Abstract
Early adversity, for example poor caregiving, can have profound effects on emotional development. Orphanage rearing, even in the best circumstances, lies outside of the bounds of a species-typical caregiving environment. The long-term effects of this early adversity on the neurobiological development associated with socio-emotional behaviors are not well understood. Seventy-eight children, who include those who have experienced orphanage care and a comparison group, were assessed. Magnetic resonance imaging (MRI) was used to measure volumes of whole brain and limbic structures (e.g., amygdala, hippocampus). Emotion regulation was assessed with an emotional go-nogo paradigm, and anxiety and internalizing behaviors were assessed using the Screen for Child Anxiety Related Emotional Disorders, the Child Behavior Checklist, and a structured clinical interview. Late adoption was associated with larger corrected amygdala volumes, poorer emotion regulation, and increased anxiety. Although more than 50% of the children who experienced orphanage rearing met criteria for a psychiatric disorder, with a third having an anxiety disorder, the group differences observed in amygdala volume were not driven by the presence of an anxiety disorder. The findings are consistent with previous reports describing negative effects of prolonged orphanage care on emotional behavior and with animal models that show long term changes in the amygdala and emotional behavior following early postnatal stress. These changes in limbic circuitry may underlie residual emotional and social problems experienced by children who have been internationally adopted.
According to data published by the U.S. Department of State, approximately twenty thousand infants and children are adopted from abroad each year - a trend that has increased steadily over the past fifteen years. With this growing number of adopted children in the U.S. has come a new wave of studies on the effects of orphanage rearing and growing concerns on the long-term consequences of such early experiences (Ames, 1990). As outlined by Gunnar et al. (2000), the social-emotional behavioral domain, including attachment relationships (Smyke, Dumitrescu, & Zeanah, 2002), peer interactions, and emotion regulation (Hodges & Tizard, 1989), is a common area of concern for previously institutionalized (PI) children. In a sample of Romanian children, emotional behavior with peers was disrupted even in children adopted before four months of age who showed resilience in other non-emotional developmental domains (Ames, 1997). Diagnostically, PI children have an increased prevalence of anxiety disorders (Ellis, Fisher, & Zaharie, 2004). Such emotional difficulties may reflect a more general problem of heightened emotional reactivity. These socio-emotional profiles may be ameliorated by living with post-adoption families, but subtle effects tend to remain well into adolescence (Hodges et al., 1989), a finding that suggests that there may be long-term changes in neural systems associated with processing emotional information following orphanage care.
Even in the best institutions, orphanage care is outside the bounds of species-typical caregiving. As described by Gunnar and colleagues (2000,2007), the caregivers are paid employees who rotate shifts and are in charge of an overwhelming number of children (sometimes as high as 20 children:1 caregiver), resulting in continuous instability of caregiving for an infant, and the caregiving in an orphanage lacks in terms of both quality and quantity (Smyke, Koga, Johnson, Fox, Marshall, Nelson, Zeanah, & Group, 2007). Lack of a stable caregiver is a potent stressor for the human infant (Johnson, 2002). In fact, unstable caregiving in an otherwise enriched environment (e.g., high staff–to-child ratios, stimulating toys), is sufficient to alter social and emotional behavior years after adoption (Hodges & Tizard, 1989). Studying the emotional development of children who had been institutionalized during infancy and have subsequently been removed from this environment allows us to ask questions regarding the long-term correlates of early adversity (which is often difficult to separate from later-life adversity) in a human sample.
Impact of adversity on emotional processing
Individuals with a history of maltreatment have difficulty regulating their emotions in the context of threatening stimuli (Pollak, Vardi, Putzer Bechner, & Curtin, 2005). There is evidence to suggest that the difficulties arise in part because of enhanced processing of negatively valenced information. Maltreated children show a greater attentional bias for negatively valenced stimuli in a dot-probe task (Dalgleish, Moradi, Taghavi, Neshat-Doost, & Yule, 2001), a processing bias for threatening stimuli, as measured by decreased amount of perceptual information needed to recognize threat (Pollak & Sinha, 2002), and an increased amplitude of the N170, an event-related potential sensitive to facial expressions (Blau, Maurer, Tottenham, & McCandliss, 2007), for fear faces (Parker & Nelson, 2005). The emotional weight negatively-valenced stimuli carry for individuals with a history of early of adversity can result in emotion regulation difficulties (Williams, Mathews, & MacLeod, 1996). Such findings suggest that life stressors increase the emotional salience of negative information, making it more difficult for these individuals to regulate behavior in the context of negative information.
This processing bias for negatively valenced information increases the risk of developing an anxiety disorder. It has been shown that both anxious adults (Bradley, Mogg, White, Groom, & de Bono, 1999; Derryberry & Reed, 2002) and anxious children (Vasey, el-Hag, & Daleiden, 1996) have this bias. Even in a non-referred population of children, higher trait anxiety produces faster search times for threatening faces (Hadwin, Donnelly, French, Richards, Watts, & Daley, 2003). Thus, both anxious individuals and those with a history of adversity are more affected by negative information in such a way that perception and orientation toward negative information is enhanced. The neurobiological systems that support these changes in behavior have largely been investigated in animal models. In this article, we present neurobiological and behavioral data that show a relationship between early-life adversity and changes in anxiety-related phenotypes within a population of children.
Animal models of deprivation have provided evidence within a laboratory-controlled setting that the quality of an environment has drastic, long-lasting effects on behavior (Greenough, Black, & Wallace, 1987; Greenough, Hwang, & Gorman, 1985; Greenough, McDonald, Parnisari, & Camel, 1986). The large numbers of changes that occur early in development make this period of life particularly sensitive to environmental effects. Poor or non-existent maternal care produces lifelong alterations in emotion regulation, as evidenced by increased stress reactivity and fearful behavior in the offspring in rodents (Caldji, Tannenbaum, Sharma, Francis, Plotsky, & Meaney, 1998; Francis, Champagne, Liu, & Meaney, 1999; Huot, Thrivikraman, Meaney, & Plotsky, 2001) and in non-human primate that experience stressors while parenting (Rosenblum, Forger, Noland, Trost, & Coplan, 2001). Other work has examined the effects of manipulating timing of maternal separation on emotion regulation and showed that while all maternally deprived juvenile rhesus monkeys show increases in anxious behaviors, the timing of maternal deprivation has specific effects on subsequent socio-emotional behavior (Nelson, Bloom, Cameron, Amaral, Dahl, & Pine, 2002). The effects of stress on emotional behavior may be mediated by morphologic and neurofunctional changes that follow stressful experiences. These neural phenotypes may, in part, account for the anxiety and emotion regulation difficulties that often characterize the behavior of PI children (Smyke et al., 2002).
Impact of adversity on limbic regions
Stressful experiences produce specific changes in the brain, particularly in limbic regions like the amygdala and hippocampus (McEwen, 2004). The amygdala, a structure implicated in processing and responding to emotional information (Davis & Whalen, 2001), has been shown to be important in learning about the emotional environment and safety of that environment. In adult animals, psychological stressors or direct administration of stress hormones increases dendritic arborization and formation of new spines (Mitra, Jadhav, McEwen, Vyas, & Chattarji, 2005; Vyas, Bernal, & Chattarji, 2003; Vyas, Mitra, Shankaranarayana Rao, & Chattarji, 2002) and activity of the amygdala (Rosen, Hamerman, Sitcoske, Glowa, & Schulkin, 1996; Rosen & Schulkin, 1998). Early-life stress has long term effects, and rat pups who were separated from their mothers during the neonatal period show greater amygdala response to stress as adults than non-separated rats (Sanders & Anticevic, 2007). In contrast, stress decreases hippocampal arborization in rodents (Vyas et al., 2002). Decreased hippocampal volumes have been observed in adult humans who have experienced high levels of stress or trauma (Bremner, Randall, Scott, Bronen, Seibyl, Southwick, Delaney, McCarthy, Charney, & Innis, 1995; Bremner, Randall, Vermetten, Staib, Bronen, Mazure, Capelli, McCarthy, Innis, & Charney, 1997; Gurvits, Shenton, Hokama, Ohta, Lasko, Gilbertson, Orr, Kikinis, Jolesz, McCarley, & Pitman, 1996), although not all studies show this association (Bonne, Brandes, Gilboa, Gomori, Shenton, Pitman, & Shalev, 2001; De Bellis, Keshavan, Clark, Casey, Giedd, Boring, Frustaci, & Ryan, 1999). Smaller hippocampal volumes have not been reported in children with early-life stress; in fact, one report shows larger hippocampal volume in previously stressed children (Tupler & De Bellis, 2006). Animal models suggest that differences in hippocampal volumes attenuate once the stressor has ended, unlike the stress-related growth in amygdala volume, which seems more resistant to recovery (Vyas et al., 2004). Based on this recovery effect and the lack of evidence of smaller hippocampal volume in previously stressed children, we predict that amygdala changes, but not necessarily hippocampal ones, will be observed in PI children who have been adopted out of orphanages into families.
Consistent with these data from animal models of stress, neuroimaging studies show that stress and trauma affect amygdala structure and function in humans. Previously stressed adults have exaggerated amygdala responses to threatening stimuli relative to nonstressed comparison groups (Liberzon, Taylor, Amdur, Jung, Chamberlain, Minoshima, Koeppe, & Fig, 1999; Rauch, Whalen, Shin, McInerney, Macklin, Lasko, Orr, & Pitman, 2000; Shin, Wright, Cannistraro, Wedig, McMullin, Martis, Macklin, Lasko, Cavanagh, Krangel, Orr, Pitman, Whalen, & Rauch, 2005). Given the amygdala’s role in monitoring the environment for emotional significance (Dolan & Vuilleumier, 2003), these findings would predict more anxious and vigilant behavior in stressed individuals.
