Abstract
Cannabinoid CB1 inverse agonists suppress food-motivated behaviors, but may also induce psychiatric effects such as depression and anxiety. To evaluate behaviors potentially related to anxiety, the present experiments assessed the CB1 inverse agonist AM251 (2.0 – 8.0 mg/kg), the CB1 antagonist AM4113 (3.0 – 12.0 mg/kg), and the benzodiazepine inverse agonist FG-7142 (10.0 – 20.0 mg/kg), using the open field test and the elevated plus maze. Although all three drugs affected open field behavior, these effects were largely due to actions on locomotion. In the elevated plus maze, FG-7142 and AM251 both produced anxiogenic effects. FG-7142 and AM251 also significantly increased c-Fos activity in the amygdala and nucleus accumbens shell. In contrast, AM4113 failed to affect performance in the plus maze, and did not induce c-Fos immunoreactivity. The weak effects of AM4113 are consistent with biochemical data showing that AM4113 induces little or no intrinsic cellular activity. This research may lead to the development of novel appetite suppressants with reduced anxiogenic effects.
Keywords: rimonabant, anxiety, depression, cannabinoid, aversive motivation, open field, plus maze
1.0 Introduction
Drugs that impair cannabinoid transmission are known to suppress food intake in animals and humans. CB1 inverse agonists such as SR141716 (rimonabant) and AM251 reduce food intake in a number of different animal models under conditions of food-deprivation and satiation, and after acute or chronic treatment (Arnone et al., 1997; Colombo et al., 1998; Williams and Kirkham, 1999; Shearman et al., 2003; Wiley et al., 2005; McLaughlin et al., 2003, 2005; Gardner and Mallet, 2006; Salamone et al., 2007). Clinical trials with CB1 inverse agonists rimonabant and taranabant have shown these drug to be effective at reducing body weight and waist circumference (Curioni and Andre, 2006; Despres et al., 2005; Pi-Sunyer et al., 2006; Van Gaal et al., 2005; Addy et al., 2008). However, these same clinical trials also provide evidence of serious psychiatric side effects including depression and anxiety. A meta-analysis of rimonabant in obesity (RIO) studies performed by the Food and Drug Administration (FDA) showed that 26% of subjects treated with 20 mg rimonabant reported adverse psychiatric events versus 14% of those treated with placebo (US Food and Drug Administration Advisory Committee, 2007). The significantly higher incidence of psychiatric events observed with rimonabant was a key factor in the decision of the FDA to deny approval of the drug for treatment of obesity in the United States. Clinical trials of taranabant, another CB1 inverse agonist, were similarly plagued with adverse psychiatric events. Onset of psychiatric adverse events occurred early in the clinical trials, and dropouts related to psychiatric adverse events were frequent (Addy et al., 2008). In the FDA’s meta-analysis of clinical trials using rimonabant, the most commonly reported adverse psychiatric event was anxiety (US Food and Drug Administration Advisory Committee, 2007). Several animal models of anxiety have been utilized in the testing of various CB1 inverse agonists. Although the outcomes from these tests are inconsistent (see Akinshola et al., 1999; Haller et al., 2002), many of these animal studies have found CB1 inverse agonists to produce behavioral signs of anxiety (Arevalo et al., 2001; McGregor et al., 1996; Navarro et al., 1997; Rodriguez de Fonseca et al., 1996; Haller et al., 2004; Rogers et al., 2005).
A novel CB1 antagonist, AM4113, was shown to induce less intrinsic biological activity in comparison with CB1 inverse agonists such as AM251 and rimonabant as demonstrated by in vitro cAMP accumulation assays (Chambers et al., 2007; Sink et al., 2008a). Like CB1 inverse agonists, AM4113 attenuated feeding and food-motivated behaviors (Sink et al., 2008a, 2008b, 2009). AM4113 has also been assessed in behavioral tests of spontaneous locomotion and induction of nausea (Sink et al., 2008a, Chambers et al., 2007; Jarbe et al., 2008). However, the effects of AM4113 on anxiety-related behaviors have not yet been characterized. Thus, the present experiments were undertaken to evaluate the potential anxiogenic behavioral effects of AM4113 and AM251. For evaluation of anxiety-related behavioral effects, two exploration-based tests of anxiety were employed: the elevated plus maze, and the open field. Anxiety-related behavioral effects evoked by AM4113 and AM251 were compared with those produced by a well-characterized anxiogenic drug, the benzodiazepine inverse agonist, FG-7142 (Evans and Lowry, 2007). A second goal of these studies was to compare the neural activation patterns induced by AM251 and AM4113, as measured using c-Fos expression, within anxiety- and feeding-related brain areas. C-Fos is an immediate early gene product that encodes a transcription factor involved in the coupling of extracellular signals to long-term phenotypic cellular changes by regulating the expression of later-onset gene products (Morgan and Curran, 1991; Sheng and Greenberg, 1990). It is a powerful tool for mapping neuronal activation in part because of low basal level of expression (Hughes et al., 1992), because c-Fos is inducible by a wide array of pharmacological treatments and external stimuli (Morgan and Curran, 1989), and also because c-Fos is expressed in a region- and cell-specific manner that depends upon the type of stimulus (Wisden et al., 1990). Previous studies reported that injections of 10.0 mg/kg SR141716 (rimonabant) induced c-Fos activation in several brain structures, including prefrontal cortex, nucleus accumbens shell, central amygdala, paraventricular nucleus of the hypothalamus, and central gray area (Alonso et al., 1999; Rodriguez de Fonseca et al., 1997). Also, increased c-Fos expression within the dorsal striatum and nucleus accumbens shell, as well as paraventricular, arcuate and lateral hypothalamic nuclei, has been reported to occur after injections of AM251 (Rueda-Orozco et al., 2008; Sinnayah et al., 2008). To date, there have been no studies of c-Fos activation using AM4113. The present work compared c-Fos activation produced by the well-characterized CB1 inverse agonist AM251 with the novel drug, AM4113, within the striatum, hypothalamus, and amygdala. These structures were examined because of their roles in food intake, locomotor activity, and anxiety, all functions that are modified by drugs that act on CB1 receptors (Sink et al., 2008a, 2008b, 2009; McLaughlin et al., 2003, 2005; Chambers et al., 2007; Jarbe et al., 2008). The anxiogenic benzodiazepine inverse agonist, FG-7142, was also included in this experiment for comparison of activation patterns within structures related to anxiety.
Based on the results of previous studies of anxiety-related behavior and c-Fos expression using CB1 inverse agonists, and in vitro evidence that AM4113 has little intrinsic activity at CB1 receptors compared to AM251 (Sink et al., 2008a, Chambers et al., 2007), it was hypothesized that AM251 would produce some anxiety-like behavior and moderate neural activation, and that the overall patterns of behavioral and neural activation effects produced by AM4113 would be weaker than those elicited by AM251.
2.0 Experimental Procedures
2.1 Animals
A total of 297 animals were used for these experiments. Adult male Sprague–Dawley rats (Harlan Sprague–Dawley, Indianapolis, IN; 300–325 g, 3–4 months age) were pair-housed in a colony maintained at 23°C, with a 12-h light/dark cycle (lights on 07:00). Food and water was available ad libitum in the home cages. Animal protocols were approved by the University of Connecticut Institutional Animal Care and Use Committee, and the studies were conducted according to NIH guidelines for animal care and use.
