Abstract
A major obstacle to understanding the functional importance of dimerization between Class A G protein-coupled receptors (GPCRs) has been the methodological limitation in achieving control of the identity of the components comprising the signaling unit. We have developed a functional complementation assay that enables such control and illustrate it for the human dopamine D2 receptor. The minimal signaling unit, two receptors and a single G protein, is maximally activated by agonist binding to a single protomer, which suggests an asymmetrical activated dimer. Inverse agonist binding to the second protomer enhances signaling, whereas agonist binding to the second protomer blunts signaling. Ligand-independent constitutive activation of the second protomer also inhibits signaling. Thus, GPCR dimer function can be modulated by the activity state of the second protomer, which for a heterodimer may be altered in pathological states. Our novel methodology also makes possible the characterization of signaling from a defined heterodimer unit.
The most intriguing questions about the functional mechanisms of G protein-coupled receptors (GPCRs) center on the role of dimerization, its physiological significance, and its pharmacological consequences1. Many results from the literature are tantalizing because they describe effects that have been attributed to activating one receptor in the presence of another2 and the ability to modulate activity of one receptor by ligands targeting the second receptor3,4. Compelling as these examples are, it has thus far been difficult to construct a mechanism that would coherently explain all these phenomena. For most GPCRs, a major obstacle has been methodological, especially the inability to control the identity of the components of the G protein signaling unit that must include the interacting receptors and G proteins. Here we present a mechanism for rhodopsin-like Class A GPCRs that we were able to identify using a novel approach that enabled us to control the identity of the participants in the signaling complex.
In Class C GPCRs such control has been possible because of the unique cell biology of the GABAB receptor. The R2 subunit does not signal by itself in response to GABA (1), but is essential for surface expression of the R1 subunit and therefore for signaling of the heterodimeric complex5. Therefore, the only species on the surface that can signal must contain R1 and R2, which allows the study of defined heterodimers. These receptors have been shown to function through a “transactivation” mechanism in which a GABA-binding R1 signals through interactions of R2 with G protein5. A clever adaptation of the endoplasmic reticulum (ER) retention signal from the GABAB receptor has enabled controlled cell surface expression and study of signaling by defined metabotropic glutamate receptor (mGluR) “hetero”-dimers6, which have been inferred to signal through trans-activation as well as through cis-activation, in which the agonist-bound receptor interacting directly with G protein6. Such an approach to engineered ER retention signals has not yet been successful in Class A receptors, but Class A glycoprotein hormone receptors with large extracellular binding domains also appear to be capable of both trans- and cis-activation7.
To date the native functional signaling unit in other Class A rhodopsin-like receptors remains unclear. Indeed, both rhodopsin8 and the β2-adrenergic receptor (B2AR)9 have been shown to signal efficiently to G proteins when reconstituted into lipid nanodiscs containing only a single receptor. Thus, after solubilization and reconstitution, these GPCRs can function alone. Nevertheless, such studies cannot determine whether these receptors do function alone in vivo, and this question must be addressed directly through an exploration of signaling in their native organization. A number of studies have shown that coexpression of two different Class A GPCRs can lead to signaling properties that differ from their properties when expressed alone10,11. However, it is not possible from such studies to differentiate downstream integration of signaling from an actual heteromeric signaling unit in which the two protomers interact directly to modulate signaling.
Evidence for association of conformational change at a homodimer interface with activation state12 supports state-dependent communication between protomers and a potential role for inter-protomer modulation of signaling. However, in contrast to findings for the Class C GABAB and mGlu receptors and the Class A receptors with large N-terminal binding sites, TSH, FSH, and LH receptors, results for the rhodopsin-like leukotriene B4 receptor BLT1 support the existence of cis- but not trans-activation, with no functional role identified for the second protomer, despite evidence that it changes conformation in response to agonist binding to its dimer partner13,14.
Receptor-G protein fusion constructs, in which the C-terminus of a GPCR is fused to the N-terminus of Gα, have been used to explore receptor signaling15–18. Coexpression of such GPCR-G protein fusions with a second GPCR has been used to study heterodimer signaling; in such a scenario the unfused GPCR can activate the G protein fused to a coexpressed GPCR15–17. However, the participants in the signaling unit are not identifiable in this experimental protocol because coexpression of GPCRs leads to a combination of different signaling units consisting of both homodimers and heterodimers. Indeed, a tethered G protein fused to a single membrane-spanning segment can be activated efficiently by a coexpressed GPCR16,19, suggesting that a GPCR-G protein fusion construct might provide G protein for activation by another receptor, or another dimer of receptors, without directly participating in the relevant dimeric signaling unit. The long cytoplasmic tails and flexible linkers through which G proteins have been fused to GPCRs are likely to allow promiscuous interactions that exacerbate this problem. Indeed, the tether attaching the B2AR to fused Gs can be dramatically shortened with preserved function20, but it remains unknown whether the G protein in this case is activated by the receptor to which it is covalently attached, or by another receptor.
Here, we have developed a functional complementation assay that allows us to control the components of the human dopamine D2 receptor (D2R) signaling unit and thus to explore the dimeric functional unit and the individual contributions from each GPCR protomer to G protein signaling. Our system reports directly on receptor-G protein interactions, which allows us to rule out downstream crosstalk as the mechanism of modulation of G protein function upon coexpression of different partner receptors. This novel methodology allowed us to propose a mechanistic explanation for the reciprocal modulation of protomer functions in a dimeric signaling complex. The minimal signaling unit, consisting of two GPCRs and a single heterotrimeric G protein, appears to be maximally activated by agonist binding to a single protomer, which suggests an asymmetrical activated dimer. Indeed, agonist binding to the second protomer blunts signaling, whereas inverse agonist binding to the second protomer enhances signaling. Such allosteric modulation of one protomer by the state of the other also has important ramifications for pharmacological manipulation of GPCR heterodimers. That a non-binding constitutively active receptor blunts signaling of a coexpressed wild type (WT) receptor highlights the importance of the conformational state of the second protomer. Therefore, GPCR heterodimer function will be modulated not only by ligand binding to the second protomer, but also by its ligand-independent constitutive activity; both types of modulation may be altered in pathological states.
Results
Engineering a luminescence readout for D2R activation
To isolate signaling of the D2R, a prototypical Go/Gi coupled receptor, from endogenous G proteins and to control each of the components of the signaling complex, we engineered Flp-In T-REx-293 cells to stably express aequorin (AEQ cells) (see Methods). Aequorin produces luminescence in a calcium-dependent manner in the presence of the substrate coelenterazine21 (2), and it has been used to create a sensitive luminescence readout for GPCR-mediated PLC activation22. In these cells, endogenous muscarinic or purinergic receptors signaled robustly via endogenous Gq, resulting in strong agonist-induced (ACH (3) and ATP (4), respectively) luminescence signals (Supplementary Fig. 1a online). In contrast, when D2R was stably expressed in AEQ cells, treatment with the aqonist quinpirole (5) did not lead to luminescence, consistent with a lack of D2R coupling to Gq (Fig. 1a, Supplementary Fig. 2 online).
Figure 1. Functional complementation of two “non-functional receptors”.
We used an aequorin assay that couples Gq (or Gqi5) activation to a luminescence readout. (a) The agonist quinpirole did not lead to D2R-induced Gq activation. (b) D2R when coexpressed with free Gqi5 or (c) D2R fused with Gqi5 via a linker (D2-linker-Gqi5) led to quinpirole-induced luminescence. (c) A nonfunctional Gα deficient fusion construct, D2-linker-Gqi5G208A failed to produce luminescence. (d) Free Gqi5 rescued the function of D2-linker-Gqi5G208A. (e) Free Gqi5 failed to rescue the function of non-linker D2R-Gqi5, which unlike D2R-linker-Gqi5, did not signal when expressed alone. (f) Coexpressing D2R with D2R-Gqi5 (12 hour tetracycline induction) restored signaling, despite the inability of either construct to signal in this assay when expressed alone. Activation data represent luminescence relative to that seen with 0.1% triton treatment. The mean±SEM of at least 3 experiments, each conducted in triplicate, are shown. The symbols used in (Fig. 1–Fig. 4 and Fig. 6) are explained in detail in (Supplementary Fig. 2 online).
