Abstract
Although fear conditioning is an important psychological construct implicated in behavioral and emotional problems little is known about how it develops in early childhood. Using a differential, partial reinforcement conditioning paradigm, this longitudinal study assessed skin conductance conditioned responses in 200 children at ages 3, 4, 5, 6, and 8 years. Results demonstrated that in both boys and girls: (1) fear conditioning increased across age, particularly from ages 5 to 6 years, (2) the three components of skin conductance fear conditioning that reflect different degrees of automatic and controlled cognitive processes exhibited different developmental profiles, and (3) individual differences in arousal, orienting, and the unconditioned response were associated with individual differences in conditioning, with the influence of orienting increasing at later ages. This first longitudinal study of the development of skin conductance fear conditioning in children both demonstrates that children as young as age 3 years evidence fear conditioning in a difficult acquisition paradigm, and that different sub-components of skin conductance conditioning have different developmental trajectories.
Keywords: Classical conditioning, Fear, Skin conductance, Child development, Age
For decades classical fear conditioning has been a critically important construct in the fields of cognitive science, clinical science, and neuroscience. Fear conditioning has been applied to a number of clinical conditions, the most salient being anxiety, phobias, and conduct disorder (Field, 2006; Raine, Venables, & Williams, 1996). Despite its potential importance, there has been surprisingly little research on the development of fear conditioning in normal children. Given that many serious mental illnesses are being increasingly viewed as resulting from long-term disturbances in neural development (Rich et a., 2006) and the association between fear conditioning and psychopathology (Pine et al., 2001), addressing this critical gap in the literature on fear conditioning could provide a new basic science platform upon which to better understand how such pathological behavior arises in the course of development. The purpose of the current study was to examine whether there are developmental changes in skin conductance (SC) fear conditioning during early childhood in a longitudinal community sample of female and male children.
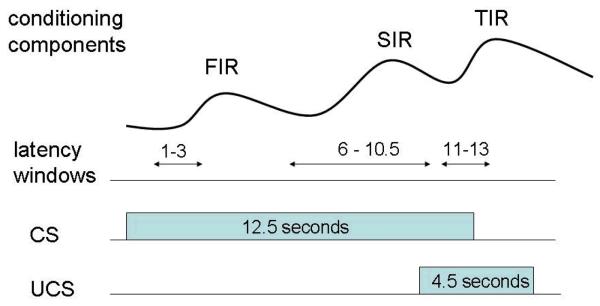
Fear conditioning in humans has been most typically studied using autonomic measures, including cardiovascular and SC responses (Fanselow & Poulos, 2005; Öhman, in press). Specifically, SC measures have characteristics that make them informative about conditioning using relatively few trials, as well as having the advantage for child research as being non-invasive, inexpensive, and minimally threatening. The fact that SC measures are viewed as primary indicators of conditioning is indicated by the fact that they are frequently used to confirm the presence of conditioning in fMRI studies of classical conditioning (Büchel & Dolan, 2000). SC fear conditioning in humans has been traditionally delineated by three separate components of the conditioned fear response (referred to as the first interval response, the second interval response, and the third interval response), a separation achieved by lengthening the CS (conditioned stimulus)-UCS (unconditioned stimulus) interval in order to allow sufficient time to assess separable SC components (Grings, 1969; Prokasy & Ebel, 1967). As illustrated in Figure 1, the first interval response occurs shortly following the onset of the CS, the second interval response begins shortly preceding the onset of the UCS, and the third interval response begins shortly following the omission of the UCS on a non-reinforced trial (for further descriptions and theoretical interpretations, see Dawson & Schell, 1987; Öhman, 1983).
Figure 1.
Schematic diagram illustrating stimuli durations, latency windows (in seconds from the onset of the CS), and the three conditioning components. FIR = first interval response, SIR = second interval response, TIR = third interval response.
In terms of the functional neuroanatomy of classical conditioning, one key structure consists of the amygdala (Öhman, in press). Animal (Davis, 1992), human lesion (LaBar, LeDoux, Spencer, & Phelps, 1995), and fMRI studies (Critchley, Mathias, & Dolan, 2002; Knight, Nguyen, & Bandettini, 2005; Pine et al., 2001) have all implicated this structure in classical fear conditioning. It has been suggested that sensory input projects to the lateral amygdala where synaptic plasticity takes place, and that projections from the amygdala's central nucleus to the brain stem regulate the learning of autonomic fear responses (Davis, 2000; Knight et al., 2005; LeDoux, 2000). While to our knowledge there appears to be no functional imaging research on amygdala activation in children, adult research has shown significant amygdala activation during the development of the conditioned fear response (Carter, O'Doherty, Seymour, Koch, & Dolan, 2006; Tabbert, Stark, Kirsch, & Vaitl, 2006). Taken together, research indicates that the amygdala is one of the most important structures in regulating autonomic fear conditioning, although other structures including the medial prefrontal cortex (Barbas, Saha, Rempel-Clower, & Ghashghaei, 2003), the middle frontal gyrus (Carter et al., 2006), the hippocampus (Bast, Zhang, & Feldon, 2003; Knight, Smith, Cheng, Stein, & Helmstetter, 2004), and the insula (Shi & Davis, 2001) are likely involved.
An important question concerns how fear conditioning develops across age in organisms. Babies as young as three months show skin potential aversive conditioning (Ingram & Fitzgerald, 1974), but to date only one study has been conducted on the development of autonomic measure of conditioning in children. Block, Sersen, and Wortis (1970) using a 95 dB UCS and a 600 Hz difference between CS+ and CS− in a relatively small sample found that 6-11 year old children (N = 38) showed evidence for cardiac discrimination conditioning. In contrast, 4-6 year olds (N = 17) showed only partial evidence for conditioning, while 2-4 year olds (N = 22) showed no conditioning; degree of conditioning showed a linear increase with age with the 6-11 year olds showed a 36% increase in the degree of conditioning compared to the 2-4 year olds. The 6-11 year olds and 4-6 year olds did not differ significantly, although this may be due to the smaller samples sizes in these groups. The potential importance of this developmental issue is twofold: 1) understanding how fear conditioning develops in normal children can provide a foundation to understanding abnormal development in conditionability which may in turn aid understanding of the etiology of later childhood and early adolescent clinical disorders, including phobia, anxiety, and antisocial behavior (Field, 2006; Raine et al., 1996), and 2) understanding the developmental trajectory of fear conditioning may help shed light on our knowledge of developmental functional neuroanatomy, especially the amygdala which has been found to be critically involved in fear conditioning (Öhman, in press).
