Abstract
A zirconia (ZrO2) adsorption-based immunoassay by electrochemical quartz crystal microbalance (EQCM) has been initially developed, aiming at the detection of phosphorylated acetylcholinesterase (Phospho-AChE) as a potential biomarker for bio-monitoring exposures to organophosphate (OP) pesticides and chemical warfare agents. Hydroxyl-derivatized monolayer was preferably chosen to modify the crystal serving as the template for directing the electro-deposition of ZrO2 film with uniform nanostructures. The resulting ZrO2 film was utilized to selectively capture Phospho-AChE from the sample media. Horseradish peroxidase (HRP)-labeled anti-AChE antibodies were further employed to recognize the captured phosphorylated proteins. Enzyme-catalytic oxidation of the benzidine substrate resulted in the accumulation of insoluble product on the functionalized crystal. Ultrasensitive EQCM quantification by mass-amplified frequency responses as well as rapid qualification by visual color changes of product could be thus achieved. Moreover, 4-chloro-1-naphthol (CN) was studied as an ideal chromogenic substrate for the enzyme-catalytic precipitation. Experimental results show that the developed EQCM technique can allow for the detection of Phospho-AChE in human plasma with a detection limit of 0.020 nM. Such an EQCM immunosensing format opens a new door towards the development of simple, sensitive, and field-applicable biosensor for biologically monitoring low-level OP exposures.
Keywords: Phosphorylated acetylcholinesterase, Zirconia, Enzyme-catalytic precipitation, EQCM, Organophosphate exposures
1. Introduction
Organophosphates (OPs), which are commonly used as pesticides and chemical warfare agents, are highly neurotoxic. How to detect these toxic agents in the environment and public places or workplaces especially to evaluate the human health risk of OP exposures has been a very active research area.(Noort et al. 2002; Noort et al. 2006; Worek et al. 2005) After the tragic “9.11” event, public concerns about the development of rapid and effective analysis techniques for OP monitoring have been increasing steadily.(Noort et al. 2002; Noort et al. 2006; Worek et al. 2005) At present, the detection of OPs in environmental and biological samples is routinely carried out using analytical techniques, such as gas or liquid chromatography and mass spectrometry.(Fidder et al. 2002; Noort et al. 2006; Polhuijs et al. 1997; Read and Black 1999) The methods, however, generally require extensive labor, lengthy turnaround time, and large-sized analysis settings, which may prohibit them from being used for rapid analysis under field conditions. Recently, portable OP pesticide kits have become commercially available, but present qualitative or semi-quantitative results in addition to complicated handling procedures.(Liu and Lin 2005) Alternatively, considerable efforts have been contributed to the development of a diverse range of biosensors for OP detections in both environmental and biological systems.(Liu and Lin 2005; Mulchandani et al. 1999; Pavlov et al. 2005; Sacks et al. 2000; Schulze et al. 2004; Simonian et al. 2005) These portable devices are thought to well meet the requirements of decentralized point–of-care tests or field detections with portable instrument and simple operation.(Cousino et al. 1997; Pumera et al. 2007; Zhang 2007)
It is recognized that once entering the human or animal body, OPs can covalently bind with cholinesterase (ChE) to conduct the enzyme inhibition effects, at the meantime, they may be decomposed by organophosphorus hydrolase to form inactive phosphonic acids that are renally excreted.(He 1999) Accordingly, common biomarkers for probing internal OP dosimetry mainly include free OPs in blood, or their metabolites in urine, and ChE inhibition-based biological effects.(He 1999; He et al. 2002; Noort et al. 2002; Wessels et al. 2003) However, the high reactivity of OPs with the enzymes suggests that the blood levels of free OPs will be inherently low (typically seen in the range of nanogram per liter or parts per trillion in blood (Wessels et al. 2003). Ultra-sensitive methodologies for detecting free OPs in body are thereby required. Moreover, OP metabolite level in urine is also considered a sensitive indicator of OP exposures,(He 1999; Liu et al. 2005) however, it may be challenged by the urine output varies and the fact that not all toxicant specific metabolites are derived solely from OPs. Also, the enzyme inhibition-based OP quantification may have even more disadvantages. (Simonian et al. 2005; Wessels et al. 2003) For example, a baseline enzyme level is also required to be determined prior to the sample tests due to the fluctuations in enzyme levels for diverse individuals. Therefore, exploring a more effective and sensitive biomarker for evaluation of OP exposures is of considerable interest.
