Enhanced gene transfection activity is observed when using a new helper lipid with DOTAP, compared to DOPE.
The delivery of nucleic acids to a cell offers the potential to correct a defective gene or introduce a new gene for a specific biological activity.1–7 As such, in vitro gene delivery is widely used in research laboratories, and in vivo gene therapy holds promise for the cure of genetic diseases including cancer. The current delivery approaches include viral vectors, synthetic cationic vectors, calcium phosphate particles, surface-mediated vectors, and electroporation.1,2,8,9 Of these, synthetic cationic vectors offer the advantages of minimal toxicity, non-immunogenicity, ease of synthesis, and large nucleic acid payloads; but suffer from low transfection activities.1–4,10–20 This low activity reflects inefficiencies in the overall transfection pathway.21 Today, many synthetic cationic vectors such as 1,2-dioleoyloxy-3-(trimethylammonio)propane (DOTAP; 1) are used in conjunction with “helper” phospholipids which allow fusion of the bilayer with the membrane of the endosome, to increase the transfection efficacy.22,23 These helper lipids are typically zwitterionic lipids such as dioleoylphosphatidylethanolamine (DOPE; 2) or dioleoylphosphatidylcholine (DOPC; 3). Herein, we describe the synthesis and characterization of a new helper lipid that displays enhanced gene transfection activity when used with DOTAP.
The zwitterionic phospholipid 4 is shown in Fig. 1. The complete synthetic procedure to lipid 4 can be found in the ESI†. Briefly stated, benzyl formate was added to an octane solution of dodecanoic diacid in the presence of Dowex 50WX2, and the resulting monobenzylated fatty acid was coupled to 3-(tert-butyldiphenylsilyl)glycerol in the presence of DCC and DMAP in CH2Cl2. The silyl group was removed with n-tetrabutylammonium fluoride in tetrahydrofuran. The resulting compound was reacted with chloro-oxo-dioxaphospholane in THF at 0 °C for 18 h, and then the reaction mixture was transferred to a pressure tube and heated to 60 °C for 1 day with trimethylamine in acetonitrile to give compound 4.
Fig. 1.
Cationic and zwitterionic amphiphiles under investigation.
Given the structural similarity between 4 and lipids 2 and 3, it is likely that 4 will form bilayers. A differential scanning calorimeter (DSC) trace of hydrated amphiphile 4 shows a phase-transition temperature at ∼44 °C. Next, we prepared vesicles of 4, 4/DOTAP, and DOPC/DOTAP. The milky aqueous suspension of 4, 4/DOTAP, or DOPC/DOTAP was extruded through a 100 nm polycarbonate membrane using a mini-extruder and after 20 extrusions, a homogeneous liposome solution was observed (see ESI for details†). The average diameter, determined by dynamic light scattering, of the liposomes prepared from 4, 4/DOTAP, and DOPC/DOTAP was 106, 127, and 139 nm, respectively. Transmission electron micrographs of 4 and 4/DOTAP showed vesicular organizations in both samples of several hundred nanometres. The structure of the bilayers formed by 4 was investigated at 25 °C by X-ray diffraction. The diffraction patterns of oriented multilayers of 4 show a lamellar structure with a d spacing of 6.1 nm. We were unable to obtain patterns from pellets of 4/DOTAP. In the presence of DNA, the 4/DOTAP/DNA assembly d spacing increases to 7.6 nm. This 1.5 nm increase in repeat period is similar to that observed for other bilayers containing cationic lipids when DNA is incorporated between adjacent bilayers.16
The propensity of the lipids to bind DNA was measured via an ethidium bromide displacement fluorescence assay. This assay entailed measuring the reduction of the fluorescence intensity of the DNA-intercalated ethidium bromide, as this fluorescent probe is displaced by the cationic amphiphile. The fluorescence intensity decreases upon addition of DOTAP, 4/DOTAP, DOTAP/DOPE, and DOPC/DOTAP. The results obtained with DOTAP, DOTAP/DOPE, and DOPC/DOTAP are consistent with previous reports. A 1 : 1 assembly is formed between the lipids and DNA. The zwitterionic lipids 4, DOPC, and DOPE do not displace EtBr, consistent with the unfavorable electrostatic interactions with the anionic DNA (Fig. 2).
Fig. 2.
