Abstract
Background
Psychotic symptoms in Alzheimer Disease (AD+P) identify a heritable phenotype associated with a more severe course. We recently found an association of AD+P with depression symptom severity. Reports have shown an association of a serotonin-2A receptor (HTR2A) gene T102C polymorphism with AD+P and with depression during AD. We examined the interaction of this common genetic polymorphism with depression and increased psychosis risk.
Methods
Subjects with possible or probable AD or Mild Cognitive Impairment (MCI) without psychosis at study entry were genotyped for the HTR2A T102C polymorphism and reassessed every 6 months until psychosis onset. Psychotic and depressive symptoms were rated using the CERAD Behavioral Rating Scale (CBRS). Cox proportional hazard models with time dependent covariates were used to examine associations with psychosis onset.
Results
A total of 324 Caucasian subjects completed at least one follow-up exam. Depressive symptom severity was a strong predictor of psychosis onset. Neither psychosis onset nor depression severity was associated with the HTR2A genotype. Genotype interacted with depression severity to moderate the risk of AD+P onset. This did not result from an interaction of HTR2A genotype with antidepressant use.
Conclusion
Psychosis onset in AD is strongly associated with severity of depressive symptoms, an association that may be modified by HTR2A genotype.
Keywords: Neurodegenerative disorders, Mood, Genetic Linkage, genotype, Alzheimer Disease
Introduction
Psychotic symptoms in Alzheimer Disease (AD+P) identify a distinct phenotype that is characterized clinically by more severe cognitive impairment, more rapid functional decline, and premature institutional placement (Ballard et al., 1997; Drevets and Rubin, 1989; Jeste et al., 1992; Levy et al., 1996; Lopez et al., 1991; Lopez et al., 1997; McShane et al., 1997; Paulsen et al., 2000b; Paulsen et al., 2000a; Rockwell et al., 1994; Stern et al., 1987) compared to the course of AD without psychosis. The AD+P phenotype is familial (Sweet et al., 2002a), with an estimated heritability of 60–70% (Bacanu et al., 2005). AD+P demonstrates linkage (Bacanu et al., 2002), is associated with selected genes (Go et al., 2005; Holmes et al., 1998; Holmes et al., 2001; Nacmias et al., 2001; Sweet et al., 1998; Sweet et al., 2005), and is associated with distinct post-mortem neurochemical changes (Sweet et al., 2001; Sweet et al., 2002b).
We recently reported (Wilkosz et al., 2006) an association of antidepressant use with increased risk of AD+P onset. Subsequent analyses indicated that antidepressant use was a proxy for a true association of AD+P with depressive disorders, mediated by current depression symptom severity. The association between depressive symptoms and psychosis in AD has been observed in some, but not all studies (Bassiony et al., 2002; Gormley and Rizwan, 1998; Mizrahi et al., 2006), and the biological basis for the co-occurrence of depressive symptoms and psychosis in AD is not yet clearly understood.
Several reports(Assal et al., 2004; Holmes et al., 1998; Nacmias et al., 2001; Rocchi et al., 2003) have shown an association of the serotonin-2A receptor (HTR2A) gene T102C polymorphism (rs6313) with psychosis in AD and also with depression in a cohort of late onset AD subjects (Holmes et al., 2003). The association of HTR2A with depression and psychosis may not be restricted to AD. In a meta-analysis of 31 case-control association studies, Abdolmaleky et al (2004), found a significant association of rs6313 and schizophrenia. This polymorphism has also been associated with mood disorders (Arias et al., 2001a; Arias et al., 2001b; Arranz et al., 1997).
In general it has been the frequency of the C allele of rs6313 that has been increased in subjects with schizophrenia and with psychotic symptoms in AD (Abdolmaleky et al., 2004; Holmes et al., 1998; Nacmias et al., 2001; Polesskaya and Sokolov, 2002; Pritchard AL et al., 2006; Rocchi et al., 2003) as well as with depression in AD (Holmes et al., 2003). The T(102)C substitution does not alter the amino acid sequence of the HTR2A receptor; both alleles code for a serine in codon 34 (Warren et al., 1993). Nonetheless, this polymorphism is associated with changes in HTR2A expression. In human postmortem studies, mRNA expression of the rs6313 (C) allele in the temporal cortex of normal and schizophrenic heterozygotes was significantly lower than mRNA expression of the rs6313 (T) allele (Polesskaya and Sokolov, 2002). Similarly, total levels of HTR2A mRNA and protein in normal and schizophrenic postmortem individuals with the CC genotype, as well as the combined CC + TC genotype groups, were lower than in individuals with the TT genotype (Polesskaya and Sokolov, 2002).
