Abstract
Objective
To examine the trajectory of marital adjustment, satisfaction, and dissolution among 121 hematopoietic stem cell transplant (HSCT) couples – as a function of role (patient or spouse) and gender.
Methods
Prospective, longitudinal design. Participants completed the Dyadic Adjustment Scale at six time points: pre-transplant (4–7 days after beginning medical workup prior to starting transplant), 6 months post-transplant, and 1, 2, 3 and 5 years post-transplant. They also reported on marital status over time.
Results
Participants ranged in age from 23–68 (52% female patients and 48% female spouses). Ninety-eight patients received an allogeneic transplant; 23 received an autologous transplant. Marital dissolution was uncommon (4 divorces since the transplant among 55 participating 5-year survivors, 7%). Dyadic satisfaction was stable over time for both male and female patients and male spouses, but not for female spouses who reported reductions in satisfaction at all time points relative to baseline.
Conclusions
Couples were by and large resilient. However, caregiver-specific gender differences indicate an increased risk for relationship maladjustment and dissatisfaction among female spouses and ultimately, female spouse/ male patient couples.
Keywords: caregiver, marital satisfaction, gender, hematopoietic stem cell transplant, oncology, cancer
It is well-accepted that chronic illness and its treatment impact not just the patient but also the family caregiver [1]. This is particularly true when the primary caregiver is the patient’s spouse. Research from the broader caregiving literature suggests that partners, relative to other informal caregivers, devote more time to care, experience greater caregiving burden, and evidence higher rates of psychiatric symptoms [2,3].
To date, research on cancer and marriage has focused largely on three areas [4]: the effects of caregiving on spouses [see reference 5 for a literature review]; general marital quality; and distress among patients and spouses, comparing patient and spouse distress levels and/or examining effects of gender. With respect to the “Who experiences more distress: patients or spouses?” question, some investigations indicate that patients and spouses are equally as distressed [6]. Others indicate that patients are more distressed than spouses [7–9] or conversely, that spouses are more distressed than patients [10–13]. For still others, the nature of the disparity changes over time. For example, Oberst and Scott [14] found that spouses were more distressed prior to hospital discharge following surgery, but patients were more distressed 10 days post-discharge, largely due to unanticipated physical symptoms. With respect to gender, Hagedoorn and colleagues [15] conducted an extensive review and meta-analysis of studies examining effects of gender and role (patient versus spouse) on distress. They concluded that gender matters more than role. Females reported higher levels of distress than males, regardless of whether they were the patient or the spouse. The authors also noted that gender and role are often confounded, as in studies involving heterosexual samples of breast cancer patients and their husbands [16–20] or prostate cancer patients and their wives [21–23].
Regarding general marital quality, research finds that couples are generally able to adapt to a diagnosis of cancer [4]. Adaptation has been examined in both objective and subjective ways, and multiple terms have been used, at times interchangeably: marital stability, dissolution, quality, adjustment, and satisfaction. Dissolution and stability refer to breakdown, i.e., separation or divorce. A perception exists among both the lay public and health care professionals that cancer patients (in particular, female cancer patients) are often deserted by their partners [24]. However, the data generally do not bear this out [24–29]. One notable exception comes from an abstract by Glantz and colleagues, in which the odds of divorce were higher among female versus male patients with brain cancer [30]. Spanier [31] describes marital adjustment as “an ever-changing process with a qualitative dimension which can be evaluated at any point in time on a dimension from well adjusted to maladjusted” (p. 17). We see this as akin to marital quality, and distinct from satisfaction, a component of overall adjustment [31]. Again, the picture is largely positive in that adjustment and satisfaction levels among cancer patients and spouses are on par with general population norms [13,22,32–35]. However, much of the work has been cross-sectional in design [36,37], with few prospective, longitudinal investigations conducted (affording examination of potential changes in relationship outcomes relative to treatment and recovery phases) [10,19,32,35].
The present study focused on three marital outcomes (dissolution, adjustment and satisfaction), each as a function of role and gender, building upon the previously mentioned work on role and gender with application to a relationship-focused outcome. We employed a prospective, longitudinal design and included couples in which one member of the dyad received a hematopoietic stem cell transplant (HSCT). HSCT is used in the treatment of hematologic malignancies and other blood disorders as well as some solid tumors [38]. It affords the administration of high doses of chemotherapy with or without total body irradiation, followed by the infusion of healthy stem cells. The procedure is highly demanding, with multiple known early and late medical and psychosocial sequelae [39–42]. Because of the nature of treatment, the process can be highly demanding for caregivers as well, exacting a toll in terms of the time required for caregiving, financial sacrifices involved [43], and high burden of care, including medical management [44–46]. Partners are further impacted because treatment is known to adversely affect sexual functioning and fertility among patients [47–52].
Few studies have examined the effects of HSCT on marital adjustment or satisfaction. Langer and colleagues [10] found that partners reported reductions in relationship satisfaction as compared to both patients and controls, not prior to the transplant but 6 months and 1 year post-transplant, and that female gender was a risk factor for such dissatisfaction. A study by Bishop and colleagues yielded similar results in that partners reported less satisfaction as compared to both patients and controls [37]. However, this study employed a cross-sectional design, with a wide range of post-transplant years (1.9–19.4), and the researchers did not report satisfaction values by gender.
Aims of the present investigation were two-fold: (1) to examine the trajectory of relationship adjustment and satisfaction over time as a function of role and gender, adjusting for pre-transplant emotional distress, and (2) to provide a simple count of the number of divorces among participating survivors. Given the aforementioned previous work on HSCT couples [10,37], we hypothesized long-term reductions in relationship satisfaction among spouses in comparison to patients and, in particular, among female spouses. Analyses regarding marital dissolution were exploratory and descriptive.
METHODS
Participants
Participants were enrolled in a randomized controlled trial designed to improve physical and cognitive limitations, and manage emotional and family changes associated with HSCT. Inclusion criteria for patients were: the impending receipt of an HSCT for a hematologic malignancy, age > 21, and the ability to speak and comprehend English. Patients with a major psychiatric disorder not in remission were excluded (determined by medical record review or clinical team report of psychiatric problems). While distress was measured for research purposes, patients were not screened for distress.
Once enrolled, patients were asked to nominate a caregiver to the study, ideally a spouse if the patient was married. Inclusion criteria for caregivers were: age ≥ 18, the ability to speak and comprehend English, patient consent, presence at the transplant site in the month prior to returning home from transplant, and cohabitation with the patient for at least 3 months following discharge. Caregiver participation was not a requirement for patient enrollment. As with patients, caregivers were not screened for distress.
The present analyses included only legally married patients at pre-transplant for whom the spouse was also participating in the study. This resulted in the exclusion of 2 homosexual and 8 heterosexual cohabiting couples. We chose to exclude cohabiting couples because one aim of the study was to examine relationship dissolution and research suggests that this has a different likelihood in unmarried couples. Bouchard [53] followed a sample of 117 married couples and 109 cohabiting couples for 2 years. Cohabiting couples were more likely to report dissolution at 2 years as compared to married couples. In a study of relationship quality among several types of cohabiting couples without children (heterosexual married couples, heterosexual unmarried couples, and gay and lesbian couples), Kurdek and Schmitt [54] found that relative to married couples, the other types of couples perceived fewer barriers to leaving the relationship.
