Abstract
BACKGROUND:
Experimental human stem cell transplantation to the heart has begun, but the mechanisms underlying benefits seen in pre-clinical models, both at the site of cell injection and at more distant regions, remain uncertain. We hypothesize that these benefits may be best understood first at the level of key intracellular signaling cascades in the host myocardium that may be responsible for functional and structural preservation of the heart.
STUDY DESIGN:
Western blot and ELISA were used to assess key pathways that regulate cardiac myocyte survival and hypertrophy in both the infarct/borderzone (I/BZ) and remote myocardium (RM) of C57/B6 mouse hearts subjected to coronary artery ligation, with subsequent injection of either vehicle or bone marrow-derived adult mesenchymal stem cells (MSC).
RESULTS:
Improved left ventricular function with MSC transplantation was associated with a relative preservation of Akt phosphorylation (activation) and of phosphorylation of downstream mediators of cell survival and hypertrophy. There was no significant difference in activation of MAP kinase p38, and activation of the anti-apoptotic MAP kinase ERK was lower at one week after MSC treatment but rose above controls by week 2. Similar changes were observed in both the I/BZ and the RM.
CONCLUSION:
Stem cell transplantation in the post-MI murine myocardium is associated with preservation of Akt signaling. Together with a possible later increase in ERK activation, this signaling change may be responsible for cardioprotection. Further focused investigation may identify elements in transplantation regimens that optimize this mechanism of benefit, and that may increase the likelihood of human clinical success.
Introduction
Early experiences with translation of experimental stem cell transplantation for human heart disease have met with only rare, limited success. In contrast, the wide variety of successful pre-clinical models has indicated that the growth of new myocardial tissue from donor cells, likely to occur in only a small subset of these models and stem cell types, may not be a primary mechanism of benefit in many cases. For example, reductions in infarct size and improvements in ventricular function have been documented even in the absence of electrical coupling between donor and host cells, and even when only a very small percentage of donor cells can be found to survive. Attention has therefore been focused on two possible alternative mechanisms of benefit: 1) induction of angiogenesis 1-5 and 2) paracrine or immunomodulatory amelioration of cardiac remodeling 5-11. Given the rare survival of transplanted cells even when benefits have been observed, and observations of success with a variety of donor cells that are unlikely to yield vascular cell differentiation, it is likely that both of these theories boil down to a paracrine or other secondary influence of cell transplantation within the recipient host tissue.
While some researchers have begun to analyze the secretion of paracrine factors by various target donor cells in vitro 9-11, extrapolation of these observations to the complex in vivo setting is a daunting challenge. We have therefore postulated instead that the benefits observed in experimental cardiac cell transplantation may be first understood in terms of biological changes instigated in the host myocardial cells. Although some studies have explored signaling within the donor stem cells, few reports have addressed changes in signaling within the host myocardium. Nevertheless, much has been learned in recent years regarding the roles of various intracellular signaling pathways in regulating cardiac myocyte fate 12-14. Interaction between these pathways helps determine an ongoing “decision” between cell survival and apoptosis; it also governs phenotypic changes that can lead either to pathologic or physiologic cardiac hypertrophy 15-21. Two signaling cascades share an interesting role in cardiac myocytes: both the PI3K/Akt and MAP kinase pathways contain arms that have been associated with cell survival and with induction of “physiologic” hypertrophy, while other limbs have been implicated instead in cardiac myocyte apoptosis and the transition from cardiac hypertrophy to ventricular dilatation 15-21.
In this study, we have begun to dissect changes in cardiac signaling that comprise the myocardium's response to the transplantation of adult, bone marrow-derived mesenchymal stem cells (MSCs) early after acute myocardial infarction in mice. This model has been consistently found to yield measurable structural and functional benefit despite the absence of new, donor-derived myocardial muscle coupled to host tissue7,22-24. We hypothesized that either an increase in PI3K/Akt activation or a shift in the balance between the p38 and ERK arms of the MAP kinase pathway would help explain the reduction in cardiac myocyte apoptosis and the improvement in ventricular function that have been clearly documented in past reports. Such an observation would in turn represent a first step toward elucidation of the intermediate mechanistic steps between the transplantation of stem cells and the benefit derived by the host myocardium, and may allow a more rational approach both to further analysis of the direct role of donor cells, and to strategies for human translation that are more likely to yield early clinical success.
