 deletion syndrome: genomic organization and deletion endpoint analysis. Hum Mol Genet. 2000;9:489–501. doi: 10.1093/hmg/9.4.489. [DOI] [PubMed] [Google Scholar]
- Shashi V, Keshavan MS, Howard TD, Berry MN, Basehore MJ, Lewandowski E, et al. Cognitive correlates of a functional COMT polymorphism in children with 22q11.2 deletion syndrome. Clin Genet. 2006;69:234–238. doi: 10.1111/j.1399-0004.2006.00569.x. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 2007;59:22–33. [PubMed] [Google Scholar]
- Sobin C, Kiley-Brabeck K, Karayiorgou M. Lower prepulse inhibition in children with the 22q11 deletion syndrome. Am J Psychiatry. 2005;162:1090–1099. doi: 10.1176/appi.ajp.162.6.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Paulsen J, Braff DL, Butters N, Geyer MA, Swenson MR. Impaired prepulse inhibition of acoustic and tactile startle response in patients with Huntington’s disease. J Neurol Neurosurg Psychiatry. 1995;58:192–200. doi: 10.1136/jnnp.58.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe J, Tregellas JR, Martin LF, Freedman R. Effects of nicotine on hippocampal and cingulate activity during smooth pursuit eye movement in schizophrenia. Biol Psychiatry. 2006;59:754–761. doi: 10.1016/j.biopsych.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Thaker GK. Neurophysiological endophenotypes across bipolar and schizophrenia psychosis. Schizophr Bull. 2008;34:760–773. doi: 10.1093/schbul/sbn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaker GK, Wonodi I, Avila MT, Hong LE, Stine OC. Catechol-O-methyltransferase polymorphism and eye tracking in schizophrenia: a preliminary report. Am J Psychiatry. 2004;161:2320–2322. doi: 10.1176/appi.ajp.161.12.2320. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Tanabe JL, Miller DE, Ross RG, Olincy A, Freedman R. Neurobiology of smooth pursuit eye movement deficits in schizophrenia: an fMRI study. Am J Psychiatry. 2004;161:315–321. doi: 10.1176/appi.ajp.161.2.315. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet IM, Leroy H, van Megen HJGM. De M.I.N.I. Internationaal Neuropsychiatrisch Interview, Nederlandse Versie 5.0.0. Leiden University Medical Center, Department of Psychiatry; The Netherlands: 2000. [Google Scholar]
- Vorstman JAS, Morcus MEJ, Duijff SN, Klaassen PWJ, Heinemande Boer JA, Beemer FA, et al. The 22q11.2 deletion in children: high rate of autistic disorders and early onset of psychotic symptoms. J Am Acad Child Adolesc Psychiatry. 2006;45:1104–1113. doi: 10.1097/01.chi.0000228131.56956.c1. [DOI] [PubMed] [Google Scholar]
- Wechsler D, Kort W, Compaan EL, Bliechrodt N, Resig WCM, Schittekatte M, et al. WISC-III NL, Handleiding. Psychological Corporation; London: 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


