Abstract
Background
Individuals with schizophrenia show deficits in cognitive functioning, as evidenced by deficits on neurocognitive tasks such as the Wisconsin Card Sorting Task (WCST). Studies of risk/reward decision-making in individuals with schizophrenia have yielded mixed results, and few studies have examined systematically the relationship between these domains and their relationship with clinical factors.
Method
Thirty-two smokers with schizophrenia, ten non-smokers with schizophrenia, nine non-psychiatric non-smokers and ten non-psychiatric smokers were administered computerized versions of the Iowa Gambling Task (IGT) and the WCST. Smokers were allowed to smoke ad libitum during designated breaks in order to prevent deprivation.
Results
Subjects with schizophrenia performed significantly worse than non-psychiatric controls on both the IGT and the WCST, and performance on these tasks was significantly correlated across subject groups. Among women with schizophrenia, smokers performed significantly better than non-smokers on the IGT.
Conclusions
Individuals with schizophrenia performed worse than controls on the IGT, suggesting impairments in risk/reward decision-making. Correlations between IGT and WCST performance suggest a shared element underlying task performance, such as a deficit in set-shifting or perseverance. Further research is needed to establish the relationship between cigarette smoking and IGT performance in schizophrenia.
Keywords: Schizophrenia, decision-making, gambling, nicotine, executive functioning, dorsolateral prefrontal cortex (dlPFC), ventromedial prefrontal cortex (vmPFC)
1. Introduction
1.1. Executive function deficits in schizophrenia: the Wisconsin Card Sorting Task (WCST)
Deficits in neuropsychological function related to schizophrenia include executive functions governed largely by dorsolateral prefrontal cortical (dlPFC) circuits (Berman et al., 1995; Berman, Zec, & Weinberger, 1986). Impairments on the Wisconsin Card Sorting Task (WCST; Heaton, 1981) have been widely reported in schizophrenia (Berman et al., 1986; Berman et al., 1995). The WCST assesses executive functions associated with dlPFC function, including concept-formation, problem-solving and cognitive flexibility (Berman et al., 1986; Berman et al., 1995; Marenco et al., 1993; Rezai et al., 1993).
1.2. Risk/reward decision-making and the ventromedial prefrontal cortex (vmPFC): The Iowa Gambling Task (IGT)
The Iowa Gambling Task (IGT; Bechara et al., 1994) has been used to study risk/reward decision-making in schizophrenia (Beninger, 2003; Cavallaro et al., 2003; Wilder et al., 1998) and other psychiatric populations, including those with substance use disorders (SUDs; Tucker, 2004; Verdejo-Garcia et al., 2007). The IGT was originally designed in order to study decision-making in individuals with vmPFC lesions (Bechara et al., 1994). These people display disadvantageous, risky decision-making despite prior knowledge/experience of associated negative consequences (Damasio, 1994; Fellows & Farah, 2005; Hilz et al., 2006). Subsequent research demonstrating significant performance impairments among patients with vmPFC lesions has validated the IGT as an indirect measure of vmPFC function and as an operational measure of risk/reward decision-making (Bechara et al., 1994; Fellows and Farah, 2005).
Disadvantageous risk/reward decision-making has been hypothesized to be influenced by somatic markers of emotion (Bechara et al., 1994), and individuals with schizophrenia evidence irregularities in emotion processing; e.g., affective flattening or negative symptoms (American Psychiatric Association, 1995). Disadvantageous risk/reward decision-making is significantly associated with measures of daily functioning – such as unemployment - in other patient populations (Bechara, 2003). The relevance of risk/reward decision-making to clinical aspects of schizophrenia is less well understood. However, the frequent engagement in risky behaviors such as smoking, substance abuse and problem gambling by individuals with schizophrenia suggests a clinically relevant deficit in risk/reward decision-making (Desai & Potenza, In Press).
1.3. IGT performance in schizophrenia
Multiple studies of neuropsychological performance in schizophrenia have included the IGT. Whereas seven studies have found no significant differences in IGT performance between individuals with schizophrenia and healthy control subjects (Cavallaro et al., 2003; Evans et al., 2005; Martino et al., 2007; Turnbull et al., 2006; Rodriguez-Sanchez et al., 2005; Sevy et al., 2007; Wilder et al., 1998), six studies have reported impaired IGT performance in individuals with schizophrenia (Beninger et al., 2003; Kester et al., 2006; Lee et al., 2007; Nakamura et al., 2008; Ritter et al., 2004; Shurman et al., 2005).
These seemingly conflicting findings may relate to multiple factors including sample heterogeneities or influences of variables affecting IGT performance. Whereas some studies have excluded patients with current SUDs, only one has systematically controlled for the presence of an SUD (Sevy et al., 2007). Only two of the studies finding significant between-group performance differences in IGT performance have controlled for type of antipsychotic medication, with one finding an association between typical antipsychotic drug use status and better IGT performance (Beninger et al., 2003; Shurman et al., 2005). None of the studies reporting a lack of significant differences in IGT performance between individuals with schizophrenia and healthy control subjects controlled for medication status (Cavallaro et al., 2003; Evans et al., 2005; Martino et al., 2007; Rodriguez-Sanchez et al., 2005; Sevy et al., 2007; Turnbull et al., 2006; Wilder et al., 1998).
Disturbances in affect and executive function have been widely reported in schizophrenia. However, the relationship between IGT performance and schizophrenia is less clear. Sampling heterogeneities and the presence of potentially confounding factors may have contributed to the seemingly conflicting findings of IGT performance in schizophrenia.
1.4. Cigarette smoking and neuropsychological performance in schizophrenia
Tobacco is the most commonly abused substance amongst individuals with schizophrenia (George & Krystal, 2000), and the prevalence of smoking in clinical samples with schizophrenia (58-88%) is much higher than in the US general population (20-22%; Fiore & Jaen, 2008; Kalman et al., 2005). Several studies suggest that nicotine effects of cigarette smoking may partially ameliorate performance deficits on neuropsychological tasks, particularly those related to dlPFC function, in individuals with schizophrenia (Depatie et al., 2002; George et al., 2002; George et al., 2006; Levin et al., 1996; Sacco et al., 2005; Sacco et al., 2006). For example, smoking abstinence is associated with poorer visuospatial working memory (VSWM) performance for smokers with schizophrenia, but not for non-psychiatric smokers (George et al., 2002; Sacco et al., 2005). A significant correlation between impaired performance on dlPFC-associated tasks and failure to quit smoking has been reported among smokers with schizophrenia but not among control smokers (Dolan et al., 2004).