According to the biochemical mechanism established for the phosphorylation of ChE (i.e., AChE), (Kwong 2002; Mileson et al. 1998) the inhibition event may produce very stable enzyme complexes with structurally precise phosphoserine esters.(George et al. 2003) The so produced phosphorylated ChE is assumed to be a new biomarker correlating to the severity of original OP exposures. However, the detection of such a new biomarker can be challenged by the unavailability of specific recognition elements or receptors, i.e., antibodies. Recent researches have demonstrated that some transitional metal oxides such as zirconia (ZrO2) can have a strong affinity for phosphoric groups and are used as capturing agents for selectively enriching free OP and phosphorylated peptides.(Kweon and Hakansson 2006; Liu and Lin 2005) In this current work, we try to introduce synergistically ZrO2 nanoparticles and horseradish peroxidase (HRP) labeled anti-AChE antibodies (HRP-anti-AChE) as selective sorbents and immunorecognition receptors to detect phosphorylated AChE (Phospho-AChE) as a potential biomarker for bio-monitoring OP exposures. Electrochemical quartz crystal microbalance (EQCM) was employed as the real-time transducer. Phospho-AChE is selectively captured by ZrO2 nanoparticles electro- deposited on the crystals, and then recognized by HRP-anti-AChE. The so bound HRP will catalyze the H2O2-induced oxidation of 4-chloro-1-naphthol (CN) substrate to form an insoluble precipitation on the crystals, thus causing frequency changes (Δf) that are proportional to the concentrations of targeting Phospho-AChE. The main advantage of this enzyme-catalytic precipitation protocol lies in that the accumulation of an insoluble product on the crystals can lead to greatly increased mass changes as reflected by Δf (Alfonta et al. 2000; Ebersole and Ward 1988; Patolsky et al. 1999; Su and Li 2001). It can achieve much higher detection sensitivity than some traditional EQCM methods with mass changes directly originating from the adsorption of the analytes or even the enzyme-labeled antibodies. To our best knowledge, this is the first report regarding the development of ZrO2 adsorption-based EQCM immunosensor for the determination of Phospho-AChE for bio-monitoring the exposures to OP pesticides and chemical warfare agents.
2. Experimental
2.1. Reagents
Human acetylcholinesterase (AChE), bovine serum albumin (BSA), CN, paraoxon, zirconium oxychloride (ZrOCl2), 2-mercaptoethanol (ME), N-hydroxysuccinimide (NHS), 1- ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (EDC), polyethylene glycol (PEG, MW 10 KD), 2-(N-morpholino)ethanesulfonic acid (MES) buffer, hydroxylamine, 3,3′5,5′-tetramethylbenzidine dihydrochloride (TMB), Tris-HCl stock buffer (1.0 M), Tween-20, and hydrogen peroxide were purchased from Sigma-Aldrich. Polyclonal anti-AChE antibodies were purchased from Abcam. Human plasma was purchased from Golden West Biologicals, Inc.. The washing buffer was prepared with 0.02 M phosphate buffer saline (PBS, pH 7.2) containing 0.5 % BSA, 0.25 % Tween-20 and 0.15 M NaCl. The BSA-PEG blocking buffer consisted of 3.0 % BSA and 1.0 % PEG. The CN-H2O2 substrate mixture was prepared by freshly mixing CN solution in ethanol and H2O2, with final concentrations of 1.0 mM and 5.0 mM, respectively. Other reagents were of analytical reagent grade.
2.2. Apparatus
CHI 400 A electrochemical analyzer, crystals (9 MHz, gold electrodes), and EQCM cell system were bought from CH Instruments (Austin, TX), in which the EQCM cell consists of a detection cell and Ag/AgCl reference electrode and Pt wire counter electrode. SEM (JEOL JSM-5900 LV) was used for observing the microstructure of the crystal surface with different modifications. Disposable PD-10 desalting columns packed with Sephadex G-25 medium (Amersham Bioscience Corp.) were utilized to purify the protein solution. Centrifugation was performed using a Sorvall RC 26 plus (Kendro Laboratory Product). Vortex mixer with touch-on-off function of mixing (Barnstead International, Iowa) was used for sample and reactant mixture.
2.3. Electro-deposition of ZrO2 nanoparticle film
The electro-deposition of ZrO2 film onto the gold electrode of the crystals was conducted in the EQCM cell system. The crystal was first cyclic-potential scanned within the potential range 0.5 – 1.5 V in freshly prepared 0.2 M H2SO4 until a voltammogram characteristics of the clean polycrystalline gold was established. Then, it was washed with distilled water and dried by nitrogen. Following that, 50 μl ME (20 mM) was dropped on the gold electrode to be assembled overnight at 4 °C. ZrO2 nanoparticles were deposited onto the mercaptoethanol-derivatized in an aqueous electrolyte of 5.0 mM ZrOCl2 and 0.1 M KCl by cycling the potential between −1.1 and + 0.7 V (versus Ag/AgCl) at a scan rate of 20 mV s−1 for 5 consecutive scans. The gold electrodes modified with ZrO2 nanoparticles were rinsed with DI-water and dried in N2 for further experiments. For purpose of comparison, ZrO2 film was also deposited on bare gold electrode of the crystal in the same way above. SEM images were recorded to characterize comparably the surface topologies of the ZrO2 films so formed.
2.4. Preparation of Phospho-AChE
Human AChE (1.1 μM) was mixed with 7.5 mM paraoxon to be incubated overnight at 37 °C. The enzyme activity was measured with the modified Ellman colorimetric method, (Ellman et al. 1961) showing the complete inhibition of AChE activity. Furthermore, the mixture was dialyzed against 0.02 M PBS buffer (pH 7.2) overnight at 4 °C in order to remove the possibly excessive paraoxon. Moreover, the resultant adducts were concentrated by using ultra-filtration to a final volume of ~ 0.40 ml, and stored at − 20 °C for future usage. The protein concentration of the Phospho-AChE solution was determined to be ~ 3.0 μM by spectrophotometry at 280 nm.
