Abstract
Objectives
Patients undergoing lower extremity bypass are at high risk for surgical site infections (SSI). We examine lower extremity bypasses by graft origin and body mass index (BMI) classification to analyze differences in postoperative mortality and SSI occurrence.
Methods
The 2005-2007 National Surgical Quality Improvement Program (NSQIP), a multi-institutional risk-adjusted database, was queried to compare perioperative mortality (30-day), overall morbidity, and SSIs after lower extremity arterial bypass for peripheral arterial disease. Bypass was stratified by graft origin as aorto-iliac, femoral, or popliteal. Patient demographics, comorbidities, operative, and post-operative occurrences were analyzed.
Results
There were 7,595 bypasses performed (1,596 aorto-iliac, 5,483 femoral, and 516 popliteal origin). Mortality was similar regardless of bypass origin (2.8%, 2.4%, & 2.7%, P=.57). Surgical site infections occurred in 11% of overall cases (10%, 11%, & 11%, P=.47). Graft failure was significantly associated with postoperative SSI occurrence (OR 2.4, 95%CI 1.9-3.1, P<.001) as was postoperative sepsis (OR 6.5, 95%CI 5.1-8.3, P<.001). Independent predictors of mortality were age, aorto-iliac bypass origin, underweight, normal weight, or morbid obesity (compared to overweight and obese), end stage renal disease, poor preoperative functional status, preoperative sepsis, chronic obstructive pulmonary disease, hypoalbuminemia, and cardiac disease. Independent predictors of SSI were obesity, diabetes, poor preoperative functional status, a history of smoking, and female gender.
Conclusions
Surgical site infections occur frequently after lower extremity bypass regardless of bypass origin and are associated with early graft failure and sepsis. Obesity predicts postoperative SSI. Mortality risk was greatest in the underweight followed by morbidly obese and normal weight patients, while overweight and mild-moderate obesity were associated with the lowest mortality.
Introduction
The incidence of obesity within the United States is currently >25% and has been growing substantially over recent years.1 Studies within general surgery literature have been mixed with regards to outcomes associated with obesity. Some have shown an increased risk of mortality for obese patients while others fail to find this link and some even find that the underweight patients fare the worst. For example, a recent National Surgical Quality Improvement Program (NSQIP) analysis found, within abdominal oncologic operations that underweight patients were at the greatest mortality risk but that obesity was predictive of surgical site infections.2
Within vascular literature, studies that analyze the impact of obesity are generally limited to institutional data.3-7 In a series of 300 LE bypass procedures within our institution, we found that short and long term mortality, patency, and limb salvage were similar, but postoperative surgical site infection (SSI) was greater with obesity.3 Within other institutional studies, two found obesity to not be a predictor of wound infection, another found a strong relationship, and one found the relationship to be limited to distal bypass wounds only.4-7
Related to this, SSIs have been shown to increase length of stay, add to the overall cost of a hospitalization and postoperative care, and decrease postoperative quality of life.3,4,7-9 Within lower extremity bypasses, SSIs may be common due to the presence of a groin wound, a large vein harvest wound, or from contamination of a distal incision from a pre-existing ulceration or gangrenous infection. The impact of SSIs upon mortality and major morbidity is variable.3,4,7,8 The Prevent III trial investigators found wound complications to impact mortality and amputation, however other studies have not found a relationship to mortality.3,7,8
We sought to further investigate the impact of obesity and the occurrence and effects of SSI on lower extremity bypass surgery using the large cohort available within the NSQIP. This database allows us to analyze weight classifications by body mass index (BMI) class and has reliable postoperative SSI occurrence data collected by a trained nurse clinician reviewer.
Methods
ACS-NSQIP
We analyzed data within the NSQIP database from 2005-2007. This database is a multi-institutional national, prospectively collected database created to allow quality control review of outcomes. Originally developed for VA centers, it was expanded to the private sector in a pilot study of 14 academic medical centers. The pilot study concluded in 2004 and enrollment was expanded beginning in Oct of 2004. Since then the program has steadily grown to currently include 227 participating community and academic medical centers across the US. Dependent upon its overall volume, each center contributes all or a portion of operative cases performed, with selection occurring in a rotating manner to ensure random representation of procedures and outcomes. Clinical nurse reviewers are specially trained for data review and recording patient information. Primary and concurrent procedures are recorded by Current Procedural Terminology,4th edition (CPT-4) coding. A comprehensive list of preoperative comorbidity data is collected along with operative and perioperative information. 30-day postoperative outcomes including post-hospitalization information is collected from hospital course, clinic visits, and/or followup phone contact.