The relationship between tobacco smoking and task performance related to more ventral components of the PFC is less clear. No studies of IGT performance in schizophrenia have controlled for cigarette smoking. Elements of tasks related to dlPFC and vmPFC function may share similarities. For example, an impaired ability to shift sets on the WCST (leading to perseverative errors) may share features with continuing to select cards from disadvantageous decks on the IGT resulting in a correlation between WCST and IGT performance. If nicotine acts on both dorsal and ventral regions of the PFC in schizophrenia, cigarette smoking might be associated with improved performance on both WCST and IGT performance. As such, influences of nicotine exposure on WCST performance may extend to IGT performance. If nicotine does indeed influence IGT performance, it is additionally possible that some of the seemingly conflicting findings of IGT performance in schizophrenia may be attributable to the drug's influences on neuropsychological performance.
1.5. Gender differences in schizophrenia: relationship to tobacco smoking and cognitive function
Gender differences in IGT performance have been reported. One study reported significantly lower IGT scores among female versus male healthy controls (Reavis & Overman, 2001). Non-performance related gender-differences in regional brain activation during IGT performance have additionally been reported (Bolla et al., 2004). Recent studies suggest that estradiol may partially mediate schizophrenia-associated symptoms (Bergemann et al., 2007) and that estradiol may influence the expression of nicotinic acetylcholine receptors (nAChRs; Centeno et al., 2006). Among women with schizophrenia, estrogen levels have been positively correlated with neuropsychological performance (Hoff et al., 1997) and negatively correlated with Brief Psychiatric Rating Scale (BPRS) and Positive and Negative Syndrome Scale (PANSS) scores (Bergemann et al., 2007). The nAChRs subtypes α4β2 and α7 thought to mediate nicotinic effects on cognitive processes including attention and memory are regulated by sex steroids such as estradiol (Curtis et al., 2002). Estradiol administration increases the expression of low-affinity α7 nAChRs on CA3 neurons in the hippocampus, stimulates serotonergic neurons in the raphe nucleus and noradrenergic neurons in the locus coereleus (Centeno et al., 2006) and increases nAChR activity, potentiating cholinergic responses (Curtis et al., 2002). As such, sex differences related to tobacco smoking and IGT performance might be predicted, particularly amongst individuals with schizophrenia.
1.6. The current investigation
In this study, we compared the performance of schizophrenia patients versus healthy controls on the IGT and WCST while accounting for smoking status. We predicted that: (1) individuals with schizophrenia would perform worse than controls on the IGT; (2) among individuals with schizophrenia, there would be a beneficial main effect of cigarette smoking on IGT performance; (3) among individuals with schizophrenia, there would be a main effect for type of antipsychotic medication (typicals vs. atypicals) on IGT performance; (4) there would be a significant correlation between WCST and IGT performance among smokers with schizophrenia, and (5) heterogeneities among diagnostic groups will be partially accounted for by gender effects.
2. Method
2.1. Participants
A total of n = 61 subjects were studied: Non-psychiatric non-smokers (CNS) (n = 10), non-psychiatric smokers (CS) (n = 9), non-smokers with schizophrenia (SNS) (n = 10) and smokers with schizophrenia (SS) (n = 32) participated in this study.
2.1.1. Inclusionary and exclusionary criteria
Inclusion criteria for schizophrenia patients were: (1) a confirmed diagnosis of schizophrenia or schizoaffective disorder assessed via the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I), (2) treatment with anti-psychotic medication (both typical and atypical), (3) no illicit drug use in the previous 6 months, and (4) confirmed to be stable by a Master's or Ph.D. level clinician. Inclusion criteria for control participants were: (1) absence of a non-tobacco-related Axis I disorder, assessed via the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I), and (2) no illicit drug use in the previous 6 months.
Inclusion criteria for smokers were: (1) a score of ≥5 on the Fagerström Test for Nicotine Dependence (FTND), and (2) a minimum of 10 cigarettes smoked per day (with an expired breath carbon monoxide (CO) level ≥10 ppm). In order to be classified as a non-smoker, individuals had to have not smoked for at least 6 months prior to the study (with biochemical verification of non-smoking status by CO level <10 ppm). The FTND is a six-item measure of nicotine dependence (Heatherton et al., 1991). The reliability of this measure has been demonstrated in both schizophrenia and non-psychiatric populations (Weinberger et al., 2007).
2.2. Demographic and clinical assessments
In order to obtain demographic and clinical characteristics, all participants were administered the Shipley Institute of Living Scale to assess IQ (Shipley, 1940; Shipley et al., 1970), the Beck Depression Inventory (BDI) to assess depression (Beck & Steer, 1987), and the BPRS to assess other psychiatric symptomatology (Overall & Gorham, 1962). Subjects with schizophrenia were administered the PANSS (Kay et al., 1987).
2.3. Neuropsychological assessments
2.3.1. Risk/reward decision-making assessment: the Iowa Gambling Task (IGT)
All participants were administered a computerized version of the IGT (Bechara et al., 1994) using standardized instructions. During task performance, participants select cards from one of four decks in order to win money. Rewards and losses during the IGT are determined by fixed schedules of reward and punishment: Decks A & B yield high-risk/high-reward outcomes, and continued selection from these decks over time results in losing money. Decks C & D yield low-risk/low-reward outcomes, and continued selection from these decks over time results in winning money (Bechara, et al., 1994). Variables of interest for the IGT were total net score ((C + D) – (B + A)) and performance over time (i.e. number of disadvantageous cards selected per quintile) as done previously (Bechara et al., 1994).
2.3.2. The Wisconsin Card Sorting Task (WCST)
Participants were administered the computerized WCST (Heaton, 1981) using standardized instructions. Primary variables of interest were those associated with general conceptual and problem solving abilities (i.e. percent perseverative errors, percent perseverative responses, categories completed; Paolo et al., 1995), impairments on which have been reported in previous studies of the WCST in schizophrenia (Berman et al., 1986; Berman et al., 1995).
2.4. Procedures
All assessments were administered at the Connecticut Mental Health Center (CMHC). The IGT and the WCST were administered as part of a larger neuropsychological battery. Tasks were administered in one-hour blocks and participants were randomized to one of three test-order schedules (e.g. 1st WCST, 2nd IGT…) in order to control for potential serial or position effects. CO tests were administered upon arrival to confirm smoking status (smoker or non-smoker). Prior to the initiation of testing and after each hour of testing all subjects were required to take scheduled breaks. Smokers were not required to smoke during breaks, but were permitted to smoke ad libitum (i.e. allowed to smoke as many cigarettes as wanted). This was done in order to replicate as closely as possible subjects' naturalistic smoking behaviors (see Sacco et al., 2005). All participants were compensated for their time.