Since polymorphisms in the HTR2A gene, in addition to conferring susceptibility for psychosis in AD may also be associated with depression in AD, we sought to better understand the interrelationships of HTR2A genotype, psychosis and depressive symptoms in late-onset AD. Specifically, given our prior observation that depression symptom severity was predictive of psychosis onset in AD (Wilkosz et al., 2006), we examined possible interactions of HTR2A genotype and depressive symptoms on AD+P onset. We proposed our analyses (Kraemer et al., 2001) to answer the following questions as illustrated in Figure 1: Is HTR2A genotype a risk factor for psychosis in AD independently of depression symptom severity as a risk factor for psychosis in AD? Alternatively, is HTR2A genotype a risk factor for both AD+P and depressive symptoms, i.e., is depression symptom severity a proxy for HTR2A genotype as a risk factor for psychosis in AD; or, does depression symptom severity mediate the effect of HTR2A genotype to influence this risk? Finally, does HTR2A genotype moderate the effect of depression symptom severity and its association with AD+P?
Figure 1. Interactions of Genetic Variation at a Particular Locus (GV) and Depression (Dep) on Risk of Psychotic Symptoms in Alzheimer Disease (AD+P).
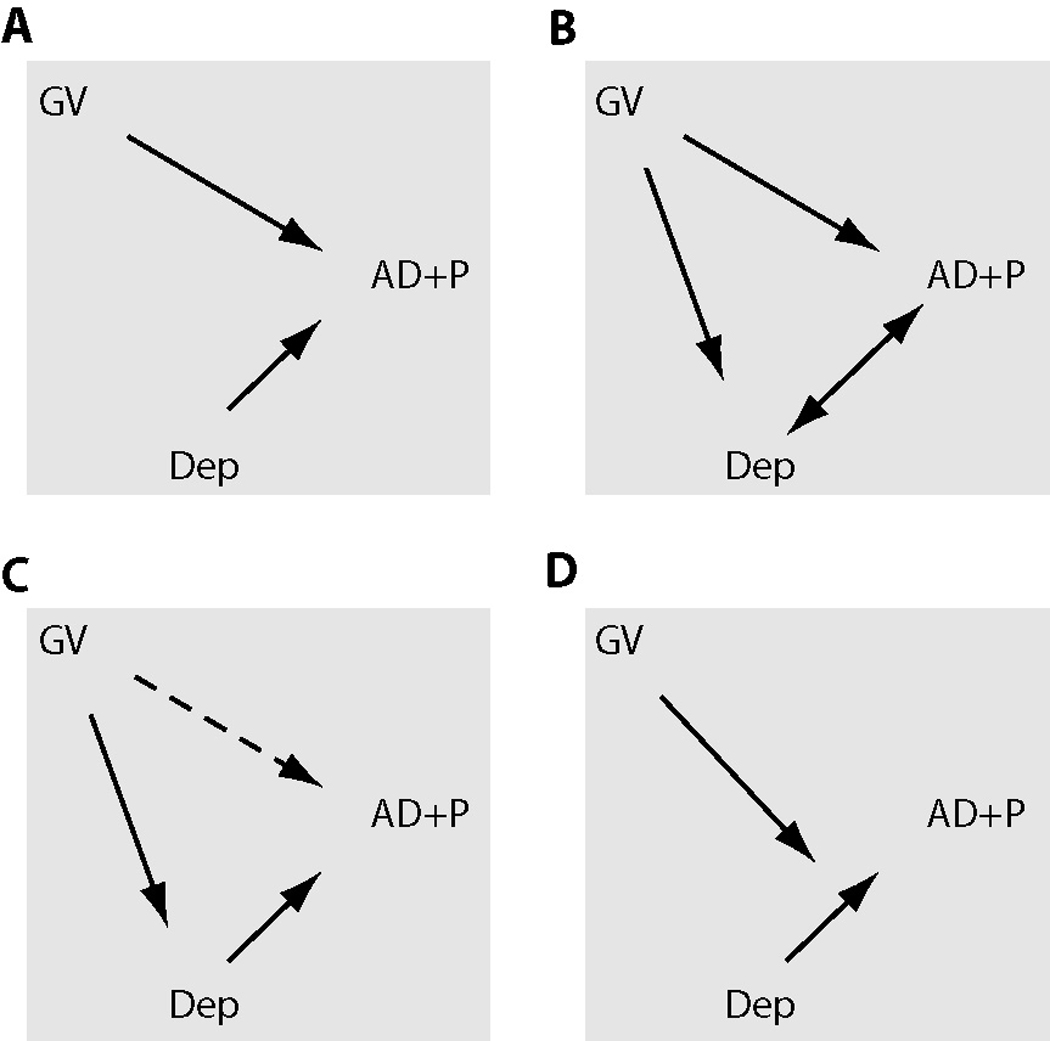
Correlations are shown as bold arrows, dashed arrows are correlations that weaken or disappear after consideration of the other risk factor. A. GV and Dep are independent risk factors for AD+P. B. GV yields both AD+P and Dep, i.e. Dep is a proxy for GV as a risk factor for AD+P. C. Dep mediates the effect of GV on AD+P risk. D. GV moderates the effect of Dep on AD+P risk.