A large literature suggests that the amygdala is the mediating agent between environmental stress and subsequent self-regulation difficulties, like anxiety and mood disorders. Amygdala volumes are atypically larger and more reactive in anxious children and adolescents relative to typically developing individuals (De Bellis, Casey, Dahl, Birmaher, Williamson, Thomas, Axelson, Frustaci, Boring, Hall, & Ryan, 2000; MacMillan, Szeszko, Moore, Madden, Lorch, Ivey, Banerjee, & Rosenberg, 2003; Thomas, Drevets, Dahl, Ryan, Birmaher, Eccard, Axelson, Whalen, & Casey, 2001), they are larger in adults at the first episode of depression relative to non-depressed adults (Frodl, Meisenzahl, Zetzsche, Bottlender, Born, Groll, Jager, Leinsinger, Hahn, & Moller, 2002), and amygdala volumes positively correlate with levels of anxiety in adults (Barros-Loscertales, Meseguer, Sanjuan, Belloch, Parcet, Torrubia, & Avila, 2006). Taken together, the behavioral and neurobiological evidence suggests that adversity is followed by atypical amygdala development and these differences result in greater reactivity to emotional information. Better understanding of how the environment impacts the neurobiological systems that support emotional reactivity provides insight into the etiology of anxious behaviors following stress. However, the varying times and durations of the stressful events in most studies make it unclear how timing played a role in the observed effects, and imaging studies of stressed or anxious populations do not consistently find larger amygdale volumes (Bremner et al., 1997; Lindauer, Vlieger, Jalink, Olff, Carlier, Majoie, den Heeten, & Gersons, 2004; Schmahl, Vermetten, Elzinga, & Bremner, 2003; Wignall, Dickson, Vaughan, Farrow, Wilkinson, Hunter, & Woodruff, 2004). The strength of studying a group of internationally adopted children is that the timing of the adverse experience is known and is temporally discrete.
To get at this issue of timing, studies of PI children have often made distinctions between early and late adoptions because duration of institutionalization affects outcome. The cut-off age has varied from study to study and has included early infancy (O'Connor & Rutter, 2000), one year old (van Ijzendoorn & Juffer, 2006), and two years old (Gunnar & van Dulmen, 2007; Rutter & O'Connor, 2004). These varying ages suggest that there may not be one critical cut-off age of adoption, but the impact of the cut-off age may differ depending on the outcome measure. The current study places the split between early and later adoptions at the median age of adoption.
The current study used structural MRI to examine the development of limbic structures including the amygdala and hippocampus (controlling for cortical size) with relative specificity to a control structure (caudate), following a discrete period of early-life stress. In addition we measured emotion regulation with an emotional go-nogo behavioral paradigm (Hare, Tottenham, Davidson, Glover, & Casey, 2005; Hare, Tottenham, Galvan, Voss, Glover, & Casey, 2008; Ladouceur, Dahl, Williamson, Birmaher, Axelson, Ryan, & Casey, 2006), shown to recruit the amygdala (Hare et al., 2005; 2008), and measured clinical status using parental reports of individual differences in anxiety symptoms and internalizing behaviors. We hypothesized that longer exposure to orphanage-rearing would be associated with larger amygdala volume and greater difficulties in regulating behavior in the context of emotional information. Moreover, we predicted that amygdala volume would be related to these behavioral difficulties and to higher levels of anxiety.
Method
Subjects
The seventy-eight children (38 PI children and 40 comparison, never institutionalized children) who participated are described in Table 1. Of the 38 total PI children, 53% met criteria for at least one psychiatric disorder, and specifically 18% of the PI children met criteria for an anxiety disorder. Supplemental figure 1 shows that diagnosis was equally represented across the three analyses for the total sample, the sample with MRI data, and the sample with behavioral data. The PI children in our sample had parents whose yearly income was comparable to the parents of the comparison group (see Table 2), and both groups had a household income that was well above the median annual household income in the United States ($48,201; US Census Bureau, 2006), a high socio-economic profile similar to that described in a Minnesotan population (Hellerstedt, Madsen, Gunnar, Grotevant, Lee, & Johnson, 2008). Age adopted out of the orphanage was negatively correlated with time spent with family (r(36)=−0.39, p<.02), positively correlated with duration of time in the orphanage (r(36) = 0.91, p<10−6), but showed no relationship with age at testing (r(36) = 0.22, ns). Not all children completed both behavioral and MRI portions of the study as a result of a number of factors (e.g., motion artifact, fatigue, running out of time during the session) and thus the participants that were entered into each analysis are described below in detail.
Table 1.
Characteristics for the previously institutionalized (PI) and comparison participants. Note: there is overlap between participants in the whole sample, those that provided imaging data, and those that provided behavioral data.
| Whole Sample† | Imaging Data | Behavioral Data | ||||||
|---|---|---|---|---|---|---|---|---|
| PI (N = 38) |
Comparison (N=40) |
PI (N = 34) |
Comparison (N=28) |
PI (N = 19) |
Comparison (N=27) |
|||
| Sex | 29 female; 9 male |
29 female; 11 male |
26 female; 8 male |
23 female; 5 male |
13 female; 6 male |
16 female; 11 male |
||
| Mean Age in months (SD); range | 99.7 (22.4); 59.6–142.8 |
114.3 (25.6); 72.3–189.2 |
101.6 (22.2); 62.7–142.8 |
113.6 (26.5); 72.3–189.2 |
t(60)=1.94, ns |
101.5 (20.0); 60.0–134.0 |
115.3 (26.7); 72.3–189.2 |
t(44)=1.78, ns |
| Mean (SD) IQ; range | 103.8(14.5); 77–127 |
110.3 (15.6); 76–145 |
103.4 (14.6); 77–127 |
109.4 (16.1); 76–145 |
t(57)=1.48, ns †† |
103.3 (15.2); 77–127 |
111.3 (14.4); 76–145 |
t(44)=1.82, ns |
| Country of Origin | 8 Eastern Europe; 30 Asia |
7 Eastern Europe; 27 Asia |
5 Eastern Europe; 14 Asia |
|||||
| Mean Age Orphaned in months (SD); range | 2.5 (5.4); 0–28 |
2.7 (5.7); 0–28 |
3.1 (6.9); 0–28 |
|||||
| Mean Age Adopted in months (SD); range | 19.2 (13.1); 2.5–60 |
18.8 (13.3); 2.5–60 |
23.9 (15.1); 2.5–60 |
|||||
| Mean time with family in months (SD); range | 80.2 (23.5); 39.0–134.3 |
82.5 (22.5); 40.7–134.3 |
77.9 (22.7); 39.0–114.8 |
|||||
Within the manuscript, PI and comparison children from the whole sample are never directly compared, and therefore, we do not examine the differences in the demographic information in this table.
missing IQ on 3 control subjects
Table 2.
Yearly household income of the families of the comparison and PI children.
| Yearly Income | Comparison | PI |
|---|---|---|
| 10,000–40,000 | 14% | 10% |
| 40,001–85,000 | 7% | 22% |
| 85,001–150,000 | 29% | 28% |
| 150,001 – 200,000 | 36% | 18% |
| more than 200, 000 | 14% | 24% |
Neuroimaging data
Imaging data were successfully collected from 62 children (34 PI and 28 comparison). As seen in Table 1, the groups were similar to each other in terms of sex and age. Because age at adoption is a variable of interest, this variable was examined continuously and also examined dichotomously by forming two subgroups of the PI children, split at median age of adoption (15 months). This split resulted in a group of 17 early adopted children (15 girls, 2 boys; 16 from Asia, 1 from Eastern Europe) and 17 late adopted children (10 girls, 7 boys; 11 from Asia, 6 from Eastern Europe), and these two groups did not differ in age at testing (t(32)=0.06, ns; early adopted mean age = 101.4 months, SD = 21.3 & late adopted mean age =101.8 months, SD = 23.7). The comparison group was also divided into two subgroups that were made as similar as possible to the two PI subgroups on age and sex (14 children (13 girls, 1 boy) in the comparison group for the early-adopted PI children and 14 children (10 girls, 4 boys) in the comparison group for the late-adopted PI children)1. The mean age of the comparison group for the early adopted children was 117.8 (SD=21.3) months, which was older than the early adopted PI children by approximately 16 months (t(29) = 2.13, p< 0.05). The mean age of the comparison group for the late adopted children was 109.4 (SD=31.1) months, which was not significantly different from the late adopted children ((t(29) = 0.77, ns). The early adopted PI group had been living with their families for an average of 92.0 months (SD = 21.9; range: 55.4–134.3) at the time of testing, which was significantly longer than the late adopted PI group had been living with their families (mean: 73.0 months; SD=19.4; range: 43.1–97.8) at the time of testing (t(32) = 2.68, p <0.05).
Behavioral data
Complete behavioral data were collected from 46 children (19 PI and 27 comparison), and characteristics of these children are provided in Table 1. As seen in Table 1, the groups were similar to each other in terms of sex and age.
As with the neuroimaging analyses, PI children were identified as either early- or late-adopted based on a 15-month age of adoption cut-off, which resulted in a group of late-adopted PI children (n=12); it was our opinion that the remaining early adopted children (n=7) were too few to perform statistical tests. Of the 12 late adopted PI children (8 female and 4 male), 7 were born in Asia and 5 were born in Eastern Europe. Mean age at testing was 98.8 months old (SD=22.8), which was not significantly different from the 12 children (8 female, 4 male) used in the comparison group (mean age = 111.1 months (SD = 23.9); t(22) = 1.28, ns).
There were 30 children (15 PI (10 girls, 5 boys), and 15 comparison (10 girls, 5 boys)) who provided complete neuroimaging and data from the behavioral task. Average age of the PI children who completed the behavioral task and the imaging procedure was 107.44 months (SD = 17.87), which was not significantly different from the comparison children (mean age: 114.80 months; SD = 29.18; t(28) = 0.83, ns). Twelve of these PI children were from Asia, and three were from Eastern Europe.