Another gap in the literature is that there appear to be no developmental studies on the differential trajectories of the three components of SC fear conditioning (first, second, and third interval response). Regarding psychological correlates, the first interval response is considered to be an orienting response to the onset of the reinforced CS+ based on its learned signal significance, and reflects more attentional than associative processes (Prokasy & Raskin, 1973). The second interval response in contrast not only depends on the learned significance of the CS but also on the expected temporal relationship between the CS onset and UCS onset. Thus, the second interval response reflects a forward-looking process of expectancy (Öhman, 1979) or strategies to attenuate responsiveness to the aversive stimuli (Cheng, Richards, & Helmstetter, 2007; Petrovic, Carlsson, Petersson, Hansson, & Ingvar, 2004). The third interval response also reflects the learned significance of the CS and is also considered to represent an orienting response to the omission of the expected UCS. Some researchers have argued that these SC responses occur in most conditioning paradigms only in subjects aware of the CS-UCS relation and not in those unaware of the stimulus relation (Dawson & Furedy, 1976; Dawson & Schell, 1987), although it has also been argued that conscious discovery of the CS-UCS contingency may not be a prerequisite for conditioning (Schell, Dawson, & Marinkovic, 1991). In order to measure the third interval response, one must use a partial reinforcement paradigm, including some CS+s without the UCS, and its relation to awareness has been somewhat less studied. Given the related but somewhat different psychological mechanisms involved in the three components of SC conditioning, it was expected that different developmental trajectories of the first, second, and third interval responses would be observed in children from ages 3 to 8 years.
Another issue requiring investigation concerns the role of individual differences in arousal, orienting, and defensive responding (i.e., the unconditional response, UCR) in contributing towards individual differences in fear conditioning. Sokolov (1963) theorized that orienting (i.e., the capacity to allocate attention to, and process, novel environmental stimuli) facilitates the formation of the conditioned response and was indeed a prerequisite for conditioning. Based on this theorizing, adult research has shown that individual differences in orienting correlate with individual differences in conditioning (Ingram, 1978), and studies have also demonstrated this to be true in infants (Ingram & Fitzgerald, 1974). In contrast, there appears to be little research on this issue in young children. With respect to the UCR, it is known that experimentally increasing the strength of the UCS increases conditionability (Davey, 1992; Hosoba, Iwanaga, & Seiwa, 2001; Prokasy & Kumpfer, 1973). Individual differences exist in the degree to which children respond to the UCS, with some giving larger UCRs than others, at least in part reflecting the psychological impact of the UCS on the child (Morrow, Boring, Keough, & Haesly, 1969). Similarly, experimental manipulations of arousal in adults have shown that increased arousal causes increased conditionability (Critchley et al., 2002). The questions of the extent to which these individual differences within a group are associated with individual differences in degree of conditioning in that group are to our knowledge unanswered in the child literature. Specifically, given the significant role of orienting in conditioning particularly in the first and the third interval response (Öhman, 1972; Prokasy & Raskin, 1973), and given that SC orienting has been found to increase significantly throughout childhood (Gao, Raine, Dawson, Venables, & Mednick, 2007), it was expected that the influences of SC orienting on conditioning would increase across time.
The present study aims to address a significant gap in the literature by assessing SC fear conditioning longitudinally in 200 children at ages 3, 4, 5, 6, and 8 years. A 10 second inter-stimulus interval (ISI) was employed in order to differentiate the first, second, and third interval response and to assess for any differences in developmental trajectories across these three components of conditioning. SC measures of arousal, orienting, and defensive responding were also taken to assess whether they help account for changes in conditioning across ages. It was hypothesized that: (1) fear conditioning would generally increase from 3 to 8 years of age, (2) the developmental profile for conditioning would follow the profile previously established for orienting in this same sample (Gao et al., 2007), (3) the three components of SC conditioning should show similar but different developmental trajectories, and (4) individual differences in arousal, orienting, and the UCR will be associated with individuals differences in conditioning, with higher conditioning in those with increased arousal, orienting, and UCR, and a stronger association between orienting and conditioning at later ages compared to earlier ages. Because there is very little prior research in gender differences in conditionability in children, no specific hypothesis was generated on this issue.
Method
Participants
The population from which the participants were drawn consisted of 1,795 children from the country of Mauritius, a tropical island in the southwest Indian Ocean. All children born in 1969–1970 in two towns (Quatre Bornes and Vacoas) were recruited when aged 3 years. The ethnic makeup of the sample was as follows: 68.7% Indian, 25.6% African, and 5.6% other (Chinese, English, or French descent). Females made up 45.9% of the sample. Two hundred children (100 boys and 100 girls) were selected from the original sample based on their sex, race, and age 3 years SC activity. Full details of sample selection are given in Venables (1978). Psychophysiological measures were taken at ages 3, 4, 5, 6, and 8 years. Informed consent was obtained from the parents of the children. To assess whether this population of 200 was representative of the initial population (N = 1,795), comparisons were made between those included in the study and the rest of the sample on demographic measures. Participants and nonparticipants did not differ on sex, χ2 (1) = 0.490, p > .488, ethnicity, χ2 (4) = 0.465, p > .501, and social adversity (assessed by nine indicators of family functioning – see Raine, Yaralian, Reynolds, Venables, & Mednick, 2002 for full details), t(1,110) = 0.730, p > .327.
Experimental design
The conditioning paradigm consisted of a set of 12 tones (9 CS+ and 3 CS−). The CS+ tone was reinforced by a loud noise UCS, and a different tone served as the un-reinforced CS−. A 66% partial-reinforcement schedule was used, with 6 of the 9 presentations of the CS+ randomly reinforced. The CS+ was a 1000 Hz, 60 dB, 12.5 sec tone with rise and fall time of 25 msec whereas CS− was a 500 Hz, 60 dB, 12.5 sec tone with rise and fall time of 25 msec. The UCS was an auditory stimulus of 90 dB intensity and 4.5 sec duration recorded from white noise played in a tin can with metal jangling objects (e.g., keys), thus adding low and high frequency components to the stimulus. From a human subject standpoint, this stimulus was used in order to produce as noxious a sound as possible without causing unnecessary upset to the child and avoiding the use of a higher intensity sound which might cause physiological damage. The onset of the UCS was 10 s after the onset of the CS+, with a randomized ITI (inter-trial interval) of 38 s (range 34-42 s). Three un-reinforced CS+/CS− pairs (trial 3/4, 7/8, and 10/12) with complete SC conditioning components (see below) were used in the current study. A schematic diagram illustrating the stimuli durations and SC response onset latency windows is provided in Figure 1. Further details of the complete standard auditory stimuli used are given in Raine, Venables, Mednick, and Mellingen (2002) and Venables (1978).
Prior to the conditioning paradigm, an orienting paradigm was administered in which six neutral tones (three 75-dB tones of 1 s duration at 1000 Hz frequency, followed by three 75-dB tones of 1 s duration at 1311 Hz frequency) were presented through headphones. For four of the five ages (3, 4, 5, and 6), data were also available on SC measures that all preceded in time the onset of conditioning trials: (1) initial SC level, a widely-used index for arousal, recorded prior to the onset of the first orienting tone, (2) SC orienting responses averaged across the initial six orienting trials, and (3) the UCR to the very first UCS during the conditioning section (see Gao et al. (2007) for full details).
All subjects were tested in a sound-insulated cubicle with a controlled temperature of 30°C. A dehumidifier was used to minimize fluctuations in humidity. Stimuli were delivered through padded headphones. The child was seated on a chair at each phase except at age 3, when the subject was seated on the mother's lap throughout testing. It was established by asking the mothers at age 3 that the mother could not hear any of the stimuli presented to the child.
Skin conductance recording and data reduction
SC data were collected using a Grass Type 79 polygraph with a constant voltage system, Beckman miniature silver/silver chloride electrodes, and an electrolyte consisting of 0.5% KCl in 2% agar–agar (for further details, see Raine, Venables, et al., 2002; Venables, 1978). SC was recorded from the medial phalanges of the index and middle fingers of the left hand.