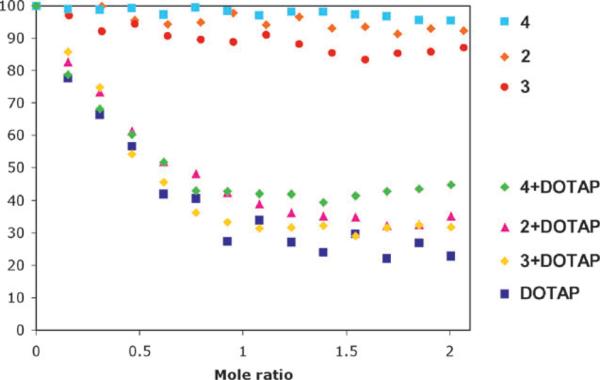
Ethidium bromide displacement assay for the amphiphiles under investigation. N = 3. Error bars per point range from 5 to 10%.
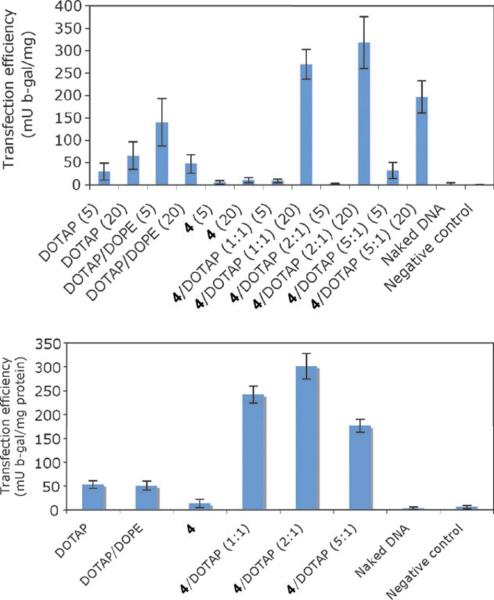
Transfection experiments with 4, 4/DOTAP, DOPE/DOTAP and DOTAP using the reporter gene, β-galactosidase (β-gal, pVax-LacZ1, Invitrogen), were performed with Chinese hamster ovarian (CHO) cells and L6 human muscle cells. The DOPE/DOTAP system was used instead of DOPC/DOTAP since the former is reported to be more active.22 The reporter gene was first mixed with the lipids in potassium phosphate buffer (PBS) at room temperature. We tested two different lipid/DNA ratios (5 : 1 and 20 : 1) while keeping the zwitterionic/DOTAP ratio constant at 1 : 1. Next, the lipid/DNA complexes were added to the cells. The amount of DNA used was the same as used in the naked DNA control (no lipid), and the negative control was compound 4 without DNA. After incubation at 37 °C and 5% CO2 for 2 h, the medium was removed and fresh growth medium was added. Transfection efficiencies were assessed after 48 h using the β-galactosidase enzyme assay in conjunction with a standard curve. The efficiency of each transfection was calculated as β-gal activity normalized to total protein. The zwitterionic lipid 4 by itself does not transfect DNA. As expected upon addition of DOPE to DOTAP, the transfection efficacy increased.22 In the presence of 4/DOTAP at a ratio of 1 : 1 (with amphiphile/DNA ratio of 20 : 1), the transfection increased ∼400% when compared to DOPE/DOTAP under the same conditions (Fig. 3). Increasing the 4/DOTAP ratio from 1 : 1 to 2 : 1 afforded higher activity, but further increases in the ratio yielded less transfection. With these encouraging results, we evaluated the 4/DOTAP vector system for gene transfection in L6 cells. As shown in Fig. 3, the use of the zwitterionic lipid 4 and DOTAP increased the transfection level by about four-fold compared to DOPE/DOTAP. Cytotoxicity experiments were also performed with CHO cells, and none of the amphiphiles showed significant cytotoxicity, with results similar to the non-treated cells (see ESI†).
Fig. 3.
(Top) gene transfection results after 48 h in CHO cells (cation/zwitterion ratio in parentheses) and (bottom) gene transfection results after 48 h in L6 cells (cation/zwitterion ratio in parentheses). Amphiphile/DNA ratio 1 : 20; N = 3.
In summary, we have synthesized and characterized a new helper zwitterionic phospholipid for use in the delivery of nucleic acids into cells. When combined with DOTAP, this phospholipid affords an increase in gene delivery as measured by new protein expression in two different cell lines. Mechanistic studies including cell trafficking experiments are ongoing to elucidate the pathway and the role this new helper lipid plays in gene transfection. The investigation of new synthetic vector systems provides opportunities to prepare specific amphiphilic structures, study their self-assembly properties, characterize their interactions with nucleic acids, and evaluate new strategies for increasing transfection efficiency.