Study Design
Left anterior descending (LAD) coronary ligation and MSC transplantation
Male C57BL/6 mice (6-8weeks old) were anesthetized with 1.5% isoflurane, and ventilated on a Harvard Rodent Respirator. An anterior thoracotomy was performed and the proximal LAD artery was ligated approximately one third the distance from the base to the apex of the heart. Within 30 minues after ligation, 1×105 culture-expanded MSCs resuspended in 10 μl of normal saline were injected directly into the myocardium around the infarct border zone. The chest was then closed and animals were allowed to recover. For sham operations, 10 μl of saline were injected without cells. The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996), and were approved by the Institutional Animal Care and Use Committee of the San Francisco Veterans Affairs Medical Center
Isolation and characterization of mouse MSC
Mouse MSC were obtained as previously described 25. Bone marrow was collected from 6-8 week-old male C57Bl/6 or C57-GFP transgenic (C57Bl/6 TgN[ACT6EGFP]) mice (Jackson Laboratory). In brief, the femurs and tibiae were excised using sterile technique and were clipped to expose the bone marrow. Cells were diluted with growth medium (αMEM supplemented with 10% fetal bovine serum, 10% horse serum, glutamine 2mmol/L and penicillin/streptomycin and filtered through a 70 μm nylon mesh (Cell strainer, Falcon, Franklin Lakes, NJ). After centrifugation at 200 g for 10 min, the cell pellet was resuspended and plated in T75 flasks. The cells were incubated for 3 days and the non-adherent cells were removed by washing. For subculture, the adherent cells were split upon reaching confluence at a ratio of 1:2 or 1:3.
For detection of surface antigens, MSCs were trypsinized, centrifuged and incubated for 30 minutes at 4°C with phycoerythrin (PE)- or fluoresceinisothiocyanate (FITC)-conjugated antibodies against murine Sca-1, CD29, CD 31, CD44, CD117, CD90.2, CD34, CD45, VCAM-1 and CD11b according to the manufacturer's instruction (ebioscience, CA). Background fluorescence was subtracted after analyzing unstained cells. Stained cells were analyzed by fluorescence activated cell sorting (FACScan, Becton Dickinson, Mountain View, CA)
In vitro differentiation
Osteogenic differentiation was induced by treating cultured MSCs with 10nmol/L dexamethasone (Sigma), 200μmol/L ascorbic acid 2-phosphate(Sigma) and 10mmol/L β-glycerophosphate (Sigma) 24. Calcium deposition was documented via washing cultures with PBS, fixation with 4% paraformaldehyde in room temperature PBS for 20 minutes, and staining for 5 minutes with Alizarin Red S (Sigma).
To induce adipogenic differentiation, MSCs were treated with 1μmol/L dexamethasone (Sigma), 10 μg/ml insulin (Sigma), 100 μmol/L indomethacin (Sigma) and 0.5mmol/L 3-isobutyl-methylxanthine (IBMX, Sigma). Cells were fixed with 4% paraformaldehyde and stained with Oil Red O (Sigma) solution for 5 minutes.
Echocardiography
Transthoracic echocardiography was performed with an Acuson Sequioa 512 with a 13-MHz probe. A two-dimensional short-axis view of the left ventricle was obtained at the level of the papillary muscles, and two-dimensionally targeted M-mode tracings were recorded to determine LV fractional shortening.
ELISA and Western blot
Myocardial tissues were divided into two separate specimens representing infarct/borderzone (I/BZ) and remote, uninfarcted myocardium (RM), and were homogenized in a lysis buffer containing 0.13mol/L KCl, 1mmol/L EDTA, 1mmol/L EGTA, 1mmol/L Na3(VO4), 5mmol/L NaF, 20mmol/L HEPES and Protease inhibitor cocktail tablet (Roche Diagnostics, Mannheim, Germany). BCA Protein Assay Reagent Kit (Pierce, Rockford, IL) was used to measure protein concentration. Phospho-ERK1/2, Phospho-Akt, Phospho-GSK-3α/β, and Phospho-p70S6 Kinase levels were then assayed by using commercially available ELISA kits (R&D Systems, Minneapolis, MN). Samples containing equal amounts of protein were separated by Bis-Tris Gels and transferred to PVDF membranes (Invitrogen, Carlsbad, CA). Blots were probed with primary antibodies specific for p38, ERK1/2, Akt, p70S6 kinase, GSK, BAD, and □-actin(Cell Signaling, Beverly, MA), and then with horseradish peroxidase–conjugated secondary antibodies. SuperSignal West Femto Maximum Sensitivity substrate (Pierce, IL) was used for the chemiluminescent visualization of proteins. Expression of each protein was normalized to the level of α-actin to offset the variation from sample loading.
