Abstract
Autoimmune diseases comprise a group of about 85 heterogeneous conditions that can affect virtually any organ and tissue in the body. Many autoimmune diseases change significantly during pregnancy: some ameliorate, some worsen, and others are unaffected. Two autoimmune diseases present prominently in relation to pregnancy: postpartum autoimmune thyroiditis and autoimmune hypophysitis. This article will review the current state of knowledge of the immunological changes that occur during normal pregnancy, and will explore the striking temporal association with pregnancy observed in thyroiditis and hypophysitis.
Keywords: Pregnancy, Hypophysitis, Postpartum thyroiditis
1. Historical context and scope
The immune system changes significantly during pregnancy. Indeed, it is still a mystery why the mother does not reject the fetus who carries paternal antigens (semi-allograft) and thus should be subjected to the standard laws of transplantation. This immunological paradox of pregnancy has fascinated scientists for generations. In 1953 Medawar proposed three reasons for the lack of fetus rejection: the fetus is antigenically immature; the fetus is anatomically separated from the mother; the maternal immune system is “paralyzed” or “inert” during pregnancy. It is now well established that pregnant women make antibodies directed against paternal antigens, indicating the fetus is not antigenically immature [1]. And, in fact, the MHC antisera used extensively for tissue typing are obtained from multiparous women. Second, cells traffic between mother and fetus during pregnancy, giving rise to microchimerism, with quantitatively greater transfer in the mother-to-fetus direction [2], proving that the fetus is not anatomically sequestered from the mother. Finally, the maternal immune system is not wholly suppressed during pregnancy since it is capable of mounting a response against live infectious agents or vaccines [3]. Medawar's postulates have, thus, been largely abandoned, but have stimulated the quest for fresh understanding of how the fetus evades maternal immune recognition. Modern studies focus on the fetal placenta and the maternal uterine decidua.
2. Placental mechanisms of immunoregulation
A brief review of placental anatomy is necessary to envision the site of action of immunoregulatory mechanisms (Table 1). The placenta is a dish-shaped fetal organ required to maintain pregnancy. The placenta, at term, is composed of three major zones called the basal plate, lacunar system, and chorionic plate.
Table 1.
Mechanisms that induce tolerance at the placenta-decidua interface
| Syncytiotrophoblast expresses a unique pattern of MHC molecules |
| Expression HLA-G and release of HLA-G1 |
| Expression of HLA-E |
| Expression of HLA-C |
| Lack of HLA-A, HLA-B, HLA-DP, HLA-DQ and HLA-DR |
| Syncytiotrophoblast expresses high levels of indolamine 2,3-dioxygenase |
| IDO reduces the local concentration of tryptophan below the level required for T cell activation |
| Syncytiotrophoblast expresses Fas ligand |
| Induction of apoptosis on Fas positive T cells |
| Syncytiotrophoblast expresses regulatory proteins (CD46, CD55, and CD59) that lower complement activity |
| Decidua is infiltrated by uterine natural killer cells |
| Decidua is infiltrated by regulatory T cells |
The basal plate is the maternal surface of the placenta; it makes contact with the inner lining of the uterus (endometrium), which during pregnancy takes on the name of decidua basalis. After delivery of the placenta, some decidua basalis is left in the uterus and some is part of the basal plate. The basal plate is a mixture of maternal cells [uterine natural killer lymphocytes (70%), T lymphocytes (10%), macrophages and other myeloid cells (20%)] and fetal extravillous trophoblast cells (see below), all embedded in extracellular debris, fibrinoid, and blood clots. The surface of the basal plate is subdivided into 10-40 areas called cotyledons by a system of incomplete grooves. The grooves correspond histologically to the pillars that protrude into the lacunar system and subdivide it into 10-40 lobes.