Methods
Subjects
All patients were initially examined at the Alzheimer Disease Research Center (ADRC) of the University of Pittsburgh Medical Center, Pittsburgh, PA between 05/05/2000 – 03/29/ 2004. Subjects completed baseline assessment including standardized neurological and psychiatric evaluations, cognitive testing, laboratory studies and brain imaging as previously described (Becker et al., 1994; Lopez et al., 1997; Sweet et al., 1998). Following evaluation, subjects were diagnosed with possible or probable AD using criteria of the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer Disease and Related Disorders Association (McKhann et al., 1984). The rate of autopsy confirmation of AD in subjects diagnosed with possible or probable AD exceeds 90% in our center (Lopez et al., 2000a; Lopez et al., 2000b). Standardized criteria for a diagnosis of Mild Cognitive Impairment (MCI) were also used (Lopez et al., 2003). Psychiatric diagnoses were made according to Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV) criteria (1994). For those subjects able to return to the clinic, diagnostic assessments were repeated annually. Psychiatric medications were recorded at each visit. Psychiatric medications were categorized as cognitive enhancers, antipsychotics, antidepressants, and sedative-hypnotics (including benzodiazepines, sleeping aids, and barbiturates). All data collected in this study were obtained with protocols approved by the Institutional Review Board of the University of Pittsburgh.
Assessment of Psychosis and Depression
The psychiatric evaluation included ratings on the 1996, 46 item version of the CERAD Behavioral Rating Scale (CBRS) (Tariot et al., 1995). The CBRS measures the extent of behavioral pathology in persons with dementia, and was designed to be administered to an informant (Mack et al., 1999). Informants for behavioral ratings in this study were predominantly spouses (53.2%), children (32.7%), or other family members (3.7%). The majority of informants (65.3%) lived with subjects, all informants had contact with the subject at least 1–2 days/week, and 84.5% had contact with subjects 3–4 days/week or more.
Psychosis was defined by the presence of persistent hallucinations or delusions occurring 3 or more times in the past month, operationalized for the CBRS as a score of 2, 3, or 4.. A delusion was defined as a false belief based on incorrect inference about external reality, resistant to persuasion or contrary evidence, and not attributable to social or cultural mores. Hallucinations were defined as sensory perceptions for which there was no basis in reality. Discrete hypnogogic and hypnopompic hallucinations, as well as symptoms occurring only during an episode of delirium, were not rated.
Depression symptom severity was defined as the sum of CBRS items for depressed appearance, hopelessness, crying, guilt, poor self-esteem, suicidal ideation, anhedonia, failure to initiate activities, anergia, change in sleep, change in appetite and change in weight. For the survival curve, depression symptom scores were assigned using the median score to separate the subjects into high depression severity scores at or above the median, and low depression severity scores below it.
The CBRS was conducted at baseline, at annual visits, and between annual visits by telephone interview of the informants. Inter-rater reliability for determination of psychosis presence using the CBRS during the study was established at study baseline and at midpoint, using videotapes of 10 CBRS interviews. Reliability was calculated for each study rater in comparison to the “gold standard” rating generated by the geriatric psychiatrist who conducted the videotaped interviews. For the presence of psychosis for all raters, the mean (S.D.) kappa was high (0.98 (0.08)). Test-retest reliability for the presence of psychosis comparing telephone and in-person CBRS administration yielded a kappa of 0.63. Inter-rater reliability for ratings of depression severity revealed a mean (S.D) intraclass correlation coefficient of 0.90 (0.05). The corresponding value of test-retest reliability comparing telephone and in-person CBRS administration was 0.84 (0.01).