Analyses were performed to examine the association between internalizing behaviors/anxiety and amygdala volume across PI and comparison children (complete magnetic resonance imaging (MRI) and Child Behavior Checklist (CBCL; Achenbach, 1991) data were obtained from 43 children (30 PI (22 girls & 8 boys; mean age = 101.50 (SD= 22.72); 24 born in Asia & 6 born in Eastern Europe)); 13 comparison (9 girls & 4 boys; mean age = 112.34 months (SD = 30.52)), and complete MRI and SCARED data were obtained from 25 children (22 PI (17 girls & 5 boys; 103.81 months (SD = 22.02); 17 born in Asia & 5 born in Eastern Europe); 3 comparison (1 girl & 2 boys; mean age = 152.54 (SD = 33.74)))2.
Measures
Structured Interview
Diagnostic information was obtained using a structured interview with the parent (Schedule for Affective Disorders and Schizophrenia for School-Age Children – Parent version; Orvaschel, 1994). In addition, all subjects participated in IQ testing using the WASI (Wechsler, 1999).
Internalizing and Anxiety Questionnaires
Parents completed the CBCL (Achenbach, 1991), an instrument that measures internalizing and externalizing behaviors on a continuum and has been shown to be reliable and valid, and they also completed the Screen for Child Anxiety Related Emotional Disorders (SCARED), a parent-report instrument that measures anxious behaviors on a continuum and has been shown to be reliable and valid (Birmaher, Brent, Chiappetta, Bridge, Monga, & Baugher, 1999).
Behavioral Task
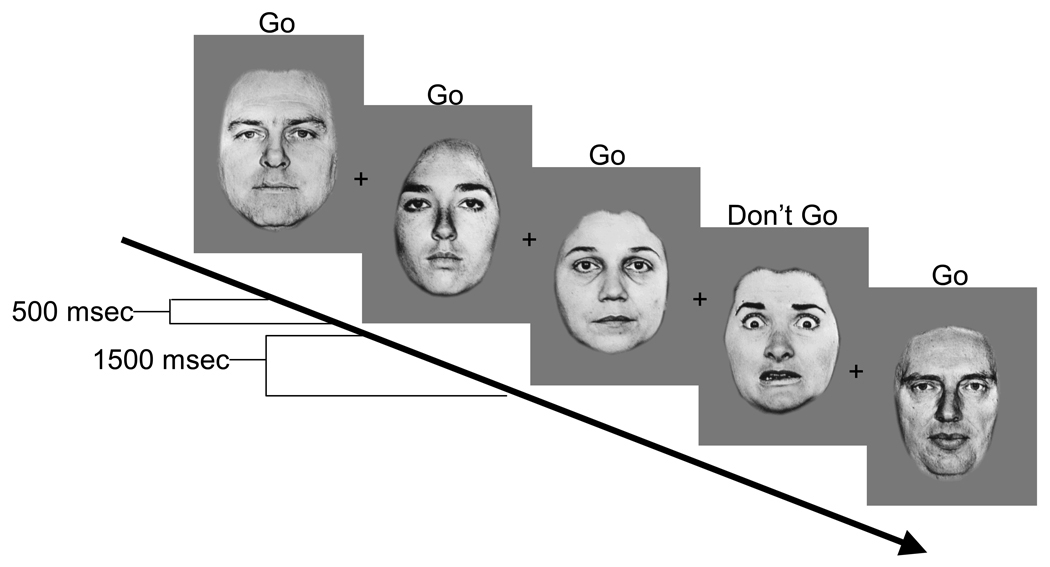
The emotional go-nogo paradigm was employed to measure self-regulation in emotional contexts. The task requires the subject to either rapidly detect emotional (either positively or negatively valenced) faces (e.g., happy) amidst a series of neutral face distracters or to detect neutral faces amidst a series of emotional face distracters (Hare et al., 2005; 2008; Ladouceur et al., 2006). Faces with negative (fearful, sad, or angry) or positive (happy) valence were paired with neutral faces, and depending on the block, either the emotional or the neutral faces were the distracter stimuli (Figure 1). The set of stimuli consisted of pictures of eight adults (Ekman & Friesen, 1976). Stimulus duration was 500 milliseconds with an interstimulus interval of 1500 msec. Targets were presented with 70% probability. These data were collected outside of the scanner environment. Children were instructed at the beginning of each of 8 randomized blocks of 30 trials to press a button to a given facial expression but told not to press to the other expression. They were provided with practice blocks to ensure they could perform the task.
Figure 1.
Temporal layout of one block where subjects were instructed to only press a button for the neutral faces and withhold pressing to other expressions. In this example, neutral faces are the target stimuli and faces with negative valence are the distracter stimuli.
MRI Acquisition
Magnetic resonance imaging was performed using a GE 1.5 Tesla Unit and a 3 Tesla Unit3 (Signa System, General Electric Medical Systems, Milwaukee, WI). A sagittal scout series verified patient position. A three-dimensional spoiled gradient recalled acquisition was used to obtain 124 contiguous images with slice thickness of 1.5 mm in the coronal plane (time of echo = 5, time of repetition = 25, flip angle = 40°, acquisition matrix = 256 × 256, number of excitations =1, field of view = 24 cm).
Procedure
Parents of participants gave written consent in accordance with procedures approved by the Institutional Review Board of the Weill Cornell Medical College. In addition, children provided both verbal and written assent to participate. Participants and their parents participated in a two day protocol. On the first day the behavioral task was administered to children, the structured interview and questionnaires were administered to parents, and children practiced MRI scanning within a mock scanner. On the second day, MRI scanning occurred while children were occupied by a movie of their choosing.
Morphometric Analysis
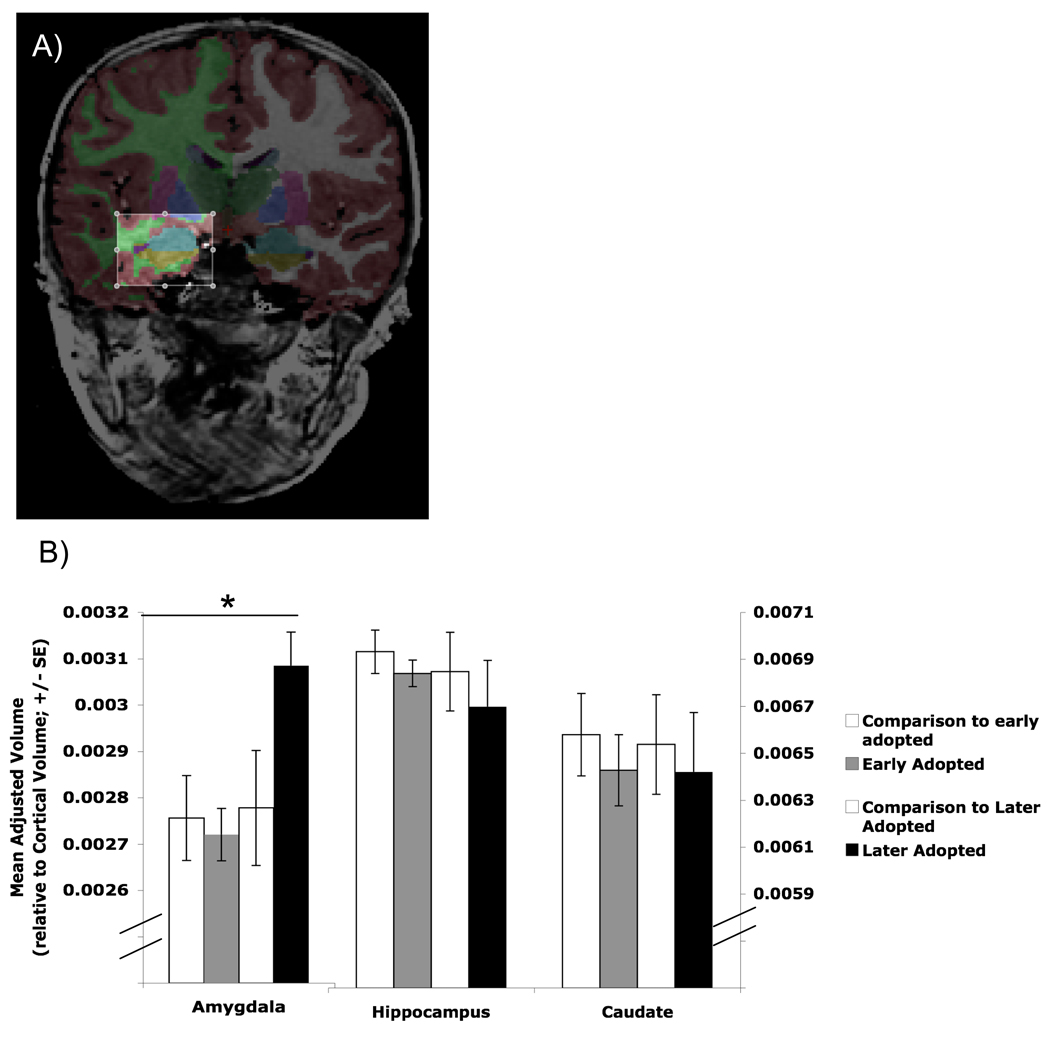
The automated procedures for volumetric measures of the different brain structures are described in detail by Fischl et al. (2002; 2004). This procedure automatically assigns a neuroanatomical label to each voxel in an MRI volume based on probabilistic information estimated from a manually labeled training set. The classification technique employs a non-linear registration procedure that is robust to anatomical variability, including the reduced ventricle size of a pediatric population. The segmentation uses three pieces of information to disambiguate labels: (1) the prior probability of a given tissue class occurring at a specific atlas location, (2) the likelihood of the image given what tissue class, and (3) the probability of the local spatial configuration of labels given the tissue class. The technique has previously been shown to be comparable in accuracy to manual labeling. The segmentations were visually inspected for accuracy by a single operator, and edited when necessary. Figure 2a shows the anatomical segmentation of the amygdala from neighboring structures, which is often difficult to segment manually.