Three types of classically conditioned SC responses were scored: (a) the first interval response which was elicited by the CS, within a latency window of 1 to 3 sec post-CS onset, (b) the second interval response elicited prior to the UCS, with a latency window of 6 - 10.5 sec post-CS onset, and (c) the third interval response with a latency window of 11-13 sec post-CS onset on nonreinforced trials (see Figure 1). In line with prior studies on children (Fowles, 2000), responses greater than 0.05 μS with onsets within the appropriate latency window were scored as SC responses.
Statistical analyses
Three sets of analyses were conducted. First, to test for the presence of conditioning, an analysis of variance (ANOVA) with Stimulus (CS+ and CS−) and Age (3, 4, 5, 6, and 8 years) as within-subject factors and Sex as the between-subjects factor was conducted on the average conditioning and each of the three components (first, second, and third interval response), respectively. Effect sizes were reported using Cohen's d (Cohen, 1988).
Second, latent growth curve modeling was used to elucidate the developmental profiles of conditioned SC responses over time. Difference scores were calculated in which responses to CS− were subtracted from those to the CS+ for first, second, and third interval response, respectively. Difference scores of zero reflect no differential learning and difference scores above zero reflect greater responses to the CS+ relative to the CS−. In addition, conditioned SC responses were averaged across the three components (first, second, and third interval response) to generate an index for average conditioning. Latent growth curve models were then fitted for the average SC conditioning responses and each of the three components, respectively.
In a classical growth curve model, the score of each individual (Y[t]) is composed of the following components:
(1) the y0,n(intercepts), which are latent scores representing the individual's initial level, (2) the ys,n (slopes), which are latent scores representing the individual's linear change over time, (3) the Age[t]n, a set of basis coefficients that define the timing or shape of the profile over time, and (4) the e[t]n, which are unobserved but independent errors of measurements. On the next level, the latent scores are decomposed into parameters representing a mean and a variance term:
where μ0 and μs represent the fixed group means for intercept and slope, d0,n and ds,n imply individual deviations around these means, and a set of random variance and covariance terms (σ02, σs 2, σ0s) are used to describe the distribution of individual deviations. Usually only one random error variance (σe 2) is assumed.
Three models were fitted for each SC measure of conditioning to determine the shape of profiles and the moderating effect of sex. The first model was a no-growth model (M0), which assumed no slope component and only three parameters were estimated: an initial level mean (μ0), an initial variance (σ0 2), and an error variance (σe 2). Second, a linear growth model (M1) was fitted, in which a linear pattern of change over time was assumed and a fixed basis coefficient was formed by taking A[t] = [(Age[t] − 3)/5], or fixed values of A[t] = [0, .2, .4, .6, 1]. This linear scaling was chosen to permit a practical interpretation of the slope parameters in terms of a per-5-year change. Three more parameters were estimated: a slope mean (μs), a variance (σs 2), and a correlation (ρ0s). The third model was a latent growth model (M2), in which some of the loadings, A[t], were free to vary. We fixed A[3] = 0 and A[8] = 1, but the three other coefficients were estimated from the data. In order to examine whether the change between any two ages was same, the loadings were fixed to be equal between them. The results of the new model fitting were then compared with the original model to assess whether a significantly better fit was observed. Within each model, sex was included and coded using effects coding such that boys were coded 1 and girls −1 to examine sex differences in intercept and slope parameters.
Data were analyzed using Version 2 of Mplus (Muthén & Muthén, 1998). One of the benefits of its use is a maximum likelihood estimation procedure that uses all observations within the data set, including those with data missing at one or more waves. This reduces the sampling bias that occurs in approaches such as listwise or pairwise deletion that excludes participants with incomplete data. Expectation maximization (EM) algorithm (Little & Rubin, 1987) was used to estimate means, variances, and covariances among manifest variables using all data available for each participant. The likelihood ratio (χ2) and root mean square error approximation (RMSEA) were used to evaluate the fit of each model. Mean magnitudes of the SC responses to each stimulus were recorded at ages 3, 4, 5, 6, and 8 years. Trials with no measurable response were counted as zero in the current study. A square root transformation (using SQRT (magnitude + 0.0001)) was used before conducting the inferential statistical analyses to attain normality (Venables & Christie, 1980).
Third, to assess the possible role of individual differences in arousal, orienting, and the UCR in explaining individual differences in conditionability, measures of these three processes (which preceded conditioning in time in this experiment) were correlated with the average conditioning score for each of the four years where these data were available (ages 3, 4, 5, and 6). In addition to correlational analyses, linear regression using the stepwise method was used to assess the relative importance of the three processes at each age.
Results
Attrition in this 5-year longitudinal study was low, as indicated by the fact that complete data were available on 172 (86.0%) of the 200 children originally tested at age 3. Information about the pattern of missing data has been reported in Gao et al. (2007). Analyses indicated no biases in drop-out of subjects (Gao et al., 2007). Descriptive statistics and correlations between average conditioning and each conditioning sub-component within each age and between ages are listed in Table 1.
Table 1.
Observed Descriptive Statistics and Correlations between Average Classical Conditioning, First Interval Response, Second Interval Response, and Third Interval Response within Each Age (First Half) and between Ages (Second Half)
| Age 3 (N = 200) | Age 4 (N = 195) | Age 5 (N = 190) | Age 6 (N =184) | Age 8 (N = 172) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ave. | FIR | SIR | TIR | Ave. | FIR | SIR | TIR | Ave. | FIR | SIR | TIR | Ave. | FIR | SIR | TIR | Ave. | FIR | SIR | TIR | |
| Mean | .067 | .073 | .060 | .067 | .057 | .034 | .060 | .076 | .069 | .073 | .080 | .053 | .096 | .105 | .085 | .099 | .100 | .101 | .088 | .112 |
| SD | .071 | .106 | .097 | .096 | .059 | .071 | .080 | .107 | .068 | .112 | .102 | .083 | .081 | .111 | .132 | .106 | .075 | .122 | .099 | .128 |
| FIR | .675** | 1 | .273** | .110 | .805** | 1 | .336** | .556** | .699** | 1 | .221** | .101 | .705** | 1 | .229** | .319** | .664** | 1 | .273** | .018 |
| SIR | .787** | 1 | .460** | .647** | 1 | .292** | .739** | 1 | .273** | .733** | 1 | .240** | .677** | 1 | .130 | |||||
| TIR | .700** | 1 | .853** | 1 | .600** | 1 | .697** | 1 | .616** | 1 | ||||||||||
|
| ||||||||||||||||||||
| Age 4 | ||||||||||||||||||||
| Ave. | .095 | |||||||||||||||||||
| FIR | .123 | |||||||||||||||||||
| SIR | −.040 | |||||||||||||||||||
| TIR | . | .076 | ||||||||||||||||||
| Age 5 | ||||||||||||||||||||
| Ave. | .211** | .142* | ||||||||||||||||||
| FIR | .077 | .046 | ||||||||||||||||||
| SIR | .126 | −.023 | ||||||||||||||||||
| TIR | .064 | .093 | ||||||||||||||||||
| Age 6 | ||||||||||||||||||||
| Ave. | .080 | .121 | .035 | |||||||||||||||||
| FIR | .144* | .031 | .170* | |||||||||||||||||
| SIR | −.040 | .033 | −.100 | |||||||||||||||||
| TIR | .086 | .101 | −.070 | |||||||||||||||||
| Age 8 | ||||||||||||||||||||
| Ave. | .126 | .076 | .091 | .360** | ||||||||||||||||
| FIR | −.006 | −.040 | .061 | .148* | ||||||||||||||||
| SIR | .079 | .015 | .119 | .185* | ||||||||||||||||
| TIR | −.052 | .056 | .008 | .099 | ||||||||||||||||
Note. Ave. = Average classical conditioning; FIR = First interval response, SIR = Second interval response, TIR = Third interval response.
p < .05
p < .01.