2.5. Data analyses
Of the sample, 14 of the 42 patients carried a diagnosis of schizoaffective disorder. Preliminary comparisons of individuals with schizophrenia versus schizoaffective disorder revealed no significant between-group differences in IGT, WCST or demographic variables such as IQ. As such, all patients were grouped together for subsequent comparisons of patients versus controls. A two-way repeated-measures ANOVA was conducted to evaluate the effects of smoking and psychiatric status on IGT performance over time (Block Trials). One-way analyses of variance (ANOVAs) were then used to further compare patients and controls on all continuous variables meeting parametric criteria. Non-parametric continuous variables were compared using Kruskal-Wallis tests. Correlational analyses were conducted using either Pearson's r or Spearman's rho, as appropriate. Partial correlations controlling for smoking status in patients versus controls and controlling for psychiatric status between smokers and nonsmokers were also conducted. Results were considered significant at the 0.05 level given the exploratory nature of the investigation.
3. Results
3.1. Descriptive statistics
There were no significant differences between patients and controls in age (Table 1). Patients and controls differed on several variables: years of education (p < 0.001, F = 17.35), IQ (p < 0.001, F = 25.994), BDI scores (x2 = 7.717, df = 1, p = 0.005) and BPRS scores (x2 = 37.498, df = 1, p < 0.001). Among subjects who smoked, there were no significant differences in CO levels, FTND scores, or number of cigarettes smoked per day. There were no significant differences in PANSS scores between smokers and non-smokers with schizophrenia.
Table 1. Demographic and clinical characteristics of subject groups compared using analysis of variance (ANOVA) and x2 tests.
| Control non-smokers | Control smokers | Non-smokers with schizophrenia | Smokers with schizophrenia | p values | F | |
|---|---|---|---|---|---|---|
| N | 12 | 9 | 10 | 32 | ||
| Percent male | 83.3% (n = 10) | 77.8% (n = 7) | 50.0% (n = 5) | 76.7% (n = 24) | ||
| Age (years) | 36.7 (13.7) | 38.0 (8.8) | 39.3 (9.6) | 41.0 (6.3) | 0.16 | 2.0 |
| Education (years) | 16.1 (2.6) | 13.2 (2.6) | 13.6 (1.2) | 11.7 (2.2) | <0.001 | 17.4 |
| IQ | 112.3 (14.8) | 96.4 (13.0) | 89.9 (11.9) | 84.5 (13.3) | <0.001 | 26.0 |
| p values | x2 | |||||
| BDI | 1.3 (1.5) | 4.6 (6.6) | 8.0 (6.9) | 9.6 (9.8) | 0.005 | 7.7 (1) |
| BPRS Total | 19.3 (2.6) | 13.4 (11.1) | 38.7 (5.0) | 34.6 (6.3) | <0.001 | 37.5 (1) |
| CO | 1.0 (0.6) | 20.6 (5.3) | 0.9 (0.6) | 25.7 (15.1) | 0.65 | 0.2 (1) |
| Cigarettes/Day | --- | 18.9 (5.4) | --- | 21.6 (9.4) | 0.67 | 0.2 (1) |
| FTND | --- | 5.8 (1.2) | --- | 6.8 (1.6) | 0.09 | 2.9 (1) |
| PANSS-Total | --- | --- | 58.7 (8.6) | 56.1 (9.5) | 0.442 | 0.6 (1) |
IQ: Shipley Institute of Living Scale
Education: Years of Education
BDI: Beck Depression Inventory
BPRS: Brief Psychiatric Rating Scale
CO: Carbon Monoxide
FTND: Fagerström Test for Nicotine Dependence
PANSS: Positive and Negative Syndrome Scale
For age, education, IQ, BDI, BPRS, and CO comparisons, values reflect comparisons of patients versus controls, not stratified by smoking status.
For cigarettes/day and FTND comparisons, p and x2 values reflect differences between smokers with and without schizophrenia.
For PANSS comparison, p and x2 values reflect differences between smokers and nonsmokers with schizophrenia.
3.2. Results of between-group analyses
3.2.1. Patients versus controls
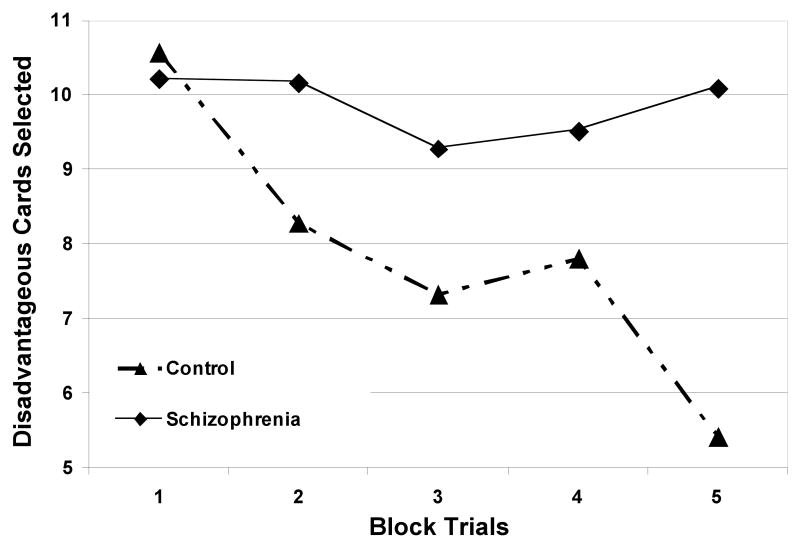
Between-group comparisons of IGT and WCST performance are listed in Table 2. Card selections on the IGT were divided into quintiles (e.g. number of disadvantageous cards per 1st 20 card selections) and analyzed using a mixed design ANOVA, which revealed a significant main effect for psychiatric status (p = 0.03, F = 5.14), but not smoking status (p = 0.38, F = 0.79), on IGT performance over time (Figure 1). Patients performed significantly worse (p = .01, F = 6.87) than did controls on the IGT, as measured by total IGT score ((C + D) – (A + B)). Whereas schizophrenia patients and control participants did not differ significantly in number of disadvantageous card selections during the 1st quintile of the IGT (p = 0.74, F = 0.11), patients made significantly more disadvantageous card selections than controls during the 5th quintile (p = 0.001, F = 11.42), suggesting reduced learning overtime on the IGT.