2.5. Preparation of HRP-anti-AChE conjugate
HRP was first activated by adding 0.5 mg HRP in 1.0 ml of activation buffer containing 0.10 M MES and 0.50 M NaCl. A total of 0.4 mg of EDC (final concentration ~ 2.0 mM) and 0.6 mg of NHS (final concentration ~ 5 mM) were added to the activated HRP solution to be reacted for 15 min at room temperature. The EDC was quenched by adding 1.4 μl of ME (final concentration of 20 mM). The excess reducing agent and inactivated cross-linker were removed with a PD-10 column. The eluent was collected and concentrated to 1.0 ml in an ultracentrifuge tube. Anti-AChE antibody was added at an equal molar equivalent to HRP in the above solution, and the reaction proceeded for 2 h at room temperature. The reaction was quenched by adding hydroxylamine at a final concentration of 10 mM. Excess hydroxylamine was removed using a PD-10 column. The eluent of HRP-anti-AChE was collected and kept in PBS buffer containing 0.5 % BSA at 4 °C for further use.
2.6. ZrO2 adsorption-based EQCM immunoassays
EQCM immunoassays were performed in the EQCM cell system in which the EQCM analyzer was connected to a laptop computer. An aliquot of 50 μl of Phospho-AChE, which was diluted to the desired concentration with acetate buffer solution (pH 4.0) containing 0.5 % BSA or with human plasma, was injected onto the above ZrO2-coated crystal and incubated for 1.5 h at 37 °C in a humidified chamber. Phospho-AChE attached crystal was then washed three times with the washing buffer. The resulting sensor was blocked for 30 min with the blocking buffer. After washing twice, 50 μl of HRP-anti-AChE (2.5 μg ml−1) was dropped onto the crystal and incubated for 1 h at 37 °C, then, rinsed with the washing buffer. Following that, 50 μl of the CN-H2O2 mixture was added onto the crystal resulting in the enzyme-catalytic precipitation of the CN substrate. The induced frequency changes (Δf) were recorded as the reaction proceeded from 30 s (after the addition of samples) until equilibrium was reached (~ 15 min). The control tests with AChE were performed accordingly. The frequency changes in all experiments were referred to the average responses with corresponding standard deviations (± SD) of triplicate measurements, unless otherwise indicated.
In addition, the investigation of pH dependent adsorption of Phospho-AChE on ZrO2 film was carried out by detecting 0.1 nM Phospho-AChE at different pH values with acetate or PBS buffer solutions.
3. Results and Discussion
3.1 Main principle and characteristics of ZrO2 adsorption-based EQCM immunoassays
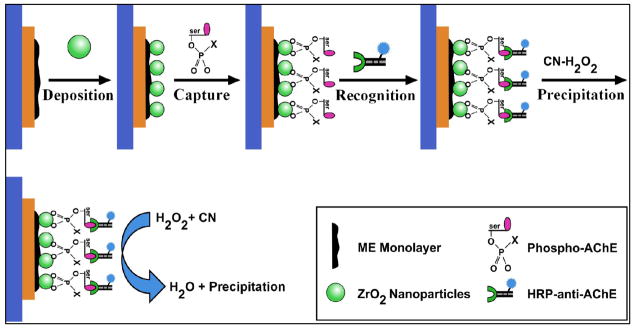
It is established that upon entering human body, OPs will bind with ChE (i.e., AChE) to conduct the irreversible enzyme inhibition yielding a stable phosphorylated ChE adduct.(George et al. 2003; Kwong 2002; Mileson et al. 1998) This phosphorylated adduct is thought to be a new biomarker which amount in body can correlate to the degree of OP exposures. Moreover, ZrO2 nanoparticles have been demonstrated with strong affinity to phosphoric groups for selectively binding OPs or phosphorylated peptides.(Kweon and Hakansson 2006; Liu and Lin 2005) In this work, we seek to utilize ZrO2 nanoparticles and HRP-anti-AChE as selective sorbents and recognition receptors, respectively, to achieve the specific detection of the Phospho-AChE using real-time EQCM. Fig. 1 illustrates the schematic principle for ZrO2 adsorption-based EQCM immunoassays for Phospho-AChE. The gold electrode of the crystal was first modified with ME monolayer and then electro-deposited with ZrO2 film. Phospho-AChE adducts were then captured onto the ZrO2-coated crystal to be further recognized by HRP-anti-AChE. The CN-H2O2 substrate was subsequently introduced onto the crystal, where enzyme could catalyze a H2O2-induced oxidation of CN to form an insoluble product. The time-dependent accumulation of the precipitation on the crystal will cause Δf proportional to the Phospho-AChE concentrations. Herein, the relationship between Δf and mass changes on the crystal can be described by the Sauerbrey equation.(Buttry and Ward 1992) Moreover, a red-purple color change can be observed synchronously, thereby facilitating a rapid qualitative assessment of Phospho-AChE as an indictor of OP exposures.
Fig. 1.
Schematic illustration of the principle and process for ZrO2 adsorption-based EQCM immunoassays for Phospho-AChE, including the ME monolayer modification, ZrO2 film electro- deposition, Phospho-AChE capture, HRP-anti-AChE recognition, and HRP-catalytic precipitation of insoluble product towards amplified EQCM immunoassays.