To couple D2R activation to a luminescence readout in these cells, we expressed a chimeric, pertussis toxin (PTX)-resistant Gq (Gqi5) that could signal from Gi-coupled receptors23 (see Methods). D2R signaled robustly when stably coexpressed with free Gqi5 or when fused at its C-terminus to Gqi5 through an 8 amino acid linker (D2-linker-Gqi5) (Fig. 1b, c). The increase in luminescence was unaffected by PTX (Supplementary Fig. 1b online), whereas a mutation in Gqi5 (Gqi5G208A) that prevents GTP-induced Gα activation24 prevented the luminescence response to D2R activation (Fig. 1c). No quinpirole response was seen when free Gqi5 was expressed without D2R (data not shown), consistent both with the absence of endogenous D2R in these cells and with the lack of other targets for quinpirole-mediated signaling.
Curiously, expression of free Gqi5 fully rescued function of D2-linker-Gqi5G208A (Fig. 1d), indicating that the linker afforded sufficient flexibility for the nonfunctional G protein to swing away and permit a free functional Gqi5 to interact and to restore agonist-mediated signaling. Therefore, we could not use the D2-linker-Gqi5 construct to monitor functional coupling of two defined protomers, since the flexibility of the linker might allow this construct to provide the Gα to another protomer (or to another dimer of protomers) without the actual participation of the fused receptor in the signaling unit.
To address this problem, we developed another D2R/Gqi5 construct in which the linker was removed and Gqi5 was fused more directly to the short cytoplasmic tail of the D2R (D2-Gqi5). This construct expressed at the plasma membrane (Supplementary Fig. 3a, b online), but agonist treatment failed to produce luminescence (Fig. 1e). We hypothesized that the lack of signaling resulted from the inability of the short-tethered Gα to be positioned appropriately for a productive interaction either with the cytoplasmic loops of the receptor to which it was fused, or with a second protomer. Indeed, in contrast to the D2-linker-Gqi5, D2-Gqi5 signaling was not rescued by free Gqi5 (Fig. 1e), most likely because the tethered Gα sterically blocks free Gqi5 from making a productive interaction with the cytoplasmic loops of the fused receptor.
Remarkably, however, coexpression in the AEQ cells of D2R (termed “Protomer A”) and D2-Gqi5 (“Protomer B”), each of which is incapable of signaling in our assay when expressed alone, led to robust agonist-mediated receptor activation (Fig. 1f), indicating that when activated the fused Gqi5 is fully capable of interacting with PLC. That this effect was mediated solely by the fused Gqi5 and not by endogenous Gi/o was demonstrated by the lack of effect of PTX treatment on activation (Supplementary Fig. 1c online). This reconstitution of a signaling unit from two “nonfunctioning” Class A GPCR protomers provided us with the unique opportunity to manipulate each protomer independently and to determine its role in signaling while eliminating the contribution of homodimers. These experiments do not rule out a higher order receptor complex, but in the simplest scenario the minimal signaling unit is composed of protomer A, protomer B and the G protein fused to protomer B (Fig. 1f), and for simplicity we will subsequently refer to this receptor complex as a “dimer”. The extremely close proximity between these protomers and the inability of protomer B to signal to its own fused G protein or to a neighboring fused G protein indicates that only one G protein serves this signaling unit of two GPCRs. Our inferences regarding the signaling unit are entirely consistent with the results from our parallel computational modeling studies (see below). These modeling studies make the essential point that the relatively large size of the G protein heterotrimer matches the cytoplasmic surfaces of at least two neighboring GPCR protomers.
Revealing asymmetry of signaling
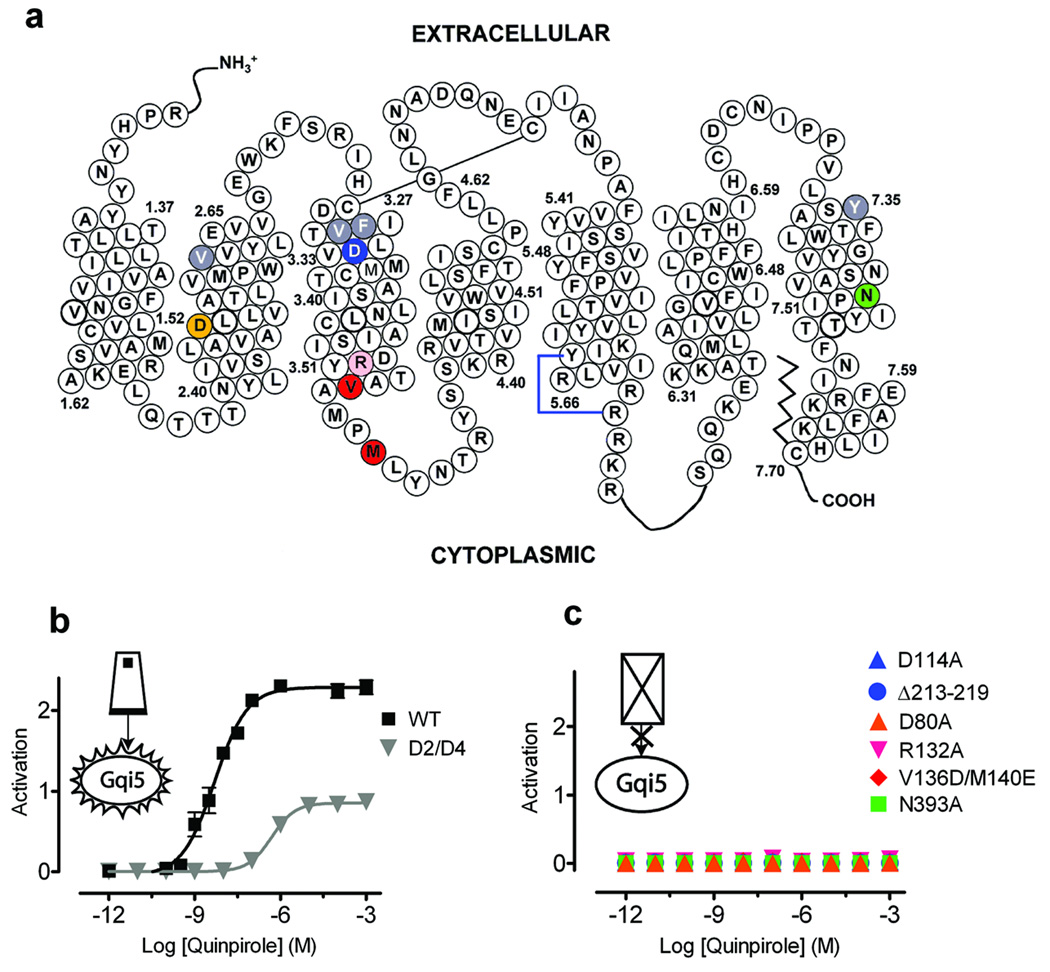
In order to manipulate experimentally the function of each protomer in the dimeric unit, we constructed a panel of D2R mutants predicted to be binding- and activationdeficient based on findings in the literature for related Class A GPCRs. These include D1143.32A, which does not bind agonists or antagonists25, as well as R1323.50A26 and V1363.54D/M1403.58E in IL227, deletion of 213–219 in IL328, and D802.50A (previously characterized in D2R)29 and N3937.49A30 in the membrane-spanning segments (Fig. 2a), all of which were expected to disrupt agonist-mediated G protein activation. We also expressed a D2R mutant V912.61F/F1103.29L/V1113.28M/Y4087.35V (termed D2/D4) (Fig. 2a), which, unlike WT D2R, is potently inhibited by the selective D4 antagonist L745,87031 (6) (Supplementary Fig. 4 online). Each of these constructs expressed at the plasma membrane (Supplementary Fig. 3 online). In contrast to the robust activation of WT, we observed a reduction in potency and a large decrease in maximal activation by quinpirole in D2/D4 when expressed with free Gqi5 (Fig. 2b). As anticipated, none of the mutants deficient in binding or signaling led to agonist-mediated luminescence when placed into an unfused D2R construct coexpressed with free Gqi5 (Fig. 2c), or when the mutations were placed in the D2-linker-Gqi5 construct and expressed alone (Supplementary Fig. 5 online).