Figure 2.
Morphometric segmentation across groups. A) Anatomical segmentation of the amygdala (in aqua) from neighboring structures. B) Adjusted volumes by group. Children who had been adopted out of the orphanage at older ages (> 15 months old) had larger amygdale volumes than early-adopted children (< 15 months old) and comparison children, who did not differ from each other.
Calculation of behavioral data
We calculated separate reaction times and % correct scores ((hits – false alarms)/total trials) × 100)4 for facial expressions with positive and negative valence. Reaction time averages were only calculated from correct trials, and outliers (more than 3 SDs from the mean) were removed.
Results
Morphometry
Overall cortical volume did not significantly differ between PI children (mean volume = 1,193 cm3, SD = 125) and comparison children (mean volume = 1,232 cm3, SD = 105; t (60) = 1.32, n.s.), nor was cortical volume related to age of adoption (F(1,33) = 1.33, n.s.). All regional volumes reported below control for total cortical volume (i.e., by dividing regional volume by cortical volume) and current age. Because diagnosis could influence the results, its role was examined as well.
Regional volumes for the PI children relative to the comparison group were examined. An alpha of 0.02 (0.05/3) was used to correct for multiple comparisons. Volumetric measurements (mean adjusted volume (SD)) did not differ for the amygdala (t(60) = 1.32, ns; 0.00277 (0.00039) & 0.00290 (0.00032)), the hippocampus (t(60) =0.71, ns; 0.00689 (0.00064) & 0.00677 (0.00070)), or the caudate (t(60) =0.64, ns; 0.0066 (0.0006) & 0.0064 (0.0009)) between comparison and PI children, respectively. However, when early adopted children were distinguished from later adopted children (i.e., less than 15 months old vs. more than 15 months old at time of adoption, respectively), a one-way ANOVA showed a difference between the four groups (early-adopted PI, late-adopted PI, comparison for early adopted PI, and comparison for late adopted PI) for the amygdala (Figure 2b; F(3,61) = 4.24, p < 0.009), but not for the hippocampus (F(3,61)= 0.32, ns) or the caudate (F(3,61)= 0.14, ns). Post-hoc tests (LSD) showed that later-adopted PI children had significantly larger adjusted amygdala volumes than the early adopted group and the comparison groups5. Adjusted amygdala volume did not differ between the early adopted children and the comparison groups6.
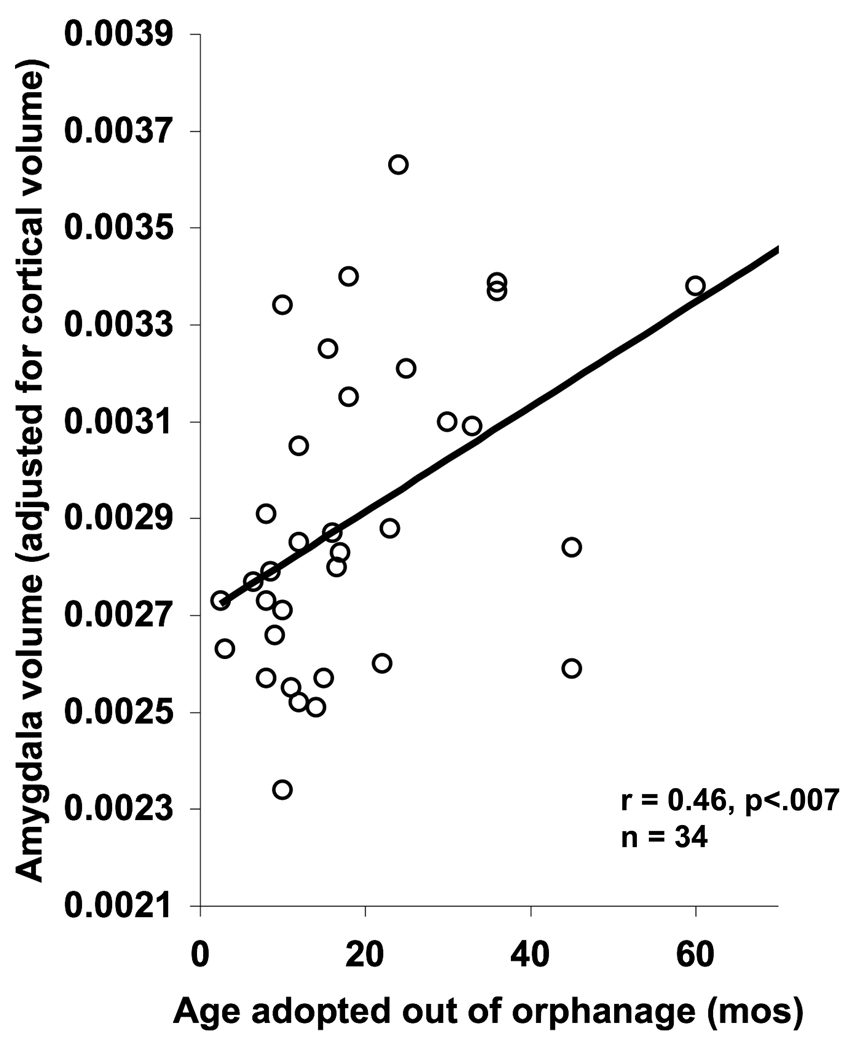
Length of time in orphanage
The association between amygdala volume and length of orphanage stay was examined with regression analysis within the PI group of children. Adjusted amygdala volume was associated with age adopted out of the orphanage (Figure 3; F (1,33) = 8.43, p < 0.007), an association which also held when current age was controlled for using partial correlation (r(31)=0.54, p<0.001)7. Because IQ was also correlated with age adopted out of the orphanage (r (32) = −0.34, p< 0.05), partial correlations were performed and showed that the association between age adopted out of the orphanage and amygdala could not be explained by IQ (r (31) = 0.37, p < 0.05). The association was not driven by children with anxiety disorders either (De Bellis et al., 2000), for when children with anxiety disorders were removed from the analysis, the association between age adopted and adjusted amygdala volume remained (F (1,25) = 7.26, p < 0.05).
Figure 3.
Age of adoption and amygdala volume. The older children were when adopted out of the orphanage, the larger their amygdala volume. This association exists even when the child adopted at 60 months old is removed from the analysis.
Behavior
Separate repeated measures ANOVAs were performed on reaction time and % correct, with valence (positive, negative) and emotional stimulus type (target, distracter) as the within subjects variables and group (PI, comparison) as the between subjects variable.
Reaction time
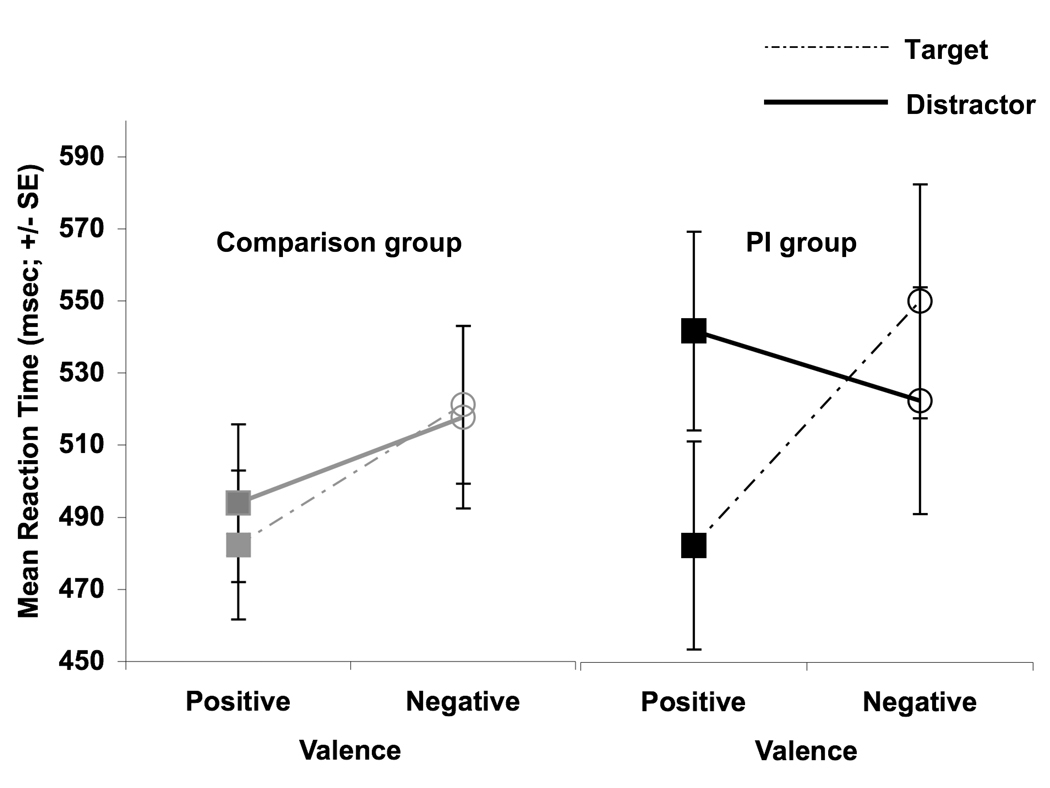
There was a main effect of valence (F (1,44) = 8.79, p < 0.005) such that mean reaction times were slower during blocks of faces with negative valence (mean = 526.04 msec, SD = 18.43) than with positive valence (mean = 498.69 msec, SD = 16.29). There was an interaction between valence and stimulus type (F (1,44) = 16.25, p <0.0001) where reaction times were fastest to faces with positive valence when they were the target stimuli (mean = 481.75 msec, SD =111.64); mean reaction time during blocks with positive valenced distractors was 511.94 msec (SD = 115.02), mean reaction time during blocks with negqatively valenced targets was 531.35 msec (122.35), and mean reaction time during blocks with negatively valenced distractors was 518.43 msec (130.04). There was an interaction between valence, stimulus type, and group (F(1,44)= 7.84, p<0.008). Post hoc t-tests showed that the PI children were more likely than the comparison children to modify reaction time depending on the valence (positive vs negative) and the stimulus type (target vs distractor) such that there whichever face in the block was relatively more negative resulted in a slowed reaction time (Figure 4). In other words, PI children tended to slow reaction times to positively valenced blocks when pressing to neutral faces (i.e., positively valenced faces were the distractor stimuli) relative to when they were pressing to positively valenced faces (t(18) = 3.43, p < 0.005). To a lesser degree, although still reliably, PI children tended to slow reaction times more to negatively valenced blocks when pressing to the negative face relative to when they were pressing for the neutral faces (i.e., negative faces were the target; t(18) = 2.16, p < 0.05). However, the comparison group showed no modulation of reaction time as a function of whether the emotional faces were the target or the distractor stimuli (positive: target vs. distractor t(26) = 1.00, ns; negative target vs. distractor t(26) = 0.26, ns). There were no other main effects or interactions. There was no relationship between reaction time to positive faces and amygdala volume (r(28) = 0.28), negative faces (r(28) = 0.10), neutral faces in the context of positive faces (r(28) = 0.08), or neutral faces in the contact of negative faces (r(28)=0.04).