Table 2. Comparisons of neurocognitive task performance across subject groups compared using analysis of variance (ANOVA) and x2 tests.
| Controls | Schizophrenia | p values | F | |
|---|---|---|---|---|
| N | 21 | 42 | ||
| IGT Total Score | 23.6 (24.1) | 5.2 (27.0) | 0.01 | 6.87 |
| IGT disadvantageous 1st quintile | 10.6 (3.9) | 10.2 (4.0) | 0.74 | 0.11 |
| IGT disadvantageous 5th quintile | 5.4 (5.7) | 10.1 (4.9) | 0.001 | 11.42 |
| WCST percent perseverative responses | 11.7 (7.2) | 19.7 (14.4) | 0.02 | 5.54 |
| p values | x2 | |||
| WCST percent perseverative errors | 11.12 (6.6) | 17.3 (11.5) | 0.03 | 4.8 (1) |
| WCST categories completed | 5.1 (1.8) | 3.4 (2.3) | 0.005 | 7.8 (1) |
IGT: Iowa Gambling Task
WCST: Wisconsin Card Sorting Task
Figure 1. IGT performance over time between patients and controls.
Card selections were divided into quintiles to assess IGT performance over time. A repeated-measures two-way ANOVA revealed a significant main effect for psychiatric status (p = 0.03, F = 5.14) on IGT performance over time.
Patients performed significantly worse than controls on the WCST (Table 2). In comparison to controls, patients had significantly higher percent perseverative errors (x2 = 4.80, df =1, p = 0.03) and perseverative responses (p = 0.02, F = 5.54) and completed significantly fewer WCST categories (x2 = 7.80, df = 1, p = 0.005).
A two-way ANOVA revealed a significant main effect of psychiatric status on the variables WCST percent perseverative responses (p = 0.003, F = 9.62), IGT total score (p = 0.009, F= 7.38), and IGT 5th quintile (p = 0.002, F = 10.47), but not on IGT 1st quintile (p = 0.75, F = 1.06) scores. There was no significant main effect for smoking status on the variables WCST percent perseverative responses (p = 0.29, F = 1.12), IGT total score (p = 0.85, F= 0.04), IGT 5th quintile (p = 0.98, F = 0.001), and IGT 1st quintile (p = 0.49, F = 0.48) scores.
3.2.2. Smokers versus nonsmokers
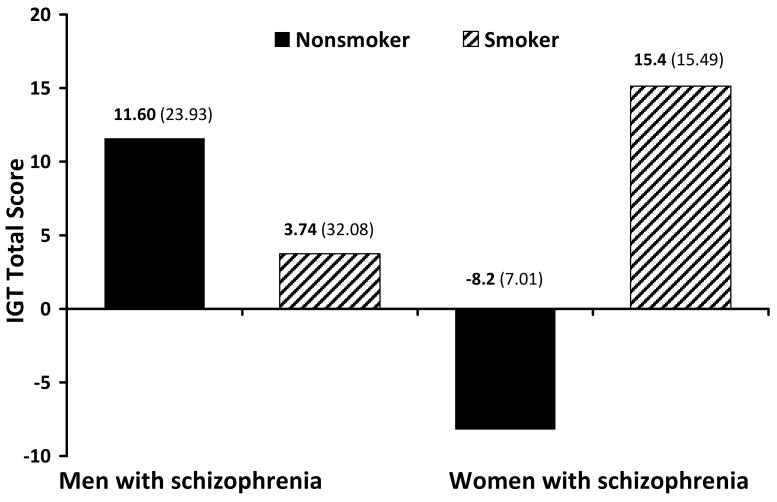
No significant differences were found between smokers with schizophrenia and non-smokers with schizophrenia on the IGT. Among women with schizophrenia, smokers had significantly better total scores on the IGT (x2 = 6.36, df = 1, p = 0.01) than did non-smokers (Figure 2). No significant differences between smokers and non-smokers were found on other neuropsychological variables for men and women with schizophrenia, respectively. Among male control subjects, smokers had significantly higher percent perseverative responses (x2= 4.36, df =1, p = 0.04) and perseverative errors (x2 = 5.82, df = 1, p = 0.02) on the WCST than did non-smokers. Due to the small number of female control participants (n = 4) enrolled in this study, comparisons of control female smokers versus non-smokers were not explored.
Figure 2. Gender differences in IGT performance among subjects with schizophrenia.
Female smokers with schizophrenia had significantly higher total IGT scores than did female nonsmokers with schizophrenia (x2 = 6.36, df = 1, p = 0.01). Male smokers with schizophrenia had lower total IGT scores than nonsmokers; however this difference was not statistically significant.
3.2.3. Antipsychotic drug status
Complete medication information was not available for all patients. Type of antipsychotic medication information was available for 37 of the 42 patients. Among these, 9 were taking typical antipsychotic medication - i.e. fluphenazine (n = 3), haloperidol (n = 3), perphanazine (n = 3) - and 28 were taking atypical antipsychotics - i.e. clozapine (n = 7), aripiprazole (n = 2), risperidone (n = 8), olanzapine (n = 9), and quetiapine (n = 2). In comparison to patients taking atypical antipsychotic drugs (n = 28), patients taking typical antipsychotic drugs (n = 9) performed better on the IGT (x2 = 4.58, df = 1, p = 0.03; Figure 3). No significant differences were found between medication groups for WCST performance. Due to small sample sizes, three-way interaction effects of gender × medication type × smoking status were not explored.
Figure 3. Medication differences in IGT performance among subjects with schizophrenia.
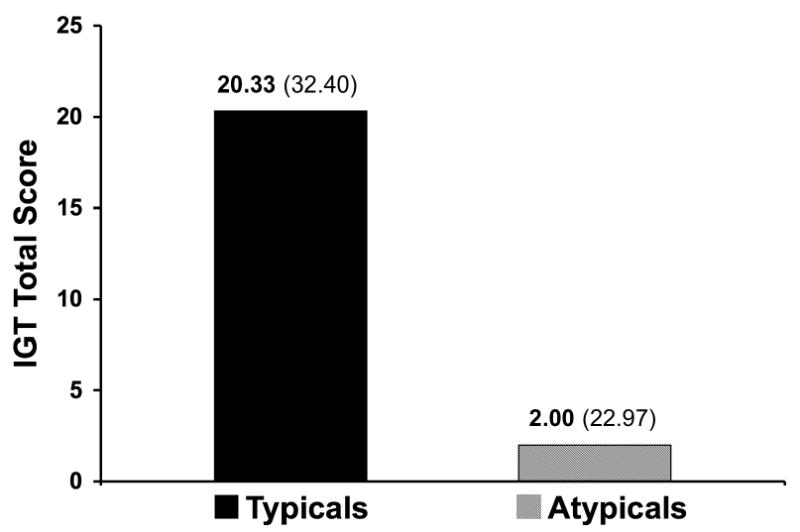
Subjects with schizophrenia taking typical antipsychotic medication had significantly higher total IGT scores than did subjects taking atypical antipsychotic medication (x2 = 4.58, df = 1, p = 0.03).