Figure 2. Characterization of D2R mutants.
(a) Schematic representation showing the positions of the mutations in the D2 receptor, with coloring corresponding to the symbols and lines in (b) and (c). (b) D2/D4, a D2 mutant with 4 amino acids substituted from the D4 receptor (V912.61F/F1103.29L/V1113.28M/Y4087.35V) making it 1000-times more sensitive to a D4-selective inhibitor (Supplementary Fig. 4 online), is activated by quinpirole, albeit with a lower potency and efficacy when compared with WT D2R. (c) All the other mutants, which are described in the text, were non-functional. Activation data were normalized as in Fig. 1. The mean±SEM of at least 3 experiments, each conducted in triplicate, are shown.
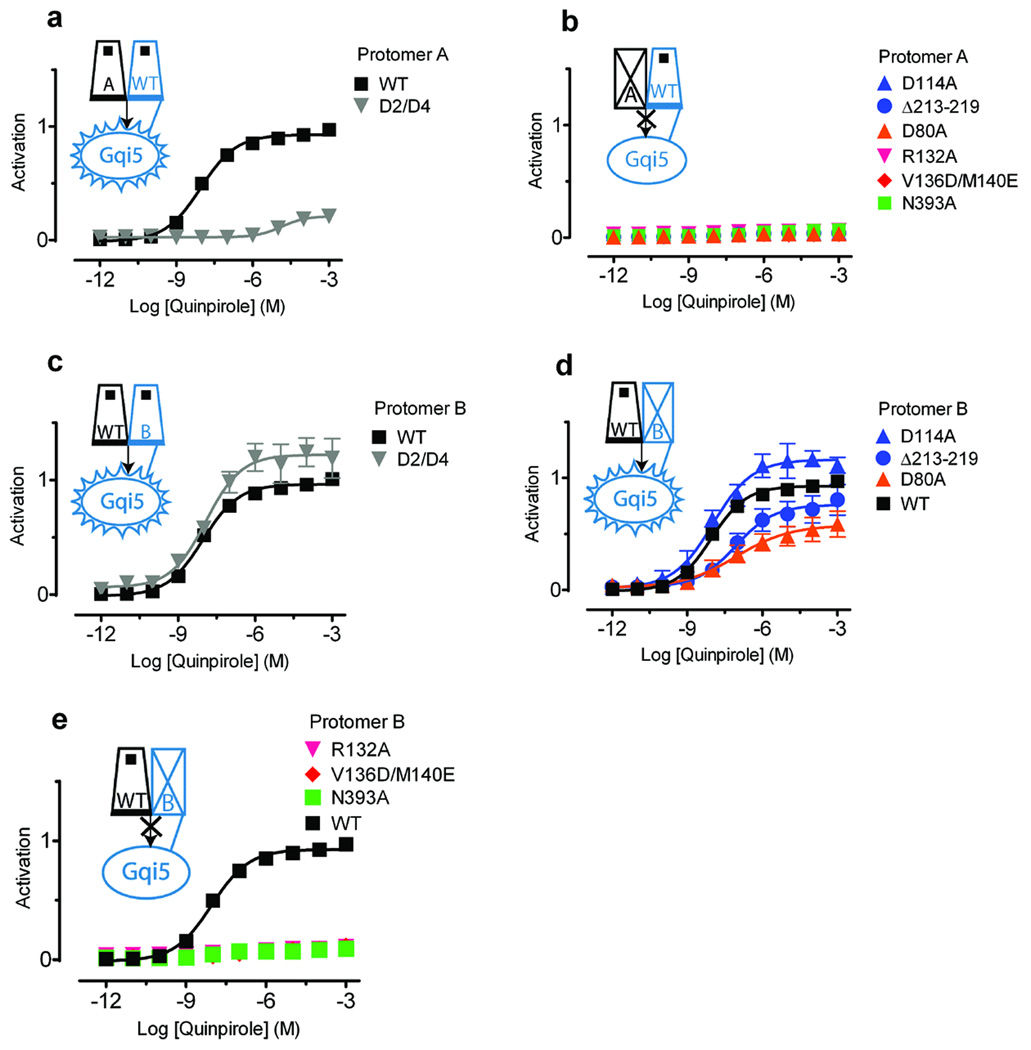
When D2/D4 was expressed as protomer A with WT D2R-Gqi5 as protomer B, we observed a reduction in potency and a large decrease in maximal activation by quinpirole (Fig. 3a), similar to its signaling properties when expressed with free Gqi5 (Fig. 2b). (These and all subsequent activation data were normalized for surface expression of protomer B; see Methods, Supplementary Fig. 3, Supplementary Fig. 6 online). Expression of any of the nonbinding or nonsignaling receptor mutants as protomer A completely prevented activation (Fig. 3b), despite the presence of WT D2R-Gqi5 in protomer B. Thus, protomer A, which must interact with the Gα provided by protomer B, appears to play a dominant role in the activation process. Note that the absence of trans-activation was not a result of our functional complementation system or lack of sufficient mobility of fused G protein; we also failed to see evidence for trans-activation even when nonbinding and noncoupling receptors (without G protein fusions) were co-expressed with free Gqi5 (data not shown).
Figure 3. Asymmetric contributions of the protomers to signaling.
(a,b) When all mutants (as protomer A) were coexpressed with WT D2R-Gqi5 (as protomer B), only (a) WT and D2/D4 were able to signal. (b) None of the other mutants were able to restore signaling when coexpressed with WT D2R-Gqi5. (c,d,e) The results differed when WT D2R (as protomer A) was coexpressed with the various mutant-Gqi5 constructs (as protomer B). (c) D2/D4-Gqi5 ( ), (d) D1143.32A-Gqi5 (
), (d) D1143.32A-Gqi5 ( ), deletion 213–219-Gqi5 (
), deletion 213–219-Gqi5 ( ), and D802.50A-Gqi5 (
), and D802.50A-Gqi5 ( ) restored the ability of unfused WT D2R to signal. (e) Coexpressing R1323.50A -Gqi5 (
) restored the ability of unfused WT D2R to signal. (e) Coexpressing R1323.50A -Gqi5 ( ), V1363.54D/M1403.58E-Gqi5 (
), V1363.54D/M1403.58E-Gqi5 ( ), or N3937.49A-Gqi5 (
), or N3937.49A-Gqi5 ( ) with WT D2R failed to rescue signaling (d). Note that D114A-Gqi5 (
) with WT D2R failed to rescue signaling (d). Note that D114A-Gqi5 ( ) and D2/D4-Gqi5 (
) and D2/D4-Gqi5 ( ) showed a higher maximal activation than WT. Activation data represent relative luminescence when compared to WT D2R coexpressed with WT D2R-Gqi5 after normalizing for surface expression of the Gqi5 fusion construct (see Methods). The mean±SEM of at least 3 experiments, each conducted in triplicate, are shown.
) showed a higher maximal activation than WT. Activation data represent relative luminescence when compared to WT D2R coexpressed with WT D2R-Gqi5 after normalizing for surface expression of the Gqi5 fusion construct (see Methods). The mean±SEM of at least 3 experiments, each conducted in triplicate, are shown.
In contrast, we observed robust agonist-mediated activation with WT D2R as protomer A and D2/D4-Gqi5 (Fig. 3c) or D114A-Gqi5 (Fig. 3d) as protomer B. These data suggest that agonist binding to protomer A is sufficient for normal activation (see below) and imply an asymmetric organization of the signaling complex comprising two GPCR protomers with G protein. When R1323.50A-Gqi5 or V1363.54D/M1403.58E-Gqi5 was expressed as protomer B with WT D2R as protomer A, no activation was observed (Fig. 3e). In contrast, although the IL3 deletion construct abolished activation when placed in protomer A (Fig. 3b), it supported signaling when coexpressed as protomer B (fused to Gqi5) along with WT D2 as protomer A (Fig. 3d). These data support a mechanism in which two GPCRs activate a single G protein through interactions that involve IL2 from both protomers whereas IL3 from only one protomer is essential for signaling. Note that the failure of R1323.50A-Gqi5 or V1363.54D/M1403.58E-Gqi5 to function with WT is not due to an inability of these protomers to interact, since we observed efficient bioluminescence resonance energy transfer as well as bimolecular luminescence and fluorescence complementation32 between these mutants and WT D2R (Supplementary Fig.7 online).