2.2 Drugs and Selection of Doses
All studies used intraperitoneal (IP) injections, with a total volume of 1.0 ml/kg. AM251 and AM4113 (synthesized at the Center for Drug Discovery, Northeastern University) were dissolved in a vehicle of dimethylsulfoxide (DMSO; Fisher, Waltham, MA, USA), Tween-80 (Fisher), and 0.9% saline in a 1:1:8 ratio. This mixture also served as vehicle for elevated plus maze and open field experiments. FG-7142 (β-carboline-3-carboxylic acid N-methylamide) obtained from Sigma Chemical (St. Louis, MO) was suspended in a solution containing 0.9% saline and 2% Tween-80. ± Fenfluramine was obtained from Sigma Chemical Co. (St. Louis, Mo., USA). Although the + isomer is thought to be the more active isomer, several studies have shown that the racemic mixture is active in suppressing food intake (see, for example, Francis et al., 1997; Voigt et al., 2000; Salamone et al., 2002). Fenfluramine was dissolved in 0.3% tartaric acid. Doses and pretreatment times for AM251, AM4113, FG-7142, and fenfluramine were chosen based upon previous research demonstrating these doses to be behaviorally active in anxiety models (FG-7142) or as food intake suppressants (AM251, AM4113, fenfluramine; Sink et al., 2008a, 2008b, 2009; McLaughlin et al., 2003; Salamone et al., 2002), and handling conditions were based upon pilot studies. Vehicle control animals in the c-Fos study received either 0.9% saline, 0.3% tartaric acid, or 1:1:8 DMSO, Tween-80, and 0.9% saline. Data obtained under the three vehicle conditions were similar, allowing the results to be pooled for statistical purposes.
2.3 Open Field
The open field apparatus consisted of a Plexiglas-covered wooden floor (115 x 115 cm) painted black with red lines spaced 23 cm apart, dividing the floor into a five-by-five grid. Walls around the perimeter measured 44 cm in height. Testing was performed in a very dimly lit room with a single red light situated at one corner of the box. The apparatus was novel to the subject at the time of testing, and each subject was tested only once. Between rats, the apparatus was wiped down with 70% isopropyl alcohol. Each rat was placed on the apparatus facing into a corner. An experimenter who was blind to the treatment condition observed the session on a video monitor located approximately 6 feet away from the box, and tallied the number of inner and outer line crossings, defined as movement of both front paws from one square to another. Outer crossings were defined as movement into one of the 16 squares adjacent to the walls, while inner crossings were defined as movement into one of the 9 central squares. Simultaneously, a video tracking system (SMART, Panlab, Barcelona, Spain) recorded the exact track and speed (cm/s) of each subject throughout the session, and discretely logged the distance (cm) and time spent in the inner region (central squares) and the outer region (the 16 squares along the perimeter of the floor). Each 10-min session was divided into two 5-min bins. All testing was conducted during the light portion of the light-dark cycle.
2.4 Elevated Plus Maze
The elevated plus maze was constructed of black Plexiglas based on the specifications of Pellow et al (1985). It consisted of a central platform measuring 15 × 15 cm, from which four arms extended outward such that the maze resembled a “plus” shape. The two opposing open arms were 45 cm long and 15 cm wide, with a 2 cm rim around the perimeter to prevent animals from falling. Perpendicular to these arms were two enclosed arms of the same dimensions with opaque walls 30 cm tall. The closed arms were fitted with removable covers. The entire maze was elevated 46 cm above a black plywood base. The apparatus was novel to the subject at the time of testing, and each subject was tested only once. Between subjects, the maze was wiped down with 70% isopropyl alcohol. Testing was performed in a room illuminated with a single 15-Watt light bulb aligned with the open arms of the maze. Two experimenters observed the test sessions from vantage points at the ends of the open arms. Number of open arm entries and time spent on open arms were recorded, and then transformed into percentages of total time and total entries respectively for analytical purposes. The percentage of time spent on open arms and percentage of open arm entries are thought to be anxiety-related measures in the elevated plus maze (Cruz et al., 1994). Time spent on the central square was not included in either open-arm time or closed-arm time, as factor analysis has shown this measure to correlate poorly with anxiety levels (Hogg, 1996). Number of head dips while on open arms is an ethological measure also thought to relate to anxiety level (Hogg, 1996; Wall and Messier, 2001). A pilot study (n = 29) was conducted to determine the effect of AM251 on various ethological measures in the plus maze, including head dips, scanning, risk assessment and grooming. It was observed that head dipping was the measure most consistently identified in the plus maze, and that AM251 produced a robust reduction of head dipping (Mean (± SEM), vehicle: 12.06 (± 2.06), 12.0 mg/kg AM251: 6.0 (± 1.29), t = 2.35, df = 27, p < 0.05); thus, head dipping (lowering the snout over the open side of the maze) was included in the full studies. The number of total arm entries was taken as a measure of locomotor activity (Cruz et al, 1994), but total number of closed arm entries also was analyzed. All testing was conducted during the light portion of the light-dark cycle.
2.5 C-Fos Immunohistochemistry and Cell Counting
For the c-Fos experiment, the animals were anesthetized with CO2 and perfused with physiological saline followed by formalin ninety min after IP injection. The brains were stored in formalin and then were cryoprotected for four days before being sliced into 50-μm sections using a cryostat and stored in wells containing Dulbecco’s phosphate buffered saline (Sigma Chemical Co, St. Louis). Tissue sections were later incubated in the primary antibody, anti-c-Fos (1:5000, Calbiochem, Germany), at 4°C for 48 hours. Subsequently, the sections were incubated in the secondary antibody, anti-rabbit horseradish peroxidase conjugate, Envision Plus (DAKO, Denmark) for 1.5 hours. The visualization of c-Fos protein expression was completed using the chromagen diaminobenzidine (DAB). The sections were then mounted onto glass microscope slides and cover-slipped. Tissue from the different treatment groups was processed together in batches in order to control for variability in the immunohistochemical reaction.
Cover-slipped slides were examined microscopically under 10X magnification to determine and digitally capture marked cells in the regions of interest. Only brain sections having uniform background staining and without extensive damage were included in the statistical analysis. Regions of interest (dorsal striatum; anterior, middle, and posterior hypothalamus, nucleus accumbens core and shell, basolateral amygdala, medial amygdala, and central amygdala) were determined by comparing tissue with adjacent Nissl sections or corresponding sections in a stereotaxic atlas (Paxinos and Watson, 1998). Because of the irregular nature of the c-Fos staining patterns observed in hypothalamic sections chosen for analysis, and because neural activation patterns did not adhere to traditional nuclear boundaries, data from multiple nuclei (paraventricular, dorsal hypothalamus, ventromedial hypothalamus) were pooled into a single measure of hypothalamic c-Fos expression. These regions were digitally imaged using SPOT software and then montaged with GNU Image Manipulation Program (GIMP). Cells were counted using three sections per region for each animal using ImageJ (a National Institute of Health (NIH) sponsored imaging program) by an observer blind to the treatment condition. The average value for the three sections (expressed as counts/mm2) was used for statistical analysis. Reliability of the counting method was checked by re-counting a sample of eight c-Fos immunostained sections, including at least one from each treatment group and from each different region under study. The Pearson product-moment correlation between these two sets of counts was r = 0.972 (p < 0.05). The serotonergic appetite suppressant fenfluramine was used as a positive control for the staining methods.