Data
The NSQIP database was queried using SAS statistical software (Version 9.1, SAS Institute Inc., Cary, NC). All lower extremity bypass procedures within the dataset were identified by CPT coding for the primary procedure. These included aorto-femoral bypasses, femoral-femoral, femoral-popliteal, femoral-infrapopliteal, and popliteal-infrapopliteal bypasses. Cohorts were divided by bypass origin as aortic, femoral, or popliteal to allow stratification of patients with and without groin wounds. Additionally, the bypass type as prosthetic graft or vein graft was identified by CPT coding. Concurrent secondary bypass procedures were identified as well. Patients with a primary International Classification of Diseases, 9th edition (ICD-9) diagnosis of abdominal or thoracic aortic aneurysm or aortic dissection (441) were excluded.
Preoperative demographic and comorbidity variables were recorded for each patient. BMI classification was assigned based upon NIH definitions of underweight (BMI ≤18.6 kg/m2), normal weight (18.7-25 kg/m2), overweight (25.1-30 kg/m2), obese class I (30.1-35 kg/m2), obese class II (35.1-40 kg/m2), and obese class III (>40 kg/m2). Obesity was defined as obese class I through III and morbid obesity was defined as obese class III. Preoperative functional status was defined as being independent versus partially or completely dependent in activities of daily living. Preoperative laboratory values of interest were noted including white blood cell count, albumin, and creatinine. Intraoperative variables included blood transfusion volume (units) and operative time (hours). Concurrent major (above the ankle) and minor (below the ankle) amputations were recorded as well. Thirty day postoperative morbidity included wound infection (superficial, deep, or organ space infection), wound dehiscence, graft failure requiring intervention or revision, and 13 other NSQIP-defined complications. Patients requiring a return to the operating room for a major surgical procedure were also recorded. Other postoperative outcomes include 30 day mortality and length of stay.
Statistical Analysis
Statistical analysis was performed using STATA statistical software (College Station, TX: StataCorp LP). Statistical significance was defined as P <.05. Demographics, comorbidities, and perioperative events and outcomes were compared between bypass origin cohorts as well as between obese and non-obese patients. Categorical variables were analyzed using the Chi-square test and continuous variables were compared using Student's T-test or ANOVA for parametric data and Wilcoxon rank-sum or Kruskal Wallis test for nonparametric data. Preoperative predictors of SSI, overall morbidity, and mortality were analyzed by univariate and multivariate logistic regression.
Results
There were at total of 7,595 lower extremity bypasses performed. The majority of these were femoral in origin. A total of 1,596 were aorto-iliac origin, 5,483 were femoral origin, and 516 were popliteal origin. Concurrent bypasses were performed in 5% of the aorto-iliac origin, 1% of femoral origin, and 3% of popliteal origin patients. The groups had significant differences with regard to age, gender, race, comorbidities, and preoperative laboratory values (Table I). Age, male gender, and black race all increased with more distal bypasses.
Table 1.
Demographics and comorbidities of patients undergoing lower extremity bypass within the National Surgical Quality Improvement Program 2005-2007.
| Aortic N= 1,596 |
Femoral N= 5,483 |
Popliteal N= 516 |
P-value | |
|---|---|---|---|---|
| Age, mean ± SD, y | 61 +/- 11 | 68 +/- 12 | 69 +/- 14 | < .001 |
| Male | 58% | 63% | 68% | < .001 |
| White Race | 86% | 79% | 73% | < .001 |
| Black Race | 10% | 17% | 22% | < .001 |
| BMI Classification (kg/m2) | ||||
| Underweight (< 18.6) | 5.4% | 4.2% | 2.9% | < .05 |
| Normal Weight (18.6-25) | 38.9% | 33.9% | 30.2% | < .001 |
| Overweight (25-30) | 32.1% | 33.3% | 33.5% | .63 |
| Obese I (30-35) | 15.6% | 16.3% | 20.2% | < .05 |
| Obese II (35-40) | 4.0% | 6.6% | 7.2% | < .01 |
| Morbid Obesity (>40) | 4.0% | 5.7% | 6.0% | < .05 |
| Comorbidities | ||||
| Prior Revascularization or Amputation | 38% | 56% | 58% | < .001 |
| Chronic Renal Failure | 2% | 7% | 14% | < .001 |
| Cardiac Disease | 30% | 40% | 43% | < .001 |
| Recent Myocardial Infarction | 2% | 2% | 2% | .94 |
| Congestive Heart Failure | 1% | 3% | 4% | > .001 |
| Hypertension on Medication | 76% | 83% | 82% | < .001 |
| Chronic Pulmonary Disease | 13% | 14% | 7% | < .05 |
| Diabetes | 20% | 41% | 62% | < .001 |
| Cerebrovascular Disease | 15% | 19% | 17% | < .01 |
| Obesity (BMI > 30kg/m2) | 24% | 29% | 33% | < .001 |