To explore the nature of the conformational changes in the dimeric receptor unit we also studied inactivating mutations within the membrane-spanning segments. The transduction-uncoupling mutants D802.50A and N3937.49A revealed additional differences in the roles of protomers A and B. When either of these mutations was placed in protomer A, signaling was abolished, consistent with the dominant role of this protomer (Fig. 3b). In contrast, when placed in protomer B, D802.50A-Gqi5 signaled when coexpressed with WT D2R as protomer A (Fig. 3d), whereas N3937.49A-Gqi5 did not (Fig. 3e). These results suggest that the nature of the conformational changes in protomer B during activation differs from those in protomer A.
The activation state of Protomer B modulates signaling
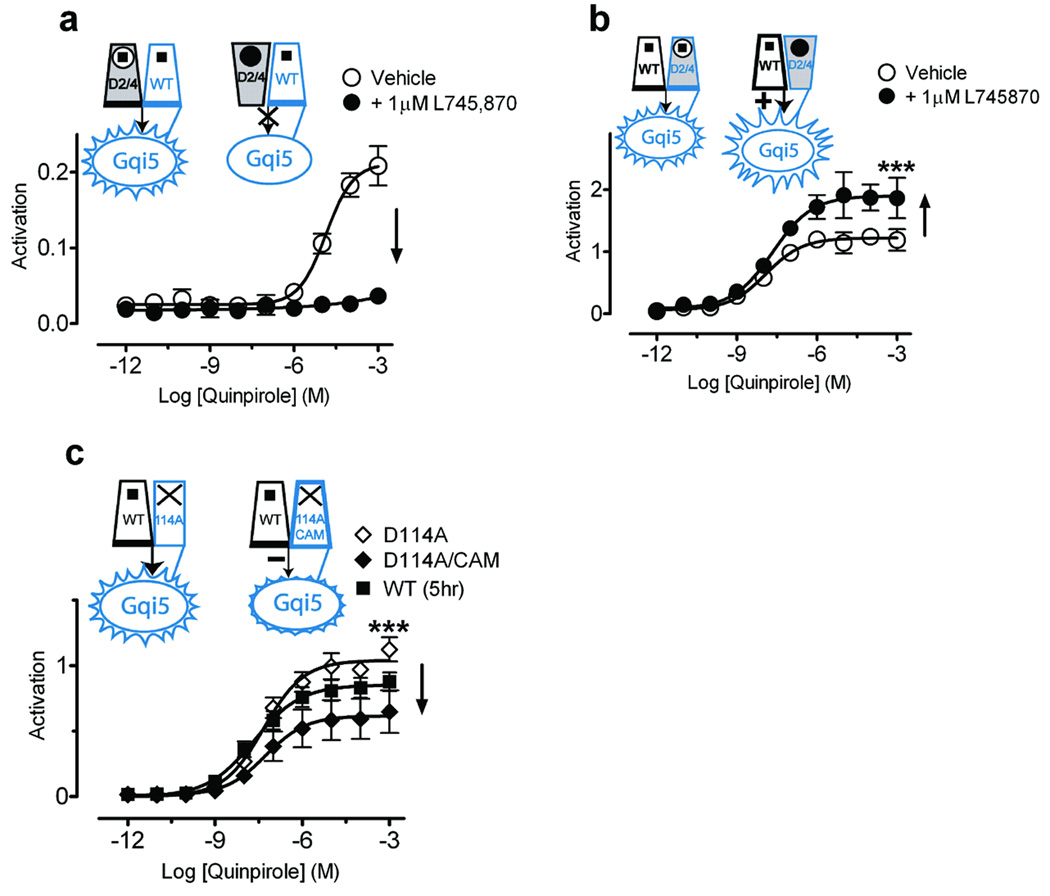
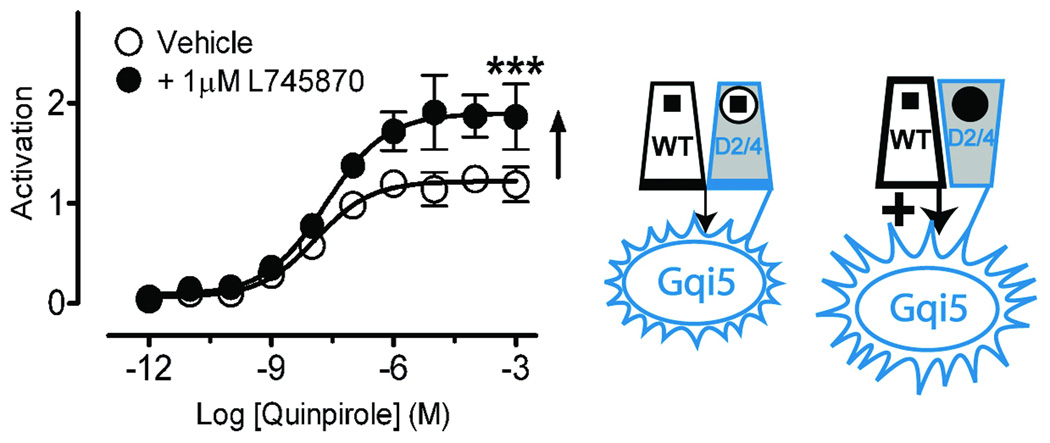
Agonist binding to only protomer A and not protomer B produced full activation, as coexpressed D2R and D1143.32A-Gqi5 were robustly activated by quinpirole (Fig. 3d). Moreover, it appeared that binding of a second agonist to protomer B might inhibit signaling, as coexpression of both WT D2R and D2R-Gqi5 led to lower maximal activation than did D2R coexpressed with D1143.32A-Gqi5 (Fig. 3d). We tested this hypothesis using the D2/D4 chimeric receptor. As predicted, when D2/D4 was expressed as protomer A with D2R-Gqi5 as protomer B, quinpirole’s ability to bind and activate was blocked by the D4-selective antagonist L745,870, reflecting the primacy of protomer A (Fig. 4a). In contrast, coexpressing D2/D4-Gqi5 as protomer B with D2R as protomer A led to robust receptor activation that was greater than that seen with WT D2R and D2R-Gqi5 (Fig. 3c) and was further enhanced in the presence of L745,870 (Fig. 4b), which blocks quinpirole binding to protomer B but not protomer A.
Figure 4. The second protomer allosterically modulates signaling.
Shown are effects on signaling with the D2/D4 construct expressed either as protomer A (a), or as protomer B (D2/D4-Gqi5) (b). (a) The D4-selective antagonist L745,870 (1 µM) totally blocked signaling of the D2/D4 construct expressed as protomer A with WT-Gqi5. (b) In contrast, L745,870 increased maximal activation for WT D2R coexpressed with D2/D4-Gqi5 to156.7±7.3% (n=9) (p<0.01*** by Student’s t-test) of that observed for D2R coexpressed with WT D2R-Gqi5 (Fig. 3a). (c) Coexpression of a constitutively active mutant (Supplementary Fig. 8 online) that was unable to bind ligand (D1143.32A/CAM-Gqi5), to enhance the fraction of protomer B in an active conformation, led to 49.6±8.4% (n=9) (p<0.01*** by Student’s t-test) of maximal activity (♦) when compared to WT D2R coexpressed with D114A-Gqi5 (◊). Activation data were normalized to surface expression as described in Fig. 3. The mean±SEM of at least 3 experiments, each conducted in triplicate, are shown.
It is the active conformation of the second protomer that inhibits signaling, and not agonist binding per se. This is evidenced by the finding that activating protomer B by constitutively activating mutations (Supplementary Fig. 8 online) in a nonbinding receptor (D1143.32A/E3396.30A/T3436.34R-Gqi5)26,33 substantially reduced the signaling efficacy of a WT protomer A (Fig. 4c). Thus, activation of the second protomer, either by ligand binding or by its inherent constitutive activity, inhibits signaling by its partner.