Genotyping
Subjects were genotyped for rs6313 using single base extension method (SnaPshot assays, Applied Biosystems (ABI)). PCR was done by combining 30ng of genomic DNA with 5pmol of each primer (Forward- 5’tctgctacaagttctggctt 3’ and Reverse- 5’ ctgcagctttttctctaggg 3’), 1× PCR buffer, 0.5mM dNTP, 1.5mM MgCl2, and 0.5U of Ampli-Taq DNA polymerase (ABI). The PCR reaction mix was incubated at 94°C for 10 min and subjected to 35 cycles of 94°C for 45 s, 60°C for 45 s and 72°C for 60 s, followed by a final extension at 72°C for 7 min. 2.5ul of PCR amplified product was treated for primer extension by incubating with 1 U exonuclease I (Amersham) and 1.25 U shrimp alkaline phosphatase (Amersham) for 1hr at 37°C on a thermocycler followed by 15 min at 85°C. Primer extension was performed in a 5 ul reaction by combining 1 µl treated PCR product with 1.5 µl SnaPshot Kit, 0.15 pmol extension primer (5’agagtcgactgtccagttaaatgcatcagaagtgttagcttctcc 3’). The reaction mix was incubated at 95°C for 2 min and subjected to 25 cycles of 95°C for 5 s, 50°C for 5 s, and 60°C for 5 s. Excess of unincorporated fluorescent ddNTPs were removed by treating with 1 U shrimp alkaline phosphatase at 37°C for 1 hr followed by 15 min at 85°C. The samples were loaded by combining 1 µl SNaPshot product and 9 µl Hi-Di formamide in a 96-well 3100 optical microamp plate (ABI), which was loaded onto a 3100 DNA sequencer (ABI). Reactions were electrophoresed on a 36-cm capillary array at 60°C by using POP4 polymer, dye set "E" and Genescan run module "SNP36POP4_default". Electrophoresis data was processed by using Genescan Analysis version 3.7 (ABI). Peak heights of the allele-specific extended primers were determined by using Genemapper 4.0 (ABI) and allele calls were made independently, blind to diagnostic status.
Statistical Analysis
324 Caucasian subjects with a diagnosis of probable or possible AD or MCI, who were without psychosis at baseline, and who were genotyped for rs6313, were included in the analysis. Survival analyses using extended Cox proportional hazard models examined the effects of age, gender, education, Mini Mental State Exam (MMSE), age of AD onset, genotype, and psychotropic medication use on time from study entry to psychosis onset. Because age, MMSE, depression score, and psychotropic medication use change over time, these variables were entered as time dependent covariates. All Cox proportional hazard models used Wald statistical testing with 1 degree of freedom and a significance level of p < .05. For group comparisons of depression symptom severity scores by genotype, classified as TT vs. TC/CC, the non-parametric Wilcoxon two-sample test was used. Grouping of TC and CC genotypes was chosen based on prior AD studies (Holmes et al., 1998).
Results
Subject Characteristics
Demographic and clinical characteristics of our subjects are presented (Table 1). The average age of the individuals in the study was 74.3. Slightly over half were female (58.0%), and the average number of years of education was 13.4. Most participants carried a diagnosis of Probable AD (73.1%), with the remainder classified as Possible AD (10.8%) or MCI (16.0%). Most participants demonstrated mild to moderate severity of cognitive impairment as reflected by a mean Mini-Mental State Examination Score of 20.1. Approximately one-third (33.9%) of the subjects exhibited psychotic behavior during follow-up, consistent with other reports. Mean duration of follow-up was 25.8 months, with a median follow-up duration of 25.2 months and a range of 5.4–57.1 months.
Table 1.
Demographics and Clinical Characteristics
| Clinical Variable | Mean (S.D.) or N (%) N=324 |
|---|---|
| Age (yrs) | 74.31 (8.72) |
| Range | 38.0–90.0 |
| Gender | |
| Female | 188 (58.0) |
| Male | 136 (42.0) |
| Education (yrs) | 13.36 (3.01) |
| Range | 4.0–23.0 |
| MMSE* | 20.09 (5.83) |
| Range | 1.0–30.0 |
| Onset Age (yrs) | 71.02 (9.08) |
| Range | 34.0–89.0 |
| Diagnosis | |
| Probable AD | 237 (73.1) |
| Possible AD | 35 (10.8) |
| MCI | 52 (16.0) |
| Depression Score | 8.09 (5.88) |
| Range | 0.0–31.0 |
| Psychosis | |
| Never | 214 (66.1) |
| Ever | 110 (33.9) |
| Duration of Follow-up (mos.) | 25.8 (13.6) |
| Range | 5.4–57.1 |
MMSE = Mini Mental State Exam
Nearly one-half (51.2%, N=166) of subjects were taking cognitive enhancers (donepezil, galantamine, rivastigmine, memantine) at study entry. Use increased to 81.2% (N=263) during follow-up. Antidepressant use was also common, with 32.4% (N=105) of subjects using antidepressants (amitriptyline, citalopram, escitalopram, trazodone, venlafaxine, doxepin, fluoxetine, nortriptyline, paroxetine, mirtazepine, nefazodone, bupropion, sertraline) at study entry. Use increased to 45.7% (N=148) during follow-up. In contrast, use of antipsychotics (haloperidol, olanzapine, risperidone, quetiapine) at study entry (2.2%, N=7) or during follow-up (6.8%, N=22), and use of sedatives/hypnotics (alprazolam, zolpidem, lorazepam, clonazepam, buspirone, temazepam) at baseline (8.9%, N=29) and during follow-up (14.5%, N=47), was uncommon.