Statistics
Data are reported as mean ± SEM. Comparisons between groups were made using Student's t test and ANOVA. A P-value of less than 0.05 was considered to denote statistical significance.
Results
Mouse MSCs
Marker expression was examined via FACS analysis after expansion of cells isolated via differential plating of adult mouse bone marrow aspirates. Consistent with previous reports of adult mesenchymal stem cells 25, these cells were found to express CD29 and SCA1 but not CD34, CD45 or CD11b. Furthermore, upon stimulation with dexamethasone, ascorbic acid and β-glycerophosphate or with dexamethasone, insulin, indomethacin and IBMX, these cells underwent differentiation into either an adipocyte or osteoblast phenotype, respectively.
Cell transplantation and functional benefit
Injection of isogeneic, bone marrow-derived MSCs into the peri-infarct region of the left ventricle was completed successfully early after LAD ligation in C57/B6 mice, with a procedural mortality of 10-15%. MSCs isolated from transgenic mice with constitutive expression of EGFP could be identified via fluorescent microscopy of thin sections of the left ventricle in all animals studied. Serial echocardiography was used to confirm a recapitulation of the functional benefit documented in previous studies of stem cell transplantation early after MI in mice (Table I). At one week after MI, a trend toward improved fractional shortening (FS) was observed in MSC-transplanted hearts. By two weeks after MI, a statistically significant 26% improvement in FS was seen in mice receiving isogeneic MSC transplantation compared to controls receiving similar injections of normal saline shortly after LAD ligation. Interestingly, this improvement in FS was observed at the level of the mitral apparatus, i.e. in the myocardium remote to the infarct and some distance from the injection of MSC, suggesting a global impact of the transplantation on myocardial biology. Ejection fraction and cardiac output, as calculated from echocardiographic measurements, were also statistically significantly improved with MSC transplantation (Table I).
TABLE 1.
| 1w | 2w | |||
|---|---|---|---|---|
| Saline | BMSC | Saline | BMSC | |
| EF (%) | 60.56±2.42 | 66.46±4.15 | 60.28±2.95 | 70.56±2.41* |
| FS (%) | 34.12±1.98 | 39.63±3.71 | 33.95±2.51 | 42.66±2.23* |
| LVEDV(μl) | 69.84±5.93 | 76.98±11.91 | 79.72±7.88 | 81.95±8.85 |
| LVESV (μl) | 28.46±3.49 | 29.38±8.67 | 32.12±4.37 | 24.94±4.20 |
| CO (ml/min) | 22.81±1.32 | 25.22±1.98* | 27.79±3.33 | 32.91±2.98* |
Changes in pathway activation
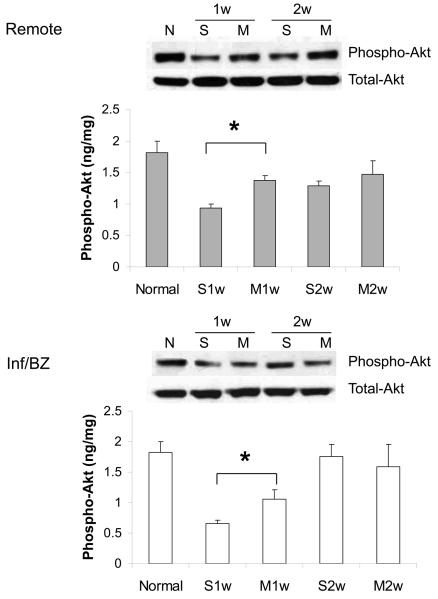
At 1 week after MI, a significant drop was observed in the level of myocardial Akt phosphorylation (i.e. activation) in saline-injected control animals compared to uninfarcted hearts. This cardioprotective signaling was significantly preserved in MSC-injected hearts at this time point, both in the infarct/borderzone and in the remote myocardium (Figure 1). By two weeks after MI, Akt phosphorylation had partially recovered in both the saline and MSC groups. Although the treatment groups were no longer statistically different, levels in the MSC group had almost returned to those of uninfarcted hearts. Western blot indicated that total Akt protein expression was similar in both groups at all time points (Figure 1).