The chorionic plate is the fetal surface of the placenta; it faces the amniotic cavity and the embryo, and gives rise to the umbilical chord. It consists of the amniotic layer and the chorion. The amniotic layer, which contacts the amniotic fluid, is a single layer of epithelial cells positioned above an avascular connective tissue stroma. The chorion is composed of a vascular stroma and trophoblast cells. Approximately 60-70 vascular villi (also called fetal lobules) emerge from the chorionic plate and protrude into the lacunar space, so that each lobe is occupied by one to four villi. Most villi are freely floating into the lacunar system, whereas others reach the basal plate anchoring the placenta to the maternal endometrium. Each villum consists of a stromal core covered by an epithelium-like layer called the villous trophoblast. The villous trophoblast has two components: cytotrophoblast and syncytiotrophoblast. The cytotrophoblast forms a complete, uninucleated layer of cells in direct contact with the basement membrane; as cells proliferate, they detach from the basement membrane, differentiate, and fuse to form the multinucleated villous syncytiotrophoblast. The syncytiotrophoblast is like a continuous blanket that envelopes the forest of villous trees. Its cells are polarized: the apical side of these cells, the one that contacts the maternal blood filling the lacunar system, has in fact numerous microvilli, which amplify the surface area about seven fold. Villous syncytiotrophoblast cells continue to differentiate after their formation, expressing proteins specific for this layer (HLA-G), but loose any proliferative activity so that the maintenance of this syncytial layer is totally dependent upon the cells from the villous cytotrophoblast.
The key mechanisms that induce tolerance at the placenta-decidua interface are summarized below.
Syncytiotrophoblast cells express a unique pattern of MHC molecules. They lack the classical MHC molecules (HLA-A, HLA-B, and class II molecules), even after interferon-gamma stimulation, but express classical HLA-C and non-classical HLA-E and HLA-G molecules [4]. This expression profile prevents NK cell activation and CD8 mediated cytotoxicity.
Syncytiotrophoblast cells and macrophages express high levels of indoleamine 2,3- dioxygenase [5], an enzyme that degrades and thus decreases the local concentration of tryptophan. T cells are extremely sensitive to tryptophan and do not proliferate when tryptophan levels drop [5].
Syncytiotrophoblasts express Fas ligand (CD95L) [6], a molecule that when bound to Fas (CD95) on activated lymphocytes induces their death, and complement regulatory proteins (like CD46, CD55, and CD59) that dampen complement activation [7].
The transformation of uterus into decidua is accompanied by a marked infiltration of NK cells [8]. In human peripheral blood, NK cells exist as two major subsets: CD56dim CD16+ and CD56bright CD16low or negative. The first and most abundant subset contains cells that are more cytotoxic, more refractory to IL-2-induced proliferation, and poor cytokine producers. In contrast, CD56bright CD16low or negative cells produce more cytokines, proliferate in response to low levels of IL-2, and have low cytotoxicity because of the absence or decreased expression of CD16. Uterine NK cells resemble the second subset because they express high levels of CD56, produce large amounts of immunoregulatory cytokines (e.g. IFNγ, GMCSF, TNF), and are less cytotoxic. Uterine NK cells likely originate from secondary lymphoid organs such as spleen and lymph nodes and then migrate to the decidua, homing through their Lselectin and α4-integrin receptors [9]. The functions of uterine NK cells are still unclear. They might play a role in guiding the invading trophoblast into the decidual stroma, and in replacing the tunica media of the spiral arteries with fibrinoid material [8]. Mice deficient in uterine NK cells are prone to fetal losses, showing hypocellular decidual stroma and unmodified spiral arteries [10].
A lot of attention has been devoted lately to CD4 CD25 Foxp3 regulatory T cells (Treg), a population capable of suppressing proliferation and activation of other T cells, either through direct cell-cell contact or by release of immunosuppressive cytokines like IL-10 and TGF-β. Treg increase very early after conception, peak during the second trimester, and decline postpartum [11]. The increase is independent of paternal antigens since it can be seen also in syngeneic pregnancies [12]. Treg accumulate in the decidua, where they represent 30% of the total CD4 positive population [13]. The proportion of decidual Treg is significantly lower in specimens from spontaneous abortion than in those from induced abortions [14], indicating that Treg contribute to preventing fetus rejection. Recently, Schumacher and colleagues have shown that Treg are attracted to the implantation site by human chorionic gonadotropin (hCG) [15]. Once Treg are generated, they can suppress other immunological processes, rather than just controlling a response to the fetus semi-allograft.