ANOVA
An ANOVA based on 172 complete cases was first conducted on the average conditioning measure (first, second, and third interval response combined). Significant main effects were observed for Stimulus, F (1, 170) = 10.503, p = .001, and Age, F (4, 167) = 38.352, p < .001. In addition, a significant Age × Stimulus interaction was found, F (4, 167) = 2.954, p = .022. Post-hoc analyses showed that CS+ elicited significantly larger responses than CS− at age 3, t (199) = 2.695, p = .008, d = 0.193, and age 8, t (171) = 3.562, p < .001, d = 0.181, but not other ages. No other significant effects were found, p > .102.
Similar analyses on each of the three components revealed a significant main effect for Stimulus for the first and third interval response, respectively, F (1, 170) = 10.982, p < .01, F (1, 170) = 3.890, p < .05, indicating significant conditioning for these two components. In contrast, a main effect of Stimulus was not observed for the second interval response, F (1, 170) = 0.339, p = .561. A significant Age × Stimulus interaction was found for the second interval response, F (4, 167) = 2.594, p < .05, but not for the first or the third interval response, F (4, 167) < 1.340, ps > .257. Further analysis showed that for the second interval response, CS+ elicited significantly larger responses than CS− at age 8 but not other ages, t (171) = 2.483, p = .014, d = 0.178, indicating significant conditioning at age 8 years. No other significant effects involving Stimulus were found (ps > .275). A main effect of Age was also found for first, second, and third interval response, respectively, F (4, 167) = 90.471, p < .001, F (4, 167) = 19.426, p < .001, and F (4, 167) = 33.027, p < .001, with SC response magnitudes increasing across ages for each component.
Growth Curve Analysis
Average Classical Conditioning
The no-growth model (M0) yielded a model likelihood of L2 = 86. The linear growth model (M1) resulted in a new likelihood of L2 = 34 and showed an improvement in fit over the baseline model (M1 vs. M0: Δχ2 = 52 on Δdf = 4, p < .05). The latent growth model (M2) produced an improvement in the model likelihood (L2 = 11) and was substantially better than both the baseline model (M2 vs. M0: Δχ2 = 75 on Δdf = 7, p < .05) and the linear growth model (M2 vs. M1: Δχ2 = 23 on Δdf = 3, p < .05). Further analyses showed that the basis coefficients at ages 6 and 8 were equal. The predicted profile of the average conditioning from ages 3 to 8 is displayed in Figure 2. Both intercept (μ0 = .064, SE = .004, p < .05) and slope mean (μs = .032, SE = .006, p < .05) were significant, indicating that in general fear conditioning was present at age 3 and increased across ages (see Table 2). Sex was not significantly related to intercept (γ0 = .001, SE = .003, p > .05) or slope (γs = −.006, SE = .005, p > .05).
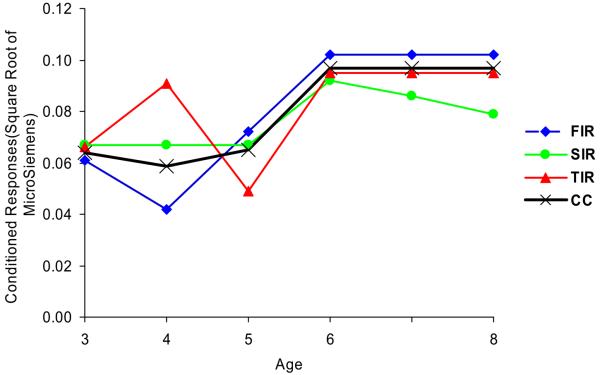
Figure 2.
Predicted developmental trajectories of average classical conditioning (CC) and the three components of conditioning from ages 3 to 8. The conditioned response is the square root of the difference between CS+ and CS−. FIR = First interval response, SIR = Second interval response, TIR = Third interval response.
Table 2.
Numerical Results from Latent Growth Curve Models Fitted to Average Conditioning, First, Second, and Third Interval Response from Ages 3 to 8 Years (N = 200)
| Parameters | Average Classical Conditioning | First Interval Response | Second Interval Response | Third Interval Response |
|---|---|---|---|---|
| Fixed effects | ||||
| Basis α [3] | 1, 0 | 1, 0 | 1, 0 | 1, 0 |
| Basis α [4] | 1, −0.158 (0.183) | 1, −0.476 (0.216) | 1, 0 | 1, 0.866 (0.240) |
| Basis α [5] | 1, 0.008 (0.152) | 1, 0.259 (0.188) | 1, 0 | 1, −0.596 (0.538) |
| Basis α [6] | 1, 1 | 1, 1 | 1, 2.154 (0.566) | 1, 1 |
| Basis α [8] | 1, 1 | 1, 1 | 1, 1 | 1, 1 |
| Level μ0 | 0.064 (0.004) | 0.061 (0.006) | 0.067 (0.004) | 0.066 (0.008) |
| Slope μs | 0.032 (0.006) | 0.041 (0.007) | 0.011 (0.006) | 0.029 (0.011) |
| Random effects | ||||
| Error σe2 | 0.004 (0.001) | 0.010 (0.001) | 0.009 (0.001) | 0.011 (0.001) |
| Level on Sex γ0 | 0.001 (0.003) | 0.007 (0.004) | 0.001 (0.004) | −0.006 (0.004) |
| Slope on Sex γs | −0.006 (0.005) | −0.006 (0.006) | −0.003 (0.005) | −0.003 (0.006) |
| Correlation ρ0s | 0.001 (0.001) | 0.001 (0.002) | 0.001 (0.002) | 0.001 (0.001) |
| Fit Indices | ||||
| Number of Parameters | 14 | 14 | 14 | 14 |
| Log Likelihood | 11 | 24 | 31 | 45 |
| RMSEA | .000 | 0.050 | 0.067 | 0.099 |
First Interval Response
The no-growth model (M0) yielded a model likelihood of L2 = 2073. The linear growth model (M1) resulted in a new likelihood of L2 = 1606 and showed an improvement in fit over the baseline model (M1 vs. M0: Δχ2 = 467 on Δdf = 4, p < .05). The latent growth model (M2) was substantially better than both the baseline model (M2 vs. M0: Δχ2 = 2049 on Δdf = 7, p < .05) and the linear growth model (M2 vs. M1: Δχ2 = 1582 on Δdf = 3, p < .05). Further analyses showed that the basis coefficients at ages 6 and 8 were equal and the coefficient at age 3 was significantly higher than that at age 4.