| Current Smoker | 66% | 45% | 23% | < .001 |
| Ever Smoker | 90% | 75% | 48% | < .001 |
| Pack Years, mean ± SD | 46 ±34 | 38 ±36 | 21 ±29 | < .001 |
| Chronic Steroid Use | 2% | 4% | 7% | < .001 |
| Prosthetic Graft | 88% | 41% | 9% | < .001 |
| Concurrent Amputation-TMA or Digit | 0.3% | 3% | 10% | < .001 |
| Symptoms | ||||
| Gangrene/Wound/Rest Pain | 42% | 62% | 82% | < .001 |
| Pre-existing Open Wound | 16% | 35% | 58% | < .001 |
| Preoperative Sepsis | 5% | 7% | 11% | < .001 |
| Laboratory Values | ||||
| Albumin, mean ± SD, g/dl | 3.9 ± 0.6 | 3.6 ±0.7 | 3.3 ±0.7 | < .01 |
| Creatinine, median, range, mg/dl | 0.9, 0.1-10.0 | 1.1, 0.2-14.9 | 1.2, 0.4-11.8 | < .001 |
| White Blood Cell, mean ± SD, K/uL | 8.5 ± 2.8 | 8.5 ±3.0 | 8.7 ±3.3 | < .001 |
| Predicted Mortality | 2.3% ± 5.4 | 3.1% ± 5.6 | 3.9% ± 5.4 | < .05 |
| Predicted Morbidity | 23.4% ± 13 | 22.0% ± 13 | 25.6% ± 13 | .35 |
Comorbidities are listed in Table I. Hypertension, cardiac disease, and diabetes were the most common comorbid conditions. Diabetes and chronic renal disease increase with more distal bypass origin. A history of peripheral vascular disease with a prior revascularization or amputation was more frequent in the infrainguinal bypasses than aorto-iliac origin bypasses. Ninety percent of the aorto-iliac origin bypass patients were prior smokers with a mean number of pack years of 46. Two-thirds of them were current smokers. Smoking was also common with femoral and popliteal origin bypasses but less so than aorto-iliac origin grafts.
Nearly one-third of all patients were classified as obese and the proportion increased with more distal bypasses. Less than 5% of the entire group was classified as underweight (BMI< 18.6kg/m2) and slightly greater than 5% were classified as morbidly obese (BMI> 40kg/m2) (Table I).
Prosthetic grafts were, as expected, used most commonly in aorto-iliac origin bypasses and rarely for popliteal origin bypasses (Table I). Conversely, the proportion of patients with either gangrene, ulceration, or rest pain was highest in the more distal bypass origin cohorts as was the proportion of patients with a pre-existing open wound. Preoperative sepsis was also increased in infrainguinal bypasses, particularly popliteal origin. A concurrent transmetatarsal or digit amputation was performed in 10% of popliteal origin bypasses, 3% of femoral, and less than 1% of aorto-iliac origin bypasses. There were no significant differences between amputations for obese (3.9%) versus non-obese (3.3%) patients (P =.68), however morbidly obese patients had a significantly higher rate of concurrent amputations (5.6% vs 3.1% non-morbidly obese, P< .01).
Predicted mortality and morbidity estimates provide an assessment of overall fitness based upon previously identified predictors of mortality and morbidity for vascular and general surgical procedures. The mean predicted mortality increases in a linear fashion with more distal origin bypasses (Table I). Predicted morbidity across bypass types is not significantly different. Compared to non-obese patients, obese patients have a lower mean predicted mortality (2.6% vs 3.2%, P< .001) but a similar morbidity (23.0% vs 22.4%, P < .05).
Intraoperative Outcomes
Mean operative time was 3.9 hours ± 1.8. Operation length by origin was 4.4 ± 1.8 for aorto-iliac, 3.7 ± 1.8 for femoral, and 4.1 ± 1.6 for popliteal (P < .001). Operation length increased incrementally with BMI classification (Table II).
Table II.
Operative time stratified by body mass index classification for lower extremity bypasses within the National Surgical Quality Improvement Program 2005-2007.
| Operative Length (Hours, mean +/- SD) |
|
|---|---|
| BMI Classification (kg/m2) | |
| Underweight (< 18.6) | 3.6 +/- 1.8 |
| Normal Weight (18.6-25) | 3.8 +/- 1.8 |
| Overweight (25-30) | 3.9 +/- 1.8 |
| Obese I (30-35) | 4.0 +/- 1.8 |
| Obese II (35-40) | 4.0 +/- 1.8 |
| Morbid Obesity (>40) | 4.2 +/- 1.8 |
Twenty-five percent of patients received intraoperative blood transfusions. The majority of transfusions were within aorto-iliac origin bypasses (46%) compared to femoral (18%), and popliteal (22%) (P< .001).
Perioperative Outcomes
Mortality was 2.5% and did not significantly differ by bypass origin. Overall morbidity was lowest after femoral origin bypasses (25%) compared to aorto-iliac (29%) and popliteal (29%) bypasses. Surgical site infections were seen in 11% of all patients. Graft failure requiring revision was more common in infrainguinal bypasses and occurred in 6% of overall cases (Table IIIA). Eighteen percent of patients required a subsequent major operation within 30 days, however no detailed surgical information is available for these procedures.
Table III.