2.6 Experiments 1–3: Open Field
For the open field experiments (1–3), rats were handled for five min five days per week for one month prior to testing. For each of the four days prior to the test day, each rat received a habituation injection of 0.3 mL isotonic saline i.p. On the day of testing, animals were administered their assigned treatments and then transported to the experiment room for habituation thirty min prior to testing. Within each individual experiment, rats were randomly assigned to one of the following treatment conditions:
| Experiment 4: FG-7142 | vehicle (n=15), 10.0 (n=13) or 20.0 (n=8) mg/kg |
| Experiment 5: AM251 | vehicle (n=15), 2.0 (n=18), 4.0 (n=15), or 8.0 (n=16) mg/kg |
| Experiment 6: AM4113 | vehicle (n=15), 3.0 (n=15), 6.0 (n=16), or 12.0 (n=16) mg/kg |
2.7 Experiments 4–6: Elevated Plus Maze
Unlike the open field experimental procedures, rats in elevated plus maze experiments (4–6) were not handled (except briefly during tail-marking) or habituated to injections prior to testing. On the day of testing, animals were transported to the experiment room as a group between one and two hours prior to test and were also injected in this room. Within each individual experiment, subjects were randomly assigned to one of the following treatment conditions:
| Experiment 1: FG-7142 | vehicle (n=11), 10.0 (n=11) or 20.0 (n=11) mg/kg |
| Experiment 2: AM251 | vehicle (n=12), 2.0 (n=12), 4.0 (n=11), or 8.0 (n=12) mg/kg |
| Experiment 3: AM4113 | vehicle (n=12), 3.0 (n=7), 6.0 (n=8), or 12.0 (n=7) mg/kg |
Experiment 7: c-Fos expression within striatum, hypothalamus, and amygdala
In order to minimize the possible effects of receiving a novel potentially stressful injection, rats were handled for seven days prior to treatment and received i.p. injections of 0.3 mL isotonic saline for four days prior to treatment. Animals were then randomly assigned to receive vehicle (n=4), 8.0 mg/kg AM251 (n=5), 12.0 mg/kg AM4113 (n=4), 20.0 mg/kg FG-7142 (n=4), or 6.0 mg/kg fenfluramine (n=4). Ninety min after drug injection the animals were anesthetized and perfused. The brains were extracted and processed using immunocytochemical methods for detection of c-Fos protein as described above. Labeled cells in regions of interest (central, medial, and basolateral amygdala, hypothalamus, nucleus accumbens shell and core, and dorsal striatum) were counted and analyzed using ImageJ software as described above.
2.8 Data Analysis
For each drug treatment, two measures of anxiety were calculated and analyzed from the open field data: the percentage of total time spent within the inner portion of the field and the ratio of distance traveled within the inner area divided by total distance traveled (i.e., the inner distance ratio). The total distance traveled was also separately analyzed. To determine if locomotor activity contributed to drug effects in the open field, analysis of covariance also was performed, using total distance traveled as the covariate. For elevated plus maze experiments, data were analyzed using one-way ANOVA. If the overall ANOVA term was significant, nonorthogonal planned comparisons using the overall error term were used to compare each treatment with the vehicle control. The alpha level for each comparison was kept at 0.05 because the number of comparisons was restricted to the number of treatments minus one (Keppel, 1982). If the overall ANOVA for a given dependent variable was not significant, linear regression with dose as the x-axis variable was also calculated. Effect size calculations (R2 values; Keppel 1991) were performed to assess the magnitude of the treatment effect (i.e., the size of the treatment effect sum of squares expressed as the proportion of total sum of squares, which is a marker of the total variance accounted for by treatment variance; for example R2 = 0.3 reflects 30% of the variance explained) across experiments and measures. Effect size analyses provide a marker of the magnitude of the treatment effect that is independent of the sample size. For the c-Fos experiment, the average counts in each brain region under a given treatment condition were log transformed to reduce variability, and then analyzed with one-way ANOVA. Each treatment condition was then compared with vehicle in separate planned comparisons.
3.0 Results
3.1 Experiments 1–3: Open Field
For each experiment, the primary anxiety-related measure used was the ratio of time spent in the inner and outer areas of the field. In addition, total distance traveled within the open field was used as a marker of locomotor activity. Table 1 shows the effects of FG-7142 on behavior in the open field. ANOVA revealed a significant overall drug treatment effect for percent of time spent in inner area [F(2,30) = 5.54; p = 0.009]. Tukey post-hoc comparisons showed differences between vehicle and both 10.0 mg/kg and 20.0 mg/kg FG-7142 (p < 0.05). There also was a significant drug treatment effect on total distance traveled [F(2,30) = 7.060; p = 0.003], with significant differences between vehicle and both 10.0 mg/kg and 20.0 mg/kg FG-7142 (p < 0.05). The results with AM251 also are summarized in Table 1. The overall ANOVA for drug treatment effect was not significant for percent time in the inner portion of the field. However, linear regression as a function of dose was significant for percent time in the inner portion [F(1,45) = 4.340, p < 0.05]; this analysis demonstrated that AM251 produced significant dose related effects on this measure. AM251 also produced significant overall effects on total distance traveled [F(3,43) = 6.034; p = 0.002]. Tukey post-hoc comparisons showed that 4.0 mg/kg and 8.0 mg/kg doses significantly decreased distance traveled compared to vehicle controls (p < 0.01). For AM4113 (Table 1) there were significant effects on percent time in the inner portion [F(3,30) = 3.87, p = 0.019] and total distance traveled [F(3,30) = 9.057, p < 0.001]. Tukey post-hoc comparisons of percent time in the inner portion showed a significant decrease between vehicle and 6.0 mg/kg AM4113 (p < 0.01), but also a significant increase between 6.0 mg/kg and 12.0 mg/kg groups (p < 0.01). Tukey tests for total distance showed significant decreases for 3.0 mg/kg (p < 0.01) and 6.0 mg/kg (p < 0.01) doses compared to vehicle, but locomotor activity at both of these doses was also significantly lower than at 12.0 mg/kg (3.0 vs. 12.0 mg/kg: p < 0.01; 6.0 vs. 12.0 mg/kg: p < 0.05).
Table 1.
Mean (± SEM) behavioral measurements from 10-minute open field sessions.
| % Time Spent in Inner Area | Total Distance Traveled (cm) | |
|---|---|---|
| FG-7142 | ||
| Vehicle | 6.80 ± 1.29 | 5010 ± 352 |
| 10.0 mg/kg | 3.30 ± 0.62* | 3078 ± 612* |
| 20.0 mg/kg | 2.54 ± 0.72* | 2747 ± 543* |
| AM251 | ||
| Vehicle | 7.04 ± 0.88 | 4780 ± 236 |
| 2.0 mg/kg | 7.19 ± 1.79 | 3927 ± 407 |
| 4.0 mg/kg | 5.46 ± 1.17 | 3380 ± 212* |
| 8.0 mg/kg | 3.92 ± 0.75# | 3166 ± 271* |
| AM4113 | ||
| Vehicle | 6.14 ± 1.02 | 5371 ± 445 |
| 3.0 mg/kg | 4.19 ± 1.06 | 2259 ± 381* |
| 6.0 mg/kg | 2.44 ± 0.69* | 2443 ± 379* |
| 12.0 mg/kg | 7.65 ± 1.24 | 4657 ± 833 |
different from vehicle, p < 0.05
significant linear dose-related regression
In order to determine the contribution of overall locomotor activity to the anxiety-related measures, analysis of covariance was performed, using the total distance traveled measure as the covariate. These analyses showed that, when the total distance traveled was accounted for (i.e., used as the covariate), there were no significant drug treatment effects on percent time in the inner area (FG-7142 [F(2,29) = 1.04, n.s.]; AM251 [F(3,43) = 1.00, n.s.]; AM4113 [F(3,29) = 0.91, n.s.]. Additional covariance analyses were performed on the ratio of inner to outer crossings (data not shown), and again, there were no significant treatment effects (FG-7142 [F(2,29) = 0.87, n.s.]; AM251 [F(3,43) = 2.02, n.s.]; AM4113 [F(3,29) = 2.29, n.s.].