The mechanisms highlighted above indicate that pregnancy is associated not with a state of immunosuppression, as previously believed, but rather with a state of selective tolerance. This selective tolerance is also transient [16]. Tafuri et al used mice transgenic for a T cell receptor recognizing the MHC molecule H-2Kb (a class I MHC molecule homologous to the human HLAA), to follow the fate of T cells recognizing paternal alloantigens. These transgenic mice have a high number of cytotoxic CD8 T cells specific for the Kb antigen. When transgenic females are bred on a Kk MHC haplotype and then mated to Kb males, they harbor semi-allogeneic (Kk/b) fetuses. These pregnant females had reduced numbers of Kb-reactive T cells and accepted tumor grafts from Kb positive donors. After delivery, the T cell phenotype and responsiveness were restored, indicating that during pregnancy maternal T cells acquire a transient state of tolerance specific for paternal alloantigens [16].
3. Effect of pregnancy on thyroid and pituitary structure and function
Thyroid function and structure change profoundly during pregnancy, modifying the thyroid function tests [17] that thus need to be interpreted with greater attention.
The demand on thyroxine (T4) production from the thyroid gland increases as a consequence of the increased estrogen levels, which induce the hepatic production of thyroxin binding globulin. This carrier protein binds the T4 produced by the thyroid, decreasing the amount of free (bioactive) T4 in the circulation. The decrease stimulates the production of thyrotropin (TSH) from the pituitary gland until the total/free T4 equilibrium is re-established. Increased T4 production (or demand in hypothyroid women) continues even after thyroxine binding globulin levels plateau at mid gestation, reflecting the high thyroid hormone turnover secondary to fetal transfer of T4 and placental inactivation of thyroid hormones (see below).
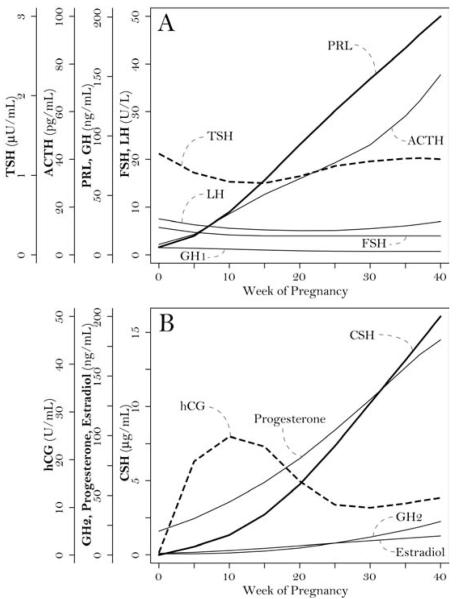
Serum TSH levels (Figure 1A) decrease during the first trimester as a consequence of the placental production of hCG. This dimeric glycoprotein, the first marker of trophoblast differentiation, stimulates the production of progesterone by the ovary. The alpha chain of hCG is identical to that of TSH, luteinizing hormone (LH), and follicle-stimulating hormone (FSH); whereas its beta chain is unique and responsible for the biologic activity. hCG also has a weak TSH-like activity so that high hCG levels during the first trimester (Figure 1B) induce a transient biochemical hyperthyroidism. TSH levels then increase during the second trimester and normalize in the third trimester.
Figure 1.
Serum levels of pituitary (A) and placental (B) hormones during pregnancy.
Iodine, the essential element for thyroid hormone synthesis, decreases during pregnancy as a consequence of increased renal clearance and redistribution to the fetus. These changes are accentuated if the mother was iodine deficient before pregnancy, and can cause goiter and hypothyroidism [17].
Maternal thyroid volume increases during pregnancy (goiter) as a consequence of the iodine deficiency indicated above, the thyroid stimulatory actions of hCG, and the increased blood flow that induces a vascular intumescence of the thyroid [17].