Mortality and complications of patients undergoing lower extremity bypass within the National Surgical Quality Improvement Program 2005-2007. A) By bypass graft origin. B) By non-obese patients versus obese patients.
| Aortic | Femoral | Popliteal | P-value | |
|---|---|---|---|---|
| Mortality | 2.8% | 2.4% | 2.7% | .57 |
| Morbidity Overall | 29% | 25% | 29% | < .01 |
| Surgical Site Infection | 10% | 11% | 11% | .47 |
| Wound Dehiscence | 2% | 1% | 2% | .12 |
| Graft Failure | 3% | 7% | 8% | < .01 |
| Sepsis | 6% | 4% | 5% | < .01 |
| Septic Shock | 6% | 2% | 1% | < .001 |
| Return to OR | 14% | 18% | 27% | < .01 |
| Non-Obese BMI<30 | Obese BMI≥30 | P-value | ||
| Mortality | 2.9% | 1.6% | < .01 | |
| Morbidity Overall | 24% | 31% | < .001 | |
| Surgical Site Infection | 9% | 16% | < .001 | |
| Wound Dehiscence | 1% | 3% | < .001 | |
| Graft Failure | 5% | 7% | < .01 | |
| Sepsis | 4% | 4% | .87 | |
| Septic Shock | 3% | 3% | .45 | |
| Return to OR | 17% | 19% | .051 | |
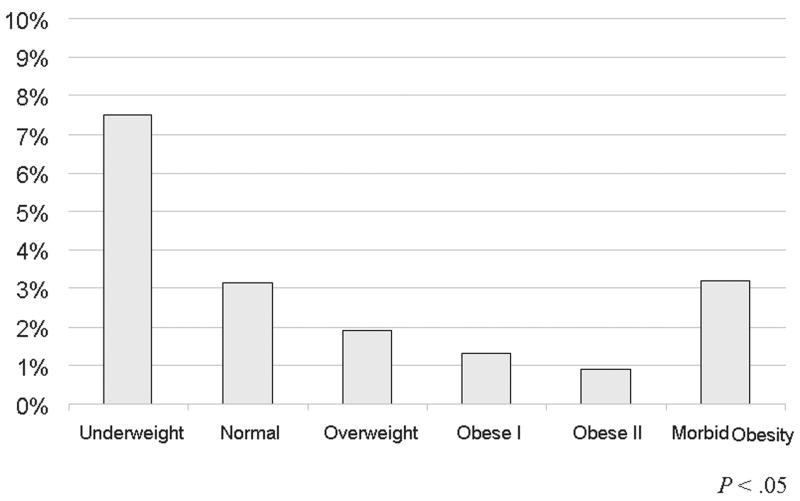
Looking at outcomes stratified by obesity, mortality was higher in the non-obese cohort (BMI< 30kg/m2) than the obese cohort (BMI> 30kg/m2) (Table IIIB). A more appropriate way of looking at mortality however, as evident by Figure 1A, is to look at mortality stratified by BMI class. The underweight group has the highest mortality, followed by morbidly obese patients and normal weight patients. Overweight, mildly obese and moderately obese patients have a lower mortality comparatively.
Figure 1.
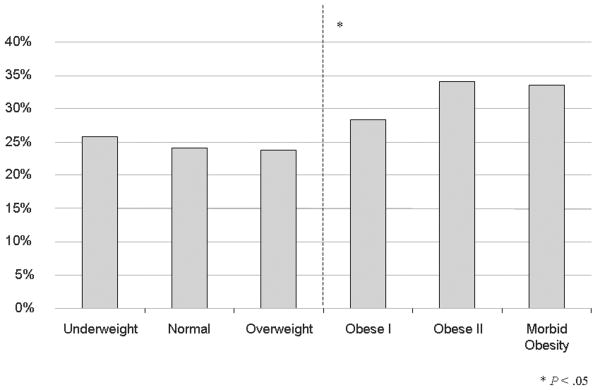
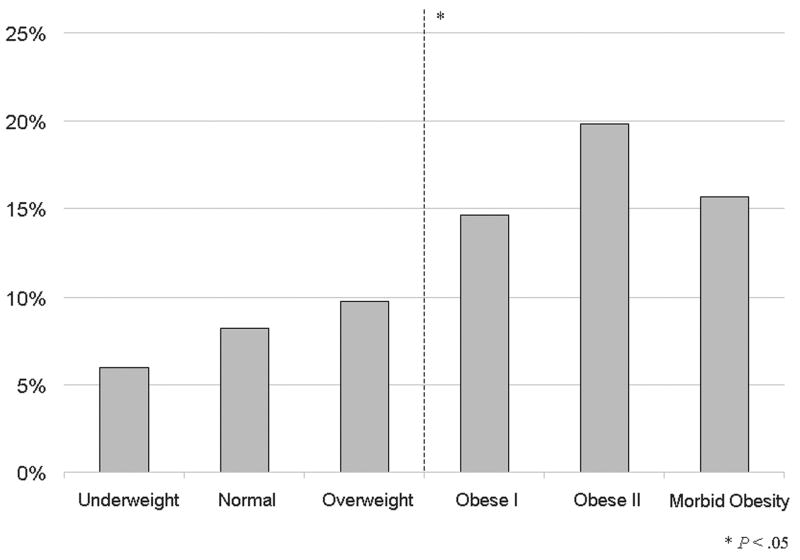
Outcomes by body mass index classification of patients undergoing lower extremity bypass within the National Surgical Quality Improvement Program 2005-2007. A) Mortality B) Morbidity C) Surgical site infections.