3.3. Results of Correlational Analyses
3.3.1. Patient versus control subjects
Among control subjects, significant correlations were found between IQ and IGT score (r = 0.53, p = 0.01), IQ and WCST percent perseverative errors (r = -0.81, p < 0.001), and between IQ and WCST percent perseverative responses (r = -0.84, p < 0.001). Among subjects with schizophrenia, significant correlations were found between IQ and WCST percent perseverative errors (r = -0.38, p =0.02), IGT score and WCST percent perseverative errors (r = -0.40, p = 0.01) and IGT score and WCST percent perseverative responses (r = -0.34, p = 0.03).
When controlling for smoking status, associations significant at p < 0.05 were found among SZ subjects between the following variables: IQ and IGT total score (r = 0.40, p = 0.03), IQ and WCST percent perseverative errors (r = -0.41, p = 0.02), IQ and WCST percent perseverative responses (r = -0.43, p = 0.02), IGT total score and percent perseverative errors (r = -0.37, p = 0.04) and IGT score and percent perseverative responses (r = -0.38, p = 0.04). When controlling for smoking status associations significant at p < 0.05 were found among control subjects between the following variables: IQ and IGT total score (r = 0.57, p = 0.02), IQ and WCST percent perseverative errors (r = -0.77, p < 0.001), IQ and WCST percent perseverative responses (r = -0.80, p < 0.001) and years of education and WCST percent perseverative responses (r = -0.71, p = 0.001).
When controlling for psychiatric status, associations significant at p < 0.05 were found among nonsmokers between IQ and IGT total score (r = 0.48, p = 0.045), WCST percent perseverative errors (r = -0.68, p = 0.002) and WCST percent perseverative responses (r = -0.68, p = 0.002). When controlling for psychiatric status, an association significant at p < 0.05 was found among smokers between IQ and IGT total score (r = 0.44, p = 0.01).
3.3.2. Smokers versus non-smokers
IQ was significantly correlated with IGT performance only among control smokers (r = 0.73, p = 0.03). IGT score was significantly and inversely correlated with both WCST percent perseverative errors and WCST percent perseverative responses only among smokers with schizophrenia (r = -0.37, p < 0.05; r = -0.41, p = 0.03). However, r values between IGT and WCST performance measures appeared to be of similar magnitudes in other groups, and there was a significant association between IGT and WCST performance measures across all subjects (r = -0.36, p = 0.005; Figure 4), suggesting that sample size may be a contributing factor in determining statistical significance of these findings.
Figure 4. Correlation between IGT total score and WCST percentage of perseverative errors across subject groups.
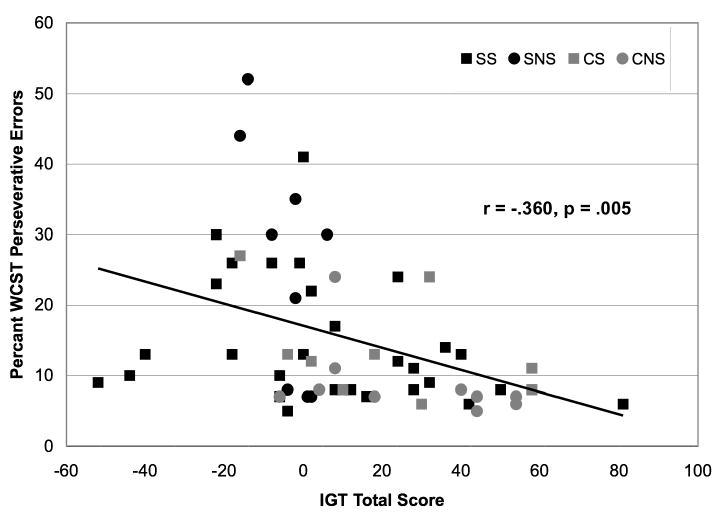
Pearson's r correlation revealed a significant inverse correlation (r = -0.360, p = 0.005) between IGT total scores and percentage of perseverative errors on the WCST across subject groups.
4. Discussion
Consistent with our primary hypothesis, individuals with schizophrenia performed significantly worse than did healthy control subjects on multiple measures of IGT performance (total score, performance over time, final quintile disadvantageous choices), indicating a significant diagnostic group effect on risk/reward decision-making. Partially consistent with our second hypothesis, cigarette smoking was significantly associated with improved IGT performance in schizophrenia. However, this effect was only observed among female patients. This finding, consistent with our fifth hypothesis, may reflect underlying sex differences in brain functioning related to neuropsychological functions like risk/reward decision-making (Cahill, 2006) or gender-related regional brain differences in response to nicotine (e.g. differences in nAChR density; Centeno et al., 2006), and further research is needed to examine these and other possibilities.
Consistent with our third hypothesis, there was a main effect for type of antipsychotic medication on IGT performance among subjects with schizophrenia. Atypical and typical antipsychotic drugs are thought to operate by shared and unique mechanisms of action, and unique features may underlie some of the medication-related findings observed in the present study. Whereas typical antipsychotics inhibit D2 DA receptors, atypical antipsychotics additionally inhibit 5-HT2A receptors (reviewed in Stahl, 2000). Typical antipsychotics bind tightly to high-affinity D2 receptors, whereas atypical antipsychotics bind more loosely to these receptors (reviewed in Seeman et al., 2001). As such, atypical antipsychotics may be less effective in blocking D2 receptors in brain regions with high endogenous DA levels (e.g. the nigrostriatal pathway), resulting in comparatively increased receptor blockade in cortical versus limbic regions (reviewed in Seeman et al., 2001). Other differential effects on cortical function between typical and atypical antipsychotic drugs have been proposed; e.g., differential influences on c-fos levels in the mPFC (Weinberger and Lipska, 1995). Our analyses were limited by the small number of patients taking typical antipsychotics, and by incomplete medication information (typical vs. atypical) in 5 of 42 schizophrenia patients. Further research with more extensive medication information and/or using a priori assignment to medication-type could help elucidate more completely the nature of a possible relationship between typical versus atypical antipsychotic treatment and IGT performance in schizophrenia. Although limited, our finding of increased IGT scores among patients taking typical antipsychotics is nonetheless consistent with one other previous report (Beninger et al., 2003) and warrants further consideration in future research.