Computational modeling of the D2R-G protein interface
Because our experimental findings require a structural context in which the new mechanisms for GPCR–G protein interactions emerging from this study can be understood, we carried out independent computational studies that combined molecular modeling with experimental data available in the literature about the modes of interaction of the component GPCRs and G protein, but without direct reference to the new findings. We used information available from the current crystal structures of GPCRs and specific data about inactive and activated states for bovine rhodopsin, because, unlike the D2R, such a structural template is accompanied by much experimental data about details of the sites and mode of interaction with G protein to guide protein-protein docking. Thus, we took advantage of experimental data from cross-linking, alanine scanning mutagenesis, and other structural and functional studies of the GPCR–G protein interfaces pointing to several amino acid residues likely to be involved in complex formation between rhodopsin and the G protein α- and βγ-subunits (Supplementary Methods online). The data derived from the literature were used not only as constraints to guide docking of transducin (Gt) to a variety of dimer models of rhodopsin (Supplementary Fig. 9 online), but also to screen for the oligomerization solution that satisfied best these constraints, as detailed in Methods.
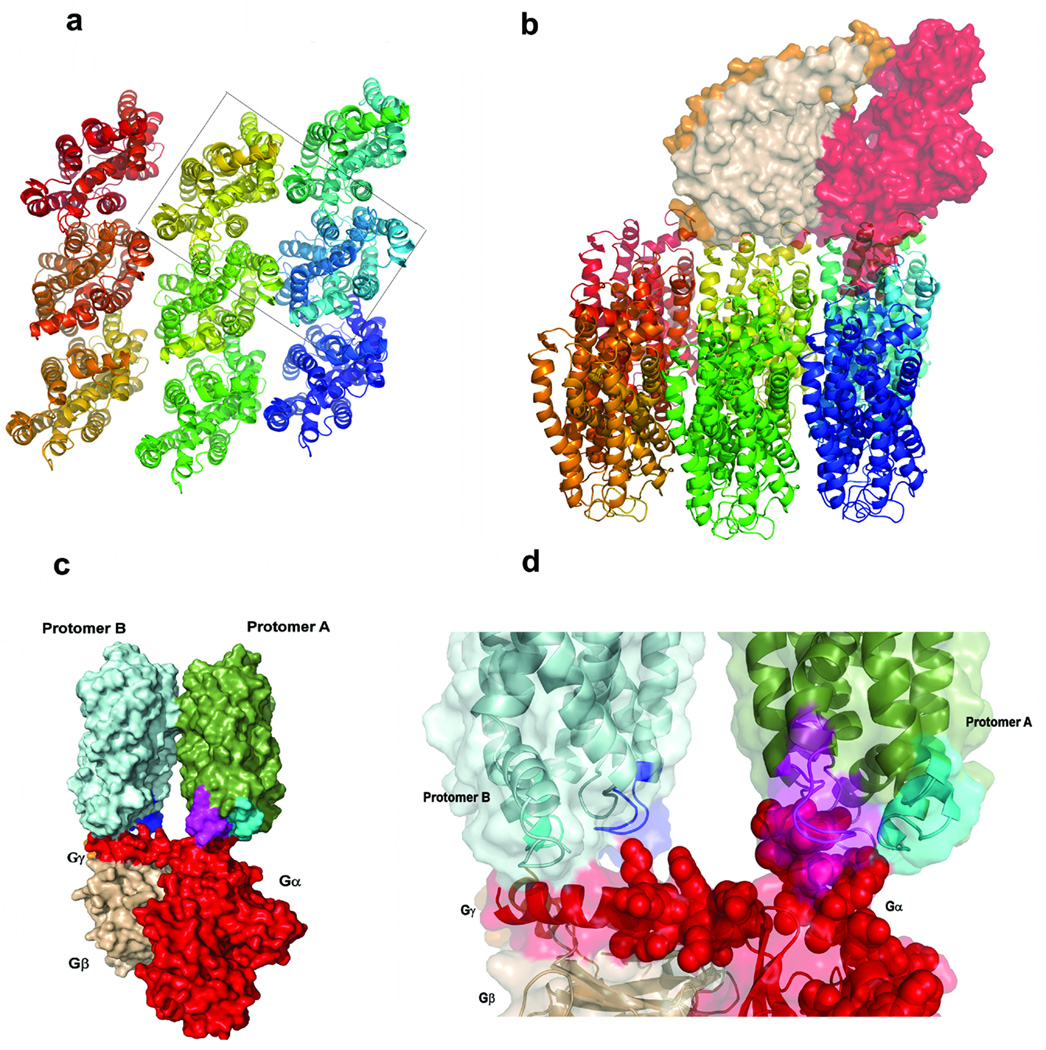
Both TM4/TM5 and TM1 have been implicated in D2R oligomerization12,32,34. In order to discriminate between a functional dimer with an interface involving TM4 and TM5 (named TM4,5 dimer) from one with a TM1 interface (TM1 dimer), we docked the molecular structure of transducin to a rhodopsin nonamer (Fig. 5a; Supplementary Fig. 9 online) subject to specific constraints for the interaction between Gt and the central rhodopsin (Supplementary Table 1 online). As described in Methods, transducin was free to rotate in any direction around this central rhodopsin to select any one of the dimeric forms in the array. The Gt could select a second monomer from the oligomeric structure in which the GPCR interface corresponds to either a TM4, 5 interface, or a TM1 interface dimer. The probability for Gt selecting either dimer interface was compared in a scan for optimal interaction carried out on the oligomeric structure shown in (Fig. 5a) and (Supplementary Fig. 9 online). The complexes resulting from this scan were considered acceptable (and counted) only if the underlying structural models satisfied at least 50% of the experimentally-based constraints (set 1 in Supplementary Table 2 online). A substantial fraction of TM4, 5 dimers (21.1%) satisfied this cutoff, but no complex with a TM1 dimer met the filtering criteria.
Figure 5. Computational model of the complex between the rhodopsin dimer and heterotrimeric Gt.
(a) Structural representation of the nonameric oligomer array; the dashed box identifies the TM4 dimer contained in Model 2. (b) Structural representation of the complex formed between transducin and the nonameric oligomer array. The optimal representative structure (defined in Methods) is shown for Model 2. (c) Closeup view of the interaction between specific residues of Gα (rendered in red, CPK representation) and the IL3 (cyan) and IL2 loops of protomer A and B (magenta and blue, respectively). (d) Side view of the complex showing Gtα (red), Gtβ (wheat), Gtγ (orange), protomer A (green), protomer B (light blue), IL2 of protomer A (magenta), IL2 of protomer B (blue), and IL3 of protomer B (cyan). Other views of the model complex are shown in (Supplementary Fig. 10 online).
A possible mode of oligomer reorganization associated with function had been suggested based on crosslinking studies in D2R34 and rhodopsin35. To evaluate the functional impact of such a reorganization, Gt was docked to the TM4, 5 and TM4 dimer alternatives (Supplementary Fig. 9 online). The Cα-Cα distances for specific interactions between rhodopsin and transducin in the optimal representative structures of the 1000 structures obtained for each alternative in this dimer docking procedure (see Methods for details) are summarized in Supplementary Table 2 online. For these optimal structures the sets of Cα-Cα distances are very similar, but the frequency of appearance of optimally positioned complexes is much higher for Model 2 (TM4 dimer; 76.1%) than for Model 1 (TM4, 5 dimer; 23.8%). This is evident from the average values of the distances (Supplementary Table 1), which are mostly larger in Model 1 than in Model 2 constructs. Thus, Model 2 is considered the better representation of the GPCR dimer complex with the G protein in the context of the oligomeric arrangement. This is consistent with the proposed transition from a TM4, 5 interface to a TM4 interface upon activation, suggested by crosslinking results for the D2R34, and indicates the relation between optimal G protein binding to the dimer and an activated state.
Notably, in the optimal G protein–dimer complex the cytoplasmic ends of TM3 and IL2 from both protomers interact with the docked G protein. This is shown in (Fig. 5) for Model 2, but holds as well for Model 1. In contrast, only IL3 from protomer A, but not from protomer B, contacts the docked Gα, consistent with our experimental results showing that an inactivating IL3 mutation is tolerated in protomer B but not in protomer A.