The predicted profile of the first interval response from ages 3 to 8 is displayed in Figure 2. Both intercept (μ0 = .061, SE = .006, p < .05) and slope mean (μs = .041, SE = .007, p < .05) were significant, indicating that the first interval response appeared at age 3 and increased across ages (see Table 2). Boys and girls did not differ in the intercept (γ0 = .007, SE = .004, p > .05) or the slope (γs = −.006, SE = .006, p > .05).
Second Interval Response
The linear growth model (M1) fit better than the baseline model (M1 vs. M0: Δχ2 = 2047 on Δdf = 4, p < .05). The latent growth model showed an improvement in fit over the baseline model (Δχ2 = 2066 on Δdf = 3, p < .05), and the linear growth model (Δχ2 = 18 on Δdf = 3, p < .05). Further analyses showed that the basis coefficients at ages 3, 4, and 5 were equal, whereas second interval response at ages 6 and 8 were higher than previous ages. The predicted trajectory of second interval response from ages 3 to 8 are displayed in Figure 2.
Based on the final latent growth model, both intercept (μ0 = .067, SE = .004, p < .05) and slope mean (μs = .011, SE = .006, p < .05) were significant from zero, indicating that second interval response appeared at age 3 and increased thereafter (see Table 2). Sex effects were found to be not significant, γ0 = .001, SE = .004, γs = −.003, SE = .005, all p > .05.
Third Interval Response
The linear growth model (M1) fit better than the baseline model (M1 vs. M0: Δχ2 = 2060 on Δdf = 4, p < .05). The latent growth model showed an improvement in fit over the baseline model (Δχ2 = 2084 on Δdf = 7, p < .05) and also the linear growth model (Δχ2 = 24 on Δdf = 3, p < .05). Further analyses showed that the basis coefficient at age 6 was equal to that at age 8. See Figure 2 for the predicted profile of the third interval response across ages.
In the final latent growth model, both intercept (μ0 = .066, SE = .008, p < .05) and slope mean (μs = .029, SE = .011, p < .05) were significant from zero, indicating that the third interval response appeared at age 3 and increased thereafter (see Table 2). No significant effects of sex were observed, γ0 = −.006, SE = .004, γs = −.003, SE = .006, all p > .05.
Role of Arousal, Orienting, and the UCR in Explaining Individual Differences in Conditionability
Correlations between SC conditioning, arousal, orienting, and UCR within each age are listed in Table 3. Increased arousal, orienting, and UCR were all significantly associated with increased conditioning in each age group. Regression analyses results for ages 3, 4, 5, and 6 years are displayed in Table 4. Linear regression using the stepwise method showed that the amount of variance explained increased from age 3 (17.2%) to 6 years (28.8%), with an exponential increase occurring from age 5 (19.5%) to age 6 (28.8%).
Table 3.
Correlations between Average Classical Conditioning and Skin Conductance Level, Orienting, and UCR within Each Age *
| Age 3 |
Age 4 |
Age 5 |
Age 6 |
Age 8 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Level | Orienting | UCR | Level | Orienting | UCR | Level | Orienting | UCR | Level | Orienting | UCR | Level | Orienting | UCR | |
| Conditioning | .377 | .374 | .280 | .291 | .392 | .389 | .346 | .382 | .407 | .429 | .462 | .412 | - | .501 | .405 |
| Level | 1 | .497 | .338 | 1 | .469 | .346 | 1 | .489 | .312 | 1 | .591 | .482 | - | - | - |
| Orienting | 1 | .538 | 1 | .704 | 1 | .338 | 1 | .674 | 1 | .640 | |||||
Note. UCR = Unconditioned response.
All p < .001.
Table 4.
Linear Regression Results
| Conditioning |
||||
|---|---|---|---|---|
| Age 3 | Age 4 | Age 5 | Age 6 | |
| Total R2 | 17.2% | 19.3% | 19.5% | 28.8% |
| Orienting | 3.2% | n.s. | 13.1% | 25.4% |
| UCR | n.s. | 19.3% | 4.1% | n.s. |
| Skin conductance level | 14% | n.s. | 2.3% | 3.4% |
Note. UCR = Unconditioned response.
Discussion
In this longitudinal study it was observed that: (1) fear conditioning increases with age, (2) somewhat different growth curves were observed for the three different conditioned fear response components, (3) for conditioning overall and all three conditioning components, there was a noticeable increase from ages 5 to 6, (4) individual differences in arousal, orienting, and UCR correlated with individual differences in conditioning at each age, with a substantial increase in the influence of these three processes from ages 5 to 6. Findings constitute the first developmental study of SC fear conditioning in early childhood, and suggest that complex automatic and controlled cognitive / emotional processes are involved in SC conditioning that increases across ages in early childhood.
A key finding of this study was the age-related changes in conditioning. As expected, conditioning significantly increased with age (see Figure 2). Though it has widely been accepted that human autonomic conditioning could be fully accounted for in terms of expectancy (e.g., Dawson, 1973; Dawson & Schell, 1987), some studies have suggested that a different process is involved in addition to orienting and conscious conditioning processes. For example, Schell et al. (1991) reported that even though subjects no longer expected the UCS after the CS+ based on their self-reports, they nevertheless continued showing reliable differential responding between the CS+ and the CS−. Several studies have argued that conscious awareness of contingency is not a necessary component for conditioning to occur and instead have proposed a two-level learning model involving automatic / emotional, and also conscious / cognitive processes (Bridger & Mandel, 1964; Mandel & Bridger, 1973). In this two-level model, one cognitive level involves the consciously learned CS-UCS association, which then results in the modification of responses related to orienting (Dawson & Schell, 1987; Öhman, 1974; Siddle, 1991). Another more automatic / emotional level involves the acquisition of conditioned defensive responses to an aversive CS, and this process does not require conscious awareness of the CS-UCS contingency and may reflect modification of a primitive fear network involving amygdala and the right cerebral hemisphere (Hodes, Cook, & Lang, 1985; Hugdahl, 1995). Consequently the increase in conditioning with age is likely to reflect not just an increase in basic (automated) attentional processes, but also increased conscious, controlled cognitive processes related to conscious contingency awareness.
One of the surprising findings was that conditioning was observed as early as age 3, despite the facts that (i) the UCS was not particularly intense (90 dB), (ii) a partial reinforcement schedule was used, (iii) the difference between the CS+ and CS− involved only a modest frequency change of 500 Hz, and (iv) a short number of conditioning trials were used. These combined conditions would be anticipated to make it relatively difficult to observe conditioning in young children, and yet evidence for significant conditioning was nevertheless observed. The implication for future developmental studies is that researchers can employ only modestly aversive and temporally short (7.5 minutes) paradigms to obtain a measure of fear conditioning in children, with minimal risk from a human subjects perspective. The belief that this is not entirely possible with young children may have deterred developmental researchers from implementing fear conditioning paradigms with vulnerable child populations. Finally, although ANOVA results suggested that SC conditioning emerges no earlier than age 8 years, latent growth curve modeling demonstrated that children show conditioning as early as age 3 years. This discrepancy may possibly be due to the listwise deletion used in ANOVA, whereas the EM algorithm used in latent growth curve modeling is considered to lead to less biased estimation and more reliable results.