Consistent with our fourth hypothesis, IGT and WCST performance were significantly correlated, particularly in smokers with schizophrenia. Although not statistically significant, the magnitude of the correlations was robust for other groups in the study, and this relationship was significant for the overall sample. Previous research suggests a double-dissociation between WCST and IGT performance in schizophrenia (Ritter et al., 2004). Different prefrontal regions are thought to mediate performance on these tasks, with dlPFC and vmPFC functioning relating to WCST and IGT performance, respectively (Bechara et al., 1994; Berman et al., 1986). The correlation between IGT and WCST measures suggests there may exist an element underlying performance on the two tasks, e.g., perseveration in the selections of disadvantageous cards on the IGT and perseverative choices on the WCST. These findings augment previous research demonstrating impairments in set-shifting on a modified version of the IGT among individuals with schizophrenia, especially among those high in negative symptoms (Turnbull et al., 2006).
Patients and controls differed significantly on several potentially confounding variables (i.e. BDI score, IQ, years of education). Correlational analyses were conducted to explore the relationship between these variables and IGT and WCST performance. Among control participants both years of education and IQ scores were significantly correlated with IGT and WCST performance, however no such association was found among schizophrenia patients; however, after dividing diagnostic groups based on smoking status the association between IQ and WCST and IGT performance remained only among control smokers. Years of education was still significantly associated with WCST performance, but not with IGT performance, among both control groups. Among schizophrenia patients divided by smoking status, there was a significant association between IQ and WCST performance only among nonsmokers. Due to the relatively small sample size of certain groups (e.g. control smokers), these data should be considered exploratory. Our findings nonetheless warrant further investigation, as performance on the IGT has not been uniformly associated with IQ (e.g. Kester, 2006).
This study has several limitations. Due to the higher than average prevalence of cigarette smoking in schizophrenia (Kalman et al., 2005), relatively few nonsmokers with schizophrenia were available for participation (Sacco et al., 2006). It is possible that such limitations in recruitment of certain populations (e.g. nonsmokers with schizophrenia, non-psychiatric smokers) may have limited statistical power. As such, the lack of significant differences between smokers and nonsmokers with schizophrenia may reflect a Type II error. Similarly, the numbers of female control smokers and non-smokers were small, limiting analyses of gender-by-smoking interaction effects in this population. Findings of a differential effect of antipsychotic medication (typical vs. atypical) on IGT performance should be interpreted cautiously, as detailed medication information for all subjects with schizophrenia was not available. Another potential limitation of this study is the restriction of ad libitum smoking to scheduled breaks – as opposed to allowing in situ smoking during testing. Finally, given the exploratory nature of this investigation and the potential clinical relevance of the findings, a liberal threshold of p<0.05 uncorrected for significance testing was selected. Future investigations involving larger samples are needed to investigate further the statistical and clinical relevance of these findings.
The study has multiple strengths including a sample of 63 subjects characterized on multiple demographic, clinical and neurocognitive features. This is the first study to compare the IGT performance of individuals with schizophrenia versus control subjects while controlling for cigarette smoking status. Previous research on the neuropsychological effects of cigarette smoking in schizophrenia has focused predominantly on measures of sustained attention and executive function. To our knowledge, this is the first study of risk/reward decision-making in schizophrenia controlling for cigarette smoking.
5. Conclusion and Future Directions
Our findings support previous research demonstrating deficits in both IGT and WCST performance in schizophrenia and that performance on these tasks may be related. Further research is needed to establish the relationship between risk/reward decision-making, other neuropsychological processes and cigarette smoking in schizophrenia. Future incorporation of imaging modalities may help to identify gender differences in regional brain activation during task performance and investigate potential differences in nAChR density.
Table 3. Pearson's r and Spearman's rho correlations between neurocognitive measures among patients and controls.
| Controls | Schizophrenia | |||
|---|---|---|---|---|
| N | 21 | 42 | ||
| r | p values | r | p values | |
| BPRS, IGT score | 0.15 | 0.54 | -0.10 | 0.58 |
| PANSS, IGT score | -- | -- | -0.16 | 0.32 |
| IQ, IGT score | 0.53 | 0.01 | 0.26 | 0.12 |
| IQ, WCST percent perseverative responses | -0.84 | 0.00 | -0.31 | 0.06 |
| Education, IGT score | 0.24 | 0.31 | 0.10 | 0.54 |
| Education, WCST percent perseverative responses | -0.75 | 0.00 | 0.10 | 0.56 |
| IGT score, WCST percent perseverative responses | -0.41 | 0.07 | -0.34 | 0.03 |
| rho | p values | |||
| IQ, WCST percent perseverative errors | -0.81 | 0.00 | -0.38 | 0.02 |
| Education, WCST percent perseverative errors | -0.78 | 0.00 | -0.01 | 0.95 |
| IGT score, WCST percent perseverative errors | -0.45 | 0.05 | -0.40 | 0.01 |
| BDI, IGT score | -0.35 | 0.12 | 0.05 | 0.75 |
| BDI, WCST percent perseverative responses | 0.20 | 0.39 | -0.12 | 0.46 |
| BDI, WCST percent perseverative errors | 0.23 | 0.34 | -0.12 | 0.43 |
BPRS: Brief Psychiatric Rating Scale
IGT: Iowa Gambling Task
PANSS: Positive and Negative Syndrome Scale
IQ: Shipley Institute of Living Scale
Education: Years of Education
WCST: Wisconsin Card Sorting Task
BDI: Beck Depression Inventory
Table 4. Spearman's rho correlations between neurocognitive measures among subject groups.