The thyroid hormones produced by the mother enter the fetus via the placenta, which regulates this transfer through the expression of deiodinases [18]. The placenta expresses high levels of deiodinase 3, the inactivating enzyme, thus avoiding an unrestricted transfer of maternal thyroid hormones to the fetus. It is worth noting that in the first trimester the small amount of T4 escaping placental inactivation represents the only source of thyroid hormone in the fetus, before the appearance of a functional thyroid. This source is fundamental for the correct development of fetal brain. Low maternal T4 concentration due to hypothyroidism or iodine deficiency may lead to irreversible fetal brain damage [19]. Considering the pregnancy-related changes in thyroid function, most notable in the first trimester, gestation-specific reference intervals for TSH and T4 are recommended.
Pituitary structure and function also change significantly during pregnancy [20]. Pituitary weight increases by about 30% over the pregestational values of 0.5-1.0g [21], so that the mean pituitary height assessed by MRI increases from 5.5 to about 10 mm, peaking on day 3 after delivery [22]. Pituitary enlargement results from the hyperplasia and hypertrophy of the prolactin-secreting cells (lactotropes). Prolactin (PRL) is in fact the only anterior pituitary hormone that increases progressively during pregnancy (Figure 1A). No significant changes are seen for LH, FSH, and growth hormone (GH1) (Figure 1A). Corticotrophin (ACTH) increases progressively during pregnancy, peaking in labor (Figure 1A).
The increased estrogen levels modify the blood flow to the pituitary, so that more blood derives from the systemic circulation and less from the portal system [23].
4. Pregnancy and postpartum autoimmune thyroiditis
The occurrence of thyroid dysfunctions after delivery was published as early as 1825 by Dr. Caleb Hillier Parry, who reported the case of a woman with Graves disease appearing 3 months postpartum, and 1888 by Dr. Horatio Bryan Donkin, who described a severe hypothyroidism 7 months after delivery. Postpartum autoimmune thyroiditis (PPAT) is a common endocrinological disorder that uniquely manifests itself within one year after delivery. The prevalence of 8% in the general population increases to 20% in women with type 1 diabetes or a family history of thyroid disease, and to 42% in women with a history of prior PPAT [24]. Symptoms may be subtle and confused with postpartum depression. Most patients (40%) become hypothyroid, a third hyperthyroid, either because of destructive thyrotoxicosis (24%) or Graves disease (11%), and the remaining patients (25%) develop a biphasic form characterized by an initial hyperthyroidism followed by hypothyroidism. PPAT is transient in most women but evolves to permanent hypothyroidism in 20% of the patients or, more rarely, to Graves disease.
The presence of serum antibodies against thyroperoxidase during the first trimester is the best predictor of PPAT development. In 2000, RC Smallridge reviewed 10 studies assessing the utility of this testing [25]. The odds of developing PPAT were on average 33-fold greater (range 10-59) in women with thyroperoxidase antibodies than in those without. Thyroperoxidase antibodies were absent in the majority of healthy women who did not develop PPAT (0.94 average specificity, range 0.90-0.98), and present in the majority of women who develop PPAT (0.71 average sensitivity, range 0.45-0.89). These sensitivity and specificity values, however, translated to a mediocre positive predictive value (average 0.57, range 0.31-0.78) so that a systematic screening of all pregnant women for thyroperoxidase antibodies is currently not recommended [26].
The mechanisms underlying PPAT remain unknown. Women who develop PPAT have a higher CD4/CD8 ratio in the peripheral blood [27] and the thyroid [28], and a greater number of activated T cells [29], suggesting a heightened immune activity during and after pregnancy, which could trigger PPAT.
Microchimerism is another possible explanation. Fetal immune cells cross the placenta and home to the maternal thyroid gland, triggering an autoimmune reaction akin to the graft-versus-host reaction [30]. Fetal cells have been reported in the thyroid of patients with autoimmune thyroid diseases, although studies looking specifically at PPAT are lacking. Ando and Davies propose that after delivery, when placental tolerogenic mechanisms are lost, intrathyroidal fetal immune cells are activated and initiate a graft-versus-host reaction against maternal antigens resulting in the activation of maternal autoreactive T cells which could eventually modulate autoimmune thyroid diseases in the postpartum [30]. In fact, a study on systemic sclerosis found that fetal T cells in the peripheral blood and skin lesions of women with systemic sclerosis are capable of proliferating in response to maternal MHC [31].