Discussion
We find that agonist binding to a single protomer maximally activates a signaling unit comprising two Class A GPCRs and a single G protein. Whereas activation of the second protomer inhibits the functional response, inverse agonist binding to the second protomer enhances signaling (Fig. 6). Our results are consistent with studies in the Class C mGluR using allosteric modulators that act within the transmembrane region to show that the inactive state of a protomer caused by inverse agonist binding results in more efficient activation of the adjacent protomer36,37. These findings are more difficult to reconcile with other findings in the mGluR showing that although one agonist can activate the dimeric signaling unit, two agonists are required for full activation38, suggesting differences in the mechanisms of these receptors, which have very different agonist binding sites. Our findings are, however, fully consistent with the proposed function of GABAB receptors, in which only R1 binds GABA39 with efficient signaling by the complex. A similar scenario also seems likely for rhodopsin’s ability to respond to single photons, which requires robust activation by a single protomer in a dimeric unit. Indeed in this case, the strong inverse agonist 11-cis-retinal (7) in the binding pocket of second protomer would in fact optimize signaling, just as we observe in configuration 4 in (Fig. 6). Our findings also suggest that optimal signaling in a heteromeric GPCR would result from co-stimulation with an agonist to one protomer and an inverse agonist to the other.
Figure 6. Cartoon of different D2R dimer activation states, with activation data for these states, from the perspective of agonist-mediated activation of protomer A.
Bound agonist is represented by a black square. Activation is represented by a trapezoid with a bold base. Extent of activation is indicated by increasingly bold trapezoid boundaries. The inverse agonist bound state is represented by an inverted trapezoid. (1) Neither protomer is activated. (2) Protomer A binds agonist and protomer B is constitutively active (or in the case of a heterodimer, is occupied by protomer B’s agonist). (3) Protomer A binds agonist, whereas protomer B cannot bind (or in a heterodimer, is not agonist-bound). Note that although protomer B is not activated by ligand, it can isomerize to the active state, which would result in configuration (2). (4) Protomer A binds agonist, whereas protomer B is stabilized in the inactive state by inverse agonist. Experimentally determined maximal activation representing these idealized conformations: (1) no ligand, (2) WT D2R coexpressed with D114A/CAM-Gqi5, (3) WT D2R coexpressed with D114A-Gqi5, (5) WT D2R coexpressed with D2/D4-Gqi5 in the presence of the selective D4 antagonist, L745,870. Activation data are normalized to that of WT D2R coexpressed with WT D2R-Gqi5, which is indicated by a dotted line to indicate the potential range of enhanced and reduced signaling achievable by modulation of the “heterodimer” partner.
Our data and models suggest that the way in which the two protomers contribute to the activated complex with the G protein is not symmetrical, and that activation requires different conformational changes in each protomer. Existing evidence for ligand-induced conformational changes in a second non-binding protomer13,40 is consistent with the proposal of conformational changes in both protomers. We previously demonstrated an activation-related conformational change at the TM4 dimer interface34 that also would be consistent with movement of either one or both TM4s. Our present finding that transduction-deficient mutants in different TMs differentially affect the ability of protomer B to rescue function is consonant with the importance of conformational changes in this protomer. Interestingly, the apparent negative cooperativity of ligand binding observed in a number of class A GPCRs41 may well relate to this proposed asymmetry of the signaling unit. For example, in cells expressing chemokine receptor heterodimers, a selective ligand for one protomer leads to dissociation of ligand bound to the other protomer42, consistent with transmission of an altered conformation across the dimer interface, and with a decreased propensity for simultaneous agonist binding to both protomers.
In summary, although a single B2AR or rhodopsin molecule can efficiently activate G protein when reconstituted into a nanodisc, a second protomer is present in vivo and profoundly modulates G protein activation of the first protomer, as we have shown with our functional complementation studies. Importantly, we show that this allosteric modulation of signaling results from a direct interaction of the receptor dimer with the G protein, rather than from a downstream effect. This is likely to explain many of the surprising observations concerning the mutual modulation of heteromeric receptor oligomers by ligand binding to one protomer or the other. Moreover, we demonstrate that the constitutive activity of a protomer will modulate the activity of the dimeric signaling unit in which it participates. Thus, inverse agonists at one protomer in a heterodimer are likely to be allosteric potentiators of the signaling of its heterodimer partner, whereas agonists of one protomer will be allosteric inhibitors of the second protomer, offering a mechanistic explanation for the often befuddling observations regarding pharmacological effects of ligands acting on heterodimers. Moreover, our model suggests that modulators might be found that are specific for heterodimers and not homodimers, but heretofore it has not been possible to screen for such compounds without the interference of homodimer-mediated signaling. Indeed, it is possible that findings of functional selectivity, that is, different agonists for a given receptor having different effects on different downstream effectors, might reflect differential pharmacological effects on different heteromeric species43. The novel methodology we present here makes it possible to identify signaling from a defined heterodimer, and thus to identify modulators of heterodimer function. The modulatory mechanism we characterized and the approach that made this possible offer a new understanding of GPCR signaling in units composed of at least two GPCRs; applied to specific systems the approach will make it possible to understand the effects of drugs that target each protomer of such a signaling unit, either identical or different.
METHODS
Materials
The D2R agonist quinpirole hydrochloride and the D4R antagonist L745,870 (3-(4-[4-Chlorophenyl]piperazin-1-yl)-methyl-1H-pyrrolo[ 2,3-b]pyridine trihydrochloride ) were from Sigma-Aldrich.
DNA constructs
Expression plasmids expressing Signal Peptide Flag-tagged D2short WT44 and mutant receptors were created using standard molecular biology procedures, as described in Supplementary Methods online. Receptor constructs were fused directly through their C-terminus, or through an 8 amino acid linker, to a PTX-resistant Gqi5 (Supplementary Fig. 2 online).
Cell culture and transfection
Flp-In T-REx 293 cells (Invitrogen) were cultured and transfected, and stable lines were selected as described in (Supplementary Methods online).
Cell Surface Expression assay
An aliquot of the cell solution used for the aequorin assay (see below) was used to determine receptor cell surface expression as described in Costagliola et al.45 with anti-Flag M2 (Sigma) or anti-Myc (Mt. Sinai hybridoma facility) as primary and R-phycoerythrin goat anti-mouse IgG (Invitrogen) as secondary antibodies using a Guava Easycyte (Guava technologies).
Aequorin assay
A functional assay based on luminescence of mitochondrial aequorin following intracellular Ca++ release was performed21,46. Cells were seeded in a 15 cm dish and grown in antibiotic-free medium for ∼48 hr until mid-log phase. 1 µg/ml tetracycline was added to the medium for 3–24 hours prior to harvest to induce the expression of the transfected D2R in pcDNA5/FRT/TO. Cells were dissociated and pelleted at 0.6 g for 3 min. After washing once with DMEM medium (Invitrogen) supplemented with 0.1% BSA), cells were resuspended in this medium at a final concentration of 5 × 106 cells/ml in the presence of 5 µM coelenterazine h. After 4 hr rotating at room temperature in the dark, the cell solution was diluted 10-fold, followed by 1 hr incubation under the same conditions. Concentration-response curves were obtained by injecting 50 µL of cell solution into wells of a 96-well microplate containing 50 µL of a 2X concentration of the desired compound in medium. Luminescence signals from the first 15 seconds after injection were read by a POLARstar optima reader (BMG). Total response was defined as the signal resulting from injecting 50 µL cell solution into 50 µL assay medium containing 0.1% triton, which raises the Ca++ concentration directly by membrane permeabilization.
To normalize for different levels of surface expression levels of the Flag-D2R-Gqi5 mutant constructs, the Emax at each expression level (Supplementary Fig. 6c online) was plotted as a function of different levels of expression of Flag-tagged wt D2R-Gqi5, the expression of which was controlled by varying the time after tetracycline induction (Supplementary Fig. 6a online). The level of Myc-D2R remained essentially unchanged (Supplementary Fig. 6b online). The standard curve was fit to a 1 site rectangular hyperbola using nonlinear regression in Graph Pad PRISM 4.0 (Supplementary Fig. 6d online). The luminescence response of the various Flag-D2R-Gqi5 constructs was normalized using this standard curve to account for the effects of different expression levels, with activation of 1 defined as that observed after 12 hour of tetracycline induction of WT D2R-Gqi5. The Flag detection was approximately 5-fold more sensitive than that of Myc; thus, the excess of Myc-tagged protomer A, which cannot signal on its own, ensures that normalization based on surface expression of the Flag-tagged Gqi5-fused protomer B accurately reflects the productive signaling entities, each of which must contain a protomer A and a protomer B.