3.2 Experiments 4–6: Elevated Plus Maze
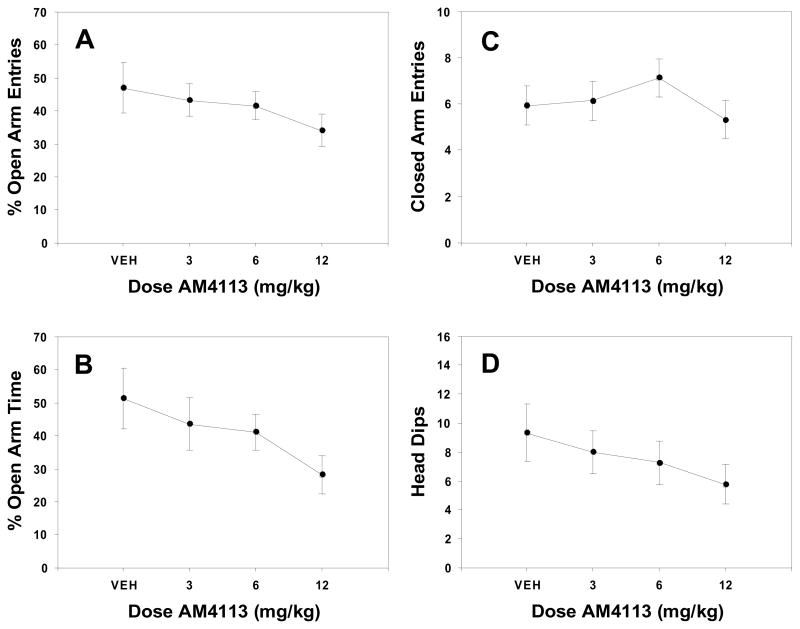
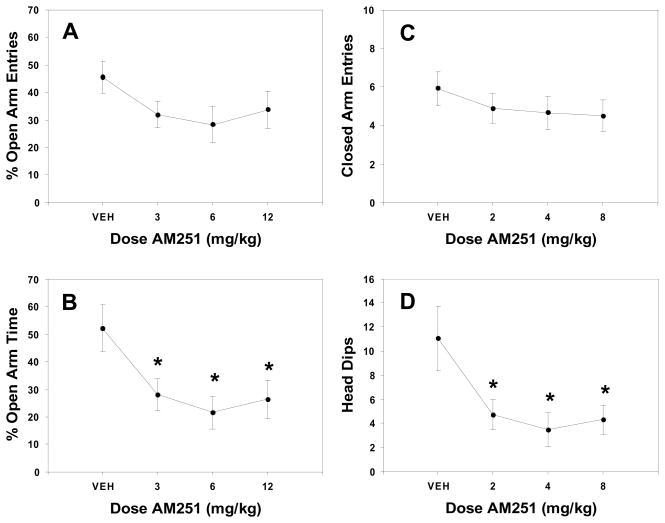
Figures 1–3 summarize the results from the three elevated plus maze experiments. FG-7142 produced overall significance in one-way ANOVAs for percentage of entries that were onto the open arms [Figure 1A; F(2,33) = 7.219, p = 0.003; R2= 0.3], percentage of time spent on open arms [Figure 1B; F(2,33) = 7.031, p = 0.003; R2 = 0.3], and head dips below the platform level on the open arms [Figure 1D; F(2,33) = 6.465, p = 0.004; R2 = 0.28], but not for closed arm entries [Figure 1C; F(2,33) = 0.239, n.s.] or total arm entries [F(2,33) = 0.905, n.s.; data not shown]. For the three variables for which overall significance was found, planned comparisons showed that both 10.0 and 20.0 mg/kg produced significant decreases (p < 0.05). AM251 (Figure 2) produced a significant overall effect for percent time spent on open arms [Figure 2B; F(3,60) = 3.801, p = 0.015; R2 = 0.16] and head dips [Figure 2D; F(3,60) = 4.744, p = 4.744; R2 = 0.19], and planned comparisons showed that every dose produced significant decreases compared to vehicle (p < 0.05). There was no significant effect of AM251 on activity-related measures, including closed arm entries [F(3,60) = 1.215, n.s.; Figure 2C] and total arm entries [F(3,60) = 0.559, n.s.; data not shown]. For AM4113, none of the measures of behavior on the elevated plus maze were significant as determined either by ANOVA or by linear regression analyses (figure 3A–D), and the effect sizes for percent open arm entries, percent time on open arms, and head dips were extremely low (R2 = 0.05, 0.08 and 0.04, respectively).
Figure 1.
Mean (± SEM) effects of FG-7412 on measures in the elevated plus maze. A) Percentage of open arm entries B) Percentage of time spent on open arms C) Total arm entries D) Head dips. FG-7142 produced significant decreases in all measures except total arm entries. *significantly different from control at p = 0.05.
Figure 3.
Mean (± SEM) effects of AM4113 on measures in the elevated plus maze. A) Percentage of open arm entries B) Percentage of time spent on open arms C) Total arm entries D) Head dips. None of the doses of AM4113 produced significant effects on any of the measures employed.
Figure 2.
Mean (± SEM) effects of AM251 on measures in the elevated plus maze. A) Percentage of open arm entries B) Percentage of time spent on open arms C) Total arm entries D) Head dips. AM251 produced significant decreases in percentage of time spent on open arms and number of head dips. *significantly different from control at p = 0.05.
3.3 Experiment 7: c-Fos expression
Table 2 shows the c-Fos-immunopositive cell counts following drug or vehicle treatment; ANOVA on log-transformed counts showed significant overall effects for all regions under investigation (basolateral amygdala [F(4,17) = 13.3, p < 0.001], central nucleus of the amygdala [F(4,17) = 13.0, p < 0.001], and medial amygdala [F(4,17) = 5.7, p < 0.004], hypothalamus [F(4,17) = 4.82, p < 0.01], dorsal striatum [F(4,17) = 30.5, p < 0.001], nucleus accumbens core [F(4,17) = 9.84, p < 0.001] and nucleus accumbens shell [F(4,17) = 14.64, p < 0.001]). Expression of c-Fos in the vehicle conditions was generally very low, except within the hypothalamus, where there appeared to be higher levels of baseline staining. Planned comparisons examining c-Fos immunoreactivity within each region were performed to contrast each drug with vehicle. FG-7142 significantly increased c-Fos expression in all three amygdala regions and nucleus accumbens shell. AM251 increased c-Fos expression in central amygdala, dorsal striatum, and nucleus accumbens shell. AM4113 produced no significant effects in any region. Fenfluramine, which was used as a positive control, produced significant increases over vehicle for all regions studied except the basolateral amygdala.