1.
Akt phosphorylation (i.e. activation) in the remote region and the infarct/borderzone of infracted mouse hearts treated with either saline (S) or MSC (M) after LAD ligation, measured either 1 week (1w) or 2 weeks (2w) after LAD ligation. (n=6-8, *P < 0.05)
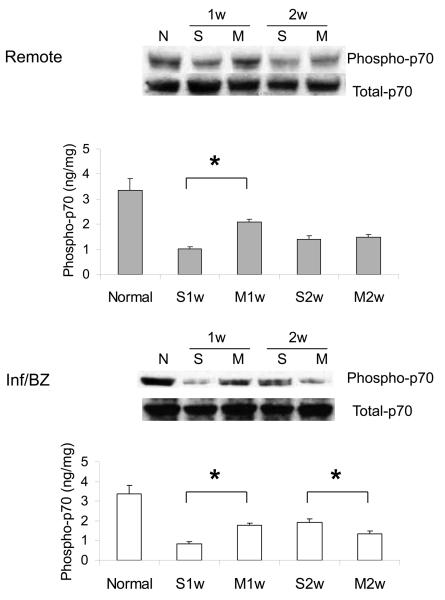
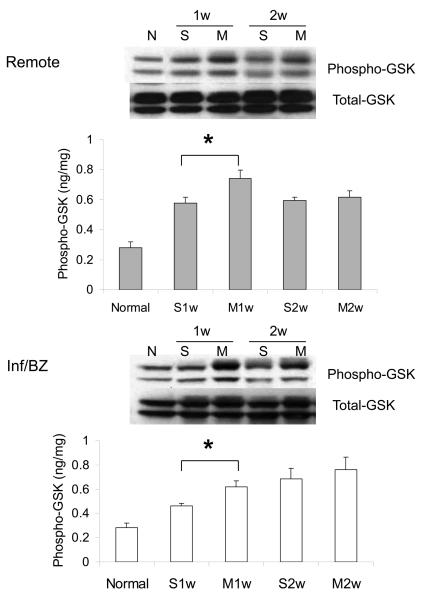
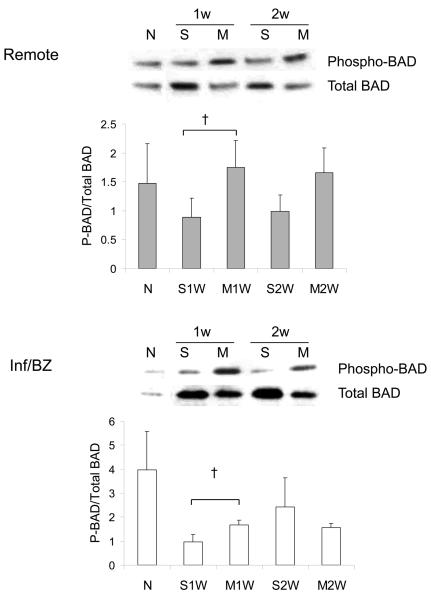
Phosphorylation of two key downstream mediators of Akt signaling was also increased at week 1 after MSC transplantation compared to saline injection, again in both the infarct/borderzone and the remote uninfarcted regions of the heart. These mediators were p70S6 kinase, a key mediator of cell hypertrophy, and of GSK-3β, which regulates, among other processes, mitochondrially-mediated apoptosis (Figure 2A,B). MSC transplantation has been associated with a reduction in myocardial apoptosis rates after acute MI 26. Akt can exert an anti-apoptotic effect in numerous cell types, including cardiac myocytes, via phosphorylation of BAD, a pro-apoptotic member of the Bcl-2 family. A qualitative, though not statistically significant, increase in the ratio of phosphorylated to total BAD protein was observed at 1 week after MSC transplantation, when an increase in Akt activation was also observed (Figure 2C).
2.
Phosphorylation of Akt downstream intermediates p70S6 kinase (A) and GSKβ-3 (B) in the remote region and the infarct/borderzone of infracted mouse hearts treated with either saline (S) or MSC (M) after LAD ligation, measured either 1 week (1w) or 2 weeks (2w) after LAD ligation. (n=6-8, *P < 0.05). A trend toward increased phosphorylation (inactivation) of pro-apoptotic BAD (n=6, †P = 0.1) was also observed at week 1.