For other outcomes including overall morbidity, wound infection, and graft failure, obese patients have a higher proportion (Table IIIB). Stratifying morbidity by BMI classes does not change the dichotomous outcome of morbidity or SSI incidence (Figure 1B & 1C). Obesity nearly doubles the incidence of SSI.
On univariate analysis, SSIs were not associated with an increased incidence of mortality (OR 0.7; 95%CI 0.4-1.2, P=.21), however they were associated with an increased incidence of graft failure requiring intervention or revision (2.4; 1.9-3.1, P< .001), subsequent major operation (3.7; 3.1-4.3, P< .001), sepsis (6.5; 5.1-8.3, P< .001), and septic shock (2.4; 1.8-3.4, P< .001).
Median length of stay was significantly longer if a patient was diagnosed with an SSI (8 vs. 6 days, P< .001).
Predictors of Mortality
On multivariate analysis, significant predictors of mortality were age and the weight categories seen to have increased mortality previously (Table IV). Underweight patients had a 3 ½ fold increased of death while morbidly obese patients had a 2-fold risk and normal weight patients had a 70% increased risk. This is compared to a referent group containing overweight, mild and moderate obese patients. Aorto-iliac bypass origin, while not a significant factor when looking at mortality by bypass origin prior to adjustment for the different cohort characteristics, was a significant predictor on multivariate analysis. Additionally, the comorbidities of end stage renal disease, poor preoperative functional status, preoperative sepsis, COPD, hypoalbuminemia, and cardiac disease were also predictive of mortality. Gender was not a significant predictor.
Table IV.
Multivariate predictors of mortality, surgical site infection, and morbidity after lower extremity bypass within the National Surgical Quality Improvement Program 2005-2007. *Age adjusted
| Predictors of Mortality After Lower Extremity Bypass | |||
|---|---|---|---|
| OR | 95% CI | P Value | |
| Age (per decade) | 1.3 | 1.2-1.4 | < .001 |
| Aorto-Iliac Bypass Origin | 2.3 | 1.6-3.4 | < .001 |
| Underweight | 3.5 | 2.1-5.9 | < .001 |
| Normal Weight | 1.7 | 1.2-2.4 | < .01 |
| Morbid Obesity | 1.9 | 1.01-3.6 | < .05 |
| End Stage Renal Disease | 4.8 | 3.3-7.0 | < .001 |
| Poor Functional Status | 2.4 | 1.7-3.4 | < .001 |
| Preoperative Sepsis | 2.1 | 1.4-3.2 | < .001 |
| COPD | 1.8 | 1.2-2.5 | < .01 |
| Albumin < 3.5g/dl | 1.7 | 1.2-2.3 | < .01 |
| Cardiac Disease | 1.4 | 1.02-1.9 | < .05 |
| Predictors of SSI After Lower Extremity Bypass* | |||
| Obesity | 1.7 | 1.4-2.1 | < .001 |
| Diabetes | 1.5 | 1.2-1.8 | < .001 |
| Poor Functional Status | 1.5 | 1.2-1.8 | < .001 |
| Ever Smoker | 1.4 | 1.1-1.7 | < .01 |
| Female Gender | 1.3 | 1.1-1.6 | < .01 |
| Predictors of Morbidity After Lower Extremity Bypass* | |||
| Preoperative Sepsis | 2.0 | 1.6-2.5 | < .001 |
| Poor Functional Status | 1.9 | 1.6-2.2 | < .001 |
| Aorto-Iliac Bypass Origin | 1.7 | 1.4-2.0 | < .001 |
| Obesity | 1.4 | 1.2-1.6 | < .001 |
| Female Gender | 1.3 | 1.2-1.5 | < .001 |
| Cardiac Disease | 1.3 | 1.1-1.5 | <. 001 |
| COPD | 1.3 | 1.1-1.5 | < .01 |
| Gangrene/Wound/Rest Pain | 1.3 | 1.1-1.5 | < .001 |
| Vein Graft (vs. Prosthetic) | 1.2 | 1.1-1.4 | < .01 |
| Cerebrovascular Disease | 1.2 | 1.1-1.4 | < .01 |
| Albumin < 3.5g/dl | 1.2 | 1.02-1.4 | < .05 |
| Ever Smoker | 1.2 | 1.03-1.4 | < .05 |
Predictors of SSI
Multivariate age-adjusted predictors of SSI were obesity, diabetes, poor functional status, smoking, and female gender (Table IV). Obesity was the strongest predictor with a 70% increased risk. Neither bypass graft material nor having a groin incision (as you would see in either the aorto-iliac origin or femoral cohorts) was a predictive factor for SSI.