Genetic susceptibility has also been reported in PPAT, with candidate genes similar to those reported for Hashimoto thyroiditis (MHC class II DR3) [27]. In keeping with this susceptibility, PPAT associates with other autoimmune manifestations. For example, Manetti and colleagues have reported that pituitary antibodies are 5 times more common in PPAT cases than in healthy controls (27% versus 5% prevalence) [32].
5. Pregnancy and autoimmune hypophysitis
Autoimmune hypophysitis (AH) is an endocrine disease of more recent characterization. Even in the original reports in the 1960s, a striking temporal association with pregnancy was recognized. This association is clear in the form of AH that affects the anterior pituitary lobe. Of the 215 women thus far reported with adenohypophysitis at ages between 15 and 45 years, 149 (69%) manifested AH during late pregnancy or postpartum. The association is much weaker for infundibulo-neurohypophysitis and pan-hypophysitis [33]. AH usually presents in late pregnancy or early postpartum with neurologic symptoms due to compression of meninges (headache) or optic chiasm (visual disturbances). Development of AH during pregnancy seems to have no adverse effects on the fetus or on the ability to become pregnant in the future [34-36]. In addition, when a subsequent pregnancy occurs in a patient with AH, the risk of disease recurrence is not increased by the history of AH. Numbers, however, are small and symptom recurrence has been reported during subsequent pregnancies. Sinha et al., for example, reported a woman who developed AH during her first pregnancy (at week 32 of gestation), and then experienced a recurrence during her second pregnancy (at week 16) [35].
Perhaps the most compelling evidence of a mechanistic link between pregnancy and increased susceptibility of the pituitary gland to develop hypophysitis comes from a recent case report of ovarian teratoma [37]. Derived from pluripotent germ cells, teratomas can give rise to ectopic tissues (such as teeth, hair, and jawbone) in the organ where they reside. It is exceedingly rare for a teratoma to give rise to anterior pituitary tissue. Schreiber-Facklam and colleagues described a 30-year old woman during the 19th week of pregnancy who developed acute abdominal pain. Ultrasound showed a 4 cm cyst in the left ovary, with normal uterus, placenta, and fetus. The patient underwent emergency laparoscopy to remove the ovarian mass. Histology showed a mature cystic teratoma with an island of adenohypophyseal tissue. Remarkably, this tissue was infiltrated by numerous T and B lymphocytes, faithfully reproducing the histological appearance of AH.
The mechanisms discussed above do not explain why the incidence of AH actually rises during pregnancy. Based on the tolerance mechanisms described above, one would predict suppression or amelioration of AH during pregnancy rather than exacerbation. A possible explanation for the link between pregnancy and AH is molecular mimicry, that is the existence of an autoantigen expressed both in the pituitary gland and the placenta.
O'Dwyer and colleagues first proposed gamma enolase as a candidate for this molecular mimicry. The enzyme catalyzes the interconversion of 2-phosphoglycerate and phosphoenolpyruvate, the penultimate step in glycolysis. This highly conserved protein [38] exists in mammals as a dimer, made by the combination of three different subunits (alpha, beta, and gamma). Enolase 1 or alpha (made of two alpha subunits encoded by human chromosome 1) is expressed in every cell. Enolase 2 or gamma (made of two gamma subunits encoded by chromosome 12) is expressed mainly in the brain including hypothalamus and pituitary, but also in T lymphocytes and pancreatic islets. Enolase 3 or beta (made of two beta subunits encoded by chromosome 17) is expressed mainly in the tongue and skeletal muscle, but also in thyroid, liver, and heart. O'Dwyer and colleagues reported that gamma enolase was expressed also in the placenta, and was recognized by the serum of a patient with peripartum AH [39].
Lupi and colleagues described more recently a peptide (KDLEEGIQTLMGRL) recognized by the serum of AH patients but not controls [40]. This peptide is found in both the pituitary growth hormone (GH1) and the placenta chorionic somatomammotropin hormones (CSH1 and CSH2) [40]. The somatomammotropins, formerly known as placental lactogens, are two nearly identical glycoproteins of 217 amino acids differing only at the third position (proline in CSH1 and alanine in CSH2). It is possible that immune recognition of CSH spreads during pregnancy to GH1, causing the clinical appearance of AH. Notably, CSHs are the hormones that reach the highest concentrations during pregnancy (Figure 1B), providing ample antigenic supply to a primed immune response. More studies need to be performed to confirm that indeed the peptide is recognized specifically by AH patients and is capable of inducing an experimental model of hypophysitis when injected into animals.