The different components of SC conditioning showed somewhat different growth patterns across age, with more erratic and complex development of the first and third interval response at early ages compared to the relatively more linear development of the second interval response. In attempting some initial understanding of these differences, the first and third interval response are thought to reflect conditioned orienting responses, whereas the second interval response is thought to reflect a partially separate expectancy or preparatory learning process (Öhman, 1979). In an fMRI study measuring SC responses to fear stimuli, Williams et al. (2000) found that fear stimuli which elicit an SC response were associated with activation of the amygdala and medial frontal cortex, whereas fear stimuli which did not evoke SC responses activated a hippocampal-lateral network. They argued that while the amygdala-medial frontal network was preferentially involved in the visceral experience of threat, the hippocampal-lateral frontal network reflected activation of declarative and contextual processing of threatening stimuli. First and third interval response may therefore more reflect amygdala-medial frontal automated processing while the second interval response may reflect more controlled hippocampal-lateral prefrontal activation.
Although conditioning generally increased with age for the second interval response (see Figure 2), this was not true for the first interval response. In particular, conditioning of the first interval response was unexpectedly higher at age 3 than at age 4 (see Figure 2). One possible explanation for this finding is that the first test session was particularly stressful for the young children, which may also partly contribute to the large variance of the assessment at age 3 (see Table 1). Anecdotal reports from adult participants of this longitudinal study to research staff and reported to one of the authors (AR) indicate that they particularly remembered testing in the small cubicle at age 3 where conditioning took place, and that this was generally a stressful experience. Animal and human research has shown that stress facilitates the development of classical conditioning (Cordero, Venero, Kruyt, & Sandi, 2003; Jackson, Payne, Nadel, & Jacobs, 2006). In contrast, during testing the following year at age 4, some habituation to the stress of the laboratory testing would be anticipated, resulting in a reduction in conditioning (first interval response – see Figure 2). Alternatively, the child sat on the mother's lap at age 3 years, but not thereafter, and this may have facilitated arousal, which in turn, facilitated conditioning. It is noted, however, that this stress / arousal explanation cannot be applied adequately to the third interval response, which increased from 3 to 4 years.
The findings of different developmental trajectories for the first and second interval response in the current study are consistent with previous studies showing dissociation of these two components. For example, Backs and Grings (1985) recorded first and second interval responses in a long ISI conditioning paradigm and found that only the first interval response increased as a function of the objective probability of UCS occurrence, indicating preparation for the impending aversive event. Cheng et al. (2007) used fMRI and concurrent SC measurements and found that the first interval response is associated with activation of the amygdala, while the second interval response is associated with deactivation of amygdala. In addition, the deactivation of amygdala during the second interval response has been argued to represent strategies that attenuate responsiveness to the aversive UCR (Cheng et al., 2007; Petrovic et al., 2004). Similarly, Hare argued that psychopaths give a larger-than-normal cardiovascular anticipatory fear response that attenuates the responsiveness of the UCR (Hare, 1978). It is worth noting that in the current study ANOVA results have suggested that the second interval response, relative to the first and the third interval response, may show later development (at age 8). Therefore, the second interval response may be an autonomic indicator of the capacity to cope with stress, and the later development of this component may suggest that this emotion regulation capability requires more resources and emerges relatively later in children. Future developmental studies measuring both first and second interval responses and emotion regulation could further test the prediction that the second interval response is more related to emotion-regulation.
For conditioning in general, it can be seen from Figure 2 that conditioning showed a marked increase from ages 5 to 6 years. Indeed, inspection of Figure 2 indicates that this increase was the most consistent part of the growth curve across the three conditioning components. The increase in conditioning from ages 5 to 6 years is partly consistent with findings documenting the growth of the cerebral hemispheres across the first ten years of life, with a very pronounced increment in electroencephalographic (EEG) phase present in left temporal-frontal electrode sites from age 4 to 6 years (Thatcher, Walker, & Giudice, 1987) and the highest level of rate of EEG coherence growth between frontal and posterior lobes at age 6 (Thatcher, 1992). It is worth noting that SC orienting was also found to show a pronounced increase from ages 5 to 6 in this same sample (Gao et al., 2007). In the current sample, at age 5 years children began to enter state schools. This environmental change may constitute a mild stressor which could facilitate fear conditioning at this age. In addition, given that environmental enrichment in animals has been shown to result in neurogenesis in the hippocampus (Kempermann & Gage, 1999), and given the role of the hippocampus in both fear conditioning (Bast et al., 2003; Knight et al., 2004) and orienting (Critchley, 2002; Williams et al., 2000), the novelty of the school experience at age 5-6 may partly explain why association learning markedly increased at this age. Conceivably, neurogenesis in the hippocampus caused by novelty and environmental enrichment may contribute to both the periods of major change in EEG coherence, orienting, and conditioning at this age period.
A subsidiary question of this study concerned what psychophysiological factors are associated with fear conditionability within each age group. The availability of pre-conditioning measures of arousal, orienting, and UCR data at four of the five ages allowed us to examine the extent to which these processes are correlated with conditioning. Results demonstrated that all three processes are positively associated with individual differences in conditioning. The amount of variability they explained in conditionability increased from 17.2% at age 3 to 28.8% at age 6 (Table 4). While it cannot be concluded that higher orienting, arousal, and UCR are causally associated with better conditioning, the fact that all three measures precede the onset of the first conditioned response in this experiment rules out the possibility that higher fear conditioning causes increases in the three processes. It is instead conceivable that higher arousal, orienting and UCR partly facilitate better conditioning. For example, because two of the three conditioned responses (first and third interval response) are known to have a significant orienting component, increased orienting could reasonably be expected to facilitate better conditioning. Furthermore, children who give a larger UCR may be expected to be more fearful of the UCS; because a more aversive UCS is known to give rise to stronger fear conditioning (Prokasy & Kumpfer, 1973) children who are more responsive to the UCS may be expected to show a stronger UCR. Alternatively, third factors (e.g., individual differences in brain processes that regulate arousal and information- processing) may result in across-the-board increases in all processes.
One further developmental finding is that stepwise linear regression showed that while SC level (at age 3) and UCR (at age 4) had significant influences at these early ages, their influence decreased over time and was replaced by the strong influence of orienting whose contribution to conditioning increased from explaining 3.2% of the variance at age 3 to 25.4% at age 6 (see Table 4). This may be due to the developmental increase in orienting previously observed in this sample (Gao et al., 2007). Indeed, the developmental profiles of orienting (Gao et al., 2007) and conditioning are strikingly similar. This may be partly due to the fact that two of the three components of conditioning (first and third interval response) are orienting in nature, reflecting the potential importance of orienting in the facilitation of conditioning.
Experimental research attempting to understand the development of fear conditioning in children has clinical neuroscience and developmental neuroscience implications. As mentioned above, Rich et al. (2006) have argued that a paradigm shift is occurring in clinical neuroscience whereby psychiatric illnesses are increasingly being viewed as neurodevelopmental in nature, and that assessment of amygdala dysfunction can help elucidate the neurodevelopmental basis of bipolar disorder in children. Similarly, Pine et al. (2001) have argued strongly that fMRI paradigms should be developed to assess amygdala functioning in children, and that there is a critical need for the assessment of fear conditioning in childhood as a risk factor for anxiety disorders in adulthood. In this context, understanding the development of the amygdala-related processes such as the conditioned fear response in children may be critically important in understanding the etiology of adolescent and adult clinical conditions. Until functional imaging studies are able to more easily scan young children in the 3-8 year age range, a relative strength of the SC fear conditioning paradigm for child development researchers is that it allows for an indirect assessment of the neural networks subserving different forms of information-processing, in particular, the specific circuit generally implicated in fear conditioning which centrally involves the amygdala.