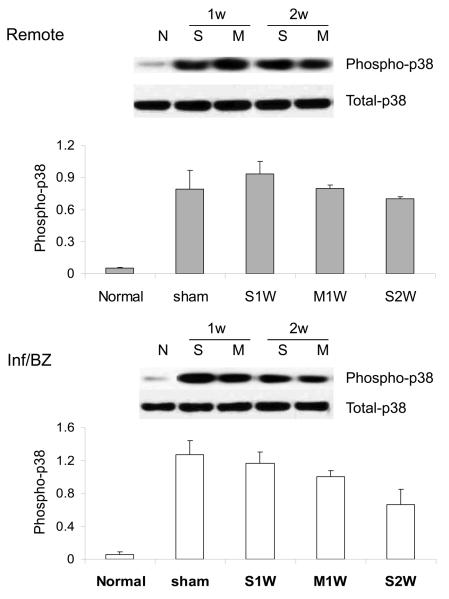
Although the role of MAP kinase p38 in the evolution of dilated cardiomyopathy remains somewhat controversial, some studies have demonstrated an association between phosphorylation (i.e. activation) of p38 and either CM apoptosis in vitro 19 or progression to dilated cardiomyopathy in vivo 20. An increase in activation of p38 was observed at both 1 and 2 weeks after MI, and, in contrast to the enhanced preservation of Akt signaling seen with MSC transplantation, there was no difference between levels of p38 activation between MSC- and saline-injected groups (Figure 3A). Isoform-specific ELISA suggested a similar pattern of increased phosphorylation of both p38 alpha and gamma isoforms that was not influenced by MSC transplantation (data not shown).
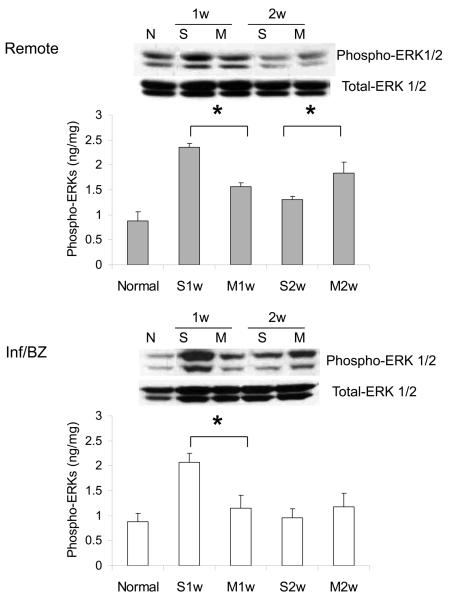
3.
Phosphorylation of p38γ (A) and ERK (B) in the remote region and the infarct/borderzone of infracted mouse hearts treated with either saline (S) or MSC (M) after LAD ligation, measured either 1 week (1w) or 2 weeks (2w) after LAD ligation. (n=4-8, *P < 0.05)
ERK activation has been associated with inhibition of apoptosis in cardiac myocytes in vitro 27 and with physiologic hypertrophy in vivo 16. ERK activation has also been documented in models of ischemia-reperfusion 16. In this study, ERK phosphorylation (i.e. activation) was increased in saline injected controls at 1 week after myocardial infarction; this increase was significantly less pronounced in hearts undergoing MSC transplantation (Figure 3B). Interestingly, ERK phosphorylation then declined significantly in control hearts, such that there was a higher level of ERK phosphorylation in MSC-treated hearts at week 2 after transplantation.
Conclusions
The results of this study have begun to suggest patterns of altered signaling in the host myocardium after isogeneic mesenchymal stem cell transplantation. These alterations may reflect the recruitment of biological pathways that result in myocardial preservation and even promote more adaptive compensatory hypertrophy, beyond any direct contribution of donor cells to myocardial function. Interestingly, the signaling changes were not limited to the regions of the myocardium into which cells were transplanted, suggesting either a more widespread paracrine effect or a shift in myocardial biology that may extend in wave-like fashion away from the site of transplantation toward more remote regions. Alternatively, a direct effect on early pathological changes in the post-MI myocardium might relieve local stresses in the infarct and borderzone regions in such a way as to relieve increased biophysical and biochemical stress on the remote myocardium, as well, resulting in an opportunity for better adaptive signaling throughout the heart.