Figure 4.
Reaction time for emotional faces as targets and distracter stimuli by group. PI children had relatively slower reaction times for neutral faces in the presence of distracter faces with positively valence and relatively faster reaction times for neutral faces in the presence of distracter faces with negative valence.
% Correct
High % correct scores on the emotional go-nogo task was an indication that the subject had high hit rates while controlling for number of false alarms. When all children were included in the analyses, there was a main effect of valence (F (1,44) = 36.34, p < 0.0001) and stimulus type (F(1,44) = 9.68, p < 0.003) on % correct such that performance was more accurate during blocks that included positive facial expressions (mean = 82.87%, SD = 1.49) than it was during blocks that included negative facial expressions (mean = 75.56%, SD = 1.37) and performance was more accurate during blocks when emotional faces were the target stimuli (mean = 81.07%, SD = 1.39) than when they were the distracter stimuli (mean = 77.43%, SD = 1.44). When only the late adopted PI children and their comparison group were included in the analysis, the main effect of valence remained (F (1,22) = 22.02, p < 0.0001) on % correct such that performance was more accurate during blocks that included positive facial expressions (mean = 82.50%, SD = 2.12) than it was during blocks that included negative facial expressions (mean = 74.72%, SD = 1.77). Additionally, there was an interaction between valence and group (F (1,22) = 4.79, p<0.05) such that the PI children made more errors than the comparison group specifically during blocks that contained negative faces but performed like the comparison group for blocks with positive faces (Figure 5). There were no other main effects or interactions on % correct.
Figure 5.
Sensitivity on the face go-nogo by group. Late adopted PI children made more errors during blocks that contained negatively valenced faces than the comparison children, but showed no group difference in responses to positive valence.
Whether there was an association between accuracy for negative faces and amygdale volume was examined for all children (PI and comparison). There was a trend for larger amygdala volumes to be associated with more errors (i.e., lower % correct) during blocks that contained negative face distracters relative to performance on the positive faces (accuracy during blocks with negative distractors – accuracy during blocks with positive distractor; r (28) = −0.34, p=0.06).
Length of time in orphanage
The association between age of adoption and behavior on the emotional go-nogo task was examined within the PI group. Separate linear regression analyses with age adopted out of orphanage were performed on reaction time and % correct scores during blocks of positive and negative faces. There were no effects of age adopted on reaction time (F(2,18) = 1.29, ns) or percent correct (F(2,18) = 0.38, ns). Because of the group differences in % correct to negative faces described above, false alarm and omission rates were also examined using linear regression within the PI group to investigate the source of the increased errors. These analyses showed that age adopted out of the orphanage was associated specifically with the number of false alarms to negative expressions (F(2,18)=4.27, p < 0.05; beta = 0.59), and not to positive expressions (beta = −0.01), and age of adoption was also not associated with errors of omission for faces with positive or negative valence (F(2,18)=0.11, ns).
Relationship between internalizing/anxious behaviors and amygdala volume
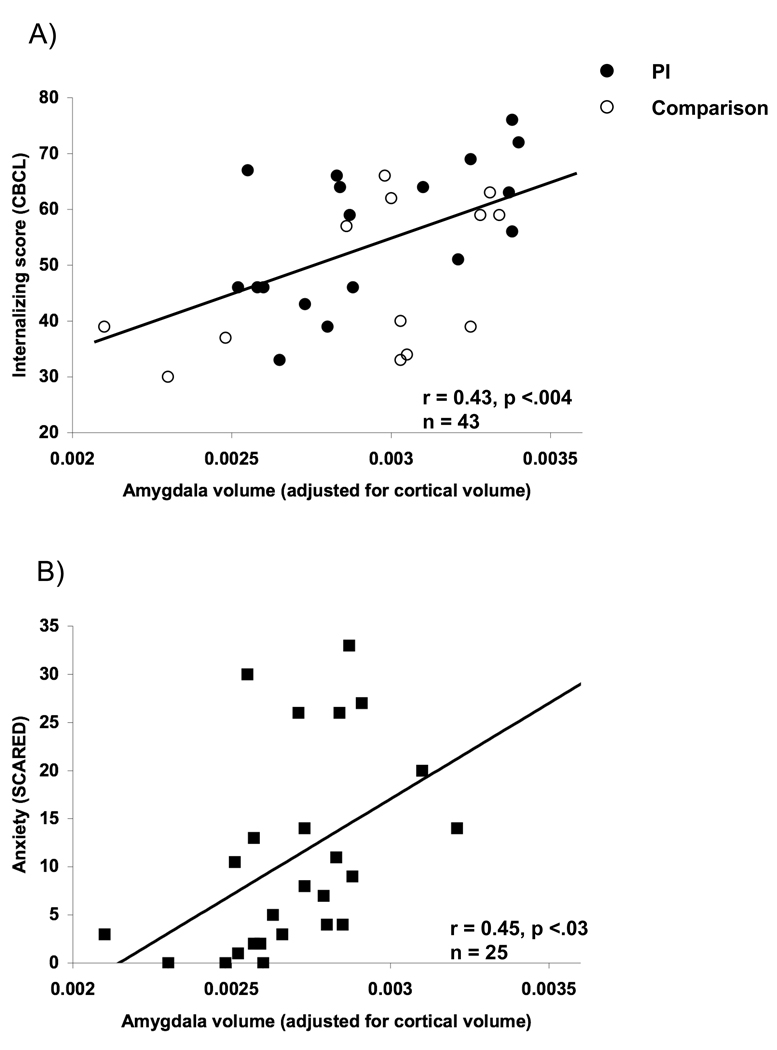
Analyses were performed to examine the association between internalizing behaviors/anxiety and amygdala volume across PI and comparison children. Internalizing behaviors/anxiety scores from the PI and comparison group were not directly compared because internalizing behaviors/ anxiety data were only obtained from a small fraction of the comparison children. Separate linear regression analyses showed that amygdala volume predicted parental ratings of internalizing behaviors as measured by the CBCL as shown in Figure 6 (F(1,42) = 9.18, p<0.004) and anxiety as measured by the SCARED (F (1,24) = 5.69, p<0.03).
Figure 6.
Amygdala volume association with internalizing behavior and anxiety. Larger amygdala volumes were associated with A) higher ratings of internalizing behaviors as measured by the CBCL and B) number of anxiety symptoms as measured by the SCARED inventory.
Discussion
The goal of this study was to examine the development of limbic structures and emotional behavior in children who experienced early-life adversity. An advantage of studying a population of children who have experienced orphanage care and have subsequently been adopted by families is that the period of that particular adversity is temporally discrete and the end date for the adversity is known. Therefore, we are able to examine the effects of timing of early-life stress on the developing emotional system. It was hypothesized that longer stays in an orphanage would be associated with atypical limbic development and associated emotion regulation difficulties, including anxiety. In general, the data from this study support this hypothesis.
We provide evidence that long periods of orphanage rearing are associated with alterations in neuroanatomical development. Specifically, children who had remained in orphanage care for the longest amount of time had amygdala volumes (cortex-corrected) that exceeded those of comparison children. These data suggest that the type of caregiving present in orphanages may act as a psychological stressor for an infant and alter the developmental trajectory of a major neuroanatomical system involved in emotion processing. What is striking about the data from the current study is that the effects of adversity are observed years after termination of the adversity, similar to what has been shown in animal models of stress and recovery (Vyas et al., 2004). In these animal studies the hippocampus, but not the amygdala, recovers from its dendritic shrinkage, and this recovery may explain why only a non-significant trend for smaller hippocampus volume was observed in the PI group. The comparison group for the early adopted PI group was approximately one year older than the early adopted PI children. Measurable amygdala development during a year was not expected and therefore, the age difference is not concerning. Moreover, amygdala volume did not differ between the early adopted group and a comparison group that did not differ in age.