In conclusion, this experimental longitudinal study documents a relatively linear increase in SC fear conditioning in early childhood from ages 3 to 8 years, suggesting a gradual development of a complex automatic and controlled cognitive / emotional process in early childhood. While the more controlled expectancy and preparatory processes reflected in the second interval response develop relatively late, the stress of first testing could have produced the accentuated conditioning observed in the more orienting-related first interval response. The marked increase in average conditioning from 5 to 6 years may be accounted for by the increasing roles of arousal, orienting, and the UCR at this time (see Table 3) and the stress and novelty / environmental enrichment associated with transition from home to formal state schools at this age. The utilization of the SC fear conditioning paradigm may provide probably the best (albeit indirect) proxy measure of amygdala functioning which can be quickly and easily assessed in young community children below functional brain scanning age.
Acknowledgments
The initial data collection was made possible by grants to Peter H. Venables from the Medical Research Council (UK) and the Welcome Trust (UK). This study was also supported by grants to Adrian Raine from NIH (Independent Scientist Award K02 MH01114 and RO1 AA10206). We thank all the local members of the Mauritius Joint Child Health Project for help with data collection.
References
- Backs RW, Grings WW. Effects of UCS probability on the contingent negative variation and electrodermal response during long ISI conditioning. Psychophysiology. 1985;22:268–275. doi: 10.1111/j.1469-8986.1985.tb01599.x. [DOI] [PubMed] [Google Scholar]
- Barbas H, Saha S, Rempel-Clower N, Ghashghaei T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neuroscience. 2003;4:25. doi: 10.1186/1471-2202-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast T, Zhang WN, Feldon J. Dorsal hippocampus and classical fear conditioning to tone and context in rats: Effects of local NMDA-receptor blockage and stimulation. Hippocampus. 2003;13:657–675. doi: 10.1002/hipo.10115. [DOI] [PubMed] [Google Scholar]
- Block JD, Sersen EA, Wortis J. Cardiac classical conditioning and reversal in the mongoloid, encephalopathic, and normal child. Child Development. 1970;41:771–785. [Google Scholar]
- Bridger WH, Mandel IJ. A comparison of GSR fear responses produced by threat and electric shock. Journal of Psychiatric Research. 1964;2:31–40. doi: 10.1016/0022-3956(64)90027-5. [DOI] [PubMed] [Google Scholar]
- Büchel C, Dolan RJ. Classical fear conditioning in functional neuroimaging. Current Opinion in Neurobiology. 2000;10:219–223. doi: 10.1016/s0959-4388(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Carter RM, O'Doherty JP, Seymour B, Koch C, Dolan RJ. Contingency awareness in human aversive conditioning involves the middle frontal gyrus. NeuroImage. 2006;29:1007–1012. doi: 10.1016/j.neuroimage.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Richards J, Helmstetter FJ. Activity in the human amygdala corresponds to early, rather than late period autonomic responses to a signal for shock. Learning and Memory. 2007;14:485–490. doi: 10.1101/lm.632007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Cordero MI, Venero C, Kruyt ND, Sandi C. Prior exposure to a single stress session facilitates subsequent contextual fear conditioning in rats: Evidence for a role of corticosterone. Hormones and Behavior. 2003;44:338–345. doi: 10.1016/s0018-506x(03)00160-0. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Electrodermal responses: What happens in the brain. Neuroscientist. 2002;8:132–142. doi: 10.1177/107385840200800209. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Fear conditioning in humans: The influence of awareness and autonomic arousal of functional neuroanatomy. Neuron. 2002;33:653–663. doi: 10.1016/s0896-6273(02)00588-3. [DOI] [PubMed] [Google Scholar]
- Davey GCL. Classical conditioning and the acquisition of human fears and phobias: a review and synthesis of the literature. Advances in Behaviour Research and Therapy. 1992;14:29–66. [Google Scholar]
- Davis M. The role of the amygdala in conditioned fear. In: Aggleton JP, editor. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. Wiley; New York: 1992. pp. 255–305. [Google Scholar]
- Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton JP, editor. The amygdala: A functional analysis. 2nd ed. Oxford University Press; New York: 2000. pp. 213–287. [Google Scholar]
- Dawson ME. Can classical conditioning occur without contingency learning? A review and evaluation of the evidence. Psychophysiology. 1973;10:82–86. doi: 10.1111/j.1469-8986.1973.tb01087.x. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Furedy JJ. The role of awareness in human differential autonomic classical conditioning: The necessary-gate hypothesis. Psychophysiology. 1976;13:50–53. doi: 10.1111/j.1469-8986.1976.tb03336.x. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM. Human autonomic and skeletal classical conditioning: The role of conscious factors. In: Davey F, editor. Cognitive processes and Pavlovian conditioning in humans. John Wiley and Sons; New York: 1987. pp. 27–57. [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annual Review of Psychology. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Field AP. Is conditioning a useful framework for understanding the development and treatment of phobias? Clinical Psychology Review. 2006;26:857–875. doi: 10.1016/j.cpr.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Fowles DC. Electrodermal hyporeactivity and antisocial behavior: Does anxiety mediate the relationship? Journal of Affective Disorders. 2000;61:177–189. doi: 10.1016/s0165-0327(00)00336-0. [DOI] [PubMed] [Google Scholar]
- Gao Y, Raine A, Dawson ME, Venables PH, Mednick SA. Development of skin conductance orienting, habituation, and reorienting from ages 3 to 8 years: A longitudinal latent growth curve analysis. Psychophysiology. 2007;44:855–863. doi: 10.1111/j.1469-8986.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- Grings WW. Anticipatory and preparatory electrodermal behavior in paired stimulation situations. Psychophysiology. 1969;5:597–611. doi: 10.1111/j.1469-8986.1969.tb02862.x. [DOI] [PubMed] [Google Scholar]
- Hare RD. Electrodermal and cardiovascular correlates of psychopathy. In: Hare RD, Schalling D, editors. Psychopathic behavior: Approaches to research. Wiley; New York: 1978. pp. 107–144. [Google Scholar]
- Hodes RL, Cook EW, III, Lang PJ. Individual differences in autonomic responses: Conditioned association or conditioned fear? Psychophysiology. 1985;22:545–560. doi: 10.1111/j.1469-8986.1985.tb01649.x. [DOI] [PubMed] [Google Scholar]
- Hosoba T, Iwanaga M, Seiwa H. The effect of UCS inflation and deflation procedures on ‘fear’ conditioning. Behaviour Research and Therapy. 2001;39:465–475. doi: 10.1016/s0005-7967(00)00025-5. [DOI] [PubMed] [Google Scholar]