Understanding the mechanisms of benefit of experimental cardiac cell transplantation models has proved difficult. Only in the case of fetal cardiomyocyte transplantation has appropriate coupling of even a small number of host and donor cells been documented 28. Furthermore, a significant proportion of surviving or “differentiated” donor cells actually represent the product of cell fusion between donor and host myocardial cells 29,30. The benefits of cell transplantation may therefore be best understood first in terms of biological changes instigated in host myocardial cells.
In contrast to this upregulation of Akt signaling, there was no change in activation of p38 MAP kinase, which has been associated in some studies with cardiac myocyte apoptosis and remodeling 19-21. We have recently observed that activation of the anti-apoptotic MAP kinase ERK is increased in the mouse myocardium after MI, and that this increase may be localized primarily to cardiac non-myocytes that are actively proliferating during both scar evolution and remodeling of the remote myocardium 31. The decrease in ERK signaling that we observed at 1 week after MI followed by MSC injection in this study may reflect a decrease in non-myocyte proliferation as a result of better overall myocyte survival and reduced remodeling with cell transplantation.
Another important discrepancy may increase the challenge of translating exciting preclinical observations into successful therapeutic strategies for treating human heart failure. Nearly all experimental data have been derived from studies of young, healthy animals. A growing body of literature, however, suggests that stem and progenitor cell populations in older, sicker individuals, i.e. those that will be targeted by clinical applications, exhibit important phenotypic differences compared to similar cells from younger, healthier ones 32-34. In addition, target tissues, such as the myocardium, in older, diseased individuals may respond differently to cell transplantation 35,36. For example, stem cells from older populations likely exhibit telomeric shortening, which can result in accelerated cell senescence. The number and function of progenitor cells have been documented to decrease both in animals and in humans with advanced age and with progressive risk factors for heart disease 37,38. Diseased and aged myocardium may be less able to recruit or retain target cells capable of contributing either directly or through paracrine mechanisms to myocardial protection or repair 39. Although direct comparisons of myocardial response to cell transplantation in male and female subjects remain to be undertaken, preliminary data suggest that important differences may exist between the sexes in terms of stem cell survival and phenotype 40-42.
It is interesting to note that the changes observed in Akt signaling may be short lived, and that a shift may take place toward enhanced ERK signaling. It is possible that the duration of transplanted cell survival may be responsible for these changing patterns of signaling activity, which may further underscore the need to understand the differences between young, healthy experimental cohorts and their human clinical counterparts. Ongoing studies will examine this time course and its implication for optimization of cell transplantation protocols. Such optimization may include enhanced or prolonged survival of donor cells, with possible implications for the time course of concomitant signaling changes that can now be explored to help improve the likelihood of successful clinical translation of this exciting new therapeutic strategy.
Acknowledgements
This work was supported by National Institutes of Health, Grant numbers:1R01HL083118; 1K08HL079239 and American Heart Associstion, Grant number: 0465090Y to MJM.
Abbreviations
- RM
Remote myocardium
- MSC
Mesenchymal stem cell
- I/BZ
Infarct/Borderzone
- LAD
Left anterior descending
- FS
Fractional shortening
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure information: Nothing to disclose.
Presented at the American College of Surgeons 94th Annual Clinical Congress, San Francisco, CA, October 2008.