Model Construction
In the absence of experimentally determined structures of D2R and Gqi5 the templates for the oligomeric model constructs were complexes between crystallographically determined structures of rhodopsin and heterotrimeric G proteins, as described in Supplementary Methods online. To enable the simultaneous probing of G protein interaction with different dimer arrangements, we constructed a rhodopsin oligomer composed of nine monomers. The activated form of the rhodopsin monomers used here was constructed by inclusion of all constraints reported for rhodopsin, as described previously47. Three dimeric interfaces were analyzed: Model 1, in which the dimers have a TM4,5 interface, Model 2, with a symmetric TM4 interface34, and Model 3, in which the dimers have a TM1 interface32.
G-Protein-Rhodopsin Docking
The docking was carried out with the HADDOCK (High Ambiguity Driven protein-protein DOCKing) software48,49. The docking process was driven by ambiguous interaction restraints (AIRs)48 to both monomers, as described in Supplementary Methods online. The constraints, established from literature-derived experimental data for the binding complex, are given in Supplementary Table 1 online. Notably, the docking protocol of Gt to such models using this set of constraints was verified by the full agreement with the complex obtained for the recent structure of opsin50 representing a putative activated form of the protein (Supplementary methods online). To select a second protomer for the complex, another docking run was made with restraints only to the central rhodopsin, allowing transducin to explore freely different orientations with respect to the rhodopsin oligomer. By application of the docking protocol consisting of randomization of orientations and rigid body energy minimization, 1000 different conformations were generated. These structures were ranked according to their average interaction energies (sum of Eelec, Evdw, EAIR), and screened using the eighteen constraints listed in (Supplementary Table 2 online), which represent the information extracted from the experimental data and translated into Cα-Cα intermolecular constraints (in Supplementary Table 2 online, set 1 refers to the interactions of the C-terminus of Gα with protomer A, set 2 to the interactions between the N-terminus of Gα and protomer A, and set 3 to the interactions between the N-terminus of Gα and protomer B). Only Cα-Cα distances <20Å were interpreted as direct rhodopsin-Gt interactions. A cutoff of 50% fulfillment of the of the interaction criteria was used for accepting valid constructs. The relative probabilities of such valid G protein complexes with the various model dimers (TM4; TM4,5; TM1) were calculated from the corresponding percentages of acceptable complexes found in the resulting set of 1000 structures retrieved from the docking procedure. The construct fulfilling the largest number of experimentally derived constraints and with the N-terminal helix of Gα parallel to the cytoplasmic face of the rhodopsin dimer, was chosen as the “optimal representative structure” for each model.
Supplementary Material
Figure 7.
Acknowledgements
We thank Céline Gáles for discussion and comments on the manuscript. Plasmids encoding apoaequorin were a gift from V. Dupriez (Euroscreen, Belgium). This work was supported in part by NIH grants DA022413 and MH054137 (J.A.J.), DA012923 (HW), the Lieber Center for Schizophrenia Research and Treatment, and an EMBO Long term fellowship (E.U.). Computational resources of the David A Cofrin Center for Biomedical Information in the Institute for Computational Biomedicine are gratefully acknowledged.
Reference List
- 1.Pin JP, et al. International Union of Basic and Clinical Pharmacology. LXVII. Recommendations for the Recognition and Nomenclature of G Protein-Coupled Receptor Heteromultimers. Pharmacol Rev. 2007;59:5–13. doi: 10.1124/pr.59.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Sartania N, Appelbe S, Pediani JD, Milligan G. Agonist occupancy of a single monomeric element is sufficient to cause internalization of the dimeric beta2-adrenoceptor. Cell Signal. 2007;19:1928–1938. doi: 10.1016/j.cellsig.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Parenty G, Appelbe S, Milligan G. CXCR2 chemokine receptor antagonism enhances DOP opioid receptor function via allosteric regulation of the CXCR2-DOP receptor heterodimer. Biochem J. 2008;412:245–256. doi: 10.1042/BJ20071689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vilardaga JP, et al. Conformational cross-talk between alpha2A-adrenergic and mu-opioid receptors controls cell signaling. Nat Chem Biol. 2008;4:126–131. doi: 10.1038/nchembio.64. [DOI] [PubMed] [Google Scholar]
- 5.Pin JP, Galvez T, Prezeau L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacology & Therapeutics. 2003;98:325–354. doi: 10.1016/s0163-7258(03)00038-x. [DOI] [PubMed] [Google Scholar]
- 6.Brock C, et al. Activation of a Dimeric Metabotropic Glutamate Receptor by Intersubunit Rearrangement. J. Biol. Chem. 2007;282:33000–33008. doi: 10.1074/jbc.M702542200. [DOI] [PubMed] [Google Scholar]
- 7.Ji IH, Lee C, Song YS, Conn PM, Ji TH. Cis-and trans-activation of hormone receptors: the LH receptor. Mol Endocrinol. 2002;16:1299–1308. doi: 10.1210/mend.16.6.0852. [DOI] [PubMed] [Google Scholar]
- 8.Bayburt TH, Leitz AJ, Xie G, Oprian DD, Sligar SG. Transducin Activation by Nanoscale Lipid Bilayers Containing One and Two Rhodopsins. J. Biol. Chem. 2007;282:14875–14881. doi: 10.1074/jbc.M701433200. [DOI] [PubMed] [Google Scholar]
- 9.Whorton MR, et al. A monomeric G protein-coupled receptor isolated in a highdensity lipoprotein particle efficiently activates its G protein. PNAS. 2007;104:7682–7687. doi: 10.1073/pnas.0611448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George SR, et al. Oligomerization of mu-and delta-opioid receptors-Generation of novel functional properties. J. Biol. Chem. 2000;275:26128–26135. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- 11.Lee SP, et al. Dopamine D1 and D2 Receptor Co-activation Generates a Novel Phospholipase C-mediated Calcium Signal. J. Biol. Chem. 2004;279:35671–35678. doi: 10.1074/jbc.M401923200. [DOI] [PubMed] [Google Scholar]
- 12.Guo W, Shi L, Javitch JA. The Fourth Transmembrane Segment Forms the Interface of the Dopamine D2 Receptor Homodimer. J. Biol. Chem. 2003;278:4385–4388. doi: 10.1074/jbc.C200679200. [DOI] [PubMed] [Google Scholar]
- 13.Mesnier D, Baneres JL. Cooperative Conformational Changes in a G-protein-coupled Receptor Dimer, the Leukotriene B4 Receptor BLT1. J. Biol. Chem. 2004;279:49664–49670. doi: 10.1074/jbc.M404941200. [DOI] [PubMed] [Google Scholar]
- 14.Damian M, Mary S, Martin A, Pin JP, Baneres JL. G protein activation by the leukotriene B4 receptor dimer: Evidence for an absence of trans-activation. J. Biol. Chem. 2008;283:21084–21092. doi: 10.1074/jbc.M710419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrillo JJ, Pediani J, Milligan G. Dimers of Class A G Protein-coupled Receptors Function via Agonist-mediated Trans-activation of Associated G Proteins. J. Biol. Chem. 2003;278:42578–42587. doi: 10.1074/jbc.M306165200. [DOI] [PubMed] [Google Scholar]
- 16.Molinari P, et al. Promiscuous Coupling at Receptor-Galpha Fusion Proteins. THE RECEPTOR OF ONE COVALENT COMPLEX INTERACTS WITH THE alpha - SUBUNIT OF ANOTHER. J. Biol. Chem. 2003;278:15778–15788. doi: 10.1074/jbc.M300731200. [DOI] [PubMed] [Google Scholar]