Another possible explanation of the link between pregnancy and AH is the transformation in structure and function that occurs in the pituitary during pregnancy (summarized in section 3). These changes may render the pituitary gland more accessible to the immune system or increase the release of pituitary antigens. Finally, pregnancy and the associated pituitary enlargement can simply unmask a pre-existing but clinically silent disease, working as an enhancing factor.
In summary, the fascinating association of AH and PPAT with pregnancy remains unexplained, but much can be learned through efforts aimed at explaining it.
Take-home messages.
• Pregnancy is associated with a selective and transient state of tolerance, rather than with generalized immune suppression.
• AH and PPAT presentation is tightly linked with pregnancy.
• The mechanisms explaining the association between pregnancy and AH or PPAT remain to be elucidated. When identified, they will advance our understanding of disease pathogenesis and likely provide new diagnostic assays or treatments.
Acknowledgements
The work was supported by NIH grant DK080351 to PC, and by the Heideneich-von-Siebold-Stipendium Georg-August-University Göttingen, Germany to AG.
Abbreviations
- AH
autoimmune hypophysitis
- PPAT
postpartum autoimmune thyroiditis
- MHC
major histocompatibility complex
- HLA
human leukocyte antigen
- hCG
human chorionic gonadotropin
- TSH
thyrotropin
- CSH
chorionic somatomammotropin hormone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van Rodd JJ, Eernisse JG, van Leeuwen A. Leukocyte antibodies in sera from pregnant women. Nature. 1958;181:1735–36. doi: 10.1038/1811735a0. [DOI] [PubMed] [Google Scholar]
- 2.Lo YM, Lau TK, Chan LY, Leung TN, Chang AM. Quantitative analysis of the bi-directional feto-maternal transfer of nucleated cells and plasma DNA. Clin Chem. 2000;46:1301–09. [PubMed] [Google Scholar]
- 3.Gall SA. Maternal immunization. Obstet Gynecol Clin North Am. 2003;30:623–36. doi: 10.1016/s0889-8545(03)00085-8. [DOI] [PubMed] [Google Scholar]
- 4.Trowsdale J, Betz AG. Mother's little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol. 2006;7:241–6. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- 5.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 6.Pongcharoen S, Searle RF, Bulmer JN. Placental Fas and Fas ligand expression in normal early, term and molar pregnancy. Placenta. 2004;25:321–30. doi: 10.1016/j.placenta.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Morgan BP, Holmes CH. Immunology of reproduction: protecting the placenta. Curr Biol. 2000;10:R381–3. doi: 10.1016/s0960-9822(00)00476-0. [DOI] [PubMed] [Google Scholar]
- 8.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2:656–63. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 9.Chantakru S, Miller C, Roach LE, Kuziel WA, Maeda N, Wang WC, Evans SS, Croy BA. Contributions from self-renewal and trafficking to the uterine NK cell population of early pregnancy. J Immunol. 2002;168:22–8. doi: 10.4049/jimmunol.168.1.22. [DOI] [PubMed] [Google Scholar]
- 10.Guimond MJ, Luross JA, Wang B, Terhorst C, Danial S, Croy BA. Absence of natural killer cells during murine pregnancy is associated with reproductive compromise in TgE26 mice. Biol Reprod. 1997;56:169–79. doi: 10.1095/biolreprod56.1.169. [DOI] [PubMed] [Google Scholar]