Design and procedure
All procedures were approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center.
Intervention
Patients were randomized to receive an intervention or standard care/ control. The intervention consisted of two group-format, 90-minute workshop sessions occurring 1–3 weeks before discharge from the transplant center to home, one on physical and cognitive difficulties for survivors and one on emotions and returning to family roles. The focus was on the survivors’ needs, but caregivers were invited to participate. The workshop format included a 15-minute introduction with video icebreaker, 60 minutes of combined didactic information and discussion, followed by a wrap-up that reviewed tips also provided in print. After return home, patients and caregivers received two 30-minute booster phone calls to facilitate their use of the workshop materials (one, approximately 1 month after return home and the other, approximately 6 months after return home).
Assessment
Participants were assessed pre-HSCT (4–7 days after beginning medical workup prior to starting transplant) and 6 months, 1 year, 2 years, 3 years and 5 years post-HSCT. Pre-transplant assessments were completed in the clinic so study staff could respond to any initial questions. Subsequent assessments were completed at home with instructions to respond without consulting anyone else and within a 24-hour time period.
To measure dissolution, participants reported on marital status at all time points except 6 months. Based on clinical experience, we did not expect to see short-term changes in marital status and therefore did not assess such. To measure marital quality, participants completed the Dyadic Adjustment Scale (DAS) at the first five time points (pre-HSCT to 3 years). At five years, participants completed only the satisfaction subscale of the DAS, to reduce responder burden. The DAS is a 32-item measure of adjustment in spousal or committed relationships [31], arguably the gold standard and sensitive to change [55]. Response formats vary, using 5-, 6- and 7-point rating scales. A total adjustment score is calculated, as are four subscales: consensus (agreement on issues such as finances, goals, friends), satisfaction (happiness with and commitment to the relationship), cohesion (doing things together), and affectional expression (displaying affection and sexual activity). We focused here on the total scale and the satisfaction subscale. Total scores have a theoretical range of 0–151, with higher numbers indicative of greater marital adjustment. As per standard cutoffs [56,57], respondents scoring below 98 were classified as relationship-maladjusted. This corresponds to 1 SD below the mean reported by Spanier [31]. Satisfaction subscale scores have a theoretical range of 0–50, with higher numbers indicative of greater satisfaction. Exemplar items include, “How often do you discuss or have you considered divorce, separation or terminating your relationship?” and “How frequently do you kiss your mate?” The developer reported psychometric properties as follows: Cronbach’s coefficient alpha of 0.96 for all 32 items and 0.94 for the 10 satisfaction items, and validity as evidenced by higher scores for married versus divorced samples [31]. In the present sample, the satisfaction subscale correlated positively with the total scale (r values ranged from 0.85–0.96 across time and role, all p values < .001).
To assess pre-transplant emotional distress, patients and spouses completed the Profile of Mood States [POMS-SF; 58]. In this measure, respondents rate the extent to which they experienced each of 30 feelings such as nervous or sad “during the past week, including today”. Ratings are made on a 0 (not at all) to 4 (extremely) scale. While six subscales are calculated (e.g., tension-anxiety, depression-dejection), we focused here on Total Mood Disturbance scores, a mean of all items administered in the Short-Form [58]. Adequate reliability and validity have been demonstrated in both clinical and non-medical samples [59].
For POMS normative comparison purposes only, we used a non-medical cohort. The POMS developers did not report norms for healthy, non-treatment adults (non-college-aged) [60]. The developer of the short-form, moreover, did not report SDs for the subscale scores or the Total Mood Disturbance score [58]; the same is true of another psychometric paper on the short-form [59]. In the present study, all five-year survivors (not just married survivors) were asked to recruit a healthy comparison to the study. The recruit was to be of the same gender, race/ ethnicity and educational status, within 5 years of age of the survivor, and without cancer history or major medical problems. This was a biologic sibling for 33 survivors, a friend for 36 survivors, and a stranger (community volunteer recruited by the research team) for 22 survivors. Healthy comparisons completed the POMS, among other measures. Total Mood Disturbance scores did not differ as a function of type of comparison (sibling, friend, community volunteer), p = .441.
Analysis
Statistical analyses were performed using SPSS 13.0 and SAS 8.0. Descriptive statistics were used to examine marital adjustment and dissolution. Linear mixed models were fit to the DAS satisfaction subscale scores. Separate models were fit for patients and spouses, with adjustment for pre-transplant distress and post-transplant intervention condition. A Toeplitz structure was assumed for covariance. Contrasts between each time point and pre-transplant were estimated for each gender, as well as between genders at each time point.
RESULTS
Figure 1 illustrates the flow of patients from eligibility screening and enrollment to subsample selection and data completion. One-hundred and forty-nine of the 199 enrolled patients were married. Among the 149, 121 had a spouse also enrolled in the study; 28 did not, primarily because the spouse remained home during transplant. The two married patient subgroups (participating spouse, non-participating spouse) were compared with respect to demographic characteristics (age, gender, education, income) and DAS scores over time. The two patient subgroups differed only with respect to gender, in that female patients were more likely to have a non-participating spouse (21 female and 7 male patients with a non-participating spouse, and 63 female and 58 male patients with a participating spouse), χ2 = 4.86, p = .027.
Figure 1.
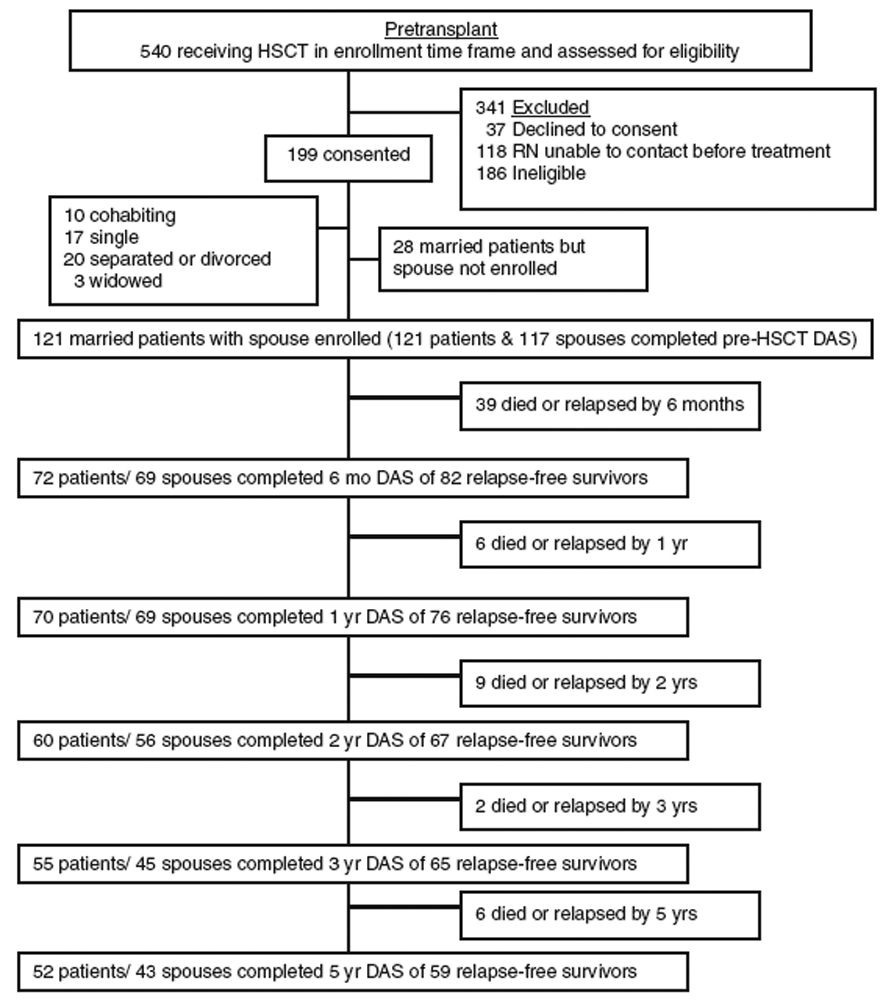
Flow chart. HSCT = hematopoietic stem cell transplant. Relapse is determined by the medical team through pathology reports. With relapse, most patients return to active treatment and their issues change dramatically relative to those in recovery. Because they also usually withdraw from quality of life research at this point, this protocol withdrew patients and spouses from the study at the time of relapse. Spouses were withdrawn when their patients died. Due to the high mortality and morbidity rates associated with HSCT, sample sizes naturally fluctuated over time.