Developmental outcome for children who experience orphanage rearing is impacted by the length of time a child is there with longer stays generally associated with psychiatric disturbances (Beckett, Maughan, Rutter, Castle, Colvert, Groothues, Kreppner, Stevens, O'Connor T, & Sonuga-Barke, 2006; Durfee, 1933; Rutter, 1998; Rutter et al., 2004). The current study examined likely biological substrates that underlie these dose-related effects on emotional behavior. There is no appropriate control group for PI children since there are a number of variables that could differ between groups. Therefore, by comparing the PI group to one another with age adopted out of the orphanage as an independent variable in the regression analyses, we in part control for this variability and compare within the PI group along this influential variable. Age adopted out predicted amygdala volume in a dose-related fashion, where longer periods of orphanage rearing were related to larger amygdala volumes (cortex-corrected), a pattern which is highly consistent with the earlier described animal models that have shown a causal link between early-life stress and subsequent emotional behavior. Although Gunnar and colleagues (2007) have shown that children adopted from Eastern Europe are at greater risk for developing behavior problems and in the current sample children from Eastern Europe tended to be adopted at older ages, the association between age adopted out and amygdala volume remained even when only children adopted from Asia were included in the analyses.
In contrast, prolonged stress typically results in a decrease in structure and function (see Bremner, 2006 and McEwen, 2007 for a review of the literature), a phenotype which shows recovery once the stressor period has ended (Vyas et al., 2004), even when that stress is experienced early in life (Yang, Hou, Ma, Liu, Zhang, Zhou, Xu, & Li, 2007). Similarly, in the current study, the difference in hippocampal measurements between PI and comparison children did not reach statistical significance, perhaps as a result of the overwhelming enrichment PI children typically receive in homes relative to orphanages. The lack of hippocampal and caudate differences also highlights the specific long-term association between stress and the amygdala. Alternatively, hippocampal changes following stress may be masked during childhood. Studies do not find stress-induced hippocampal shrinkage in children, although adults who experienced stress as children show decreased hippocampal volume (Bremner, Vythilingam, Vermetten, Southwick, McGlashan, Nazeer, Khan, Vaccarino, Soufer, Garg, Ng, Staib, Duncan, & Charney, 2003). Perhaps developmental change in hippocampal volume prevents observation of stress-induced changes in hippocampus as measured by MRI. Indeed, most of the children in the current study were female, and in typical female subjects hippocampal volume tends to show developmental change during childhood and adolescence (Giedd, Vaituzis, Hamburger, Lange, Rajapakse, Kaysen, Vauss, & Rapoport, 1996), while amygdala volumes tends to not show development change as measured by structural MRI.
We used an emotional go-nogo task to measure individual differences in one’s ability to regulate behavior during the presentation of emotionally provocative social information. As a group, all children performed better when blocks contained positive facial expressions and when emotional faces were the target stimuli rather than the distracter stimuli, as indexed by high % correct scores and fast reaction times. However, children who spent the longest amount of time in orphanage care made significantly more errors during blocks of trials that contained negatively valenced faces. The errors associated with later ages of adoption were false alarms errors when the distractor item (the item during which one was instructed to withhold pressing) was a negatively valenced face. These errors are viewed as errors in behavioral regulation (i.e., default to the prepotent response of pressing the button, which is the more frequent response) when cognitive resources were captured by emotionally salient events. The greater number of accidental responses to distracters might be the result of PI children being more affected by the emotional context of the task in general and therefore, their behavior being biased more by this information. In support of this theory, unlike with the comparison group, the amount of time needed for a PI child to press the button for targets varied greatly depending on both the valence and the stimulus type. The resulting pattern, relative slowing to neutral in the context of positive valence and relative accelerating to neutral when in the context of negatively valenced faces, is consistent with the notion that late adopted PI children are more likely than other children to be influenced by emotional contexts. This susceptibility to the emotional context is consonant with the difficulty in emotion regulation reported previously for this population of children (Hodges & Tizard, 1989) and may be the basis for emotion regulation difficulties. These statements are made with caution since children who have experienced parental neglect have shown impairments in expression recognition (Pollak et al., 2002), and these difficulties could contribute to poor performance in the face gonogo. However, in the current study age adopted out of an orphanage was specifically associated with false alarm errors, not misses, and this pattern of errors suggests that early institutionalization is associated with poor self regulation in the presence of emotionally arousing stimuli.
Poor emotion regulation is mediated by cellular growth in the amygdala in animal models of stress (Mitra et al., 2005; Vyas et al., 2002). Anxious humans (both children and adults), typically have a larger and more reactive amygdala as well as a greater processing bias for negative information (Barros-Loscertales et al., 2006; Bradley et al., 1999; Dalgleish et al., 2001; Mogg, Bradley, de Bono, & Painter, 1997; Mogg, Bradley, & Hallowell, 1994; Mogg, Kentish, & Bradley, 1993) and children with anxiety disorders (De Bellis et al., 2000; MacMillan et al., 2003). PI children tend to show more anxiety (Ellis et al., 2004) and internalizing behavior problems relative to non-adopted peers (although still showing less than children who were domestically adopted; Juffer & van Ijzendoorn, 2005). Therefore, understanding the development of these phenotypes provides a framework for understanding anxiety. In the current study larger amygdala volumes predicted higher ratings of anxiety (as measured by the SCARED) and more internalizing behaviors (as measured by the CBCL), which is a risk factor for later psychopathology including mood disorder (Hofstra, van der Ende, & Verhulst, 2002). The association between internalizing/anxious behaviors and larger amygdala volume existed for both the PI group and the comparison group, suggesting that early stress may be one of many possible routes to later anxious phenotypes. There was a trend for amygdala volume to predict more difficulties in emotional regulation (greater number of errors for faces with negative valence). The number of PI children in this study who reached diagnostic threshold for an anxiety disorder exceeded those reported within the greater population (Costello, Angold, Burns, Stangl, Tweed, Erkanli, & Worthman, 1996). Nonetheless, there were too to properly test whether the presence of an anxiety disorder was related to amygdala volume; however, the association between amygdala volume and age adopted out of the orphanage remained even when children with clinical anxiety were excluded from the analysis. While we cannot determine directionality based on these results, in the context of the animal literature, they suggest that early stress can result in amygdala hypertrophy, which produces greater reactivity to highly emotional stimuli and a more anxious phenotype. This type of adversity-driven “kindling” of the amygdala may be a significant risk factor for the development of anxiety disorders (Rosen et al., 1998). This etiological model of anxiety is supported by previous work (Thomas et al., 2001) showing that greater amygdala response to negative information correlates positively with everyday ratings of anxiety in children and adolescents.
Ethical constraints render it difficult to randomly assign children to rearing environments (Nelson, Zeanah, Fox, Marshall, Smyke, & Guthrie, 2007; Zeanah, Nelson, Fox, Smyke, Marshall, Parker, & Koga, 2003), and therefore, these data can only show a relationship between orphanage rearing and differences in emotion processing systems. There are other possible interpretations of the data. For example, longer periods in an orphanage translates into shorter periods with the family, and based on the high socio-economic status profile of families who tend to adopt internationally (Hellerstedt et al., 2008), presumably this is an environment that is an enriched on relative to an orphanage. So, although these data suggest that the effects of institutionalization were greatest for those children who remained in the orphanage the longest, it may instead be that amygdala volume and hypersensitivity to negative stimuli decrease with increasing amounts of time spent with a family. In this way, time with family can be viewed as an “intervention” for children who have experienced orphanage rearing. Although data from animal models suggest that changes in amygdala morphology are resistant to recovery (Vyas, Pillai, & Chattarji, 2004), longitudinal data will establish whether these data reflect actual plasticity and eventual normalization in emotion processing systems.
There are a number of limitations of this study inherent to research on this special population. First, the current nature of international adoption is such that parents, and thus researchers, do not have access to preadoption developmental histories, and there are many levels of privation. Given the heterogeneity of this sample, it would be impossible to define an appropriate control group for PI children. The approach taken in this paper, to rely on the continuous variable of age of adoption, has merit in that we are able to compare children within groups and make claims about the duration for which the adversity was experienced. We see this approach as a promising one for future studies with this population, and recognize that the comparison against non-adopted healthy children may be too liberal. Such comparisons are, however, informative when trying to understand how PI children may deviate from typical progressions of behavior and brain development. Because of the correlational nature of this study, it is also possible that children who are psychiatrically healthier may be more likely to be adopted out of the orphanage at earlier ages. Thirdly, although the adoption process itself may be a temporary stressor, it is a constant across the PI group, and our measures were taken years after the adoption process. Therefore, it is our opinion that the observed effects were the effects of institutionalization, not the adoption process itself. Fourth, although neuroimaging data are presented from 62 children and behavioral data are presented from 46 children, only 30 children provided complete neuroimaging and behavioral data. There remains the possibility that the children who provided neuroimaging but not behavioral data and children who provided behavioral data but not neuroimaging data are different from each other and therefore, should not be grouped together in discussion of long-term correlates of early institutionalization. Although the general pattern of increased emotionality remains consistent across all subgroups of PI children who participated in this study, conclusions about the consistency between emotional behavior and amygdala findings based on these results should be tempered. One of the goals of this study was to examine the development of limbic structures and associated behaviors following early institutional care. While this question is best addressed using a longitudinal design, the current design was cross-sectional, which tempers the strength of the conclusions that can be drawn form the data.
A final question that remains unanswered regards the age at which the adversity was experienced. Age adopted out of the orphanage predicted amygdala volume and performance on the emotional go-nogo task. It is not clear whether these effects were caused by the duration of time spent in the orphanage or the chronological age at which children experienced the stressor. Data from non-human primates that vary the age of maternal separation suggest that the experiences of later maternally-separated youngsters and the mourning they endure are fundamentally different from those who are separated soon after birth (Nelson et al., 2002). We are unable to ask this developmental question with the current design.