- 11.Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–71. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 13.Tilburgs T, Roelen DL, van der Mast BJ, de Groot-Swings GM, Kleijburg C, Scherjon SA, Claas FH. Evidence for a selective migration of fetus-specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J Immunol. 2008;180:5737–45. doi: 10.4049/jimmunol.180.8.5737. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10:347–53. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- 15.Schumacher A, Brachwitz N, Sohr S, Engeland K, Langwisch S, Dolaptchieva M, Alexander T, Taran A, Malfertheiner SF, Costa SD, Zimmermann G, Nitschke C, Volk HD, Alexander H, Gunzer M, Zenclussen AC. Human chorionic gonadotropin attracts regulatory T cells into the fetal-maternal interface during early human pregnancy. J Immunol. 2009;182:5488–97. doi: 10.4049/jimmunol.0803177. [DOI] [PubMed] [Google Scholar]
- 16.Tafuri A, Alferink J, Moller P, Hammerling GJ, Arnold B. T cell awareness of paternal alloantigens during pregnancy. Science. 1995;270:630–3. doi: 10.1126/science.270.5236.630. [DOI] [PubMed] [Google Scholar]
- 17.Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev. 1997;18:404–33. doi: 10.1210/edrv.18.3.0300. [DOI] [PubMed] [Google Scholar]
- 18.Chan SY, Vasilopoulou E, Kilby MD. The role of the placenta in thyroid hormone delivery to the fetus. Nat Clin Pract Endocrinol Metab. 2009;5:45–54. doi: 10.1038/ncpendmet1026. [DOI] [PubMed] [Google Scholar]
- 19.Morreale de Escobar G, Obregon MJ, del Rey FE. Iodine deficiency and brain development in the first half of pregnancy. Public Health Nutr. 2007;10:1554–70. doi: 10.1017/S1368980007360928. [DOI] [PubMed] [Google Scholar]
- 20.Foyouzi N, Frisbaek Y, Norwitz ER. Pituitary gland and pregnancy. Obstet Gynecol Clin North Am. 2004;31:873–92. doi: 10.1016/j.ogc.2004.08.003. xi. [DOI] [PubMed] [Google Scholar]
- 21.Elster AD, Sanders TG, Vines FS, Chen MY. Size and shape of the pituitary gland during pregnancy and post partum: measurement with MR imaging. Radiology. 1991;181:531–5. doi: 10.1148/radiology.181.2.1924800. [DOI] [PubMed] [Google Scholar]
- 22.Dinc H, Esen F, Demirci A, Sari A, Resit Gumele H. Pituitary dimensions and volume measurements in pregnancy and post partum. MR assessment. Acta Radiol. 1998;39:64–9. doi: 10.1080/02841859809172152. [DOI] [PubMed] [Google Scholar]
- 23.Elias KA, Weiner RI. Direct arterial vascularization of estrogen-induced prolactin-secreting anterior pituitary tumors. Proc Natl Acad Sci U S A. 1984;81:4549–53. doi: 10.1073/pnas.81.14.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicholson WK, Robinson KA, Smallridge RC, Ladenson PW, Powe NR. Prevalence of postpartum thyroid dysfunction: a quantitative review. Thyroid. 2006;16:573–82. doi: 10.1089/thy.2006.16.573. [DOI] [PubMed] [Google Scholar]
- 25.Smallridge RC. Postpartum thyroid disease. A model of immunologic dysfunction. Clin Appl Immunol Rev. 2000;1:89–103. [Google Scholar]
- 26.Abalovich M, Amino N, Barbour LA, Cobin RH, De Groot LJ, Glinoer D, Mandel SJ, Stagnaro-Green A. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2007;92:S1–47. doi: 10.1210/jc.2007-0141. [DOI] [PubMed] [Google Scholar]
- 27.Stagnaro-Green A, Roman SH, Cobin RH, el-Harazy E, Wallenstein S, Davies TF. A prospective study of lymphocyte-initiated immunosuppression in normal pregnancy: evidence of a T-cell etiology for postpartum thyroid dysfunction. J Clin Endocrinol Metab. 1992;74:645–53. doi: 10.1210/jcem.74.3.1740500. [DOI] [PubMed] [Google Scholar]