- 17.Pascal G, Milligan G. Functional Complementation and the Analysis of Opioid Receptor Homodimerization. Mol Pharmacol. 2005;68:905–915. doi: 10.1124/mol.105.013847. [DOI] [PubMed] [Google Scholar]
- 18.Seifert R, Wenzel-Seifert K, Kobilka BK. GPCR-G[alpha] fusion proteins: molecular analysis of receptor-G-protein coupling. Trends in Pharmacological Sciences. 1999;20:383–389. doi: 10.1016/s0165-6147(99)01368-1. [DOI] [PubMed] [Google Scholar]
- 19.Lee TW, Seifert R, Guan X, Kobilka BK. Restricting the Mobility of Gs alpha: Impact on Receptor and Effector Coupling. Biochemistry. 1999;38:13801–13809. doi: 10.1021/bi9908282. [DOI] [PubMed] [Google Scholar]
- 20.Wenzel-Seifert K, Lee TW, Seifert R, Kobilka BK. Restricting mobility of Gsalpha relative to the beta2-adrenoceptor enhances adenylate cyclase activity by reducing Gsalpha GTPase activity. Biochem. J. 1998;334:519–524. doi: 10.1042/bj3340519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rizzuto R, Simpson AWM, Brini M, Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 1992;358:325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- 22.Blanpain C, et al. Extracellular Cysteines of CCR5 Are Required for Chemokine Binding, but Dispensable for HIV-1 Coreceptor Activity. J. Biol. Chem. 1999;274:18902–18908. doi: 10.1074/jbc.274.27.18902. [DOI] [PubMed] [Google Scholar]
- 23.Conklin BR, Farfel Z, Lustig KD, Julius D, Bourne HR. Substitution of three amino acids switches receptor specificity of Gq[alpha] to that of Gi[alpha] Nature. 1993;363:274–276. doi: 10.1038/363274a0. [DOI] [PubMed] [Google Scholar]
- 24.Miller RT, Masters SB, Sullivan KA, Beiderman B, Bourne HR. A mutation that prevents GTP-dependent activation of the alpha chain of Gs. Nature. 1988;334:712–715. doi: 10.1038/334712a0. [DOI] [PubMed] [Google Scholar]
- 25.Xu W, et al. Functional role of the spatial proximity of Asp114(2.50) in TMH 2 and Asn332(7.49) in TMH 7 of the mu opioid receptor. FEBS Lett. 1999;447:318–324. doi: 10.1016/s0014-5793(99)00316-6. [DOI] [PubMed] [Google Scholar]
- 26.Ballesteros JA, et al. Activation of the beta(2)-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J. Biol. Chem. 2001;276:29171–29177. doi: 10.1074/jbc.M103747200. [DOI] [PubMed] [Google Scholar]
- 27.Moro O, Lameh J, Hogger P, Sadee W. Hydrophobic amino acid in the i2 loop plays a key role in receptor-G protein coupling. J. Biol. Chem. 1993;268:22273–22276. [PubMed] [Google Scholar]
- 28.Strader CD, et al. Mutations that uncouple the beta-adrenergic receptor from Gs and increase agonist affinity [published erratum appears in J Biol Chem 1988 Feb 25;263(6):3050] J. Biol. Chem. 1987;262:16439–16443. [PubMed] [Google Scholar]
- 29.Neve KA, et al. Modeling and mutational analysis of a putative sodium-binding pocket on the dopamine D-2 receptor. Mol Pharmacol. 2001;60:373–381. doi: 10.1124/mol.60.2.373. [DOI] [PubMed] [Google Scholar]
- 30.Urizar E, et al. An Activation Switch in the Rhodopsin Family of G Protein-coupled Receptors: The thyrotropin receptor. J. Biol. Chem. 2005;280:17135–17141. doi: 10.1074/jbc.M414678200. [DOI] [PubMed] [Google Scholar]
- 31.Simpson MM, et al. Dopamine D4/D2 Receptor Selectivity Is Determined by A Divergent Aromatic Microdomain Contained within the Second, Third, and Seventh Membrane-Spanning Segments. Mol Pharmacol. 1999;56:1116–1126. doi: 10.1124/mol.56.6.1116. [DOI] [PubMed] [Google Scholar]
- 32.Guo W, et al. Dopamine D2 receptors form higher order oligomers at physiological expression levels. EMBO J. 2008 doi: 10.1038/emboj.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson J, Lin H, Fu D, Javitch JA, Strange PG. Mechanisms of inverse agonism of antipsychotic drugs at the D2 dopamine receptor: use of a mutant D2 dopamine receptor that adopts the activated conformation. J Neurochem. 2001;77:493–504. doi: 10.1046/j.1471-4159.2001.00233.x. [DOI] [PubMed] [Google Scholar]
- 34.Guo W, Shi L, Filizola M, Weinstein H, Javitch JA. From The Cover: Crosstalk in G protein-coupled receptors: Changes at the transmembrane homodimer interface determine activation. PNAS. 2005;102:17495–17500. doi: 10.1073/pnas.0508950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kota P, Reeves PJ, RajBhandary UL, Khorana HG. Opsin is present as dimers in COS1 cells: Identification of amino acids at the dimeric interface. PNAS. 2006;103:3054–3059. doi: 10.1073/pnas.0510982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goudet C, et al. Asymmetric Functioning of Dimeric Metabotropic Glutamate Receptors Disclosed by Positive Allosteric Modulators. J. Biol. Chem. 2005;280:24380–24385. doi: 10.1074/jbc.M502642200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hlavackova V, et al. Evidence for a single heptahelical domain being turned on upon activation of a dimeric GPCR. Embo Journal. 2005;24:499–509. doi: 10.1038/sj.emboj.7600557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kniazeff J, et al. Closed state of both binding domains of homodimeric mGlu receptors is required for full activity. Nature Structural & Molecular Biology. 2004;11:706–713. doi: 10.1038/nsmb794. [DOI] [PubMed] [Google Scholar]
- 39.Kaupmann K, et al. GABAB-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- 40.Damian M, Martin A, Mesnier D, Pin JP, Baneres JL. Asymmetric conformational changes in a GPCR dimer controlled by G-proteins. EMBO J. 2006;25:5693–5702. doi: 10.1038/sj.emboj.7601449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Springael JY, Urizar E, Costagliola S, Vassart G, Parmentier M. Allosteric properties of G protein-coupled receptor oligomers. Pharmacol Ther. 2007;115:410–418. doi: 10.1016/j.pharmthera.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Springael JY, et al. Allosteric modulation of binding properties between units of chemokine receptor homo-and hetero-oligomers. Mol Pharmacol. 2006;69:1652–1661. doi: 10.1124/mol.105.019414. [DOI] [PubMed] [Google Scholar]
- 43.Urban JD, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 44.Javitch JA, et al. The fourth transmembrane segment of the dopamine D2 receptor: Accessibility in the binding-site crevice and position in the transmembrane bundle. Biochemistry. 2000;39:12190–12199. doi: 10.1021/bi001069m. [DOI] [PubMed] [Google Scholar]
- 45.Costagliola S, et al. Structure-function relationships of two loss-of-function mutations of the thyrotropin receptor gene. Thyroid. 1999;9:995–1000. doi: 10.1089/thy.1999.9.995. [DOI] [PubMed] [Google Scholar]
- 46.Brini M, et al. Transfected Aequorin in the Measurement of Cytosolic Ca2+ Concentration ([Ca2+]c) J. Biol. Chem. 1995;270:9896–9903. doi: 10.1074/jbc.270.17.9896. [DOI] [PubMed] [Google Scholar]
- 47.Niv MY, Skrabanek L, Filizola M, Weinstein H. Modeling activated states of GPCRs: the rhodopsin template. J. Comput. Aided Mol. Des. 2006;20:437–448. doi: 10.1007/s10822-006-9061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Dijk AD, et al. Data-driven : HADDOCK's adventures in CAPRI. Proteins. 2005;60:232–238. doi: 10.1002/prot.20563. [DOI] [PubMed] [Google Scholar]
- 49.van Dijk M, van Dijk AD, Hsu V, Boelens R, Bonvin AM. Information-driven protein-DNA docking using HADDOCK: it is a matter of flexibility. Nucleic Acids Res. 2006;34:3317–3325. doi: 10.1093/nar/gkl412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scheerer P, et al. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008;455:497–502. doi: 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.