References
- 1.Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–6. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 2.Tomita S, Li RK, Weisel RD, et al. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100:II247–56. doi: 10.1161/01.cir.100.suppl_2.ii-247. [DOI] [PubMed] [Google Scholar]
- 3.Kamihata H, Matsubara H, Nishiue T, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–52. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 4.Levenberg S, Golub JS, Amit M, et al. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:4391. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyahara Y, Nagaya N, Kataoka M, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–65. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 6.Behfar A, Zingman LV, Hodgson DM, et al. Stem cell differentiation requires a paracrine pathway in the heart. FASEB J. 2002;16:1558–66. doi: 10.1096/fj.02-0072com. [DOI] [PubMed] [Google Scholar]
- 7.Dai W, Hale SL, Martin BJ, et al. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112:214–23. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 8.Kofidis T, de Bruin JL, Yamane T, et al. Stimulation of paracrine pathways with growth factors enhances embryonic stem cell engraftment and host-specific differentiation in the heart after ischemic myocardial injury. Circulation. 2005;111:2486–93. doi: 10.1161/01.CIR.0000165063.09283.A8. [DOI] [PubMed] [Google Scholar]
- 9.Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–8. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 10.Niagara MI, Haider HKh, Jiang S, et al. Pharmacologically preconditioned skeletal myoblasts are resistant to oxidative stress and promote angiomyogenesis via release of paracrine factors in the infarcted heart. Circ Res. 2007;100:545–55. doi: 10.1161/01.RES.0000258460.41160.ef. [DOI] [PubMed] [Google Scholar]
- 11.Burchfield J, Iwasaki M, Koyanagi M, Urbich C, Rosenthal N, Zeiher AM, Dimmeler S. Interleukin-10 from transplanted bone marrow mononuclear cells contributes to cardiac protection following myocardial infarction. Circ Res. 2008;103:203–211. doi: 10.1161/CIRCRESAHA.108.178475. [DOI] [PubMed] [Google Scholar]
- 12.Baines CP, Molkentin JD. STRESS signaling pathways that modulate cardiac myocyte apoptosis. J Mol Cell Cardiol. 2005;38:47–62. doi: 10.1016/j.yjmcc.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Morissette MR, Rosenzweig A. Targeting survival signaling in heart failure. Curr Opin Pharmacol. 2005;5:165–70. doi: 10.1016/j.coph.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Takemura G, Fujiwara H. Morphological aspects of apoptosis in heart diseases. J Cell Mol Med. 2006;10:56–75. doi: 10.1111/j.1582-4934.2006.tb00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMullen JR, Shioi T, Zhang L, et al. Phosphoinositide 3-kinase(p110{alpha}) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100:12355–60. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bueno OF, De Windt LJ, Tymitz KM, et al. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19:6341–50. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiojima I, Sato K, Izumiya Y, et al. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:210–18. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doukas J, Wrasidlo W, Noronha G, et al. Phosphoinositide 3-kinase gamma/delta inhibition limits infarct size after myocardial ischemia/reperfusion injury. Proc Natl Acad Sci USA. 2006;103:19866–71. doi: 10.1073/pnas.0606956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Huang S, Sah VP, et al. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem. 1998;273:2161–8. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- 20.Liao P, Georgakopoulos D, Kovacs A, et al. The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy. Proc Natl Acad Sci USA. 2001;98(12):283–8. doi: 10.1073/pnas.211086598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S, Weinheimer C, Courtois M, et al. The role of the Grb2-p38 MAPK signaling pathway in cardiac hypertrophy and fibrosis. J Clin Invest. 2003;111:833–41. doi: 10.1172/JCI16290. AJ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazhari R, Hare JM. Mechanisms of action of mesenchymal stem cells in cardiac repair: potential influences on the cardiac stem cell niche. Nat Clin Pract Cardiovasc Med. 2007;4:S21–6. doi: 10.1038/ncpcardio0770. [DOI] [PubMed] [Google Scholar]