Randomization occurred post-transplant, 2 weeks prior to discharge. Thirty of the 121 couples in our analysis sample were not randomized. Twenty-two patients expired prior to randomization; 5 withdrew from the study prior to randomization; and 3 withdrew prior to randomization but were re-accrued at the one-year time point for the longitudinal aspect of the study. Forty-two couples were randomized to the intervention (20 male patient/ female spouse couples, 22 female patient/ male spouse couples) and 49 were randomized to control (20 male patient/ female spouse couples, 29 female patient/ male spouse couples).
Table 1 displays pre-transplant characteristics of the 121 married patients and participating spouses. Participants were, on average, 44 years old and predominantly Caucasian. Gender was evenly distributed, as was educational status and income. The most common patient diagnosis was chronic myeloid leukemia. Demographics for the healthy comparison sample were as follows: mean (SD) age = 47.4 (9.8); 58.2% female; 3.3% Hispanic; 97.8% Caucasian; 76.9% college-educated; and 44.0% total household income exceeding $74,999.
Table 1.
Pre-transplant characteristics of the sample
| Patients | Spouses | |
|---|---|---|
| N | 121 | 121 |
| Age, M (SD) | 43.7 (9.0) | 43.5 (9.8) |
| Age, range | 27–68 | 23–68 |
| Gender, n (%) | ||
| Male | 58 (47.9) | 63 (52.1) |
| Female | 63 (52.1) | 58 (47.9) |
| Race/ ethnicity, n (%) | ||
| Hispanic | 7 (5.8) | 6 (5.0) |
| Caucasian | 112 (92.6) | 109 (90.1) |
| Other | 2 (1.6) | 6 (4.9) |
| Educational status, n (%) | ||
| < 4-year college | 59 (48.8) | 66 (54.6) |
| 4-year college degree or more | 62 (51.3) | 55 (45.4) |
| Family income, n (%) | ||
| ≤ $59,999 | 53 (43.9) | --- |
| $60,000 – $74,999 | 24 (19.8) | --- |
| $75,000+ | 40 (33.0) | --- |
| Unknown | 4 (3.3) | --- |
| Diagnosis and stage, n (%) | ||
| Chronic myeloid leukemia | 43 (35.5) | --- |
| Acute leukemia | 22 (18.2) | --- |
| Myelodysplasia | 16 (13.2) | --- |
| Lymphoma | 14 (11.6) | --- |
| Solid tumor | 17 (14.0) | --- |
| Other | 9 (7.5) | --- |
| Type of transplant, n (%) | ||
| Autologous | 23 (19.0) | --- |
| Allogeneic | 98 (81.0) | --- |
Pre-transplant emotional distress
Participants were categorized as low or high in distress at the pre-transplant time point, using data from the healthy comparison sample. Participants scoring 1 SD above the healthy comparison mean on the POMS (M + SD = 0.94 + 0.57) were categorized as high in distress. While normal distributions would expect 15% to be elevated in distress, the percentages of highly distressed participants by role and gender were as follows: male patients (17.5), female patients (25.4), male spouses (21.7), and female spouses (36.8).
We also examined pre-transplant distress as a function of data completion status: not providing any post-transplant data, providing partial post-transplant data, or providing complete post-transplant data. Initial distress was unrelated to later data completion among both patients (χ2 = .19, p > .05) and spouses (χ2 = 3.05, p > .05).
Marital adjustment
Table 2 presents mean DAS total adjustment scores and the percentage of relationship-maladjusted participants as a function of gender and time through 3 years. Descriptively, the percentage of relationship-maladjusted participants was elevated among female spouses, not prior to transplant but at 6 months and 1–3 years post-transplant.
Table 2.
Total DAS adjustment scores as a function of time, role and gender
| Mean (SD) |
n maladjusted*/ total responding (valid %) |
|||
|---|---|---|---|---|
| Patients | Spouses | Patients | Spouses | |
| Pre-HSCT | ||||
| Males | 115.00 (10.34) | 114.92 (13.10) | 2/ 58 (3.4) | 6/ 60 (10.0) |
| Females | 117.76 (15.87) | 114.42 (12.87) | 6/ 63 (9.5) | 5/ 57 (8.8) |
| 6 months | ||||
| Males | 114.36 (13.98) | 116.90 (14.31) | 4/ 33 (12.1) | 3/ 36 (8.3) |
| Females | 117.23 (13.75) | 107.29 (17.93) | 1/ 39 (2.6) | 8/ 33 (24.2) |
| 1 year | ||||
| Males | 115.13 (12.18) | 116.89 (15.68) | 1/ 31 (3.2) | 3/ 36 (8.3) |
| Females | 117.18 (15.74) | 105.42 (18.87) | 5/ 39 (12.8) | 9/ 33 (27.3) |
| 2 years | ||||
| Males | 113.96 (15.72) | 115.29 (16.65) | 4/ 28 (14.3) | 3/ 31 (9.7) |
| Females | 114.94 (15.59) | 108.63 (16.25) | 5/ 32 (15.6) | 5/ 25 (20.0) |
| 3 years | ||||
| Males | 111.31 (15.47) | 119.55 (10.05) | 6/ 26 (23.1) | 1/ 22 (4.5) |
| Females | 118.52 (14.78) | 107.09 (15.49) | 3/ 29 (10.3) | 6/ 23 (26.1) |
Maladjusted cutoff score < 98
Marital satisfaction
Means and standard errors for patients by time and gender are displayed in Figure 2a; those for spouses are displayed in Figure 2b. Table 3 presents results of the linear mixed models for DAS satisfaction: one for patients and one for spouses. Both models controlled for pre-transplant distress and post-transplant intervention condition. A time × gender interaction was found for spouses but not patients. For male spouses, satisfaction levels did not change over time. In contrast, female spouses reported decreases in satisfaction at each time point relative to baseline. In addition, satisfaction levels were lower for female versus male spouses at multiple time points: 6 months, 1 year, and 5 years.