| Control non-smokers | Control smokers | Non-smokers with schizophrenia | Smokers with schizophrenia | |||||
|---|---|---|---|---|---|---|---|---|
| N | 12 | 9 | 10 | 32 | ||||
| rho | p | rho | p | rho | p | rho | p | |
| IQ, IGT score | 0.41 | 0.19 | 0.73 | 0.025 | 0.28 | 0.43 | 0.30 | 0.13 |
| IQ, WCST percent perseverative errors | -0.43 | 0.21 | -0.90 | 0.001 | -0.75 | 0.01 | -0.25 | 0.20 |
| IQ, WCST percent perseverative responses | -0.45 | 0.17 | -0.79 | 0.01 | -0.78 | 0.008 | -0.26 | 0.18 |
| Education, IGT score | 0.10 | 0.77 | 0.03 | 0.95 | 0.17 | 0.65 | 0.13 | 0.49 |
| Education, WCST percent perseverative errors | -0.65 | 0.04 | -0.66 | 0.06 | -0.37 | 0.29 | -0.5 | 0.78 |
| Education, WCST percent perseverative responses | -0.60 | 0.05 | -0.71 | 0.03 | -0.34 | 0.34 | -0.04 | 0.81 |
| IGT score, WCST percent perseverative errors | -0.58 | 0.08 | -0.48 | 0.20 | -0.61 | 0.64 | -0.37 | 0.05 |
| IGT score, WCST percent perseverative responses | -0.59 | 0.06 | -0.24 | 0.54 | -0.57 | 0.09 | -0.41 | 0.03 |
IQ: Shipley Institute of Living Scale
Education: Years of Education
IGT: Iowa Gambling Task
WCST: Wisconsin Card Sorting Task
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. American Psychiatric Publishing; Washington DC: 1995. [Google Scholar]
- Bechara A. Risky business: emotion, decision-making, and addiction. J of Gambling Studies. 2003;19(1):23–51. doi: 10.1023/a:1021223113233. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(13):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R. Beck depression inventory-manual. The Psychological Corporation; San Antonio: 1987. [Google Scholar]
- Beninger RJ, Wasserman J, Zanibbi K, Charbonneau D, Mangels J, Beninger BV. Typical and atypical antipsychotic medications differentially affect two nondeclarative memory tasks in schizophrenic patients: a double dissociation. Schizophr Res. 2003;61(23):281–292. doi: 10.1016/s0920-9964(02)00315-8. [DOI] [PubMed] [Google Scholar]
- Bergemann N, Parzer P, Runnebaum B, Resch F, Mundt C. Estrogen, menstrual cycle phases, and psychopathology in women suffering from schizophrenia. Psychol Med. 2007;37:1427–1436. doi: 10.1017/S0033291707000578. [DOI] [PubMed] [Google Scholar]
- Berman K, Ostrem J, Randolph C, Gold J, Goldberg T, Coppola R, et al. Physiological activation of a cortical network during performance of the Wisconsin Card Sorting Test: a positron emission tomography study. Neuropsychologia. 1995;33:1027–1046. doi: 10.1016/0028-3932(95)00035-2. [DOI] [PubMed] [Google Scholar]
- Berman K, Zec R, Weinberger D. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. II. Role of neuroleptic treatment, attention, and mental effort. Arch Gen Psychiatry. 1986;43:126–135. doi: 10.1001/archpsyc.1986.01800020032005. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nature Rev Neurosci. 2006;7:477–491. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Cavallaro R, Cavedini P, Mistretta P, Bassi T, Angelone SM, Ubbiali A, et al. Basal-corticofrontal circuits in schizophrenia and obsessive-compulsive disorder: a controlled, double dissociation study. Biol Psychiatry. 2003;54(4):437–443. doi: 10.1016/s0006-3223(02)01814-0. [DOI] [PubMed] [Google Scholar]
- Centeno ML, Henderson JA, Pau KY, Bethea CL. Estradiol increases alpha7 nicotinic receptor in serotonergic dorsal raphe and noradrenergic locus coeruleus neurons of macaques. J of Comp Neurol. 2006;497(3):489–501. doi: 10.1002/cne.21026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis L, Buisson B, Bertrand S, Bertrand D. Potentiation of Human α4β2 Neuronal Nicotinic Acetylcholine Receptor by Estradiol. Mol Pharmacology. 2002;61(1):127–135. doi: 10.1124/mol.61.1.127. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes' Error: Emotion, Reason, and the Human Brain. Penguin Books; New York: 1994. [Google Scholar]
- Depatie L, O'Driscoll GA, Holahan AL, Atkinson V, Thavundayil JX, Kin NN, et al. Nicotine and behavioral markers of risk for schizophrenia: a double-blind, placebo-controlled, cross-over study. Neuropsychopharmacology. 2002;27(6):1056–1070. doi: 10.1016/S0893-133X(02)00372-X. [DOI] [PubMed] [Google Scholar]
- Desai RA, Potenza MN. Problem and pathological gambling in patients with schizophrenia. J Clin Psychiatry. 2008 doi: 10.4088/JCP.08m04359. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan SL, Sacco KA, Termine A, Seyal AA, Dudas MM, Vessicchio JC, et al. Neuropsychological deficits are associated with smoking cessation treatment failure in patients with schizophrenia. Schizophr Res. 2004;70(23):263–275. doi: 10.1016/j.schres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Evans CE, Bowman CH, Turnbull OH. Subjective awareness on the Iowa Gambling Task: the key role of emotional experience in schizophrenia. J of Clin and Exp Neuropsych. 2005;27(6):656–664. doi: 10.1081/13803390490918354. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal damage in humans. Cerebral Cortex. 2005;15(1):58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR. A Clinical Blueprint to Accelerate the Elimination of Tobacco Use. JAMA. 2008;299(17):2083–2085. doi: 10.1001/jama.299.17.2083. [DOI] [PubMed] [Google Scholar]
- George TP, Krystal JH. Comorbidity of psychiatric and substance abuse disorders. Curr Opin Psychiatry. 2000;13:327–331. [Google Scholar]
- George TP, Termine A, Sacco KA, Allen TM, Reutenauer E, Vessicchio JC, et al. A preliminary study of the effects of cigarette smoking on prepulse inhibition in schizophrenia: Involvement of nicotinic receptor mechanisms. Schizophr Res. 2006;87(13):307–315. doi: 10.1016/j.schres.2006.05.022. [DOI] [PubMed] [Google Scholar]
- George TP, Vessicchio JC, Termine A, Sahady DM, Head CA, Pepper WT, et al. Effects of smoking abstinence on visuospatial working memory function in schizophrenia. Neuropsychopharmacology. 2002;26(1):75–85. doi: 10.1016/S0893-133X(01)00296-2. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. Br J Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heaton R. Wisconsin Card Sorting Test: Manual. Psychological Assessment Resources; Odessa, FL: 1981. [Google Scholar]
- Hilz MJ, Devinsky O, Szczepanska H, Borod JC, Marthol H, Tutaj M. Right ventromedial prefrontal lesions result in paradoxical cardiovascular activation with emotional stimuli. Brain. 2006;129(112):3343–3353. doi: 10.1093/brain/awl299. [DOI] [PubMed] [Google Scholar]
- Hoff AL, Wieneke M, Horon R, Blankfeld H, Espinoza S, Faustman WO, et al. Estrogen levels relate to neuropsychological function in female schizophrenics. Schizophr Res. 1997;24(12):107–108. [Google Scholar]