Current smoking was not a significant predictor of SSI on univariate or multivariate analysis however patients who had ever smoked had a 40% increase. Presentation with symptoms of critical limb ischemia (CLI-gangrene/open wound/rest pain) was a predictor of SSI on univariate analysis (OR 1.4, 95%CI 1.2-1.6, P< .001) however when a history of smoking was included, CLI was no longer statistically significant (OR 1.2, 95%CI 1.0-1.4, P=.09). Individually, having an open wound was also predictive of SSI on univariate analysis (OR 1.2, 95%CI 1.1-1.4, P< .001) but also lost significance after adjustment for other preoperative variables (OR 1.0, 95%CI 0.8-1.2, P=.79). Decreased albumin, leukocytosis, and increased creatinine and/or end stage renal disease did not predict SSI.
Given the variation for operation length with BMI classification identified within this study, this intraoperative variable was tested in the multivariate model to confirm obesity as a predictive factor. When operation length (hours) (OR 1.1; 95%CI 1.1-1.2, P< .001) was included in the model for SSI, the odds ratios and the statistical significance of the other predictive factors were unchanged. Similarly, the need for an intraoperative blood transfusion was associated with SSI occurrence on univariate regression analysis (OR 1.4, 95%CI 1.2-1.6, P < .001). When included in the multivariate analysis it remained significant (OR 1.3, 95%CI 1.1-1.6, P < .01) and other variables including obesity remained unchanged.
Predictors of Morbidity
Multivariate age-adjusted predictors of overall morbidity are also presented in Table IV. Preoperative sepsis and poor preoperative functional status were the strongest predictors of morbidity. Obesity conferred a 40% and female gender a 30% increased risk of morbidity. Presentation with gangrene, an open wound, or rest pain as well as having a vein versus a prosthetic graft was also associated with greater morbidity along with a number of other comorbidities. End stage renal disease was a predictor of morbidity on univariate analysis (OR 1.7, 95%CI 1.4-2.0, P< .001) however it lost significance after adjustment for other characteristics.
Discussion
Surgical site infections occur frequently after lower extremity bypass regardless of bypass origin and are associated with early graft failure, need for a subsequent operation, sepsis, and an increased length of stay. All obesity classes (BMI> 30kg/m2) were predictive of SSI even after adjusting for operation length and as well were predictive of overall morbidity. Conversely, we found that mortality has a parabolic distribution with BMI class and surprisingly, patients who were overweight, mild, or moderately obese had the lowest risk of mortality. These findings all have important clinical implications for patient selection, procedural thresholds, and perioperative management.
Obesity has not uniformly been associated with wound complications within vascular surgical literature. This may in part be to smaller sample sizes as most studies that have looked at this have been limited to institutional data. Lee et al. reported a 20 year series including nearly 1,000 cases of infrainguinal bypass procedures and found a SSI rate of 13%. Only obesity (OR 2.6, 95%CI 1.4-4.9) and postoperative incisional hematoma (OR 6.4, 95%CI 3.0-14) were associated with an increased risk.6 Conversely, in smaller series by Chang et al. (335 infrainguinal bypasses) and Nam et al. (250 infrainguinal bypasses), obesity was not found to be predictive of SSIs.5,7 Within a series of 281 cases from our own institution, where we have a high population of diabetics (72%), SSI incidence was 9.6% with obesity conferring a 2.4 fold increased risk.3
The cost of SSIs is one substantial matter that has fueled recent examination of the nationwide problem.4,9 SSI incidence additionally may effect procedural outcomes such as bypass graft failure, limb salvage, and ischemic wound complications as well as sepsis rates and return to functional health.7, 8 SSIs have been shown to be a complication in over 10% of most reported series of lower extremity bypass.3,4,6-10 A complication in 11% of the current study of over 7,000 cases, this represents a significant number of patients. This is a higher incidence than in most general surgical procedures and other vascular procedures.11-13 As we have demonstrated in the current analysis, patients with peripheral vascular disease often have many predisposing factors for infection such as diabetes and obesity and also frequently have pre-existing open wounds prior to vascular procedures. Presentation with symptoms of critical limb ischemia, including tissue loss, was not a significant predictor of SSI after adjustment for other factors, however, it was predictive of overall morbidity. The level of bypass, regardless of groin wound presence, did not affect postoperative SSI rate. Over 90% of the patients fell into this category, however, and the effect was likely tempered by the higher rates of critical limb ischemia for distal-only bypasses.