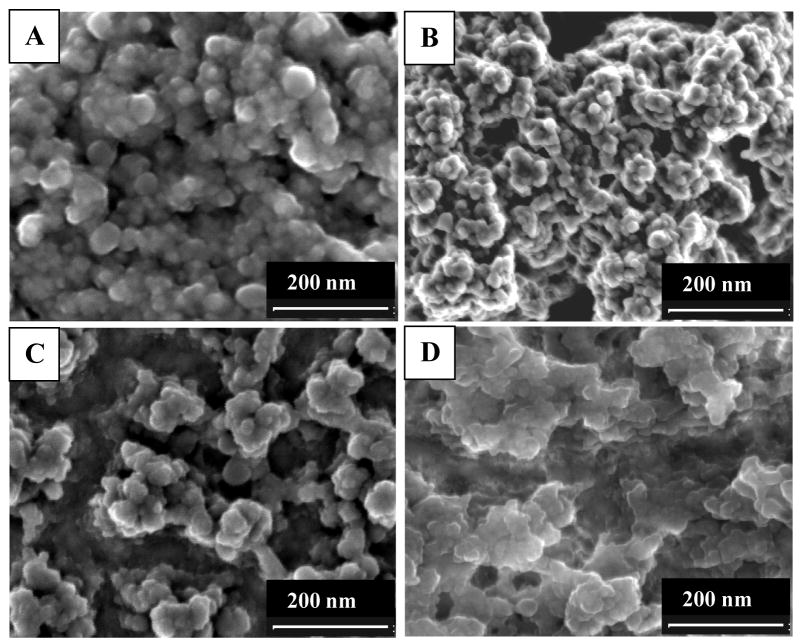
The morphological features of ZrO2 films formed on the ME-modified and bare gold electrodes were comparably investigated, including their corresponding H2O2-oxidized products formed in the subsequent immunoassays (Fig. 2). As can be seen from Fig. 2, the “coral-like islands” of ZrO2 film on the ME-modified electrode (Fig. 2A) are decorated with more uniform and smaller spherical ZrO2 particles in nanometer size, comparing with those on the bare gold electrode (Fig. 2C). Moreover, the insoluble precipitations accumulated on the ME-functionalized surface show nanometer-sized particles closely packed (Fig. 2B), in contrast to those on the bare gold surface (Fig. 2D). That is, a larger amount of enzyme-catalytic precipitation products can be obtained for the ME-functionalized surface, resulting from its better electro-deposition features of ZrO2 film.
Fig. 2.
SEM images of ZrO2 film formed on the (A) ME-functionalized and (C) bare gold electrodes of the crystals, corresponding to (B, D) the final H2O2-oxidized precipitation of CN substrate by enzyme catalysis in the immunoassays, respectively.
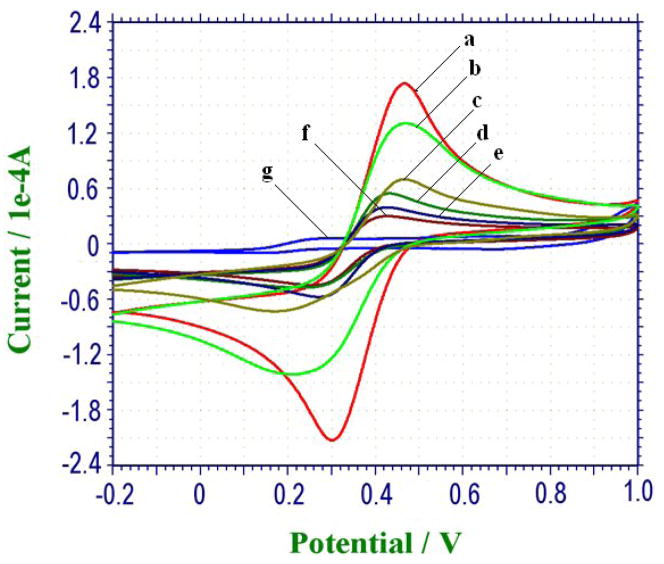
Figure 3 manifests the EQCM cyclic voltammograms (CVs) characterizing the step-by-step buildup process of the crystal using [Fe(CN)6]4−/3− as the probe couple. As can be seen from Fig. 3, a couple of quasi-reversible redox peaks of the probe were obtained at the bare gold electrode of the crystal (Fig. 3a), and a decrease in current response was envisaged upon the assembly of ME monolayer (Fig. 3b). The interfacial electron-transfer resistance of the electrode increased greatly when semi-conductive ZrO2 film was deposited (Fig. 3c). Moreover, the step-by-step insulation of the conductive surface grew with the protein or polymer modifications of Phospho-AChE (Fig. 2d), BSA-PEG (Fig. 3e), and HRP-anti-AChE (Fig. 3f). The electrical communication between the [Fe(CN)6]4−/3− probe and the electrode surface was almost inhibited completely after the H2O2-oxidized precipitation of CN by enzyme catalysis (Fig. 3g), showing a entirely depleted electrical response. Therefore, the electron-transfer kinetics indicates that ZrO2 adsorption-based EQCM immunoassays for Phospho-AChE could be achievable following the stepwise performances in the protocol established above.
Fig. 3.
Cyclic voltammograms of EQCM characterizing the stepwise buildup process of the sensor in 5 mM [Fe(CN)6]4−/3− solution (0.01 M, with 0.1 M KCl) at 100 mV s−1, where curve a-g correspond to the crystals modified with: bare, ME monolayer, ZrO2, Phospho-AChE, BSA-PEG, HRP-anti-AChE, and H2O2-oxidized precipitation of CN by enzyme catalysis.