Table 2.
c-Fos counts (Mean ± SEM counts/mm2) within each brain region (average of three sections per animal).
| Veh | AM251 (8 mg/kg) | AM4113 (12 mg/kg) | FG-7142 (20 mg/kg) | Fen (6 mg/kg) | |
|---|---|---|---|---|---|
| Amygdala | |||||
| BLA | 34.3 ± 14.0 | 23.1 ± 7.8 | 21.6 ± 7.6 | 407.7 ± 182.4* | 68.5 ± 9.0 |
| CA | 49.3 ± 19.4 | 247.8 ± 93.8* | 60.3 ± 13.0 | 631.5 ± 127.0* | 499.3 ± 82.5* |
| MA | 67.6 ± 21.1 | 61.8 ± 8.3 | 66.2 ± 9.3 | 228.0 ± 97.7* | 187.5 ± 27.4* |
| Hypothalamus | 57.9 ± 18.7 | 85.7 ± 14.5 | 58.7± 12.2 | 77.9 ± 9.7 | 182.5 ± 19.5* |
| Striatum | |||||
| DS | 3.1 ± 1.0 | 15.9 ± 5.5* | 2.4 ± 0.6 | 3.7 ± 0.8 | 351.8 ±38.7* |
| NAc Core | 21.7 ± 6.6 | 37.6 ± 6.7 | 14.6 ± 3.5 | 42.1 ± 15.5 | 199.2 ± 47.9* |
| NAc Shell | 51.8 ± 15.0 | 103.5 ± 17.8* | 46.8 ± 10.2 | 121.3 ± 14.8* | 343.7 ± 41.7* |
BLA: basolateral amygdala, CA: central amygdala, MA: Medial amygdala, DS: dorsal striatum, NAc: Nucleus accumbens;
indicates significantly different from vehicle controls (p < 0.05).
4.0 Discussion
The purpose of these experiments was to compare the anxiogenic potential of AM4113 and AM251 with a well-characterized anxiogenic substance, the benzodiazepine inverse agonist FG-7142 (Evans and Lowry, 2007; Meng and Drugan, 1993; Cole et al., 1995). In addition, neural activation patterns induced by these drugs, as measured by c-Fos immunoreactivity in amygdala, hypothalamus, and striatal areas, also were analyzed. Anxiogenic potential was assessed by testing the effects of FG-7142, AM251 and AM4113 in two rodent tests of anxiety: the elevated plus maze and the open field. These two procedures are referred to as spontaneous approach-avoidance tasks because they capitalize on psychological conflict between the fear of novel, open spaces, and the drive to explore (Wall and Messier, 2001). Rodents spending less time exploring open areas away from walls or enclosures are generally considered to be exhibiting higher levels of anxiety (Cruz et al., 1994; Wall and Messier, 2001; Prut and Belzung, 2003). Nevertheless, interpretation of activity-based measures in rodent anxiety models also is complicated by the fact that drugs may decrease locomotor activity because of an increased level of anxiety-provoked freezing, which is an ethological defensive reaction to threatening situations in rodents (Pellow et al., 1985; Rodgers, 1997; Blanchard et al., 1986), or because of motor suppressant effects. In the present studies, despite the fact that all three drugs had some effects on anxiety-related measures in the open field, they also suppressed the total distance traveled. For this reason, the open field tests also were analyzed by analysis of covariance using total distance traveled as the covariate. With this analysis, there were no significant drug treatment effects. This pattern of results suggests that the drug effects on anxiety-related measures in the open field were largely due to drug-induced suppression of locomotion, as measured by total distance traveled. Thus, it is difficult to interpret the results from the open field test in terms of their potential relevance for anxiety.
In contrast, the elevated plus maze yielded a clear pattern of results. FG-7142, a benzodiazepine inverse agonist with previously demonstrated anxiogenic properties (Evans and Lowry, 2007; Meng and Drugan, 1993; Cole et al., 1995), produced robust anxiety-related behavior in the elevated plus maze. In this task, all three anxiety-related measures (percentage of open entries, percentage of open time, and head dips) were significantly reduced in rats treated with either dose of FG-7142. FG-7142 did not affect either closed arm entries or total arm entries in the elevated plus maze, which are measures that are thought to be related to overall locomotor activity in that task. The cannabinoid antagonist/inverse agonist AM251 also induced anxiety-related behavior in the elevated plus maze. In that task, AM251 induced anxiogenic effects on some of the anxiety-related measures, including percent of time in open arms and the number of head dips. Also, both the number of closed arm entries and the total number of arm entries were unaffected, indicating a lack of effect by AM251 on locomotor activity in the elevated plus maze. These results are consistent with previous studies of CB1 inverse agonists (Arevalo et al., 2001; McGregor et al., 1996; Navarro et al., 1997; Rodriguez de Fonseca et al., 1996), including studies with AM251 in mice (Haller et al., 2004; Rodgers et al., 2005). Although the CB1 inverse agonist AM251 induced anxiogenic effects in the plus maze, the CB1 neutral antagonist AM4113 produced no significant effects on any measure in the elevated plus maze.
In the present studies, observers noted that both AM4113 and AM251 also produced effects on scratching and grooming. In the open field experiments, not a single rat treated with vehicle or FG-7142 was marked as exhibiting excessive scratching or grooming behavior. In rats treated with AM251, 3 out of 12 rats administered 2.0 mg/kg were designated as exhibiting excessive scratching and grooming, 2 out of 8 rats that received 4.0 mg/kg, and 2 out of 12 animals in the 8.0 mg/kg dose group were denoted as such. In animals treated with AM4113, 1 of 7 animals given 3.0 mg/kg, 2 of 8 animals given 6.0 mg/kg, and 4 out of 7 animals that received 12.0 mg/kg were marked as displaying excessive scratching or grooming. These observations raise the issue of whether high levels of scratching and grooming might have displaced normal exploratory behavior, rendering activity-based anxiety measures less appropriate for detecting anxiogenic effects of AM4113 and AM251. Previous studies have found excessive scratching and grooming levels to be induced by both AM4113 (Hodge et al., 2008; Jarbe et al., 2002, 2006, 2008), and AM251 (Navarro et al., 1997; Jarbe et al., 2002; Rubino et al., 2000; Vickers et al., 2003; Tallett et al., 2007a, 2007b), and it has been suggested that these behaviors are disruptive enough to produce response competition (Tallett et al., 2007a, 2007b). It is possible that excessive scratching or grooming could present themselves as side effects in some humans administered CB1 inverse agonists or antagonists. Furthermore, it should be mentioned that results from both elevated plus maze and open field studies must be interpreted with caution because the drug treatments also can induce changes in locomotor activity. It has been demonstrated that drugs that do not cause anxiety but specifically affect locomotor activity may produce outcomes in these models that appear anxiolytic or anxiogenic (Dawson et al., 1995). Both of the CB1 drugs tested in this study (AM251 and AM4113) decreased locomotor activity in the open field, and also were shown to suppress locomotion in small stabilimeter chambers (McLaughlin et al., 2003; Sink et al., 2008a). Nevertheless, neither AM251 nor AM4113 affected closed or total arm entries in the elevated plus maze, which also can be used as measures of locomotion.