What does a larger amygdala indicate on a functional level? The literature from animal models suggests that amygdala hypertrophy is associated with increased reactivity (Vyas, Jadhav, & Chattarji, 2006), consistent with the behavioral and clinical data presented in this manuscript. Decreased activity in limbic regions, including the amygdala, has been reported elsewhere (Chugani, Behen, Muzik, Juhasz, Nagy, & Chugani, 2001), although the comparison groups used in that study (adults and epileptic children) make the results difficult to interpret. To better understand the functional significance of enlarged amygdala volumes found in this population, future work will need to examine amygdala activity to emotionally provocative stimuli in this population using such techniques as functional MRI.
Much has been learned from animal models about the effects of stress on emotional behavioral and underlying neural circuitry. Because pre- and post-adoption environments differ to a great extent, studying children who have experienced orphanage rearing provides an opportunity to examine how early adversity can impact the developing emotional system in ways that are difficult to do in other stressed populations, where the effects of early and later stress cannot be easily untangled. Importantly, such studies also provide information on how and whether these systems can be altered or normalized to a typical developmental trajectory. The dose related finding directly informs policymaking by underscoring the importance of a rapid adoption process. Data like those presented in this paper should inform parents, therapists, pediatricians, agencies, and policy makers to minimize orphanage rearing experiences worldwide.
Supplementary Material
Distribution of psychiatric disorders in the sample of PI children. The distribution of children with disorders in the entire sample was representative of the subsamples of children for whom MRI data and behavioral data was obtained.
ABBREVIATIONS
- ADHD
attention deficit hyperactivity disorder
- CBCL
Child Behavior Checklist
- MRI
magnetic resonance imaging
- msec
milliseconds
- ODD
oppositional defiant disorder
- PI
previously institutionalized
- PDD
pervasive developmental disorder
- SCARED
Screen for Child Anxiety Related Emotional Disorders
- WASI
Wechsler Abbreviated Scale of Intelligence
Footnotes
Previous cross sectional data indicate that while some volumetric development occurs between the ages of 4 – 18 years old (Giedd et al., 1996), the change occurred in male children and was not observed in female children. The majority of the children in the current sample are also female, and therefore, we were less concerned with age-related changes over a 16-month period. Nonetheless, analyses are described in the Results section to address the age difference.”
SCARED data were only obtained from a subsample from of participants because this measure was collected after the start of the study.
The results obtained in this study were consistent across the two scanner environments. We provide evidence for the inter-scanner reliability in the Results section.
We also calculated sensitivity using a d-prime score showing a similar pattern of results, but d-prime cannot be accurately calculated when there is very high hit or false alarm rate.
The early adopted PI group is 16 months younger than their comparison group. Although this age difference is not expected to result in amygdala volumetric differences, we compared the age of the early adopted PI group with the age of the comparison group used for the late adopted PI group (since amygdala volume did not differ between these two groups either). Age was not significantly different (t(29) = 0.85, ns). Therefore, we are confident that the lack of volumetric difference between the early adopted PI group and their comparison group was not the artifact of an 16 month age difference.
These same statistical analyses were run separately for those data collected in the 1.5T scanner and the 3T scanner, although the number of subjects provides little statistical power. The pattern obtained when the data were collapsed is the same pattern observed when the data are split of the scanner that was used for data collection. A one-way ANOVA showed a difference between groups for the amygdala in both the 1.5T (trend: F(2,23) = 2.73, p = 0.09) and 3T scanner (F(2,37) = 7.74, p < 0.002), but not for the hippocampus (F(2,23)= 1.04, ns; F(2,37) = 0.03, ns, 1.5T and 3T scanners respectively) or the caudate (F(2,23)= .05, ns; F(2,37) = 1.18, ns, 1.5T and 3T scanners respectively).
This association between age adopted and amygdala volume was observed even when the child who was adopted at 60 months of age was removed from the analysis (F(1,32)=5.46, p<.03) and when only children from Asia were included in the analyses (F(1,26)=7.58, p<.015).
References
- Achenbach TM. Integrative Guide to the 1991 CBCL/4–18, YSR, and TRF Profiles. Burlington, VT: University of Vermont, Department of Psychology; 1991. [Google Scholar]
- Ames E. Spitz revisited: A trip to Romanian "orphanages". Canadian Psychological Association Developmental Psychology Section Newsletter. 1990;9:8–11. [Google Scholar]
- Ames E. The development of Romanian orphan-age children adopted to Canada (final report to the National Welfare Grants Program: HUman Resources Development Canada) Burnaby, BC: Simon Fraser University; 1997. [Google Scholar]
- Barbazanges A, Vallée M, Mayo W, Day J, Simon H, Le Moal M, S M. Early and later adoptions have different long-term effects on male rat offspring. Journal of Neuroscience. 1996;16(23):7783–7790. doi: 10.1523/JNEUROSCI.16-23-07783.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros-Loscertales A, Meseguer V, Sanjuan A, Belloch V, Parcet MA, Torrubia R, Avila C. Behavioral Inhibition System activity is associated with increased amygdala and hippocampal gray matter volume: A voxel-based morphometry study. Neuroimage. 2006;33(3):1011–1015. doi: 10.1016/j.neuroimage.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Beckett C, Maughan B, Rutter M, Castle J, Colvert E, Groothues C, Kreppner J, Stevens S, O'Connor TG, Sonuga-Barke EJ. Do the effects of early severe deprivation on cognition persist into early adolescence? Findings from the English and Romanian adoptees study. Child Dev. 2006;77(3):696–711. doi: 10.1111/j.1467-8624.2006.00898.x. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38(10):1230–1236. doi: 10.1097/00004583-199910000-00011. [DOI] [PubMed] [Google Scholar]
- Blau VC, Maurer U, Tottenham N, McCandliss BD. The face-specific N170 component is modulated by emotional facial expression. Behav Brain Funct. 2007;3:7. doi: 10.1186/1744-9081-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonne O, Brandes D, Gilboa A, Gomori JM, Shenton ME, Pitman RK, Shalev AY. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am J Psychiatry. 2001;158(8):1248–1251. doi: 10.1176/appi.ajp.158.8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, White J, Groom C, de Bono J. Attentional bias for emotional faces in generalized anxiety disorder. Br J Clin Psychol. 1999;38(Pt 3):267–278. doi: 10.1348/014466599162845. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Traumatic stress: effects on the brain. Dialogues Clin Neurosci. 2006;8(4):445–461. doi: 10.31887/DCNS.2006.8.4/jbremner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, Innis RB. MRI-based measurement of hippocampal volume in patients with combat- related posttraumatic stress disorder. American Journal of Psychiatry. 1995;152(7):973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, Charney DS. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse--a preliminary report. Biol Psychiatry. 1997;41(1):23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, Khan S, Vaccarino LV, Soufer R, Garg PK, Ng CK, Staib LH, Duncan JS, Charney DS. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry. 2003;106(5):924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U S A. 1998;95(9):5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhasz C, Nagy F, Chugani DC. Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. Neuroimage. 2001;14(6):1290–1301. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Angold A, Burns BJ, Stangl DK, Tweed DL, Erkanli A, Worthman CM. The Great Smoky Mountains Study of Youth. Goals, design, methods, and the prevalence of DSM-III-R disorders. Arch Gen Psychiatry. 1996;53(12):1129–1136. doi: 10.1001/archpsyc.1996.01830120067012. [DOI] [PubMed] [Google Scholar]
- Dalgleish T, Moradi AR, Taghavi MR, Neshat-Doost HT, Yule W. An experimental investigation of hypervigilance for threat in children and adolescents with post-traumatic stress disorder. Psychological Medicine. 2001;31:541–547. doi: 10.1017/s0033291701003567. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Casey BJ, Dahl R, Birmaher B, Williamson D, Thomas KM, Axelson DA, Frustaci K, Boring AM, Hall J, Ryan N. A Pilot Study of Amygdala Volumes in Pediatric Generalized Anxiety Disorder. Biological Psychiatry. 2000 doi: 10.1016/s0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, Frustaci K, Ryan ND. Developmental truamatology. Part II: Brain development. A.E. Bennet Research Award. Biological Psychiatry. 1999;45(10):1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;111(2):225–236. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Vuilleumier P. Amygdala automaticity in emotional processing. Ann N Y Acad Sci. 2003;985:348–355. doi: 10.1111/j.1749-6632.2003.tb07093.x. [DOI] [PubMed] [Google Scholar]
- Durfee H, Wolf K. Anstaltspflege und entwicklung im ersten lebensjahr. Zeitschrift fur Kinderforschung. 1933;42(3) [Google Scholar]
- Ekman P, Friesen WV. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- Ellis BH, Fisher PA, Zaharie S. Predictors of disruptive behavior, developmental delays, anxiety, and affective symptomatology among institutionally reared Romanian children. J Am Acad Child Adolesc Psychiatry. 2004;43(10):1283–1292. doi: 10.1097/01.chi.0000136562.24085.160. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23 Suppl 1:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FA, Liu D, Meaney MJ. Maternal care, gene expression, and the development of individual differences in stress reactivity. Ann N Y Acad Sci. 1999;896:66–84. doi: 10.1111/j.1749-6632.1999.tb08106.x. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl E, Zetzsche T, Bottlender R, Born C, Groll C, Jager M, Leinsinger G, Hahn K, Moller H. Enlargement of the Amygdala in Patients with a First Episode of Major Depression. Biological Psychiatry. 2002;51:708–714. doi: 10.1016/s0006-3223(01)01359-2. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. Journal of Comparative Neurology. 1996;366(2):223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Black JE, Wallace CS. Experience and brain development. Child Dev. 1987;58(3):539–559. [PubMed] [Google Scholar]