3.2 Electro-deposition of ZrO2 nanoparticles templated by hydroxyl-derivatized monolayer
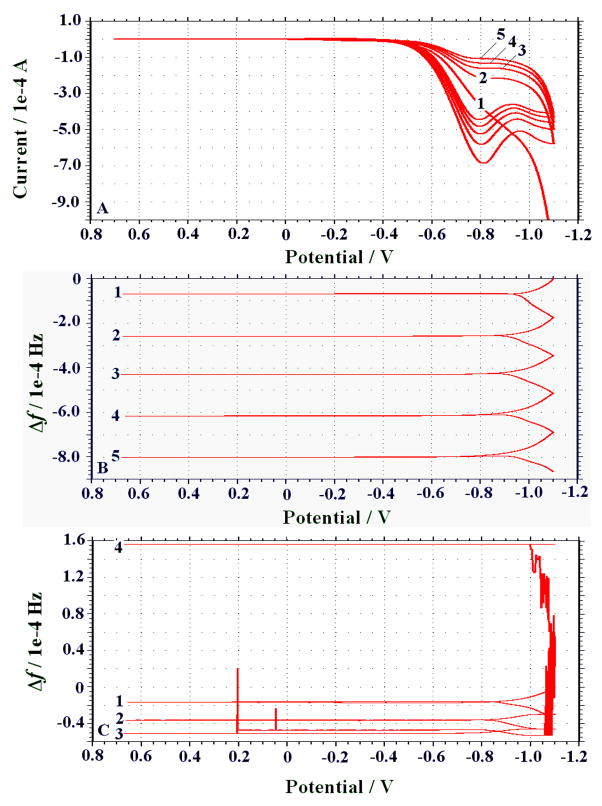
The fabrication of ZrO2 film with desired micro- to nano-structures is considered of great importance for better adsorption of phosphorylated proteins or peptides. Accordingly, the selection and preparation of micro-patterned substrates, i.e., by creating chemical templates, for inorganic material micro-fabrication are critical issues for consideration.(Azzaroni et al. 2001; Ji et al. 2002; Yu et al. 2002) In the current experiments, the gold electrode of the crystal was modified with ME monolayer serving as the template for the selective growth of ZrO2 film by electro-deposition. The ZrO2 film deposited onto bare gold electrode was also conducted for purpose of comparison. The deposition processes were monitored in real time using EQCM, simultaneously showing CVs and Δf (Fig. 4). The formation of the semi-conductive ZrO2 film on the electrodes could increase the interfacial electron-transfer resistance, in addition to a mass increase on the transducer as reflected by a decrease in resonance frequency. As can be observed from Fig. 4A, during the electro-deposition process the ZrO2 polymerization proceeded with gradually decreasing current as repetitive scanning. These CV changes can correspond to the Δf shown in Fig. 4B. Herein, resonance frequencies of the ME-modified electrode decreased reasonably with the layer-by-layer electro-deposition (~ 3510 ± 75 Hz for five scanning circles), implying that a mass increase on the crystal occurred together with a uniform formation of ZrO2 film. That is in contrast to the ZrO2 film electro-deposited onto the bare gold electrode (Fig. 4C). As shown in Fig. 4C, the Δf could decrease as ZrO2 film was deposited during the initial three scanning cycles, after which the Δf increased suddenly, presumably due to the part desorption of the ZrO2 film so deposited. Accordingly, ZrO2 film might be frequently unstable when deposited on the bare gold electrode (6.0 mm in diameter), although the general CV features are quite similar to those of the ME-modified ones (data not shown). More importantly, as aforementioned ZrO2 films formed on the ME-modified and bare electrodes showed quite different morphological characteristics of SEM images (Fig. 2). Such a phenomenon can be understood by the facts below. On the one hand, ZrOCl2 precursors can be attached to the hydroxyl-terminated monolayer via a ligand exchange reaction of the terminal alcohols of ME with chloride ligands of the precursors.(Yu et al. 2002) On the other hand, when the electrode is modified with the closely packed ME monolayer, the decreased interfacial electron transfer rate can thus allow for a slower and more organized deposition of ZrO2 nanoparticles with high stability. In addition to being the selective sorbents, the so prepared ZrO2 film can offer a larger surface area for highly loading enzyme-catalytic precipitation product yielded in the immunoassay step.
Fig. 4.
Real-time EQCM monitoring of ZrO2 film electrodeposited onto the ME-modified crystal shown by (A) electrochemical CVs and (B) Δf, comparing with (C) Δf for ZrO2 electro-deposited on bare gold electrode at a scan rate of 20 mV s−1.