Relationship of Depression Symptom Severity to Psychosis Risk
The associations of age, education, MMSE, onset age, gender and depression symptom severity with time to onset of psychosis in AD are shown (Table 2). A significant association was shown between high depression severity score and decreased time to psychosis onset. As in our prior report (Wilkosz et al., 2006), a significant association was found between lower MMSE scores and decreased time to onset of psychosis.
Table 2.
Cox Proportional Hazard Models For Risk of Psychosis Including Demographic Variables and Depression Score
| Covariates | Parameter Estimate | (S.E.)* | Hazard Ratio | χ2#, p |
|---|---|---|---|---|
| Age** | 0.033 | 0.05 | 1.03 | 0.50, 0.48 |
| Education | 0.089 | 0.04 | 1.09 | 5.39, 0.02 |
| MMSE** | −0.103 | 0.01 | 0.90 | 41.4, <.0001 |
| Onset Age | −0.027 | 0.04 | 0.97 | 0.36, 0.55 |
| Gender | −0.297 | 0.22 | 0.74 | 1.86, 0.17 |
| Depression, Severity** |
0.080 | 0.01 | 1.08 | 32.2, <.0001 |
S.E.=Standard Error
Time Dependent Covariates
Wald X2 with 1df
To determine whether results would differ if the analyses were run separately according to baseline diagnosis, we reanalyzed the data in only those individuals with a baseline diagnosis of Possible/Probable AD and found that our conclusions remained essentially unchanged: MMSE and depression severity remained strong predictors of psychosis onset (p < .0001). The MCI only group was too small to conduct meaningful comparisons, thus we did not analyze this group separately.
Relationship of HTR2A T102C to Depression and Psychosis
Analyses next examined the impact of rs6313 genotype on depression symptom severity and the risk of AD+P onset. The distribution of rs6313 genotypes and corresponding allele frequencies is shown in Table 3. The distribution of rs6313 genotypes did not deviate from Hardy-Weinberg equilibrium. Mean (SD) depression symptom severity score in TT homozygotes was 8.18 (5.62), versus 8.07 (5.94)) in rs6313 (C) allele carriers (Kruskal-Wallis χ21 =0.08, p=0.78) (Results not shown). Neither rs6313 genotype or allele frequencies were significantly associated with the presence of psychosis or with depression symptom severity.
Table 3.
Distribution of HTR2A T102C Genotype and Allele Frequencies
| Genotype | Allele | ||||
|---|---|---|---|---|---|
| TT | TC | CC | T | C | |
| Total, N (%) | 55 (17.0) | 163 (50.3) | 106 (32.7) | 273 (42.1) | 375 (57.9) |
| EverPsy, N (%) | 21 (19.1) | 56 (50.9) | 33 (30.0) | 98 (44.5) | 122 (55.5) |
| NeverPsy, N (%) | 34 (15.9) | 107 (50.0) | 73 (34.1) | 175 (40.9) | 253 (59.1) |
| High DepSev+, N (%) |
28 (17.1) | 83 (50.6) | 53 (32.3) | 139 (42.4) | 189 (57.6) |
| Low DepSev+, N (%) | 27 (16.9) | 80 (50.0) | 53 (33.1) | 134 (41.9) | 186 (58.1) |
Depression scores were assigned using the median score to separate scores into high depression severity at or above the median and low depression severity below it.