Figure 2.
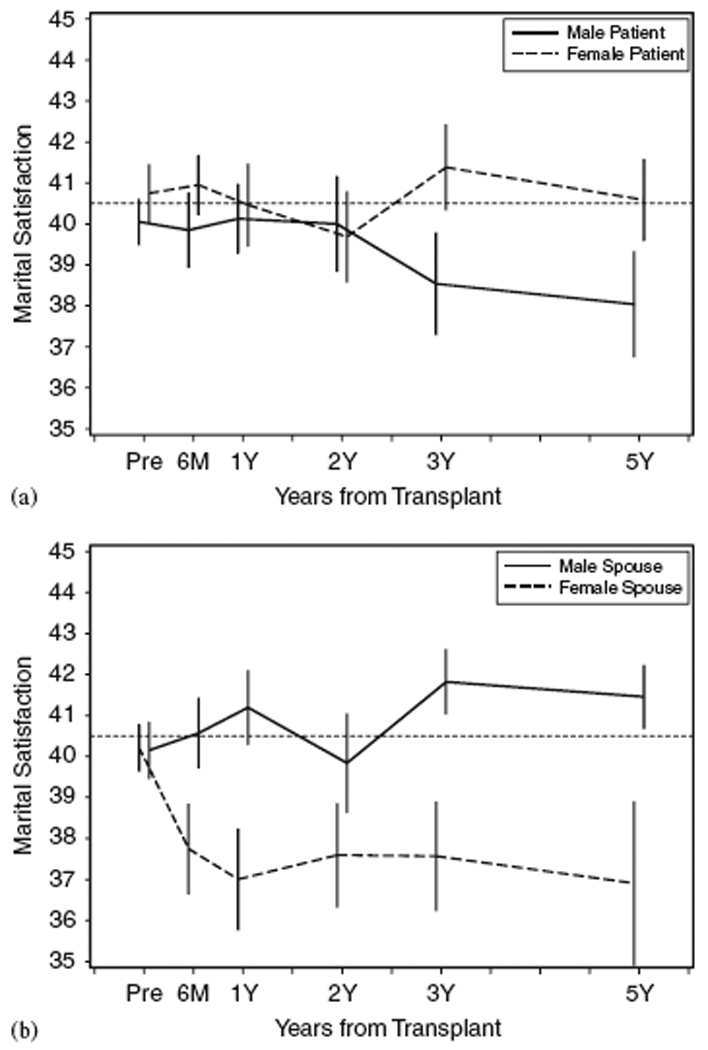
Patient (a) and spouse (b) DAS satisfaction as a function of time and gender, M + SE. Data reflect all persons responding at each time point (pairwise deletion). The dotted line at 40.5 represents the sample mean reported by the DAS scale developer [31]. Male/female differences emerged for patients at 3 years (p ≤ 0.10) and 5 years (p ≤ 0.01), and spouses at 6 months (p ≤ 0.10), 1 year and 5 years (p ≤ 0.01).
Table 3.
Results of the linear mixed models for DAS satisfaction, Mean (SE)
| Patient | Spouse | |||||
|---|---|---|---|---|---|---|
| Male | Female | M-F difference | Male | Female | M-F difference | |
| Pre-HSCT | 39.99 (0.76) | 40.72 (0.75) | −0.74 (1.01) | 41.06 (0.73) | 41.76 (0.80) | −0.70 (1.00) |
| Post-HSCT | Difference from pre-HSCT | Difference from pre-HSCT | ||||
| 6 months | −0.17 (0.73) | 0.02 (0.63) | −0.93 (1.14) | −0.52 (0.81) | −3.34 (0.91)** | 2.12 (1.22)† |
| 1 year | 0.15 (0.85) | −0.41 (0.73) | −0.18 (1.20) | −0.10 (0.84) | −3.98 (0.93)** | 3.17 (1.23)** |
| 2 years | −0.06 (0.91) | −0.98 (0.81) | 0.18 (1.26) | −1.11 (0.88) | −3.47 (1.02)** | 1.66 (1.32) |
| 3 years | −1.67 (0.95)† | −0.05 (0.87) | −2.36 (1.30)† | −0.37 (1.07) | −2.88 (1.13)** | 1.81 (1.44) |
| 5 years | −3.02 (0.94)** | −1.14 (0.90) | −2.62 (1.33)* | −0.34 (1.07) | −4.75 (1.16)** | 3.71 (1.48)** |
In both models, pre-transplant served as the referent. “M-F difference” corresponds to the difference between males and females per time point.
p < .10,
p ≤ .05,
p ≤ .01.
p value for time×sex interaction = 0.15 for patients and 0.008 for spouses. Both models adjusted for pre-transplant distress and post-transplant intervention condition. Pre-HSCT satisfaction levels refer to non-intervention and low distress. For patients, main effects of the adjustment variables were as follows: mean (SE) = 0.09 (1.14), p = 0.94 for distress, and mean (SE) = 0.05 (0.70), p = 0.94 for intervention condition. For spouses, main effects were: mean (SE) = −4.20 (0.99), p < 0.0001 for distress, and mean (SE) = 1.02 (0.80), p = 0.20 for intervention condition.
Main effects for the adjustment variables are provided in the footnote to Table 3. Intervention condition was not associated with post-HSCT satisfaction among patients or spouses. Pre-HSCT distress was not associated with satisfaction among patients, but was inversely associated with satisfaction among spouses.
Marital dissolution
Fifty-nine of the 121 initially married patients with a participating spouse survived to 5 years. Fifty-five of the 59 reported on marital status at 5 years. Fifty were still married; one was widowed; and four (2 male and 2 female) had divorced since transplant (7.3%). No changes in marital status were reported at the interceding time points, with the exception of the widowed patient at 3 years.
DISCUSSION
In this study, HSCT couples were largely satisfied and divorce was uncommon. Mean DAS scores (collapsed across gender) were commensurate with norms reported by Spanier [31]. They were also remarkably consistent with other reports of cancer patients and spouses [9,13,22,32,34,37,61,62].
The apparent resilience of marriages was offset by higher rates of relationship maladjustment among one subgroup: female spouses. Prior to the transplant, 9% of female spouses scored in the relationship-maladjusted range. This figure rose to 24% at the 6-month time point, remaining elevated thereafter. Female spouses also stood out when looking at the satisfaction data. They reported decrements in satisfaction at each time point relative to where they started. Clearly, the dissatisfaction was sustained over time. The effect appears intrapersonal for the first 2 years, in that female spouses reported relationship dissatisfaction, but their male partners did not. However, at 3 and 5 years, male patients reported reductions in satisfaction relative to pre-transplant. This suggests that the interpersonal effect of female spousal dissatisfaction (on male patients) is perhaps delayed.