In prior analyses of general and orthopedic procedures, current smoking has been shown to increase wound infection rates and some studies have shown that cessation of smoking prior to surgery could decrease this complication.14,15 We found, however, that a history of smoking but not specifically current smokers had an increased risk of SSI and overall morbidity. Additionally, unlike in prior analyses, patients with decreased preoperative albumin levels did not have higher SSI rates.11 Preoperative leukocytosis, increased creatinine and/or end stage renal failure were also not predictive of SSI occurrence however they did have an increased risk of mortality.
Appropriate selection and timely administration of antibiotics is important within this population to prevent postoperative SSI. Chang et al. cited an 8.1% failure rate for antibiotic prophylaxis despite having an established protocol in place for vascular cases.5 In the high risk population identified in this study, wound prophylaxis becomes even more important. Evidence based practice of sterile preoperative prep such as clipping versus shaving for hair removal is important as well.16
Operative time and intraoperative blood transfusions were both associated with SSI occurrence, however, given that these are intraoperative variables and a causal relationship cannot be implied, they were not included in the final multivariate model.
Cardiac literature has shown that endoscopic vein harvesting decreases the incidence of wound infections compared to traditional open saphenous vein harvesting with a particular benefit found for diabetic and obese patients.17 A similar theory of avoidance of surgical incisions can be used to support the use of endovascular interventions as a primary modality in patients at high risk for SSI when lesion characteristics allow. Particularly given the relationship found with graft failure and sepsis, avoidance of SSI after lower extremity bypass has implications even greater than its economic impact. Endovascular interventions have been shown in the BASIL trial to have a lower perioperative wound infection incidence than open bypass.18
Obesity was also found to have an association with mortality in the current analysis, however the relationship was not a simple dichotomous outcome. In prior analyses, a significant impact of obesity upon survival has generally not been shown. Obesity did not have a significant association with perioperative or long term (1 and 3 year) survival within our prior analysis of institutional bypass procedures.3 Within the Prevent III trial however, wound complications were found to have an increase risk of mortality (OR 1.4, 95%CI 1.1-1.9). Wound complications within this study included not only infections, but also postoperative hematoma, necrosis, and seromas.8 Therefore this may not be a comparable sample.
As mentioned previously, based upon our current findings as well as previous general surgical literature indicating that underweight patients may be at increased risk of mortality, comparing non-obese to obese patients for this outcome may not be appropriate.2 Underweight patients may represent patients with chronic illness or poor nutritional status not otherwise characterized by other comorbid variables. Alternatively, weight may influence medical and surgical therapies and clinical decision making. Steinberg et al. reported an “obesity paradox” within patients hospitalized with cardiac disease. They suggested that a lower observed mortality in overweight and obese patients was attributable to the finding that patients with an increased BMI were more likely to receive guideline-recommended medical treatment and invasive management of coronary artery disease.19 We suggest that future studies that include obesity as a predictive variable stratify patients by BMI class rather than into two categories.
Limitations
The major limitations of this study are due to problems with the database and its specificity. The database was developed to be best utilized as a quality improvement tool in order to compare institutions' individual outcomes with a national benchmark. As such, the database is not entirely comprehensive with every patient characteristic, intraoperative variable, and postoperative occurrence possible in regards to lower extremity bypass grafts. With CPT coding, procedure specificity for the primary surgery is acceptable, however types of preoperative or postoperative interventions or surgical procedures are not provided. Return to the operating room, regardless of reason, has been cited as a useful quality indicator across a broad spectrum of general surgical procedures therefore, as a nonspecific measure of procedure success this is still a valid estimate when used with other outcome measurements.20 It would improve the analysis however if we were able to specify if any of these were revision procedures, amputations, wound debridements, or hematoma evacuations.
Additionally, the outcomes assessed are limited to 30 day events. Wound complications generally occur within this period, however they may develop beyond this time and would not be counted. Longer term graft failure and long term mortality are factors that we are unable to assess.