- 23.Balsam LB, Wagers AJ, Christensen JL, et al. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–73. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 24.Murry CE, Soonpaa JH, Reinecke H, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–8. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 25.Peister A, Mellad JA, Larson BL, et al. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–8. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 26.Tang YL, Zhao Q, Zhang YC, Cheng L, Liu M, Shi J, Yang YZ, Pan C, Ge J, Phillips MI. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept. 2004;117:3–10. doi: 10.1016/j.regpep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Foncea R, Galvez A, Perez V, Morales MP, Calixto A, Melendez J, Gonzalez-Jara F, Diaz-Araya G, Sapag-Hagar M, Sugden PH, LeRoith D, Lavandero S. Extracellular regulated kinase, but not protein kinase C, is an antiapoptotic signal of insulin-like growth factor-1 on cultured cardiac myocytes. Biochem Biophys Res Commun. 2000;273:736–44. doi: 10.1006/bbrc.2000.3008. [DOI] [PubMed] [Google Scholar]
- 28.Rubart M, Pasumarthi KB, Nakajima H, Soonpaa MH, Nakajima HO, Field LJ. Physiological coupling of donor and host cardiomyocytes after cellular transplantation. Circ.Res. 2003;92:1217–24. doi: 10.1161/01.RES.0000075089.39335.8C. [DOI] [PubMed] [Google Scholar]
- 29.Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, Dzau VJ, Pratt RE. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006;14:840–50. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Andrade J, Lam JT, Zamora M, Huang C, Franco D, Sevilla N, Gruber PJ, Lu JT, Ruiz-Lozano P. Predominant fusion of bone marrow-derived cardiomyocytes. Cardiovasc Res. 2005;68:387–93. doi: 10.1016/j.cardiores.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Rodrigo MC, Swigart PM, Myagmar B-E, Cha J, Zhu B-Q, Yeh C-C, Karliner JS, Mann MJ, Simpson PC. Beta-blockers switch catecholamine activation of MAPKs from Maladaptive beta-adrenergic-p38 to adaptive alpha-1-adrenergic-ERK. Circulation. 2006;114:II–101. [Google Scholar]
- 32.Spyridopoulos I, Erben Y, Brummendorf TH, Haendeler J, Dietz K, Seeger F, Kissel C, Martin H, Hoffmann J, Assmus B, Zeiher AM, Dimmeler S. Telomere gap between granulocytes and lymphocytes is a determinant for haematopoetic progenitor cell impairment in patients with previous myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28:968–74. doi: 10.1161/ATVBAHA.107.160846. [DOI] [PubMed] [Google Scholar]
- 33.Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, Cheng T, DePinho RA, Sharpless NE, Scadden DT. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–6. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 34.Urbanek K, Torella D, Sheikh F, De Angelis A, Nurzynska D, Silvestri F, Beltrami CA, Bussani R, Beltrami AP, Quaini F, Bolli R, Leri A, Kajstura J, Anversa P. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci U S A. 2005;102:8692–8697. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Weel V, Seghers L, de Vries MR, Kuiper EJ, Schlingemann RO, Bajema IM, Lindeman JH, Delis-van Diemen PM, van Hinsbergh VW, van Bockel JH, Quax PH. Expression of vascular endothelial growth factor, stromal cell-derived factor-1, and CXCR4 in human limb muscle with acute and chronic ischemia. Arterioscler Thromb Vasc Biol. 2007;27:1426–32. doi: 10.1161/ATVBAHA.107.139642. [DOI] [PubMed] [Google Scholar]
- 36.Chang EI, Loh SA, Ceradini DJ, Lin SE, Bastidas N, Aarabi S, Chan DA, Freedman ML, Giaccia AJ, Gurtner GC. Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia. Circulation. 2007;116:2818–29. doi: 10.1161/CIRCULATIONAHA.107.715847. [DOI] [PubMed] [Google Scholar]
- 37.Kissel CK, Lehmann R, Assmus B, Aicher A, Honold J, Fischer-Rasokat U, Heeschen C, Spyridopoulos I, Dimmeler S, Zeiher AM. Selective functional exhaustion of hematopoietic progenitor cells in the bone marrow of patients with postinfarction heart failure. J. Am. Coll. Cardiol. 2007;49:2341–9. doi: 10.1016/j.jacc.2007.01.095. [DOI] [PubMed] [Google Scholar]
- 38.Tokalov SV, Gruner S, Schindler S, Wolf G, Baumann M, Abolmaali N. Age-related changes in the frequency of mesenchymal stem cells in the bone marrow of rats. Stem Cells Dev. 2007a;16:439–46. doi: 10.1089/scd.2006.0078. [DOI] [PubMed] [Google Scholar]
- 39.Dimmeler S, Leri A. Aging and disease as modifiers of efficacy of cell therapy. Circ Res. 2008;102:1319–30. doi: 10.1161/CIRCRESAHA.108.175943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfeiffer P, Müller P, Kazakov A, Kindermann I, Böhm M. Time-dependent cardiac chimerism in gender-mismatched heart transplantation patients. J Am Coll Cardiol. 2006;48:843–5. doi: 10.1016/j.jacc.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 41.Ogawa R, Mizuno H, Watanabe A, Migita M, Hyakusoku H, Shimada T. Adipogenic differentiation by adipose-derived stem cells harvested from GFP transgenic mice-including relationship of sex differences. Biochem Biophys Res Commun. 2004;319:511–7. doi: 10.1016/j.bbrc.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 42.Guerra S, Leri A, Wang X, Finato N, Di Loreto C, Beltrami CA, Kajstura J, Anversa P. Myocyte death in the failing human heart is gender dependent. Circ Res. 1999;85:856–66. doi: 10.1161/01.res.85.9.856. [DOI] [PubMed] [Google Scholar]