To further evaluate whether genetic variation was associated with time to onset of psychosis in AD, we included rs6313 genotype in our model, with age, education, gender, AD onset age, MMSE, and depression severity as covariates (Table 4). Genotype was not associated with time to onset of psychosis in AD subjects. Lower MMSE scores and high depression severity score remained significantly associated with time to psychosis onset. To determine whether genetic variation manifests its effects later in the course of disease when psychosis becomes more prevalent, we repeated our association analysis of HTR2A with psychosis excluding AD subjects in very early stages of disease. We obtained contingency tables in a subset of subjects with final MMSE scores of 15 or below. Results showed no association of genotype (TC/CC vs TT) (χ2 = 1.38, p =0.24) or allele (χ2 = 0.50, p = 0.48) with psychosis in this subset of subjects.
Table 4.
Cox Proportional Hazard Model For Risk of Psychosis Including Genotype
| Covariate | Parameter Estimate | (S.E.)* | Hazard Ratio | χ2#, p |
|---|---|---|---|---|
| TT vs TC/CC | −0.036 | 0.25 | 0.96 | 0.02, 0.89 |
| Age** | 0.032 | 0.05 | 1.03 | 0.48, 0.49 |
| Education | 0.089 | 0.04 | 1.09 | 5.38, 0.02 |
| MMSE** | −0.103 | 0.02 | 0.90 | 41.3, <.0001 |
| Onset Age | −0.027 | 0.04 | 0.97 | 0.34, 0.56 |
| Gender | −0.296 | 0.22 | 0.74 | 1.85, 0.17 |
| Depression Severity** |
0.080 | 0.01 | 1.08 | 32.2, <.0001 |
S.E.=Standard Error
Time Dependent Covariates
Wald X2 with 1 df
Interaction of HTR2A T102C & Depression Severity on Psychosis Risk
We further tested for a moderating effect of genotype on depression symptom severity (Table 5). When the genotype*depression symptom severity interaction term was included in our model, there was a significant interaction of genotype and depression symptoms with time to onset of psychosis (Figure 2). Depression severity score and MMSE remained significantly associated with onset of psychosis. There was also a significant association between the use of antidepressants in our subjects and onset of psychosis. Cognitive enhancers, antipsychotics, and sedative/hypnotics showed no association with psychosis onset.
Table 5.
Cox Proportional Hazard Model for Risk of Psychosis Including Genotype, Depression Score, Medications, and Genotype*Depression Interaction Term
| Variable | Parameter Estimate | (S.E.)* | Hazard Ratio | χ2#, p |
|---|---|---|---|---|
| TT vs TC/CC | 0.444 | 0.33 | 1.56 | 1.85, 0.17 |
| Age** | 0.015 | 0.05 | 1.01 | 0.10, 0.75 |
| Education | 0.076 | 0.04 | 1.08 | 3.90, 0.05 |
| MMSE** | −0.102 | 0.02 | 0.90 | 38.3, <.0001 |
| Onset Age | −0.008 | 0.05 | 0.99 | 0.03, 0.86 |
| Gender | −0.223 | 0.22 | 0.80 | 1.04, 0.31 |
| Depression Severity** |
0.101 | 0.02 | 1.11 | 24.0, <.0001 |
| Cognitive Enhancers** |
0.284 | 0.24 | 1.33 | 1.36, 0.24 |
| Antipsychotics** | −0.440 | 0.51 | 0.64 | 0.75, 0.38 |
| Antidepressants** | 0.622 | 0.21 | 1.86 | 8.78, 0.003 |
| Sedative/Hypnotics** | 0.189 | 0.29 | 1.21 | 0.41, 0.52 |
| Genotype*Depression Severity |
−0.048 | 0.02 | 0.95 | 4.35, 0.037 |
S.E.=Standard Error
Time Dependent Covariates
Wald X2 with 1 df
Figure 2. Survival Analysis For Genotype and Depression Severity Subtypes.