- 28.Jansson R, Totterman TH, Sallstrom J, Dahlberg PA. Intrathyroidal and circulating lymphocyte subsets in different stages of autoimmune postpartum thyroiditis. J Clin Endocrinol Metab. 1984;58:942–6. doi: 10.1210/jcem-58-5-942. [DOI] [PubMed] [Google Scholar]
- 29.Kuijpens JL, De Hann-Meulman M, Vader HL, Pop VJ, Wiersinga WM, Drexhage HA. Cell-mediated immunity and postpartum thyroid dysfunction: a possibility for the prediction of disease? J Clin Endocrinol Metab. 1998;83:1959–66. doi: 10.1210/jcem.83.6.4838. [DOI] [PubMed] [Google Scholar]
- 30.Ando T, Davies TF. Clinical Review 160: Postpartum autoimmune thyroid disease: the potential role of fetal microchimerism. J Clin Endocrinol Metab. 2003;88:2965–71. doi: 10.1210/jc.2002-021903. [DOI] [PubMed] [Google Scholar]
- 31.Scaletti C, Vultaggio A, Bonifacio S, Emmi L, Torricelli F, Maggi E, Romagnani S, Piccinni MP. Th2-oriented profile of male offspring T cells present in women with systemic sclerosis and reactive with maternal major histocompatibility complex antigens. Arthritis Rheum. 2002;46:445–50. doi: 10.1002/art.10049. [DOI] [PubMed] [Google Scholar]
- 32.Manetti L, Parkes AB, Lupi I, Di Cianni G, Bogazzi F, Albertini S, Morselli LL, Raffaelli V, Russo D, Rossi G, Gasperi M, Lazarus JH, Martino E. Serum pituitary antibodies in normal pregnancy and in patients with postpartum thyroiditis: a nested case-control study. Eur J Endocrinol. 2008;159:805–9. doi: 10.1530/EJE-08-0617. [DOI] [PubMed] [Google Scholar]
- 33.Caturegli P, Lupi I, Landek-Salgado M, Kimura H, Rose NR. Pituitary autoimmunity: 30 years later. Autoimmun Rev. 2008 doi: 10.1016/j.autrev.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsagarakis S, Vassiliadi D, Malagari K, Kontogeorgos G, Thalassinos N. Lymphocytic hypophysitis: late recurrence following successful transsphenoidal surgery. Endocrine. 2004;25:85–90. doi: 10.1385/ENDO:25:2:085. [DOI] [PubMed] [Google Scholar]
- 35.Sinha D, Sinha A, Pirie AM. A case of recurrent lymphocytic hypophysitis in pregnancy. J Obstet Gynaecol. 2006;26:255–6. doi: 10.1080/01443610600555311. [DOI] [PubMed] [Google Scholar]
- 36.Siddique H, Baskar V, Chakrabarty A, Clayton RN, Hanna FW. Spontaneous pregnancy after trans-sphenoidal surgery in a patient with lymphocytic hypophysitis. Clin Endocrinol (Oxf) 2007;66:454–5. doi: 10.1111/j.1365-2265.2006.02734.x. [DOI] [PubMed] [Google Scholar]
- 37.Schreiber-Facklam H, Wagner AM, Staehler K, Surbek D, Kappeler A, Zimmermann A, Vajtai I. “Exterritorial” lymphocytic hypophysitis within an ovarian teratoma during pregnancy: a possible sentinel lesion? Pathol Res Pract. 2009;205:51–6. doi: 10.1016/j.prp.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Wegner N, Wait R, Venables PJ. Evolutionarily conserved antigens in autoimmune disease: implications for an infective aetiology. Int J Biochem Cell Biol. 2009;41:390–7. doi: 10.1016/j.biocel.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 39.O'Dwyer DT, Clifton V, Hall A, Smith R, Robinson PJ, Crock PA. Pituitary autoantibodies in lymphocytic hypophysitis target both gamma- and alpha-Enolase - a link with pregnancy? Arch Physiol Biochem. 2002;110:94–8. doi: 10.1076/apab.110.1.94.897. [DOI] [PubMed] [Google Scholar]
- 40.Lupi I, Broman KW, Tzou SC, Gutenberg A, Martino E, Caturegli P. Novel autoantigens in autoimmune hypophysitis. Clin Endocrinol. 2008:68. doi: 10.1111/j.1365-2265.2008.03180.x. [DOI] [PubMed] [Google Scholar]