- Kalman D, Morisette SB, George TP. Comorbidity of smoking with psychiatric and substance use disorders. Am J Addict. 2005;14:106–123. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S, Fiszbein A, Opler L. The Positive and Negative Syndrome Scale for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kester HM, Sevy S, Yechiam E, Burdick KE, Cervellione KL, Kumra S. Decision-making impairments in adolescents with early-onset schizophrenia. Schizophr Res. 2006;85(13):113–123. doi: 10.1016/j.schres.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Kinon BJ, Lieberman JA. Mechanisms of action of atypical antipsychotic drugs: a critical analysis. Psychopharm. 1996;124:2–34. doi: 10.1007/BF02245602. [DOI] [PubMed] [Google Scholar]
- Lee Y, Kim YT, Seo E, Park O, Jeong SH, Kim SH, et al. Dissociation of emotional decision-making from cognitive decision-making in chronic schizophrenia. Psychiatry Res. 2007;152(23):113–120. doi: 10.1016/j.psychres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Levin ED, Wilson W, Rose JE, McEvoy J. Nicotine-haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmacology. 1996;15(5):429–436. doi: 10.1016/S0893-133X(96)00018-8. [DOI] [PubMed] [Google Scholar]
- Marenco S, Coppola R, Daniel D, Zigun J, Weinberger D. Regional cerebral blood flow during the Wisconsin Card Sorting Test in normal subjects studied by Xenon-133 dynamic SPECT: comparison of absolute values, percent distribution values, and covariance analysis. Psychiatry Res. 1993;50:177–192. doi: 10.1016/0925-4927(93)90029-h. [DOI] [PubMed] [Google Scholar]
- Martino DJ, Bucay D, Butman JT, Allegri RF. Neuropsychological frontal impairments and negative symptoms in schizophrenia. Psychiatry Res. 2007;152(23):121–128. doi: 10.1016/j.psychres.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Nestor PG, Levitt JJ, Cohen AS, Kawashima T, Shenton ME, et al. Orbitofrontal volume deficit in schizophrenia and thought disorder. Brain. 2008;131(1):180–195. doi: 10.1093/brain/awm265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Gorham D. The brief psychiatric rating scale. Psychol Rep. 1962;10 [Google Scholar]
- Paolo A, Tröster A, Axelrod B, Koller C. Construct validity of the WCST in normal elderly and persons with Parkinson's disease. Arch Clin Neuropsychol. 1995;10(5):463–473. [PubMed] [Google Scholar]
- Reavis R, Overman W. Adult sex differences on a decision-making task previously shown to depend on the orbital prefrontal cortex. Behav Neurosci. 2001;115:196–206. doi: 10.1037/0735-7044.115.1.196. [DOI] [PubMed] [Google Scholar]
- Rezai K, Andreasen NC, Alliger R, Cohen G, Swayze V, 2nd, O'Leary DS. The neuropsychology of the prefrontal cortex. Arch Neurol. 1993;50(6):636–642. doi: 10.1001/archneur.1993.00540060066020. [DOI] [PubMed] [Google Scholar]
- Ritter L, Meador-Woodruff J, Dalack G. Neurocognitive measures of prefrontal cortical dysfunction in schizophrenia. Schizophr Res. 2004;68(1):65–73. doi: 10.1016/S0920-9964(03)00086-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sanchez JM, Crespo-Facorro B, Perez-Iglesias R, Gonzalez-Blanch C, Alvarez-Jimenez M, Llorca J, et al. Prefrontal cognitive functions in stabilized first-episode patients with schizophrenia spectrum disorders: a dissociation between dorsolateral and orbitofrontal functioning. Schizophr Res. 2005;77(23):279–288. doi: 10.1016/j.schres.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Sacco K, Termine A, Dudas MM, Seyal AA, Allen TM, Vessicchio JC, et al. Neuropsychological deficits in nonsmokers with schizophrenia: Effects of a nicotinic antagonist. Schizophr Res. 2006;85(13):213–221. doi: 10.1016/j.schres.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Sacco KA, Termine A, Seyal A, Dudas MM, Vessicchio JC, Krishnan-Sarin S, et al. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: involvement of nicotinic receptor mechanisms. Arch Gen Psychiatry. 2005;62(6):649–659. doi: 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- Seeman P. Antipsychotic drugs, dopamine receptors, and schizophrenia. Clin Neuro Res. 2001;1:53–60. [Google Scholar]
- Sevy S, Burdick KE, Visweswaraiah H, Abdelmessih S, Lukin M, Yechiam E, et al. Iowa Gambling Task in schizophrenia: A review and new data in patients with schizophrenia and co-occurring cannabis use disorders. Schizophr Res. 2007;92(13):74–84. doi: 10.1016/j.schres.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley RE, Powell CE, Harley RJ. A method and model for automated assessment of human judgment. J of Psychol. 1970;75:87–105. doi: 10.1080/00223980.1970.9916809. 2d Half. [DOI] [PubMed] [Google Scholar]
- Shipley WC. A self-administering scale for measuring intellectual impairment and deterioration. J of Psychol. 1940;9:371–377. [Google Scholar]
- Shurman B, Horan WP, Nuechterlein KH. Schizophrenia patients demonstrate a distinctive pattern of decision-making impairment on the Iowa Gambling Task. Schizophr Res. 2005;72(23):215–224. doi: 10.1016/j.schres.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Essential Psychopharmacology: Neuroscience basis and practical applications. 2nd. Cambridge University Press; New York, NY: 2000. [Google Scholar]
- Tucker K, Potenza MN, Beauvais JE, Browndyke JN, Gottschalk PC, Kosten TR. Perfusion abnormalities and decision-making in cocaine dependence. Biol Psychiatry. 2004;56:527–530. doi: 10.1016/j.biopsych.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Turnbull OH, Evans CEY, Kemish K, Park S, Bowman CH. A novel set-shifting modification of the Iowa Gambling Task: Flexible emotion-based learning in schizophrenia. Neuropsychology. 2006;20(3):290–298. doi: 10.1037/0894-4105.20.3.290. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Benbrook A, Funderburk F, David P, Cadet JL, Bolla KI. The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug and Alcoh Dep. 2007;90(1):2–11. doi: 10.1016/j.drugalcdep.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Lipska BK. Cortical maldevelopment, anti-psychotic drugs, and schizophrenia: a search for common ground. Schizophr Res. 1995;16:87–110. doi: 10.1016/0920-9964(95)00013-c. [DOI] [PubMed] [Google Scholar]
- Weinberger A, Reutenauer E, Allen T, Termine A, Vessicchio J, Sacco K, et al. Reliability of the Fagerström Test for Nicotine Dependence, Minnesota Nicotine Withdrawal Scale, and Tiffany Questionnaire for Smoking Urges in Smokers with and without Schizophrenia. Drug and Alcoh Dep. 2007;86(23):278–282. doi: 10.1016/j.drugalcdep.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Wilder KE, Weinberger DR, Goldberg TE. Operant conditioning and the orbitofrontal cortex in schizophrenic patients: unexpected evidence for intact functioning. Schizophr Res. 1998;30(2):169–174. doi: 10.1016/s0920-9964(97)00135-7. [DOI] [PubMed] [Google Scholar]