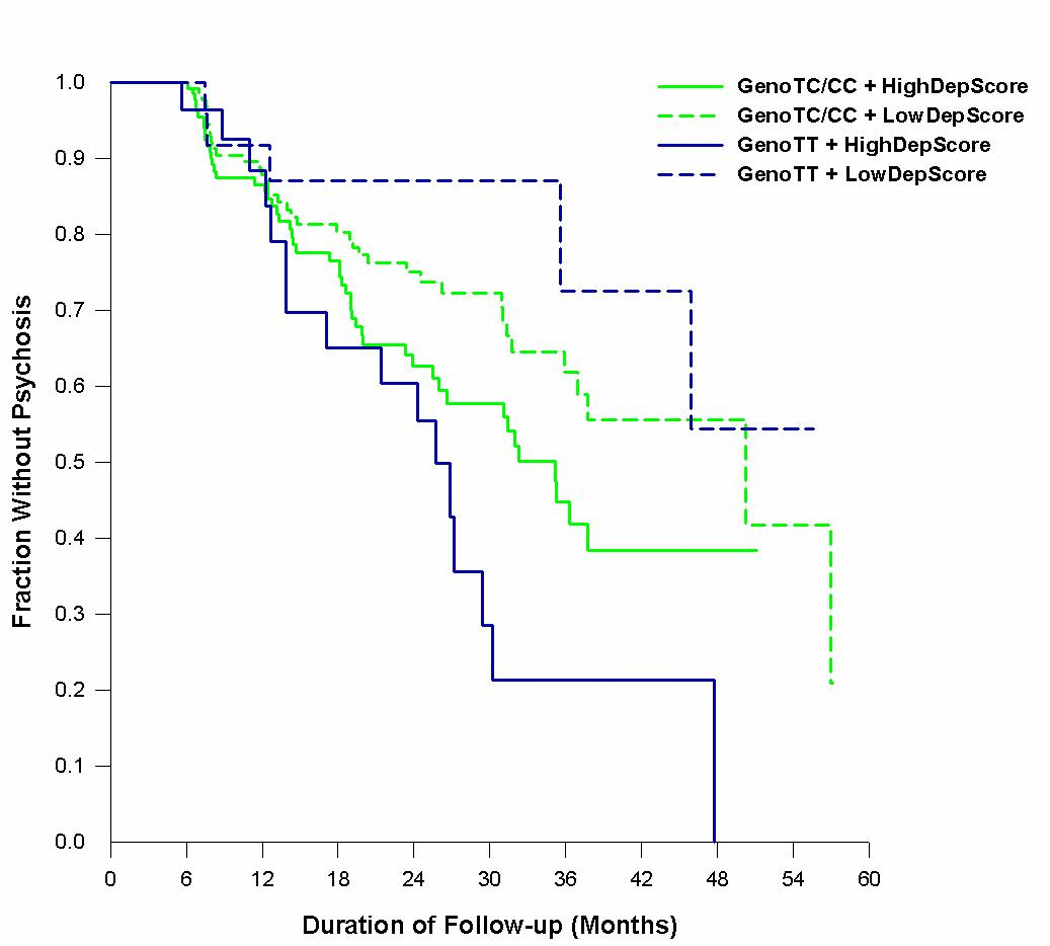
The vertical axis represents the fraction of subjects without psychosis; the horizontal axis represents the duration of follow-up of each genotype and depression severity subtype.
To examine whether rs6313 genotype might moderate the association of antidepressant use with psychosis, we included genotype, antidepressant use, depression symptom severity score and a genotype*antidepressant interaction term along with age, education, gender, AD onset age, and MMSE to determine the relationship between antidepressants and psychosis risk. When the genotype*antidepressant interaction term was included with antidepressants in our model, there was no longer a significant association of antidepressants with time to onset of psychosis (χ21=0.77, p=0.38), and no association of the genotype*antidepressant interaction term with time to onset of psychosis (χ21=0.19, p=0.66). The associations of depression symptom severity (χ21=23.6, p <.0001) and lower MMSE scores with time to onset of psychosis (χ21=36.7, p=<.0001) remained significant.
Discussion
These results confirm our previous observation of a highly significant association between severity of depression symptoms and time to psychosis onset in AD subjects. When rs6313 genotype was included in our models, there was no association of genotype with either depression symptom severity or with increased risk of psychosis. Genotype did, however, moderate the effect of depression severity on time to psychosis such that the rate of psychosis onset in the presence of increased depression symptom severity was much higher among TT homozygotes.
An association between depression and psychosis in AD has been previously documented. In a US population based study of 198 individuals with AD, Lyketsos et al. (2000) reported a latent class analysis of neuropsychiatric symptoms that identified a cluster of patients with predominantly affective symptoms, including depressed mood, together with delusions. Two large cross-sectional studies of over 1000 community-dwelling subjects with probable AD similarly reported a significant association of depression symptom severity with delusions (Bassiony et al., 2002; Mizrahi et al., 2006). In contrast, only one small cross-sectional study failed to detect such an association (Gormley and Rizwan, 1998). The current analysis both confirms and extends the results of the prior positive studies, demonstrating that depression symptom severity predicts time to onset of psychosis in AD. This relationship persists after controlling for the effect of current degree of cognitive impairment, itself a strong predictor of psychosis onset, and the effects of medication treatment, including antidepressants, cognitive enhancers, and antipsychotics.