It is intriguing to consider the mechanisms by which female spouses convey dissatisfaction to their male patient partners, who ultimately come to see the relationship in the same way. Are wives creating a self-fulfilling prophecy by behaving in ways that convey disappointment or distress? Are they protecting vulnerable patients before 3 years and waiting to convey their disappointments? Findings are interpretable in light of theory and research on relationship-focused coping [63]. Protective buffering, a form of relationship-focused coping, is defined as “hiding one’s concerns, denying one’s worries, concealing discouraging information, preventing the patient from thinking about the cancer, and yielding in order to avoid disagreement” [64, p. 275]. Indeed, evidence suggests that both cancer patients and spouses may engage in this behavior. Patient buffering of spouse has been associated with increased distress among patients [65], more so among female patients [66]. Similarly, spouse buffering of patient has been associated with increased distress among spouses [35], more so among female spouses [66]; it has also been associated with increased distress among patients [35,67]. Interestingly, Hinnen and colleagues [68] found that the more patients felt buffered by their spouses, the lower the patients’ relationship satisfaction, but this was especially true of highly assertive patients. Perhaps assertiveness is associated with a more direct communication style and consequently, patients high in this trait find it more distressing to be protected or shielded. To our knowledge, just one study has examined protective buffering among HSCT couples and employed a behavioral indicator of such, as opposed to patient- or spouse-reported buffering of partner or received buffering. Langer and colleagues [69] asked HSCT spouses to talk about their thoughts and feelings regarding the transplant and their role as a caregiver twice, once in the presence of the patient and once in the absence of the patient. Spouses’ facial expressions were deemed more positive by objective raters when the patient was present versus absent. Moreover, lexical buffering (saying more positive words when the patient was present versus absent) was associated with decreased relationship satisfaction among both patients and spouses. Additional research is needed to fully understand the communication patterns of these couples and associated effects.
Our results stand in contrast to recent meta-analytic conclusions by Hagedoorn et al. [15], in that we did not find across-the-board gender differences. This could be due to the “extremes” of HSCT. Patient prognosis can be grave, and caregiving tasks demanding, including medical care tasks. Why might caregiving pose deleterious consequences for female spouses? Several explanations have been offered, largely from the broader (non-cancer-specific) arena. One, female caregivers may simply spend more time caregiving, or engage in more personal and hands-on care tasks as compared to male caregivers [70,71]. We did not measure time spent engaged in care, nor type of care, so therefore cannot address this question. Two, male caregivers typically receive assistance from family and friends, whereas women tend to operate as sole caregiver [72]. In an intriguing study on work status, gender and caregiving, Gaugler and colleagues found that female caregivers who were employed provided more instrumental care than did male caregivers who were either employed or unemployed [73]. In other words, women were not relieved by significant others of their burden, even when combining work and caregiving. Three, evidence suggests that women in general are more strongly affected by the psychological and physical state of their partner than men, and therefore are more likely to be adversely impacted by an ill partner [64]. Four, the association between certain coping styles and relationship satisfaction may be stronger for women than men [74]. Ptacek and colleagues [74] found that active coping was positively related, and alcohol/ drug use negatively related, to marital satisfaction among female cancer survivors, but no such associations occurred for male survivors. Five, biological explanations may come into play, given fairly recent reconceptualizations of the human stress response, whereby males “fight or flee” but females “tend and befriend” (characterized by seeking out others and offering social support in the face of a stressor), a response associated with the release of oxytocin [75]. Finally, according to the gender-role socialization model [76], social norms dictate that women should be caregivers. Consequently, women are less likely to experience positive effects of caregiving, such as self-esteem, as compared to men who take on the unexpected role [77]. They also have a stronger sense of obligation [78].
Several limitations of this study merit attention. We utilized a self-report methodology. Future research will benefit from the addition of a behavioral indicator of marital quality, derived from observations of marital interactions. Future research will also benefit from the inclusion of a situation-specific measure of marital satisfaction, and perhaps more frequent assessments. Fang, Manne and Pape [32] suggest using a cancer-specific measure of marital satisfaction in addition to a general measure. Certainly, the two could yield disparate results. With respect to timing, 5 years is a relatively short period in marital relationships, and follow-up at 10 years or later could indicate further dissolution and other repercussions of the dissatisfaction within marriages of male patients and female spouses.
Our sample was likely not fully representative of the population of married HSCT patients and spouses. First, it was lacking in ethnic diversity, although this is generally true of the HSCT patient population. Second, female patient/ male spouse couples may have been under-represented. Indeed, the group of excluded married patients, those with a non-participating spouse, was comprised heavily of females. As noted by Baider and colleagues, it can be difficult to recruit husbands to behavioral studies [79]. Similarly, our data speak only to patients and spouses who enrolled, survived and participated over time. More distressed or dissatisfied participants may have declined to participate or failed to provide data. IRB regulations preclude the collection of data on participants who do not consent to a study, so we cannot address the enrollment issue. We can, however, address the attrition issue. Pre-transplant levels of emotional distress did not differentially impact post-transplant data completion.
The present findings offer implications for the care of families facing transplant. Screening is warranted, but not necessarily for everyone. Spouses merit screening for emotional distress prior to the HSCT process, as high levels were related to reductions in satisfaction. In addition, male patient/ female spouse couples merit screening for relationship adjustment, but not necessarily prior to treatment, when couples appeared unified and cohesive (though again, this may have been a function of who consented to participate). The nature of the most appropriate interventions has yet to be elucidated. The optimal intervention might be multi-modal: individual therapy for female spouses, in addition to couples’ therapy for male patient/ female spouse couples. Given the previously described deleterious consequences of hiding thoughts and feelings about illness and treatment, strategies designed to facilitate adaptive communication are suggested. Couples-based interventions with prostate cancer patients and spouses, and breast and gynecologic cancer patients and spouses, show promise on this front [21,80,81]. Similarly, individual dyad members may benefit simply from having the opportunity to express their emotions, either out loud or via journaling [82]. However, application of this paradigm to cancer is relatively new and limited. Male patients may benefit from methods that teach them to express more recognition of the care they receive. On a more pragmatic note, clinicians and medical staff should urge female caregivers to enlist help from family members and friends, and take breaks from caregiving. Finally, it may be helpful to convey to couples, perhaps as part of the treatment consent process, not just the typical medical recovery course but the psychological and interpersonal recovery course(s). Anecdotally, we often hear from both patients and spouses an expectation that things will (and should) return to “normal” within about a year after transplantation. This may be an erroneous perception, as medical and psychological recovery can take three or even five years for some patients [41]. Simple knowledge of this fact could realign expectations, enhance adaptation and prevent disappointment.
Overall, the longitudinal picture of HSCT patients and their spouses is positive in that most marriages survive transplantation and the recovery process that requires changes in the marital relationship to one of intensive caregiving by the spouse. However, female spouses are vulnerable to decreases in relationship satisfaction. We do not yet fully understand the precipitants of such decreases, or the consequences of such. Investigations designed to examine the psychological, physiological and interpersonal effects of dissatisfaction are warranted, as well as interventions to ameliorate stresses on spousal caregivers.
ACKNOWLEDGEMENTS
Supported by grants R01 CA 112631, R01 CA 78990 and R01 CA 63030 (awarded to Dr. Syrjala), and R21 CA 112477 (awarded to Dr. Langer). Thanks to Dr. Janet Abrams and all of the participating couples.