3.3. Selective adsorption of ZrO2 nanoparticles for Phospho-AChE
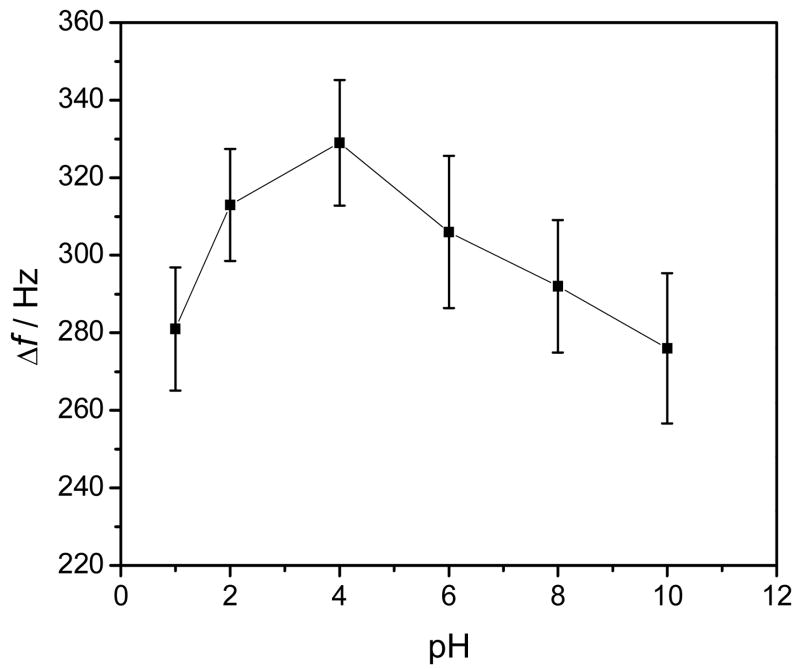
It is recognized that ZrO2 possesses amphoteric properties of either as a Lewis acid or base depending on the pH of the reaction solution, due to their unsatisfied valencies of both oxygen and zirconium atoms in the surface layer.(Kweon and Hakansson 2006; Nawrocki et al. 1993) Accordingly, high binding selectivity of phosphorylated proteins or peptides over non-phosphorylated ones should be achievable with ZrO2 upon a careful pH selection. In this current work, the adsorption abilities of ZrO2 to Phospho-AChE were studied under different pH conditions. Figure 5 manifests the experimental results. Obviously, ZrO2 film could exhibit better adsorption to Phospho-AChE under the slightly acidic environments, as reflected by relatively high Δf. Herein, ZrO2 can behave as a Lewis acid with positively charged zirconium atoms, (Kweon and Hakansson 2006) thereby providing high binding affinity for phosphoric residues of Phospho-AChE. The formation of chemical bonds between ZrO2 and phosphorous residues of Phospho-AChE may presumably occur, as schematically illustrated in Fig. 1. Note that too low pH values (i.e., pH ≤ 2.0) may cause the denaturation of proteins. The highest adsorption of phosphorylated proteins was achieved at pH 4.0, which is recommended in the current experiments.
Fig. 5.
The pH dependent adsorption of Phospho-AChE onto ZrO2 film electrodeposited on the ME-modified crystal. The detections of 0.1 nM Phospho-AChE were performed in the acetate or PBS buffer solutions with different pH values.
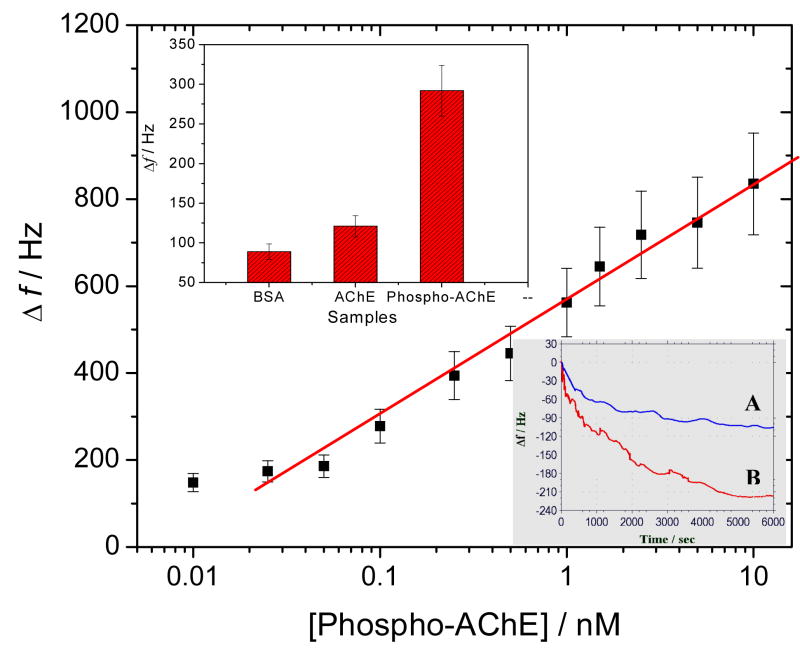
Furthermore, the adsorption selectivity of ZrO2 film to Phospho-AChE adducts was investigated by real-time EQCM monitoring, with AChE as a control (the bottom insert in Fig. 6). It can be discovered that the ZrO2 adsorptions to 0.5 nM AChE and Phospho-AChE could result in Δf of ~ 102 ± 13 Hz and ~ 220 ± 18 Hz, respectively. Obviously, non-specific adsorption of AChE on ZrO2 film may be a challenge to some degree. Accordingly, after the adsorptions of Phospho-AChE and AChE, the crystals were washed separately three times by the washing buffer. The Δf could be recovered to ~ 23 ± 7 Hz and ~ 91 ± 5 Hz, respectively. The data indicate that the harsh washing steps could allow the attached AChE to be largely removed, while most Phospho-AChE could be retained. That is, ZrO2 film can present favorably high adsorption selectivity to Phospho-AChE, if aided by the additional washing steps. Moreover, as can be seen from the bottom insert of Fig. 6, the time for the complete adsorption of Phospho-AChE on ZrO2 film is about 1.5 h.
Fig. 6.
Calibration curve plotted on a semi-log scale for detecting Phospho-AChE in plasma samples, with a detection limit of 0.020 nM. The top insert shows the comparison of final Δf responses of immunoassays for BSA (10 mg ml−1), AChE (0.1 nM) and Phospho-AChE (0.1 nM), and the bottom insert manifests the real-time EQCM monitoring for the direct adsorptions of 0.5 nM (A) AChE and (B) Phospho-AChE onto the ZrO2-coated crystals.