- Hugdahl K. Psychophysiology: The Mind-Body Perspective. Harvard University Press; Cambridge, MA: 1995. [Google Scholar]
- Ingram EM. The interstimulus interval in classical autonomic conditioning of young infants. Developmental Psychobiology. 1978;11:419–426. doi: 10.1002/dev.420110506. [DOI] [PubMed] [Google Scholar]
- Ingram EM, Fitzgerald HE. Individual differences in infant orienting and autonomic conditioning. Developmental Psychobiology. 1974;7:359–367. doi: 10.1002/dev.420070413. [DOI] [PubMed] [Google Scholar]
- Jackson ED, Payne JD, Nadel L, Jacobs WJ. Stress differentially modulates fear conditioning in healthy men and women. Biological Psychiatry. 2006;59:516–522. doi: 10.1016/j.biopsych.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. Experience-dependent regulation of adult hippocampal neurogenesis: Effects of long-term stimulation and stimulus withdrawal. Hippocampus. 1999;9:321–332. doi: 10.1002/(SICI)1098-1063(1999)9:3<321::AID-HIPO11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. The role of the human amygdala in the production of conditioned fear responses. NeuroImage. 2005;26:1193–1200. doi: 10.1016/j.neuroimage.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Knight DC, Smith CN, Cheng DT, Stein EA, Helmstetter FJ. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cognitive, Affective Behavioral Neuroscience. 2004;4:317–325. doi: 10.3758/cabn.4.3.317. [DOI] [PubMed] [Google Scholar]
- LaBar KS, LeDoux JE, Spencer DD, Phelps EA. Impaired fear conditioning following unilateral temporal lobectomy in humans. The Journal of Neuroscience. 1995;15:6846–6855. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. Wiley & Sons; New York: 1987. [Google Scholar]
- Mandel I, Bridger WH. Is there classical conditioning without cognitive expectancy? Psychophysiology. 1973;10:87–90. doi: 10.1111/j.1469-8986.1973.tb01088.x. [DOI] [PubMed] [Google Scholar]
- Morrow MC, Boring FW, Keough TE, Haesly RR. Differential GSR conditioning as a function of age. Developmental Psychology. 1969;1:299–302. [Google Scholar]
- Muthén LK, Muthén BO. Mplus user's guide. Muthén & Muthén; Los Angeles, CA: 1998. [Google Scholar]
- Öhman A. Human fear conditioning: Focus on the amygdala. In: Whalen P, Phelps E, editors. The human amygdala. Guilford; New York: in press. [Google Scholar]
- Öhman A. Factor analytically derived components of orienting, defensive, and conditional behavior in electrodermal conditioning. Psychophysiology. 1972;9:199–209. doi: 10.1111/j.1469-8986.1972.tb00754.x. [DOI] [PubMed] [Google Scholar]
- Öhman A. Orienting reactions, expectancy learning, and conditioned responses in electrodermal conditioning with different interstimulus intervals. Biological Psychology. 1974;1:189–200. doi: 10.1016/0301-0511(74)90011-8. [DOI] [PubMed] [Google Scholar]
- Öhman A. The orienting response, attention, and learning: An information- processing perspective. In: Kimmel HD, van Olst EH, Orelebeke JF, editors. The orienting reflex in humans. Erlbaum; Hillsdale, NJ: 1979. pp. 443–471. [Google Scholar]
- Öhman A. The orienting response during Pavlovian conditioning. In: Siddle DE, editor. Orienting and habituation: Perspectives in human research. Wiley; Chichester, U.K.: 1983. pp. 315–369. [Google Scholar]
- Petrovic P, Carlsson K, Petersson KM, Hansson P, Ingvar M. Context-dependent deactivation of the amygdala during pain. Journal of Cognitive Neuroscience. 2004;16:1289–1301. doi: 10.1162/0898929041920469. [DOI] [PubMed] [Google Scholar]
- Pine DS, Fyer A, Grun J, Phelps EA, Szeszko PR, Koda V, et al. Methods for developmental studies of fear conditioning circuitry. Biological Psychiatry. 2001;50:225–228. doi: 10.1016/s0006-3223(01)01159-3. [DOI] [PubMed] [Google Scholar]
- Prokasy WF, Ebel HC. Three components of the classically conditioned GSR in human subjects. Journal of Experimental Psychology. 1967;73:247–256. [Google Scholar]
- Prokasy WF, Kumpfer KL. Classical conditioning. In: Prokasy WF, Raskin DC, editors. Electrodermal activity in psychological research. Academic Press; New York: 1973. pp. 197–202. [Google Scholar]
- Raine A, Venables PH, Mednick SA, Mellingen K. Increased physiological arousal and orienting at ages 3 and 11 years in persistently schizotypal adults. Schizophrenia Research. 2002;54:77–85. doi: 10.1016/s0920-9964(01)00354-1. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH, Williams M. Better autonomic conditioning and faster electrodermal half-recovery time at age 15 years as possible protective factors against crime at age 29 years. Developmental Psychology. 1996;32:624–630. [Google Scholar]
- Raine A, Yaralian PS, Reynolds C, Venables PH, Mednick SA. Spatial but not verbal cognitive deficits at age 3 years in persistently antisocial individuals. Development and Psychopathology. 2002;14:25–44. doi: 10.1017/s0954579402001025. [DOI] [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proceeding of the National Academy of Sciences of the United States of America. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell AM, Dawson ME, Marinkovic K. Effects of the use of potentially phobic CSs on retention, reinstatement, and extinction of the conditioned skin conductance response. Psychophysiology. 1991;28:140–153. doi: 10.1111/j.1469-8986.1991.tb00403.x. [DOI] [PubMed] [Google Scholar]
- Shi C, Davis M. Visual pathways involved in fear conditioning measured with fear-potentiated startle: behavioral and anatomic studies. Journal of Neuroscience. 2001;21:9844–9855. doi: 10.1523/JNEUROSCI.21-24-09844.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddle DAT. Orienting, habituation, and resource allocation: An associative analysis. Psychophysiology. 1991;28:245–259. doi: 10.1111/j.1469-8986.1991.tb02190.x. [DOI] [PubMed] [Google Scholar]
- Sokolov EN. Perception and the conditioned reflex. Pergamon Press; Oxford: 1963. [Google Scholar]
- Tabbert K, Stark R, Kirsch P, Vaitl D. Dissociation of neural responses and skin conductance reactions during fear conditioning with and without awareness of stimulus contingencies. NeuroImage. 2006;32:761–770. doi: 10.1016/j.neuroimage.2006.03.038. [DOI] [PubMed] [Google Scholar]
- Thatcher RW, Walker RA, Giudice S. Human cerebral hemispheres develop at different rates and ages. Science. 1987;236:1110–1113. doi: 10.1126/science.3576224. [DOI] [PubMed] [Google Scholar]
- Thatcher RW. Cyclical cortical reorganization during early childhood. Brain and Cognition. 1992:24–50. doi: 10.1016/0278-2626(92)90060-y. [DOI] [PubMed] [Google Scholar]
- Venables PH. Psychophysiology and psychometrics. Psychophysiology. 1978;15:302–315. doi: 10.1111/j.1469-8986.1978.tb01383.x. [DOI] [PubMed] [Google Scholar]
- Venables PH, Christie MJ. Electrodermal activity. In: Martin I, Venables PH, editors. Techniques in psychophysiology. Wiley & Sons; New York: 1980. pp. 3–67. [Google Scholar]
- Williams LM, Brammer MJ, Skerrett D, Lagopolous J, Rennie C, Kozek K, et al. The neural correlates of orienting: An integration of fMRI and skin conductance orienting. NeuroReport. 2000;11:3011–3015. doi: 10.1097/00001756-200009110-00037. [DOI] [PubMed] [Google Scholar]