No author has a conflict of interest or financial interest in any aspect of this research or its publication.
REFERENCES
- 1.Kayser K, Watson LE, Andrade JT. Cancer as a "we-disease": Examining the process of coping from a relational perspective. Families, Systems, & Health. 2007;25:404–418. [Google Scholar]
- 2.Schulz R, Visintainer P, Williamson GM. Psychiatric and physical morbidity effects of caregiving. J Gerontology. 1990;45:181–191. doi: 10.1093/geronj/45.5.p181. [DOI] [PubMed] [Google Scholar]
- 3.Wallsten S. Elderly caregivers and care receivers: Facts and gaps in the literature. In: Nussbaum P, editor. Handbook of Neuropsychology and Aging. New York: Plenum Press; 1997. pp. 467–482. [Google Scholar]
- 4.Manne S, Badr H. Intimacy and relationship processes in couples' psychosocial adaptation to cancer. Cancer. 2008;112:2541–2555. doi: 10.1002/cncr.23450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim Y, Given BA. Quality of life of family caregivers of cancer survivors. Cancer. 2008;112:2556–2568. doi: 10.1002/cncr.23449. [DOI] [PubMed] [Google Scholar]
- 6.Northouse LL, Swain MA. Adjustment of patients and husbands to the initial impact of breast cancer. Nurs Res. 1987;36:221–225. [PubMed] [Google Scholar]
- 7.Ben-Zur H, Gilbar O, Lev S. Coping with breast cancer: Patient, spouse, and dyad models. Psychosom Med. 2001;63:32–39. doi: 10.1097/00006842-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Hinnen C, Ranchor AV, Sanderman R, et al. Course of distress in breast cancer patients, their partners, and matched control couples. Ann Behav Med. 2008;36:141–148. doi: 10.1007/s12160-008-9061-8. [DOI] [PubMed] [Google Scholar]
- 9.Northouse LL, Templin T, Mood D, Oberst M. Couples' adjustment to breast cancer and benign breast disease: A longitudinal analysis. Psycho-Oncol. 1998;7:37–48. doi: 10.1002/(SICI)1099-1611(199801/02)7:1<37::AID-PON314>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Langer S, Abrams J, Syrjala K. Caregiver and patient marital satisfaction and affect following hematopoietic stem cell transplantation: A prospective, longitudinal investigation. Psycho-Oncol. 2003;12:239–253. doi: 10.1002/pon.633. [DOI] [PubMed] [Google Scholar]
- 11.Keitel MA, Zevon MA, Rounds JB, et al. Spouse adjustment to cancer surgery: Distress and coping responses. J Surg Oncol. 1990;43:148–153. doi: 10.1002/jso.2930430305. [DOI] [PubMed] [Google Scholar]
- 12.Kornblith AB, Herr HW, Ofman US, et al. Quality of life of patients with prostate cancer and their spouses. The value of a data base in clinical care. Cancer. 1994;73:2791–2802. doi: 10.1002/1097-0142(19940601)73:11<2791::aid-cncr2820731123>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Northouse LL, Mood D, Templin T, et al. Couples' patterns of adjustment to colon cancer. Soc Sci Med. 2000;50:271–284. doi: 10.1016/s0277-9536(99)00281-6. [DOI] [PubMed] [Google Scholar]
- 14.Oberst MT, Scott DW. Postdischarge distress in surgically treated cancer patients and their spouses. Res Nurs Health. 1988;11:223–233. doi: 10.1002/nur.4770110404. [DOI] [PubMed] [Google Scholar]
- 15.Hagedoorn M, Sanderman R, Bolks HN, et al. Distress in couples coping with cancer: A meta-analysis and critical review of role and gender effects. Psychol Bull. 2008;134:1–30. doi: 10.1037/0033-2909.134.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Dorval M, Guay S, Mondor M, et al. Couples who get closer after breast cancer: frequency and predictors in a prospective investigation. J Behav Med. 2005;23:3588–3596. doi: 10.1200/JCO.2005.01.628. [DOI] [PubMed] [Google Scholar]
- 17.Feldman B, Broussard CA. The Influence of relational factors on men's adjustment to their partners' newly-diagnosed breast cancer. J Psychosoc Oncol. 2005;23:23–43. doi: 10.1300/j077v23n02_03. [DOI] [PubMed] [Google Scholar]
- 18.Kadmon I, Ganz FD, Rom M, Woloski-Wruble AC. Social, marital, and sexual adjustment of Israeli men whose wives were diagnosed with breast cancer. Oncol Nurs Forum. 2008;35:131–135. doi: 10.1188/08.ONF.131-135. [DOI] [PubMed] [Google Scholar]
- 19.Northouse L, Templin T, Mood D. Couples' adjustment to breast disease during the first year following diagnosis. J Behav Med. 2001;24:115–136. doi: 10.1023/a:1010772913717. [DOI] [PubMed] [Google Scholar]
- 20.Wimberly SR, Carver CS, Laurenceau JP, et al. Perceived partner reactions to diagnosis and treatment of breast cancer: Impact on psychosocial and psychosexual adjustment. J Consult Clin Psychol. 2005;73:300–311. doi: 10.1037/0022-006X.73.2.300. [DOI] [PubMed] [Google Scholar]
- 21.Northouse LL, Mood DW, Schafenacker A, et al. Randomized clinical trial of a family intervention for prostate cancer patients and their spouses. Cancer. 2007;110:2809–2818. doi: 10.1002/cncr.23114. [DOI] [PubMed] [Google Scholar]
- 22.Banthia R, Malcarne VL, Varni JW, et al. The effects of dyadic strength and coping styles on psychological distress in couples faced with prostate cancer. J Behav Med. 2003;26:31–52. doi: 10.1023/a:1021743005541. [DOI] [PubMed] [Google Scholar]
- 23.Lavery JF, Clarke VA. Prostate cancer: Patients' and spouses' coping and marital adjustment. Psychol Health Med. 1999;4:289–302. [Google Scholar]
- 24.Taylor-Brown J, Kilpatrick M, Maunsell E, Dorval M. Partner abandonment of women with breast cancer. Myth or reality? Cancer Pract. 2000;8:160–164. doi: 10.1046/j.1523-5394.2000.84004.x. [DOI] [PubMed] [Google Scholar]
- 25.Dorval M, Maunsell E, Taylor-Brown J, Kilpatrick M. Marital stability after breast cancer. J Natl Cancer Inst. 1999;91:54–59. doi: 10.1093/jnci/91.1.54. [DOI] [PubMed] [Google Scholar]
- 26.Carlsen K, Dalton SO, Frederiksen K, et al. Are cancer survivors at an increased risk for divorce? A Danish cohort study. Eur J Cancer. 2007;43:2093–2099. doi: 10.1016/j.ejca.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 27.Joly F, Heron JF, Kalusinski L, et al. Quality of life in long-term survivors of testicular cancer: A population-based case-control study. J Clin Oncol. 2002;20:73–80. doi: 10.1200/JCO.2002.20.1.73. [DOI] [PubMed] [Google Scholar]