FG-7142 produced strong expression of c-Fos immunoreactivity in central, basolateral, and medial regions of the amygdala, and nucleus accumbens shell. The densest counts were observed in the amygdaloid areas. This is consistent with previous studies reporting that FG-7142 significantly increased c-Fos expression in the amygdala (Salchner et al., 2006; Singewald et al., 2003). Although one must be cautious in evaluating the behavioral significance of drug-induced changes in c-Fos expression, it is worth stating that central, basolateral, and medial amygdaloid nuclei are known to mediate behaviors associated with anxiety and fear, and are activated following anxiogenic and fear-provoking stimuli (Hinks et al., 1996; Campeau et al., 1997; Duncan et al., 1996). The fact that FG-7142 increased c-Fos expression in the nucleus accumbens shell is consistent with previous data showing that this drug also increased extracellular DA in nucleus accumbens (McCullough and Salamone, 1992), and further emphasizes that this forebrain structure is responsive to aversive stimuli as well as appetitive ones (Salamone et al., 2007b). Fenfluramine was used as a positive control for the c-Fos study, and consistent with previous experiments this drug produced universally robust expression in every region under investigation except the basolateral amygdala, with the densest counts being within striatal structures (Rowland et al., 2000; Torres and Rivier, 1994; Gardier et al., 2000; Cook and Wirtshafter, 1998). AM251 increased c-Fos immunoreactivity relative to the vehicle condition in the central amygdala, dorsal striatum, and nucleus accumbens shell region. This pattern of c-Fos immunoreactivity was similar to that observed previously using a different CB1 inverse agonist, SR141716 (Alonso et al., 1999; Rodriguez de Fonseca et al., 1997). These observervations are consistent with previous reports that CB1 receptor tone is generated within the amygdala in response to stressful or anxiety-inducing stimuli (Kathuria et al., 2003), and that elevated anandamide levels in mouse amygdala were observed following exposure to a tone that was paired with footshock (Marsicano et al., 2002). By contrast, in those rats treated with the CB1 antagonist AM4113, there were no significant effects on c-Fos positive cell counts. Thus, the results of the elevated plus maze and the c-Fos activation studies indicated that AM4113 was weaker than AM251, even though both drugs were given in doses that produced comparable effects on food intake. These results are consistent with studies showing that AM4113 had little effect in tests of intrinsic activity at CB1 receptors in vitro (Sink et al., 2008a; Chambers et al., 2007), while AM251 was capable of producing the more substantial signal transduction effects that are characteristic of inverse agonism (McLaughlin et al., 2006; Sink et al., 2008a).
To summarize, the anxiogenic reference compound FG-7142 produced behavioral signs of anxiety in the elevated plus maze, as expected. The CB1 inverse agonist AM251 induced anxiogenic effects on some anxiety-related measures in the plus maze. AM251 also increased neural activation (as measured by c-Fos expression) within structures associated with anxiety or aversive conditions. In line with our hypothesis that the CB1 antagonist would produce a weaker profile of anxiety-like behaviors than the inverse agonist, AM4113 produced no significant effect in the elevated plus maze, and no significant induction of c-Fos activity. Taken together, these results may indicate that a drug such as AM4113 could have a lower clinical potential for inducing anxiety. Considering that both CB1 antagonists and CB1 inverse agonists suppress food intake (for review see Salamone et al., 2007a), these results suggest that CB1 antagonists may produce a more favorable clinical psychiatric profile (see Le Foll et al., 2009) with fewer anxiety-related side effects, relative to drugs that have stronger CB1 inverse agonist activity.
Acknowledgments
This research was supported by a grant to JDS and AM from the United States NIH/NIDA (U01DA016194).
Footnotes
CONTRIBUTORS
All authors contributed significantly to this manuscript. The work was part of the Ph.D. dissertation of K. Sink. K. Sink, K.N. Segovia, P. Randall, and M. Correa performed the behavioral studies. K. Sink, K. Segovia and J. Sink performed the c-Fos studies. V. K. Vemuri and A. Makriyannis synthesized AM 251 and AM4113. The senior author, J. Salamone, supervised the entire project.
Disclosure/Conflict of Interest: The author(s) declare that, except for income received from my primary employer, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
CONFLICT OF INTEREST
There are no conflicts of interest connected to this work. The authors declare that, except for income received from my primary employer, no financial support or compensation has been received from any individual or corporate entity for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addy C, Rothenberg P, Li S, Majumdar A, Agrawal N, Li H, Zhong L, Yuan J, Maes A, Dunbar S, Cote J, Rosko K, Van Dyck K, De Lepeleire I, de Hoon J, Van Hecken A, Depré M, Knops A, Gottesdiener K, Stoch A, Wagner J. Multiple-dose pharmacokinetics, pharmacodynamics, and safety of taranabant, a novel selective cannabinoid-1 receptor inverse agonist, in healthy male volunteers. J Clin Pharmacol. 2008;48:734–744. doi: 10.1177/0091270008317591. [DOI] [PubMed] [Google Scholar]
- Akinshola BE, Chakrabarti A, Onaivi ES. In-vitro and in-vivo action of cannabinoids. Neurochem Res. 1999;24:1233–1240. doi: 10.1023/a:1020968922151. [DOI] [PubMed] [Google Scholar]
- Alonso R, Voutsinos B, Fournier M, Labie C, Steinberg R, Souilhac J, Le Fur G, Soubrie P. Blockade of cannabinoid receptors by SR141716 selectively increases fos expression in rat mesocorticolimbic areas via reduced dopamine D2 function. Neuroscience. 1999;91:607–620. doi: 10.1016/s0306-4522(98)00675-7. [DOI] [PubMed] [Google Scholar]
- Arevalo C, de Miguel R, Hernandez-Tristan R. Cannabinoid effects on anxiety-related behaviours and hypothalamic neurotransmitters. Pharmacol Biochem Behav. 2001;70:123–131. doi: 10.1016/s0091-3057(01)00578-0. [DOI] [PubMed] [Google Scholar]
- Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, Le Fur G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Flannelly KJ, Blanchard DC. Defensive behavior of laboratory and wild Rattus norvegicus. J Comp Psychol. 1986;100:101–7. [PubMed] [Google Scholar]
- Campeau S, Falls WA, Cullinan WE, Helmreich DL, Davis M, Watson SJ. Elicitation and reduction of fear: Behavioural and neuroendocrine indices and brain induction of the immediate-early gene c-fos. Neuroscience. 1997;78:1087–1104. doi: 10.1016/s0306-4522(96)00632-x. [DOI] [PubMed] [Google Scholar]
- Chambers AP, Vemuri VK, Peng Y, Wood JT, Olszewska T, Pittman QJ, Makriyannis A, Sharkey KA. A neutral CB1 receptor antagonist reduces weight gain in rat. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2185–93. doi: 10.1152/ajpregu.00663.2007. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Hillmann M, Seidelmann D, Klewer M, Jones GH. Effects of benzodiazepine receptor partial inverse agonists in the elevated plus maze test of anxiety in the rat. Psychopharmacology (Berl) 1995;121:118–126. doi: 10.1007/BF02245598. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci. 1998;63:PL113–7. doi: 10.1016/s0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- Cook DF, Wirtshafter D. Quinpirole attenuates striatal c-fos induction by 5-HT, opioid and muscarinic receptor agonists. Eur J Pharmacol. 1998;349:41–47. doi: 10.1016/s0014-2999(98)00184-8. [DOI] [PubMed] [Google Scholar]
- Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994;49:171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- Curioni C, Andre C. Rimonabant for overweight or obesity. Cochrane Database Syst Rev. 2006;4:CD006162. doi: 10.1002/14651858.CD006162.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson GR, Crawford SP, Collinson N, Iversen SD, Tricklebank MD. Evidence that the anxiolytic-like effects of chlordiazepoxide on the elevated plus maze are confounded by increases in locomotor activity. Psychopharmacology (Berl) 1995;118:316–323. doi: 10.1007/BF02245961. [DOI] [PubMed] [Google Scholar]
- Despres JP, Golay A, Sjostrom L Rimonabant in Obesity-Lipids Study Group. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Knapp DJ, Breese GR. Neuroanatomical characterization of fos induction in rat behavioral models of anxiety. Brain Res. 1996;713:79–91. doi: 10.1016/0006-8993(95)01486-1. [DOI] [PubMed] [Google Scholar]
- Evans AK, Lowry CA. Pharmacology of the beta-carboline FG-7,142, a partial inverse agonist at the benzodiazepine allosteric site of the GABA A receptor: Neurochemical, neurophysiological, and behavioral effects. CNS Drug Rev. 2007;13:475–501. doi: 10.1111/j.1527-3458.2007.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis J, Dourish CT, Cooper SJ. Devazepide attenuates dl-fenfluramine-induced suppression of gastric emptying but not food intake in the 17 h food-deprived rat. Physiol Behav. 1997;62:545–50. doi: 10.1016/s0031-9384(97)80332-0. [DOI] [PubMed] [Google Scholar]
- Gardner A, Mallet PE. Suppression of feeding, drinking, and locomotion by a putative cannabinoid receptor ‘silent antagonist’. Eur J Pharmacol. 2006;530:103–106. doi: 10.1016/j.ejphar.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Gardier AM, Moratalla R, Cuellar B, Sacerdote M, Guibert B, Lebrec H, Graybiel AM. Interaction between the serotoninergic and dopaminergic systems in d-fenfluramine-induced activation of c-fos and jun B genes in rat striatal neurons. J Neurochem. 2000;74:1363–1373. doi: 10.1046/j.1471-4159.2000.0741363.x. [DOI] [PubMed] [Google Scholar]
- Haller J, Bakos N, Szirmay M, Ledent C, Freund TF. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur J Neurosci. 2002;16:1395–1398. doi: 10.1046/j.1460-9568.2002.02192.x. [DOI] [PubMed] [Google Scholar]
- Haller J, Varga B, Ledent C, Freund TF. CB1 cannabinoid receptors mediate anxiolytic effects: convergent genetic and pharmacological evidence with CB1-specific agents. Behav Pharmacol. 2004;15:299–304. doi: 10.1097/01.fbp.0000135704.56422.40. [DOI] [PubMed] [Google Scholar]
- Hinks GL, Brown P, Field M, Poat JA, Hughes J. The anxiolytics CI-988 and chlordiazepoxide fail to reduce immediate early gene mRNA stimulation following exposure to the rat elevated X-maze. Eur J Pharmacol. 1996;312:153–161. doi: 10.1016/0014-2999(96)00471-2. [DOI] [PubMed] [Google Scholar]
- Hodge J, Bow JP, Plyler KS, Vemuri VK, Wisniecki A, Salamone JD, Makriyannis A, McLaughlin PJ. The cannabinoid CB1 receptor inverse agonist AM 251 and antagonist AM 4113 produce similar effects on the behavioral satiety sequence in rats. Behav Brain Res. 2008;193:298–305. doi: 10.1016/j.bbr.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- Hughes P, Lawlor P, Dragunow M. Basal expression of fos, fos-related, jun, and krox 24 proteins in rat hippocampus. Brain Res Mol Brain Res. 1992;13:355–357. doi: 10.1016/0169-328x(92)90219-2. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, Andrzejewski ME, DiPatrizio NV. Interactions between the CB1 receptor agonist delta 9-THC and the CB1 receptor antagonist SR-141716 in rats: Open-field revisited. Pharmacol Biochem Behav. 2002;73:911–919. doi: 10.1016/s0091-3057(02)00938-3. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, Lemay BJ, Olszewska T, Vemuri VK, Wood JT, Makriyannis A. Intrinsic effects of AM4113, a putative neutral CB1 receptor selective antagonist, on open-field behaviors in rats. Pharmacol Biochem Behav. 2008;91:84–90. doi: 10.1016/j.pbb.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbe TU, Ross T, DiPatrizio NV, Pandarinathan L, Makriyannis A. Effects of the CB1R agonist WIN-55,212-2 and the CB1R antagonists SR-141716 and AM-1387: Open-field examination in rats. Pharmacol Biochem Behav. 2006;85:243–252. doi: 10.1016/j.pbb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and Analysis: A Researcher’s Handbook. Prentice-Hall; Englewood Cliffs NJ: 1982. [Google Scholar]
- Keppel G. Design and analysis: a researcher’s handbook. New Jersey: Prentice-Hall, Englewood Cliffs NJ; 1991. pp. 110–139. [Google Scholar]
- Le Foll B, Gorelick DA, Goldberg SR. The future of endocannabinoid-oriented clinical research after CB1 antagonists. Psychopharmacology. 2009;205:171–174. doi: 10.1007/s00213-009-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Salamone JD. Anxiogenic drugs beta-CCE and FG 7142 increase extracellular dopamine levels in nucleus accumbens. Psychopharmacology (Berl) 1992;109:379–382. doi: 10.1007/BF02245888. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Issakidis CN, Prior G. Aversive effects of the synthetic cannabinoid CP 55,940 in rats. Pharmacol Biochem Behav. 1996;53:657–664. doi: 10.1016/0091-3057(95)02066-7. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Qian L, Wood JT, Wisniecki A, Winston KM, Swezey LA, Ishiwari K, Betz AJ, Pandarinathan L, Xu W, Makriyannis A, Salamone JD. Suppression of food intake and food-reinforced behavior produced by the novel CB1 receptor antagonist/inverse agonist AM 1387. Pharmacol Biochem Behav. 2006;83:396–402. doi: 10.1016/j.pbb.2006.02.022. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston K, Swezey L, Wisniecki A, Aberman J, Tardif DJ, Betz AJ, Ishiwari K, Makriyannis A, Salamone JD. The cannabinoid CB1 antagonists SR 141716A and AM 251 suppress food intake and food-reinforced behavior in a variety of tasks in rats. Behav Pharmacol. 2003;14:583–588. doi: 10.1097/00008877-200312000-00002. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston KM, Limebeer CL, Parker LA, Makriyannis A, Salamone JD. The cannabinoid CB1 antagonist AM 251 produces food avoidance and behaviors associated with nausea but does not impair feeding efficiency in rats. Psychopharmacology (Berl) 2005;180:286–293. doi: 10.1007/s00213-005-2171-0. [DOI] [PubMed] [Google Scholar]
- Meng ID, Drugan RC. Sex differences in open-field behavior in response to the beta-carboline FG 7142 in rats. Physiol Behav. 1993;54:701–705. doi: 10.1016/0031-9384(93)90079-u. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in neurons: Role of cellular immediate-early genes. Trends Neurosci. 1989;12:459–462. doi: 10.1016/0166-2236(89)90096-9. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: Involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Navarro M, Hernandez E, Munoz RM, del Arco I, Villanua MA, Carrera MR, Rodriguez de Fonseca F. Acute administration of the CB1 cannabinoid receptor antagonist SR 141716A induces anxiety-like responses in the rat. Neuroreport. 1997;8:491–496. doi: 10.1097/00001756-199701200-00023. [DOI] [PubMed] [Google Scholar]
- Paxinos G. The rat nervous system. Academic Press; Sydney: 2004. [Google Scholar]