Conclusions
Obese patients are at high risk for morbidity including SSI following lower extremity bypass. Morbidly obese patients additionally are at increased risk of mortality along with underweight and normal weight patients whereas overweight patients, mild, and moderately obese patients have a lowered risk. Patients at high risk for either mortality or morbidity including SSIs should be considered for endovascular therapy when appropriate.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nationwide (States, DC, and Territories)- 2007. Behavioral Risk Factor Surveillance System Survey Data. Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2007. Overweight and obesity. Centers for Disease Control and Prevention (CDC) [Google Scholar]
- 2.Mullen JT, Davenport DL, Hutter MM, Hosokawa PW, Henderson WG, Khuri SF, et al. Impact of body mass index on perioperative outcomes in patients undergoing major intra-abdominal cancer surgery. Ann Surg Onc. 2008;15:2164–72. doi: 10.1245/s10434-008-9990-2. [DOI] [PubMed] [Google Scholar]
- 3.Patel VI, Hamdan AD, Schermerhorn ML, Hile C, Dahlberg S, Campbell DR, et al. Lower extremity arterial revascularization in obese patients. J Vasc Surg. 2007;46:738–42. doi: 10.1016/j.jvs.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 4.Kent KC, Bartek S, Kuntz KM, Anninos E, Skillman JJ. Prospective study of wound complications in continuous infrainguinal incisions after lower limb arterial reconstruction: incidence, risk factors, and cost. Surgery. 1996;119:378–83. doi: 10.1016/s0039-6060(96)80135-8. [DOI] [PubMed] [Google Scholar]
- 5.Chang JK, Calligaro KD, Ryan S, Runyan D, Dougherty MJ, Stern JJ. Risk factors associated with infection of lower extremity revascularization: analysis of 365 procedures performed at a teaching hospital. Ann Vasc Surg. 2003;17:91–6. doi: 10.1007/s10016-001-0337-8. [DOI] [PubMed] [Google Scholar]
- 6.Lee ES, Santilli SM, Olson MM, Kuskowski MA, Lee JT. Wound Infection After Infrainguinal Bypass Operations: Multivariate Analysis of Putative Risk Factors. Surgical Infections. 2000;1:257–263. doi: 10.1089/109629600750067183. [DOI] [PubMed] [Google Scholar]
- 7.Nam JH, Gahtan V, Roberts AB, Kerstein MD. Influence of Incisional Complications on Infrainguinal Vein Bypass Graft Outcome. Ann Vasc Surg. 1999;13:77–83. doi: 10.1007/s100169900224. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen LL, Brahmanandam S, Bandyk DF, Belkin M, Clowes AW, Moneta GL, et al. Female gender and oral anticoagulants are associated with wound complications in lower extremity vein bypass: an analysis of 1404 operations for critical limb ischemia. J Vasc Surg. 2007;46:1191–7. doi: 10.1016/j.jvs.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimick JB, Chen SL, Taheri PA, Henderson WG, Khuri SF, Campbell DA. Hospital costs associated with surgical complications: a report from the Private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199:531–7. doi: 10.1016/j.jamcollsurg.2004.05.276. [DOI] [PubMed] [Google Scholar]
- 10.Robison JG, Ross JP, Brothers TE, Elliot BM. Distal wound complications following pedal bypass: analysis of risk factors. Ann Vasc Surg. 1995;9:53–9. doi: 10.1007/BF02015317. [DOI] [PubMed] [Google Scholar]
- 11.Neumayer L, Hosokawa P, Itani K, El-Tamer M, Henderson WG, Khuri SF. Multivariable predictors of postoperative surgical site infection after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg. 2007;204:1178–87. doi: 10.1016/j.jamcollsurg.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Hutter MM, Lancaster RT, Henderson WG, Khuri SF, Mosca C, Johnson RG. Comparison of risk-adjusted 30-day postoperative mortality and morbidity in Department of Veterans Affairs hospitals and selected university medical centers: vascular surgical operations in men. J Am Coll Surg. 2007;204:1115–26. doi: 10.1016/j.jamcollsurg.2007.02.066. [DOI] [PubMed] [Google Scholar]
- 13.Johnson RG, Wittgen CM, Hutter MM, Henderson WG, Mosca C, Khuri SF. Comparison of risk-adjusted 30-day postoperative mortality and morbidity in Department of Veterans Affairs hospitals and selected university medical centers: vascular surgical operations in women. J Am Coll Surg. 2007;204:1137–46. doi: 10.1016/j.jamcollsurg.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 14.Sorenson LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg. 2003;238:1–5. doi: 10.1097/01.SLA.0000074980.39700.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moller AM, Villebro N, Pedersen T, Tonnesen H. Effect of preoperative smoking intervention on postoperative complicatations: a randomized clinical trial. Lancet. 2002;359:114–7. doi: 10.1016/S0140-6736(02)07369-5. [DOI] [PubMed] [Google Scholar]
- 16.Tanner J, Woodings D, Moncaster K. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD004122.pub3. CD004122. [DOI] [PubMed] [Google Scholar]
- 17.Carpino PA, Khabbaz KR, Bojar RM, Rastegar H, Warner KG, Murphy RE, et al. Clinical benefits of endoscopic vein harvesting in patients with risk factors for saphenectomy wound infections undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2000;119:69–75. doi: 10.1016/s0022-5223(00)70219-4. [DOI] [PubMed] [Google Scholar]
- 18.Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–34. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 19.Steinberg BA, Cannon CP, Hernandez AF, Pan W, Peterson ED, Fonarow GC. Medical therapies and invasive treatments for coronary artery disease by body mass: the “obesity paradox” in the Get With the Guidelines database. Am J Cardiol. 2007;100:1331–5. doi: 10.1016/j.amjcard.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 20.Birkmeyer JD, Hamby LS, Birkmeyer CM, Decker MV, Karon NM, Dow RW. Is unplanned return to the operating room a useful quality indicator in general surgery. Arch Surg. 2001;136:405–10. doi: 10.1001/archsurg.136.4.405. [DOI] [PubMed] [Google Scholar]