- 28.Syrjala KL, Langer SL, Abrams JR, et al. Late effects of hematopoietic cell transplantation among 10-year adult survivors compared with case-matched controls. J Clin Oncol. 2005;23:6596–6606. doi: 10.1200/JCO.2005.12.674. [DOI] [PubMed] [Google Scholar]
- 29.Syse A, Kravdal O. Does cancer affect the divorce rate? Demogr Res. 2007;16:469–492. [Google Scholar]
- 30.Glantz M, Cole B, Mills L, et al. High incidence of marital disruption in women but not men with primary brain tumors. P Am Soc Clin Oncol. 2001;20:227. [Google Scholar]
- 31.Spanier GB. Measuring dyadic adjustment: New scales for assessing the quality of marriage and similar dyads. J Marriage Fam. 1976;38:15–28. [Google Scholar]
- 32.Fang CY, Manne SL, Pape SJ. Functional impairment, marital quality, and patient psychological distress as predictors of psychological distress among cancer patients' spouses. Health Psychol. 2001;20:452–457. [PubMed] [Google Scholar]
- 33.Ganz PA, Rowland JH, Desmond K, et al. Life after breast cancer: Understanding women's health-related quality of life and sexual functioning. J Clin Oncol. 1998;16:501–514. doi: 10.1200/JCO.1998.16.2.501. [DOI] [PubMed] [Google Scholar]
- 34.Manne S, Ostroff J, Sherman M, et al. Couples' support-related communication, psychological distress, and relationship satisfaction among women with early stage breast cancer. J Consult Clin Psychol. 2004;72:660–670. doi: 10.1037/0022-006X.72.4.660. [DOI] [PubMed] [Google Scholar]
- 35.Manne SL, Norton TR, Ostroff JS, et al. Protective buffering and psychological distress among couples coping with breast cancer: The moderating role of relationship satisfaction. J Fam Psychol. 2007;21:380–388. doi: 10.1037/0893-3200.21.3.380. [DOI] [PubMed] [Google Scholar]
- 36.Manne SL, Alfieri T, Taylor KL, Dougherty J. Spousal negative responses to cancer patients: The role of social restriction, spouse mood, and relationship satisfaction. J Consult Clin Psychol. 1999;67:352–361. doi: 10.1037//0022-006x.67.3.352. [DOI] [PubMed] [Google Scholar]
- 37.Bishop MM, Beaumont JL, Hahn EA, et al. Late effects of cancer and hematopoietic stem-cell transplantation on spouses or partners compared with survivors and survivor-matched controls. J Clin Oncol. 2007;25:1403–1411. doi: 10.1200/JCO.2006.07.5705. [DOI] [PubMed] [Google Scholar]
- 38.Horowitz MM. Uses and growth of hematopoietic cell transplantation. In: Thomas ED, Blume KG, Forman SJ, editors. Hematopoietic Cell Transplantation. Malden: Blackwell Science; 1999. pp. 12–18. [Google Scholar]
- 39.Andrykowski MA, Bishop MM, Hahn EA, et al. Long-term health-related quality of life, growth, and spiritual well-being after hematopoietic stem-cell transplantation. J Clin Oncol. 2005;23:599–608. doi: 10.1200/JCO.2005.03.189. [DOI] [PubMed] [Google Scholar]
- 40.Socié G, Stone JV, Wingard JR, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. New Engl J Med. 1999;341:14–21. doi: 10.1056/NEJM199907013410103. [DOI] [PubMed] [Google Scholar]
- 41.Syrjala KL, Langer SL, Abrams JR, et al. Recovery and long-term function after hematopoietic cell transplantation for leukemia or lymphoma. JAMA. 2004;291:2335–2343. doi: 10.1001/jama.291.19.2335. [DOI] [PubMed] [Google Scholar]
- 42.Syrjala KL, Martin P, Deeg J, Boeckh M. Medical and psychosocial issues in transplant survivors. In: Chang AE, Ganz PA, Hayes DF, et al., editors. Oncology: An Evidence-Based Approach. New York: Springer-Verlag; 2006. pp. 1902–1928. [Google Scholar]
- 43.Meehan KR, Fitzmaurice T, Root L, et al. The financial requirements and time commitments of caregivers for autologous stem cell transplant recipients. J Support Oncol. 2006;4:187–190. [PubMed] [Google Scholar]
- 44.Eldredge D, Nail L, Maziarz R, et al. Explaining family caregiver role strain following autologous blood and marrow transplantation. J Psychosoc Oncol. 2006;24:53–74. doi: 10.1300/J077v24n03_03. [DOI] [PubMed] [Google Scholar]
- 45.Foxall MJ, Gaston-Johansson F. Burden and health outcomes of family caregivers of hospitalized bone marrow transplant patients. J Adv Nurs. 1996;24:915–923. doi: 10.1111/j.1365-2648.1996.tb02926.x. [DOI] [PubMed] [Google Scholar]
- 46.Gaston-Johansson F, Lachica EM, Fall-Dickson JM, Kennedy MJ. Psychological distress, fatigue, burden of care, and quality of life in primary caregivers of patients with breast cancer undergoing autologous bone marrow transplantation. Oncol Nurs Forum. 2004;31:1161–1169. doi: 10.1188/04.ONF.1161-1169. [DOI] [PubMed] [Google Scholar]
- 47.Claessens JJ, Beerendonk CC, Schattenberg AV. Quality of life, reproduction and sexuality after stem cell transplantation with partially T-cell-depleted grafts and after conditioning with a regimen including total body irradiation. Bone Marrow Transplant. 2006;37:831–836. doi: 10.1038/sj.bmt.1705350. [DOI] [PubMed] [Google Scholar]
- 48.Hammond C, Abrams JR, Syrjala KL. Fertility and risk factors for elevated infertility concern in 10-year hematopoietic cell transplant survivors and case-matched controls. J Clin Oncol. 2007;25:3511–3517. doi: 10.1200/JCO.2007.10.8993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayden PJ, Keogh F, Ni Conghaile M, et al. A single-centre assessment of long-term quality-of-life status after sibling allogeneic stem cell transplantation for chronic myeloid leukaemia in first chronic phase. Bone Marrow Transplant. 2004;34:545–556. doi: 10.1038/sj.bmt.1704638. [DOI] [PubMed] [Google Scholar]
- 50.Humphreys CT, Tallman B, Altmaier EM, Barnette V. Sexual functioning in patients undergoing bone marrow transplantation: A longitudinal study. Bone Marrow Transplant. 2007;39:491–496. doi: 10.1038/sj.bmt.1705613. [DOI] [PubMed] [Google Scholar]
- 51.Syrjala KL, Roth-Roemer SL, Abrams JR, et al. Prevalence and predictors of sexual dysfunction in long-term survivors of marrow transplantation. J Clin Oncol. 1998;16:3148–3157. doi: 10.1200/JCO.1998.16.9.3148. [DOI] [PubMed] [Google Scholar]
- 52.Syrjala KL, Kurland BF, Abrams JR, et al. Sexual function changes during the 5 years after high-dose treatment and hematopoietic cell transplantation for malignancy, with case-matched controls at 5 years. Blood. 2008;111:989–996. doi: 10.1182/blood-2007-06-096594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bouchard G. Cohabitation versus marriage: The role of dyadic adjustment in relationship dissolution. J Divorce Remarriage. 2006;46:107–117. [Google Scholar]