- Paxinos G, Watson C. The rat brain in sterotaxic coordinates. Academic Press; Sydney: 1998. [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J RIO-North America Study Group. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-north america: A randomized controlled trial. JAMA. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ. Animal models of ‘anxiety’: Where next? Behav Pharmacol. 1997;8:477–96. doi: 10.1097/00008877-199711000-00003. discussion 497–504. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Evans PM, Murphy A. Anxiogenic profile of AM-251, a selective cannabinoid CB1 receptor antagonist, in plus-maze-naïve and plus-maze-experienced mice. Behav Pharmacol. 2005;16:405–413. doi: 10.1097/00008877-200509000-00013. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Carrera MR, Navarro M, Koob GF, Weiss F. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276:2050–2054. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Rubio P, Menzaghi F, Merlo-Pich E, Rivier J, Koob GF, Navarro M. Corticotropin-releasing factor (CRF) antagonist [D-Phe12,Nle21,38,C alpha MeLeu37]CRF attenuates the acute actions of the highly potent cannabinoid receptor agonist HU-210 on defensive-withdrawal behavior in rats. J Pharmacol Exp Ther. 1996;276:56–64. [PubMed] [Google Scholar]
- Rowland NE, Roth JD, McMullen MR, Patel A, Cespedes AT. Dexfenfluramine and norfenfluramine: Comparison of mechanism of action in feeding and brain fos-ir studies. Am J Physiol Regul Integr Comp Physiol. 2000;278:R390–9. doi: 10.1152/ajpregu.2000.278.2.R390. [DOI] [PubMed] [Google Scholar]
- Rubino T, Vigano D, Zagato E, Sala M, Parolaro D. In vivo characterization of the specific cannabinoid receptor antagonist, SR141716A: Behavioral and cellular responses after acute and chronic treatments. Synapse. 2000;35:8–14. doi: 10.1002/(SICI)1098-2396(200001)35:1<8::AID-SYN2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Rueda-Orozco PE, Montes-Rodriguez CJ, Soria-Gomez E, Mendez-Diaz M, Prospero-Garcia O. Impairment of endocannabinoids activity in the dorsolateral striatum delays extinction of behavior in a procedural memory task in rats. Neuropharmacology. 2008;55:55–62. doi: 10.1016/j.neuropharm.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Arizzi MN, Sandoval MD, Cervone KM, Aberman JE. Dopamine antagonists alter response allocation but do not suppress appetite for food in rats: Contrast between the effects of SKF 83566, raclopride, and fenfluramine on a concurrent choice task. Psychopharmacology (Berl) 2002;160:371–380. doi: 10.1007/s00213-001-0994-x. [DOI] [PubMed] [Google Scholar]
- Salamone JD, McLaughlin PJ, Sink K, Makriyannis A, Parker LA. Cannabinoid CB1 receptor inverse agonists and neutral antagonists: Effects on food intake, food-reinforced behavior and food aversions. Physiol Behav. 2007a;91:383–388. doi: 10.1016/j.physbeh.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007b;191:461–82. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Salchner P, Sartori SB, Sinner C, Wigger A, Frank E, Landgraf R, Singewald N. Airjet and FG-7142-induced fos expression differs in rats selectively bred for high and low anxiety-related behavior. Neuropharmacology. 2006;50:1048–1058. doi: 10.1016/j.neuropharm.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Shearman LP, Rosko KM, Fleischer R, Wang J, Xu S, Tong XS, Rocha BA. Antidepressant-like and anorectic effects of the cannabinoid CB1 receptor inverse agonist AM251 in mice. Behav Pharmacol. 2003;14:573–582. doi: 10.1097/00008877-200312000-00001. [DOI] [PubMed] [Google Scholar]
- Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T. Induction of c-fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol Psychiatry. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Sink KS, Vemuri VK, Wood J, Makriyannis A, Salamone JD. Oral bioavailability of the novel cannabinoid CB1 antagonist AM6527: Effects on food-reinforced behavior and comparisons with AM4113. Pharmacol Biochem Behav. 2009;91:303–6. doi: 10.1016/j.pbb.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, McLaughlin PJ, Wood JA, Brown C, Fan P, Vemuri VK, Pang Y, Olzewska T, Thakur GA, Makriyannis A, Parker LA, Salamone JD. The novel cannabinoid CB(1) receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology. 2008a;33:946–55. doi: 10.1038/sj.npp.1301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Vemuri VK, Olszewska T, Makriyannis A, Salamone JD. Cannabinoid CB1 antagonists and dopamine antagonists produce different effects on a task involving response allocation and effort-related choice in food-seeking behavior. Psychopharmacology (Berl) 2008b;196:565–74. doi: 10.1007/s00213-007-0988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnayah P, Jobst EE, Rathner JA, Caldera-Siu AD, Tonelli-Lemos L, Eusterbrock AJ, Enriori PJ, Pothos EN, Grove KL, Cowley MA. Feeding induced by cannabinoids is mediated independently of the melanocortin system. PLoS ONE. 2008;3:e2202. doi: 10.1371/journal.pone.0002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallett AJ, Blundell JE, Rodgers JR. Acute anorectic response to cannabinoid CB1 receptor antagonist/inverse agonist AM 251 in rats: Indirect behavioural mediation. Behav Pharmacol. 2007a;18:591–600. doi: 10.1097/FBP.0b013e3282eff0a9. [DOI] [PubMed] [Google Scholar]
- Tallett AJ, Blundell JE, Rodgers RJ. Grooming, scratching and feeding: Role of response competition in acute anorectic response to rimonabant in male rats. Psychopharmacology (Berl) 2007b;195:27–39. doi: 10.1007/s00213-007-0880-2. [DOI] [PubMed] [Google Scholar]
- Torres G, Rivier C. Induction of c-fos in rat brain by acute cocaine and fenfluramine exposure: A comparison study. Brain Res. 1994;647:1–9. doi: 10.1016/0006-8993(94)91391-9. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration Advisory Committee. FDA; Rockville: 2007. [accessed September 2008]. FDA briefing document: zimulti (rimonabant) Tablets, 20 mg. [WWW document]. URL ( http://www.fda.gov/OHRMS/DOCKETS/AC/07/briefing/2007-4306b1-00-index.htm) [Google Scholar]
- Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S RIO-Europe Study Group. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Webster LJ, Wyatt A, Dourish CT, Kennett GA. Preferential effects of the cannabinoid CB1 receptor antagonist, SR 141716, on food intake and body weight gain of obese (fa/fa) compared to lean zucker rats. Psychopharmacology (Berl) 2003;167:103–111. doi: 10.1007/s00213-002-1384-8. [DOI] [PubMed] [Google Scholar]
- Voigt JP, Wenz D, Voits M, Fink H. Does increased endogenous CCK interact with serotonin to reduce food intake in rats? Peptides. 2000;21:1895–901. doi: 10.1016/s0196-9781(00)00329-6. [DOI] [PubMed] [Google Scholar]
- Wall PM, Messier C. Methodological and conceptual issues in the use of the elevated plus-maze as a psychological measurement instrument of animal anxiety-like behavior. Neurosci Biobehav Rev. 2001;25:275–286. doi: 10.1016/s0149-7634(01)00013-6. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Burston JJ, Leggett DC, Alekseeva OO, Razdan RK, Mahadevan A, Martin BR. CB1 cannabinoid receptor-mediated modulation of food intake in mice. Br J Pharmacol. 2005;145:293–300. doi: 10.1038/sj.bjp.0706157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Anandamide induces overeating: Mediation by central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1999;143:315–317. doi: 10.1007/s002130050953. [DOI] [PubMed] [Google Scholar]
- Wisden W, Errington ML, Williams S, Dunnett SB, Waters C, Hitchcock D, Evan G, Bliss TV, Hunt SP. Differential expression of immediate early genes in the hippocampus and spinal cord. Neuron. 1990;4:603–614. doi: 10.1016/0896-6273(90)90118-y. [DOI] [PubMed] [Google Scholar]