- Greenough WT, Hwang HM, Gorman C. Evidence for active synapse formation or altered postsynaptic metabolism in visual cortex of rats reared in complex environments. Proc Natl Acad Sci U S A. 1985;82(13):4549–4552. doi: 10.1073/pnas.82.13.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, McDonald JW, Parnisari RM, Camel JE. Environmental conditions modulate degeneration and new dendrite growth in cerebellum of senescent rats. Brain Res. 1986;380(1):136–143. doi: 10.1016/0006-8993(86)91437-x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Bruce J, Grotevant HD. International adoption of institutionally reared children: Research and policy. Development and Psychopathology. 2000;(12):677–693. doi: 10.1017/s0954579400004077. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, van Dulmen MH. Behavior problems in postinstitutionalized internationally adopted children. Dev Psychopathol. 2007;19(1):129–148. doi: 10.1017/S0954579407070071. [DOI] [PubMed] [Google Scholar]
- Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, Gilbertson MW, Orr SP, Kikinis R, Jolesz FA, McCarley RW, Pitman RK. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry. 1996;40(11):1091–1099. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwin JA, Donnelly N, French CC, Richards A, Watts A, Daley D. The influence of children's self-report trait anxiety and depression on visual search for emotional faces. J Child Psychol Psychiatry. 2003;44(3):432–444. doi: 10.1111/1469-7610.00133. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Davidson MC, Glover GH, Casey BJ. Contributions of amygdala and striatal activity in emotion regulation. Biological Psychiatry. 2005;57(6):624–632. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellerstedt WL, Madsen NJ, Gunnar MR, Grotevant HD, Lee RM, Johnson DE. The International Adoption Project: Population-based Surveillance of Minnesota Parents Who Adopted Children Internationally. Matern Child Health J. 2008;12(2):162–171. doi: 10.1007/s10995-007-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges J, Tizard B. Social and family relationships of ex-institutional adolescents. J Child Psychol Psychiatry. 1989;30(1):77–97. doi: 10.1111/j.1469-7610.1989.tb00770.x. [DOI] [PubMed] [Google Scholar]
- Hofstra MB, van der Ende J, Verhulst FC. Child and adolescent problems predict DSM-IV disorders in adulthood: a 14-year follow-up of a Dutch epidemiological sample. J Am Acad Child Adolesc Psychiatry. 2002;41(2):182–189. doi: 10.1097/00004583-200202000-00012. [DOI] [PubMed] [Google Scholar]
- Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl) 2001;158(4):366–373. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- Johnson DE. Adoption and the effect on children's development. Early Hum Dev. 2002;68(1):39–54. doi: 10.1016/s0378-3782(02)00017-8. [DOI] [PubMed] [Google Scholar]
- Juffer F, van Ijzendoorn MH. Behavior problems and mental health referrals of international adoptees: a meta-analysis. Jama. 2005;293(20):2501–2515. doi: 10.1001/jama.293.20.2501. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Williamson DE, Birmaher B, Axelson DA, Ryan ND, Casey BJ. Processing emotional facial expressions influences performance on a Go/NoGo task in pediatric anxiety and depression. J Child Psychol Psychiatry. 2006;47(11):1107–1115. doi: 10.1111/j.1469-7610.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, Koeppe RA, Fig LM. Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry. 1999;45(7):817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- Lindauer RJ, Vlieger EJ, Jalink M, Olff M, Carlier IV, Majoie CB, den Heeten GJ, Gersons BP. Smaller hippocampal volume in Dutch police officers with posttraumatic stress disorder. Biol Psychiatry. 2004;56(5):356–363. doi: 10.1016/j.biopsych.2004.05.021. [DOI] [PubMed] [Google Scholar]
- MacMillan S, Szeszko PR, Moore GJ, Madden R, Lorch E, Ivey J, Banerjee SP, Rosenberg DR. Increased amygdala: hippocampal volume ratios associated with severity of anxiety in pediatric major depression. J Child Adolesc Psychopharmacol. 2003;13(1):65–73. doi: 10.1089/104454603321666207. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2005;102(26):9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, de Bono J, Painter M. Time course of attentional bias for threat information in non-clinical anxiety. Behav Res Ther. 1997;35(4):297–303. doi: 10.1016/s0005-7967(96)00109-x. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Hallowell N. Attentional bias to threat: roles of trait anxiety, stressful events, and awareness. Q J Exp Psychol A. 1994;47(4):841–864. doi: 10.1080/14640749408401099. [DOI] [PubMed] [Google Scholar]
- Mogg K, Kentish J, Bradley BP. Effects of anxiety and awareness on colour-identification latencies for emotional words. Behav Res Ther. 1993;31(6):559–567. doi: 10.1016/0005-7967(93)90107-6. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Bloom FE, Cameron JL, Amaral DG, Dahl RE, Pine DS. An integrative, multidisciplinary approach to the study of brain–behavior relations in the context of typical and atypical development. Development and Psychopathology. 2002;14:499–520. doi: 10.1017/s0954579402003061. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science. 2007;318(5858):1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Rutter M. Attachment disorder behavior following early severe deprivation: extension and longitudinal follow-up. English and Romanian Adoptees Study Team. J Am Acad Child Adolesc Psychiatry. 2000;39(6):703–712. doi: 10.1097/00004583-200006000-00008. [DOI] [PubMed] [Google Scholar]
- Parker SW, Nelson CA. The impact of early institutional rearing on the ability to discriminate facial expressions of emotion: an event-related potential study. Child Dev. 2005;76(1):54–72. doi: 10.1111/j.1467-8624.2005.00829.x. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Sinha P. Effects of early experience on children's recognition of facial displays of emotion. Dev Psychol. 2002;38(5):784–791. doi: 10.1037//0012-1649.38.5.784. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Vardi S, Putzer Bechner AM, Curtin JJ. Physically abused children's regulation of attention in response to hostility. Child Development. 2005;76(5):968–977. doi: 10.1111/j.1467-8624.2005.00890.x. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47(9):769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Hamerman E, Sitcoske M, Glowa JR, Schulkin J. Hyperexcitability: exaggerated fear-potentiated startle produced by partial amygdale kindling. Behav Neurosci. 1996;110(1):43–50. doi: 10.1037//0735-7044.110.1.43. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Schulkin J. From normal fear to pathological anxiety. Psychol Rev. 1998;105(2):325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- Rosenblum LA, Forger C, Noland S, Trost RC, Coplan JD. Response of adolescent bonnet macaques to an acute fear stimulus as a function of early rearing conditions. Dev Psychobiol. 2001;39(1):40–45. doi: 10.1002/dev.1026. [DOI] [PubMed] [Google Scholar]
- Rutter M. Developmental catch-up, and deficit, following adoption after severe global early privation. English and Romanian Adoptees (ERA) Study Team. J Child Psychol Psychiatry. 1998;39(4):465–476. [PubMed] [Google Scholar]
- Rutter M, O'Connor TG. Are there biological programming effects for psychological development? Findings from a study of Romanian adoptees. Dev Psychol. 2004;40(1):81–94. doi: 10.1037/0012-1649.40.1.81. [DOI] [PubMed] [Google Scholar]
- Schmahl CG, Vermetten E, Elzinga BM, Bremner DJ. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Res. 2003;122(3):193–198. doi: 10.1016/s0925-4927(03)00023-4. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdale and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62(3):273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Smyke A, Dumitrescu A, Zeanah CH. Attachment Disturbances in Young Children. I: The Continuum of Caretaking Casualty. J. AM. ACAD.CHILDADOLESC.PSYCHIATRY. 2002;41(8):972–982. doi: 10.1097/00004583-200208000-00016. [DOI] [PubMed] [Google Scholar]
- Smyke AT, Koga SF, Johnson DE, Fox NA, Marshall PJ, Nelson CA, Zeanah CH, Group BC. The caregiving context in institution-reared and family-reared infants and toddlers in Romania. Journal of Child Psychology & Psychiatry. 2007;48(2):210–208. doi: 10.1111/j.1469-7610.2006.01694.x. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PJ, Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58(11):1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Tupler LA, De Bellis MD. Segmented hippocampal volume in children and adolescents with posttraumatic stress disorder. Biol Psychiatry. 2006;59(6):523–529. doi: 10.1016/j.biopsych.2005.08.007. [DOI] [PubMed] [Google Scholar]
- van Ijzendoorn MH, Juffer F. The Emanuel Miller Memorial Lecture 2006: adoption as intervention. Meta-analytic evidence for massive catch-up and plasticity in physical, socio-emotional, and cognitive development. Journal of Child Psychology & Psychiatry. 2006;47(12):1228–1245. doi: 10.1111/j.1469-7610.2006.01675.x. [DOI] [PubMed] [Google Scholar]
- Vasey MW, el-Hag N, Daleiden EL. Anxiety and the processing of emotionally threatening stimuli: distinctive patterns of selective attention among high- and low-test-anxious children. Child Dev. 1996;67(3):1173–1185. [PubMed] [Google Scholar]
- Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res. 2003;965(1–2):290–294. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143(2):387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128(4):667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Wignall EL, Dickson JM, Vaughan P, Farrow TF, Wilkinson ID, Hunter MD, Woodruff PW. Smaller hippocampal volume in patients with recent-onset posttraumatic stress disorder. Biol Psychiatry. 2004;56(11):832–836. doi: 10.1016/j.biopsych.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Williams JM, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychol Bull. 1996;120(1):3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- Yang J, Hou C, Ma N, Liu J, Zhang Y, Zhou J, Xu L, Li L. Enriched environment treatment restores impaired hippocampal synaptic plasticity and cognitive deficits induced by prenatal chronic stress. Neurobiol Learn Mem. 2007;87(2):257–263. doi: 10.1016/j.nlm.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Nelson CA, Fox NA, Smyke AT, Marshall P, Parker SW, Koga S. Designing research to study the effects of institutionalization on brain and behavioral development: the Bucharest Early Intervention Project. Dev Psychopathol. 2003;15(4):885–907. doi: 10.1017/s0954579403000452. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of psychiatric disorders in the sample of PI children. The distribution of children with disorders in the entire sample was representative of the subsamples of children for whom MRI data and behavioral data was obtained.