Supplementary Material
Acknowledgments
This work was supported by BU and grants from the National Institutes of Health (MWG and TJM).
Footnotes
Electronic supplementary information (ESI) available: Complete experimental details, characterization data, and cytotoxicity experiments. See DOI: 10.1039/b716247b
Notes and references
- 1.Luo D, Saltzman WM. Nat. Biotechnol. 2000;18:33. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- 2.Davis ME. Curr. Opin. Biotechnol. 2002;13:128. doi: 10.1016/s0958-1669(02)00294-x. [DOI] [PubMed] [Google Scholar]
- 3.Kabanov AV, Felgner PL, Seymour LW. Self-assembling complexes for gene delivery: From laboratory to clinical trial. John Wiley and Sons; West Sussex, England: 1998. [Google Scholar]
- 4.Felgner PL. Adv. Drug Delivery Rev. 1990;5:163. doi: 10.1016/s0169-409x(97)00101-4. [DOI] [PubMed] [Google Scholar]
- 5.Putnam D. Nat. Mater. 2006;5:439. doi: 10.1038/nmat1645. [DOI] [PubMed] [Google Scholar]
- 6.Reineke TM, Grinstaff MW. MRS Bull. 2005;30:635. [Google Scholar]
- 7.Barthélémy P, Lee SJ, Grinstaff MW. Pure Appl. Chem. 2005;77:2133. [Google Scholar]
- 8.Pannier AK, Shea LD. Mol. Ther. 2004;10:19. doi: 10.1016/j.ymthe.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Prata CAH, Zhao Y, Barthélémy B, Li Y, Luo D, McIntosh TJ, Lee SJ, Grinstaff MW. J. Am. Chem. Soc. 2004;126:12744. doi: 10.1021/ja0474906. [DOI] [PubMed] [Google Scholar]
- 10.Behr JP. Acc. Chem. Res. 1993;26:274. [Google Scholar]
- 11.Huang L, Hung M, Wagner E. Nonviral vectors for gene therapy. Academic Press; New York: 1999. [Google Scholar]
- 12.Miller AD. Angew. Chem., Int. Ed. 1998;37:1768. [Google Scholar]
- 13.Remy J, Sirlin C, Vierling P, Behr J. Bioconjugate Chem. 1994;5:647. doi: 10.1021/bc00030a021. [DOI] [PubMed] [Google Scholar]
- 14.Deshmukh HM, Huang L. New J. Chem. 1997;21:113. [Google Scholar]
- 15.Legendre JY, Szoka FCJ. Pharmacol. Res. 1992;10:1235. doi: 10.1023/a:1015836829670. [DOI] [PubMed] [Google Scholar]
- 16.MacDonald RC, Ashley GW, Shida MM, Rakhmanova VA, Tarahovsky YS, Kennedy MT, Pozharski EV, Baker KA, Jones RD, Rosenzweig HS, Choi KL, Qiu R, McIntosh TJ. Biophys. J. 1999;77:2612. doi: 10.1016/S0006-3495(99)77095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robbins PD, Tahara H, Ghivizzani SC. Trends Biotechnol. 1998;16:35. doi: 10.1016/S0167-7799(97)01137-2. [DOI] [PubMed] [Google Scholar]
- 18.Miller AD. Curr. Med. Chem. 2003;10:1195. doi: 10.2174/0929867033457485. [DOI] [PubMed] [Google Scholar]
- 19.Liu YM, Wenning L, Lynch M, Reineke TM. J. Am. Chem. Soc. 2004;126:7422. doi: 10.1021/ja049831l. [DOI] [PubMed] [Google Scholar]
- 20.Chabaud P, Camplo M, Payet D, Serin G, Moreau L, Barthélémy P, Grinstaff MW. Bioconjugate Chem. 2006;17:466. doi: 10.1021/bc050162q. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y, Szoka FCJ. Biochemistry. 1996;35:5616. doi: 10.1021/bi9602019. [DOI] [PubMed] [Google Scholar]
- 22.Felgner JH, Kumar R, Sridhar CN, Wheeler CJ, Tsai YJ, Border R, Ramsey P, Martin M, Felgner PL. J. Biol. Chem. 1994;269:2550. [PubMed] [Google Scholar]
- 23.Fletcher S, Ahmad A, Perouzel E, Jorgensen MR, Miller AD. Org. Biomol. Chem. 2006;4:196. doi: 10.1039/b514532e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.