3.4. Analysis performances of EQCM immunoassay
It is established that HRP can catalyze the H2O2-induced oxidization of some chromogenic substrates, typically as CN and TMB, to form insoluble precipitates towards amplified EQCM assays.(Alfonta et al. 2000; Ebersole and Ward 1988; Patolsky et al. 1999; Su and Li 2001) Nevertheless, our experimental results show that CN is better than TMB as the substrate for enzyme-catalytic precipitation in the EQCM assays (Supporting Information).
Under the optimal experimental conditions, EQCM immunoassays for Phospho-AChE, AChE and BSA were conducted comparably (the top insert in Fig. 6). Obviously, big differences in Δf responses for the final enzyme-catalytic precipitation of CN-H2O2 substrate were obtained, indicating the high detection specificity for Phospho-AChE. Moreover, several Phospho-AChE samples with different concentrations in plasma were determined by the developed EQCM immunoassay. Figure 6 describes the calibration curve between Δf versus [Phospho-AChE] plotted on a semi-log scale. As is shown in Fig. 6, a linear response is obtained over the Phospho-AChE concentrations ranging from 0.025 to 10 nM, with a detection limit of 0.020 nM, as estimated by the “S/N = 3” rule. In addition, a change in red-purple color could also be apparently accompanied when the CN-H2O2 mixture was added onto the resulting crystal surface for ~ 10 to 15 min. Rapid qualitative assessments can thus be expected allowing for the visual screening of samples as a result of OP exposures.
After each immunoassay, the used crystals were washed by using 8.0 M urea for 15 ~ 20 min. It was observed that such a urea regeneration step could cause a gradual increase in frequency responses (data not shown). The adsorbed substances from Phospho-AChE to the final enzyme-catalytic products could be thus peeled off the ZrO2-modified crystals. In addition, five repetitive measurements for 0.5 nM Phospho-AChE were carried out for the ZrO2-modified crystal so regenerated, yielding reproducible frequency responses with an RSD of 17.6 %. The above data indicate that the developed EQCM immunoassay can show high sensitivity and reproducibility for detection of Phospho-AChE. Note that human plasma contains about 8.0 ng ml−1 (≈ 0.12 nM) AChE. (Brimijoin and Hammond 1988) Accordingly, the developed immunosensing strategy can be tailored as a new method for bio-monitoring the low OP exposures. (Wang et al. 2008)
4. Conclusions
In this work, an EQCM immunoassay using ZrO2 nanoparticles as selective sorbents has been successfully developed for the first time for detection of Phospho-AChE as a new biomarker for bio-monitoring OP exposures. The synergistic applications of ZrO2 nanoparticles and HRP labeled anti-AChE antibodies have been verified to well circumvent the specific recognition of targeting Phospho-AChE. H2O2 oxidization of the benzidine substrate by HRP catalysis could produce an insoluble precipitation on the crystal resulting in greatly amplified EQCM responses. Herein, the electro-deposition of ZrO2 film onto the hydroxyl-derivatized crystal could achieve uniform nanostructures with better adsorptions for Phospho-AChE, as compared with those on bare gold electrode. The so deposited ZrO2 nanoparticle film can provide a larger surface area for loading the enzyme-catalytic product in addition to serving as the selective sorbents. Moreover, CN was verified as the ideal chromogenic substrate suitable for enzyme-catalytic precipitation. Ultrasensitive quantitative and rapid qualitative EQCM analysis of the targets could be expected through mass-amplified frequency responses and visual color changes, respectively. This proposed EQCM immunosensing technique is simple, real-time, sensitive, and field-applicable, holding great promise of being applied as an alternative tool for onsite bio-monitoring the exposures to nerve agents and pesticides.