- 54.Kurdek LA, Schmitt JP. Relationship quality of partners in heterosexual married, heterosexual cohabiting, and gay and lesbian relationships. J Pers Soc Psychol. 1986;51:711–720. doi: 10.1037//0022-3514.51.4.711. [DOI] [PubMed] [Google Scholar]
- 55.Whisman MA, Jacobson NS. Change in marital adjustment following marital therapy: A comparison of two outcome measures. Psychol Assessment. 1992;4:219–223. [Google Scholar]
- 56.Christensen A, Atkins DC, Berns S, et al. Traditional versus integrative behavioral couple therapy for significantly and chronically distressed married couples. J Consult Clin Psychol. 2004;72:176–191. doi: 10.1037/0022-006X.72.2.176. [DOI] [PubMed] [Google Scholar]
- 57.Margolin G. Marital conflict. In: Sigel I, Brody G, editors. Methods of family research: Biographies of research projects. Volume II: Clinical populations. Hillsdal: Lawrence Erlbaum; 1990. pp. 191–226. [Google Scholar]
- 58.Shacham S. A shortened version of the Profile of Mood States. J Pers Assess. 1983;47:305–306. doi: 10.1207/s15327752jpa4703_14. [DOI] [PubMed] [Google Scholar]
- 59.Curran SL, Andrykowski MA, Studts JL. Short Form of the Profile of Mood States (POMS-SF): Psychometric Information. Psychol Assessment. 1995;7:80–83. [Google Scholar]
- 60.McNair DM, Lorr M, Droppleman LF. EDITS Manual for the Profile of Mood States. San Diego: Educational and Industrial Testing Service; 1992. [Google Scholar]
- 61.Jenewein J, Zwahlen RA, Zwahlen D, et al. Quality of life and dyadic adjustment in oral cancer patients and their female partners. Eur J Cancer Care. 2008;17:127–135. doi: 10.1111/j.1365-2354.2007.00817.x. [DOI] [PubMed] [Google Scholar]
- 62.Norton T, Manne S. Support concordance among couples coping with cancer: Relationship, individual, and situational factors. Journal of Social and Personal Relationships. 2007;24:675–692. [Google Scholar]
- 63.Coyne JC, Smith DA. Couples coping with a myocardial infarction: A contextual perspective on wives' distress. J Pers Soc Psychol. 1991;61:404–412. doi: 10.1037//0022-3514.61.3.404. [DOI] [PubMed] [Google Scholar]
- 64.Hagedoorn M, Kuijer RG, Buunk BP, et al. Marital satisfaction in patients with cancer: Does support from intimate partners benefit those who need it most? Health Psychol. 2000;19:274–282. [PubMed] [Google Scholar]
- 65.Kayser K, Sormanti M, Strainchamps E. Women coping with cancer: The influence of relationship factors on psychosocial adjustment. Psychol Women Quart. 1999;23:725–739. [Google Scholar]
- 66.Manne S, Dougherty J, Veach S, Kless R. Hiding worries from one's spouse: Protective buffering among cancer patients and their spouses. Cancer Res Ther Cont. 1999;8:175–188. [Google Scholar]
- 67.Kuijer RG, Ybema JF, Buunk BP, et al. Active engagement, protective buffering, and overprotection: Three ways of giving support by intimate partners of patients with cancer. J Soc Clin Psychol. 2000;19:256–275. [Google Scholar]
- 68.Hinnen C, Hagedoorn M, Sanderman R, Ranchor AV. The role of distress, neuroticism and time since diagnosis in explaining support behaviors in partners of women with breast cancer: Results of a longitudinal analysis. Psycho-Oncol. 2007;16:913–919. doi: 10.1002/pon.1153. [DOI] [PubMed] [Google Scholar]
- 69.Langer SL, Rudd ME, Syrjala KL. Protective buffering and emotional desynchrony among spousal caregivers of cancer patients. Health Psychol. 2007;26:635–643. doi: 10.1037/0278-6133.26.5.635. [DOI] [PubMed] [Google Scholar]
- 70.Miller B, Cafasso L. Gender differences in caregiving: Fact or artifact? Gerontologist. 1992;32:498–507. doi: 10.1093/geront/32.4.498. [DOI] [PubMed] [Google Scholar]
- 71.Neal MB, Ingersoll-Dayton B, Starrels ME. Gender and relationship differences in caregiving patterns and consequences among employed caregivers. Gerontologist. 1997;37:804–816. doi: 10.1093/geront/37.6.804. [DOI] [PubMed] [Google Scholar]
- 72.Allen SM. Gender differences in spousal caregiving and unmet need for care. J Gerontology. 1994;49:187–195. doi: 10.1093/geronj/49.4.s187. [DOI] [PubMed] [Google Scholar]
- 73.Gaugler JE, Given WC, Linder J, et al. Work, gender, and stress in family cancer caregiving. Support Care Cancer. 2008;16:347–357. doi: 10.1007/s00520-007-0331-y. [DOI] [PubMed] [Google Scholar]
- 74.Ptacek JT, Pierce GR, Ptacek JJ. Coping, distress, and marital adjustment in couples with cancer: An examination of the personal and social context. J Psychosoc Oncol. 2007;25:37–58. doi: 10.1300/J077v25n02_03. [DOI] [PubMed] [Google Scholar]
- 75.Taylor SE, Klein LC, Lewis BP, et al. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- 76.Gilligan C. In a Different Voice: Psychological Theory and Women's Development. Cambridge: Harvard University Press; 1982. [Google Scholar]
- 77.Nijboer C, Triemstra M, Tempelaar R, et al. Patterns of caregiver experiences among partners of cancer patients. Gerontologist. 2000;40:738–746. doi: 10.1093/geront/40.6.738. [DOI] [PubMed] [Google Scholar]
- 78.Collins C, Jones R. Emotional distress and morbidity in dementia carers: A matched comparison of husbands and wives. Int J Geriatr Psychiatry. 1997;12:1168–1173. [PubMed] [Google Scholar]
- 79.Baider L, Koch U, Esacson R, De-Nour AK. Prospective study of cancer patients and their spouses: The weakness of marital strength. Psycho-Oncol. 1998;7:49–56. doi: 10.1002/(SICI)1099-1611(199801/02)7:1<49::AID-PON312>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 80.Baucom DH, Porter LS, Kirby JS, et al. A couple-based intervention for female breast cancer. Psycho-Oncol. doi: 10.1002/pon.1395. In press. DOI: 10.1002/pon.1395. [DOI] [PubMed] [Google Scholar]
- 81.Scott JL, Halford WK, Ward BG. United we stand? The effects of a couple-coping intervention on adjustment to early stage breast or gynecological cancer. J Consult Clin Psychol. 2004;72:1122–1135. doi: 10.1037/0022-006X.72.6.1122. [DOI] [PubMed] [Google Scholar]
- 82.Smyth JM. Written emotional expression: Effect sizes, outcome types, and moderating variables. J Consult Clin Psychol. 1998;66:174–184. doi: 10.1037//0022-006x.66.1.174. [DOI] [PubMed] [Google Scholar]