Supplementary Material
Acknowledgments
This work is supported by the National Institutes of Health CounterACT Program through the National Institute of Neurological Disorders and Stroke (award # NS058161-01). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the federal government. The research described in this paper was performed at the Environmental Molecular Sciences Laboratory, a national scientific user facility sponsored by DOE’s Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory, which is operated by Battelle for DOE under Contract DE-AC05-76RL01830.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alfonta L, Katz E, Willner I. Anal Chem. 2000;72(5):927–935. doi: 10.1021/ac990439d. [DOI] [PubMed] [Google Scholar]
- Azzaroni O, Schilardi PL, Salvarezza RC. Nano Letters. 2001;1(6):291–294. [Google Scholar]
- Buttry DA, Ward MD. Chem Rev. 1992;92(6):1355–1379. [Google Scholar]
- Brimijoin S, Hammond P. J Neurochem. 1988;51:1227–1231. doi: 10.1111/j.1471-4159.1988.tb03091.x. [DOI] [PubMed] [Google Scholar]
- Cousino MA, Jarbawi TB, Halsall HB, Heineman WR. Anal Chem. 1997;69(17):A544–A549. doi: 10.1021/ac9717651. [DOI] [PubMed] [Google Scholar]
- Ebersole RC, Ward MD. J Am Chem Soc. 1988;110(26):8623–8628. [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Featherstone RM. Biochem Pharmacol. 1961;7(2):88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Fidder A, Hulst AG, Noort D, de Ruiter R, van der Schans MJ, Benschop HP, Langenberg JP. Chem Res Toxicol. 2002;15(4):582–590. doi: 10.1021/tx0101806. [DOI] [PubMed] [Google Scholar]
- George KM, Schule T, Sandoval LE, Jennings LL, Taylor P, Thompson CM. J Biol Chem. 2003;278(46):45512–45518. doi: 10.1074/jbc.M304781200. [DOI] [PubMed] [Google Scholar]
- He FS. Toxicol Lett. 1999;108(2–3):277–283. doi: 10.1016/s0378-4274(99)00099-5. [DOI] [PubMed] [Google Scholar]
- He FS, Chen SY, Tang XY, Gan WQ, Tao BG, Wen BY. Toxicol Lett. 2002;134(1–3):119–124. doi: 10.1016/s0378-4274(02)00180-7. [DOI] [PubMed] [Google Scholar]
- Ji JM, Li X, Canham LT, Coffer JL. Adv Mater. 2002;14(1):41–43. [Google Scholar]
- Kweon HK, Hakansson K. Anal Chem. 2006;78(6):1743–1749. doi: 10.1021/ac0522355. [DOI] [PubMed] [Google Scholar]
- Kwong TC. Ther Drug Monit. 2002;24(1):144–149. doi: 10.1097/00007691-200202000-00022. [DOI] [PubMed] [Google Scholar]
- Liu GD, Lin YH. Anal Chem. 2005;77(18):5894–5901. doi: 10.1021/ac050791t. [DOI] [PubMed] [Google Scholar]
- Liu GD, Riechers SL, Timchalk C, Lin YH. Electrochem Commun. 2005;7(12):1463–1470. [Google Scholar]
- Mileson BE, Chambers JE, Chen WL, Dettbarn W, Ehrich M, Eldefrawi AT, Gaylor DW, Hamernik K, Hodgson E, Karczmar AG, Padilla S, Pope CN, Richardson RJ, Saunders DR, Sheets LP, Sultatos LG, Wallace KB. Toxicol Sci. 1998;41(1):8–20. doi: 10.1006/toxs.1997.2431. [DOI] [PubMed] [Google Scholar]
- Mulchandani A, Mulchandani P, Chen W, Wang J, Chen L. Anal Chem. 1999;71(11):2246–2249. doi: 10.1021/ac9813179. [DOI] [PubMed] [Google Scholar]
- Nawrocki J, Rigney MP, McCormick A, Carr PW. J Chromatogr A. 1993;657(2):229–282. doi: 10.1016/0021-9673(93)80284-f. [DOI] [PubMed] [Google Scholar]
- Nineham AW. Chem Rev. 1955;55(2):355–483. [Google Scholar]
- Noort D, Benschop HP, Black RM. Toxicol Appl Pharm. 2002;184(2):116–126. [PubMed] [Google Scholar]
- Noort D, Fidder A, van der Schans MJ, Hulst AG. Anal Chem. 2006;78(18):6640–6644. doi: 10.1021/ac060954t. [DOI] [PubMed] [Google Scholar]
- Patolsky F, Zayats M, Katz E, Willner I. Anal Chem. 1999;71(15):3171–3180. doi: 10.1021/ac9901541. [DOI] [PubMed] [Google Scholar]
- Pavlov V, Xiao Y, Willner I. Nano Lett. 2005;5(4):649–653. doi: 10.1021/nl050054c. [DOI] [PubMed] [Google Scholar]
- Polhuijs M, Langenberg JP, Benschop HP. Toxicol Appl Pharm. 1997;146(1):156–161. doi: 10.1006/taap.1997.8243. [DOI] [PubMed] [Google Scholar]
- Pumera M, Sanchez S, Ichinose I, Tang J. Sens Actuators B. 2007;123(2):1195–1205. [Google Scholar]
- Read RW, Black RM. J Chromatogr A. 1999;862(2):169–177. doi: 10.1016/s0021-9673(99)00944-9. [DOI] [PubMed] [Google Scholar]
- Sacks V, Eshkenazi I, Neufeld T, Dosoretz C, Rishpon J. Anal Chem. 2000;72(9):2055–2058. doi: 10.1021/ac9911488. [DOI] [PubMed] [Google Scholar]
- Schulze H, Schmid RD, Bachmann TT. Anal Chem. 2004;76(6):1720–1725. doi: 10.1021/ac035218t. [DOI] [PubMed] [Google Scholar]
- Simonian AL, Good TA, Wang SS, Wild JR. Anal Chim Acta. 2005;534(1):69–77. [Google Scholar]
- Su XD, Li SFY. Anal Chim Acta. 2001;429(1):27–36. [Google Scholar]
- Wessels D, Barr DB, Mendola P. Environ Health Persp. 2003;111(16):1939–1946. doi: 10.1289/ehp.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worek F, Koller M, Thiermann H, Szinicz L. Toxicology. 2005;214(3):182–189. doi: 10.1016/j.tox.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Yu HZ, Rowe AW, Waugh DM. Anal Chem. 2002;74(22):5742–5747. doi: 10.1021/ac025686n. [DOI] [PubMed] [Google Scholar]
- Zhang XJJHX, Wang J. Electrochemical sensors, biosensors and their biomedical applications. Academic Press of Elsevier; San Diego, CA, USA: 2007. [Google Scholar]
- Wang H, Wang J, Timchalk C, Lin YH. Anal Chem. 2008;80 (22):8477–8484. doi: 10.1021/ac801211s. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.