There is evidence that the risk of manifesting psychosis or depressive symptoms during AD may be influenced by genetic variation. For example, we have reported that AD+P aggregates in families and estimated the heritability of psychosis in AD to be as high as 61–70%, indicating a substantial genetic component (Bacanu et al., 2005; Sweet et al., 2002a). Significant familial clustering of AD+P was similarly reported in a recent study of 513 families in which at least two members met criteria for late-onset AD (Hollingworth et al., 2006). Though this report included families from the prior analysis of familial aggregation (Sweet et al., 2002a), results were identical when independently ascertained families were examined separately. Of interest, significant familial clustering was also seen for mild (non-Major) depression in these families. In the one other study to examine this question, Tunstall et al (2000) also reported significant familial effects on depression symptoms in 106 sibling pairs with Alzheimer Disease, though there was only limited support in that cohort for clustering of psychosis.
To our knowledge, no study has examined whether familial liability to depression and psychosis are shared in AD. There is, however, substantial evidence supporting a genetic relationship between mood disorders and psychosis in bipolar disorder, schizophrenia, and schizoaffective disorder (Craddock et al., 2006; Kendler et al., 1995; Owen et al., 2005; Tsuang et al., 1993). Families with multiple cases of schizophrenia and bipolar disorder exist (Pope and Yurgelun-Todd, 1990). Other studies have shown that bipolar disorder occurs at increased rates in relatives of probands with schizophrenia (Tsuang et al., 1980), and that schizoaffective disorder occurs at increased rates in families of probands with both schizophrenia (Kendler et al., 1998) and in those of probands with bipolar disorder (Rice et al., 1987). Genetic studies showed evidence for linkage of the DISC1 gene (Blackwood et al., 2001), BNDF (Schumacher et al., 2005) (Brain Derived Neurotrophic Factor), and COMT (Badner and Gershon, 2002; Hamshere et al., 2005; Lewis et al., 2003; Potash et al., 2003; Williams et al., 2003a; Williams et al., 2003b) to a broad phenotype including schizophrenia, bipolar disorder and recurrent depression. Because specific risk variants for none of the implicated genes have been identified, a broader spectrum of clinical phenotypes with susceptibility conferred by overlapping sets of genes may be a more useful conceptualization of mood-psychosis phenotypes (Craddock et al., 2006).
One hypothesis following from the above studies is that variation in genes that interact during neurodevelopment or during early adult aging with environmental factors to lead to the development of disorders in the mood-psychosis spectrum might similarly interact with the neurodegenerative brain “environment” and eventually be expressed as AD+P.
For example, some individuals carrying mutations resulting in neurodegenerative pathology also manifest with prominent psychosis, suggesting an interaction of the disease-causing allele with the genetic background (Bird et al., 1997; Harvey et al., 1998; Poorkaj et al., 1998; Rippon et al., 2003). Recently, a number of publications has tested this hypothesis more directly, examining the associations of putative psychosis liability genes, among them COMT, NRG1, and more controversially DRD3 and HTR2A, with AD+P. A three-locus COMT haplotype has been associated with increased risk for schizophrenia in case-control and family based studies (Chen et al., 2004; Handoko et al., 2004; Shifman et al., 2002). We (Sweet et al., 2005), and others (Borroni et al., 2006), have recently found evidence for association of AD+P with haplotypes of COMT. An association of the COMT valine/methionine variant (rs4680) with AD+P has also been reported in a series of publications(Borroni et al., 2004; Borroni et al., 2005; Borroni et al., 2006). Stefansson et al (2002) first identified a core haplotype of NRG1 that was strongly associated with schizophrenia risk, which they(Stefansson et al., 2003), and others(Owen et al., 2005), have subsequently replicated across several populations. Additional associated SNP’s, including a non-synonomous SNP in exon 2, have also been associated with schizophrenia (Yang et al., 2003). We have similarly found this exon 2 SNP to be strongly associated with AD+P risk in a family-based analysis (Go et al., 2005). The risk of schizophrenia and psychosis in AD has also been associated with polymorphic variation at the dopamine receptor DRD3 gene (Craig et al., 2004; Holmes et al., 2001; Nimgaonkar, 2006; Sweet et al., 1998; Talkowski et al., 2006), and with HTR2A (Abdolmaleky et al., 2004; Assal et al., 2004; Holmes et al., 1998; Nacmias et al., 2001; Pritchard AL et al., 2006; Rocchi et al., 2003), though with less consistent results. It should be noted that in no case has a causal polymorphism been clearly identified, though our understanding of the mechanisms by which genetic variants may exert their effects is evolving.
Most prior reports of the association of HTR2A with AD+P (Holmes et al., 1998; Nacmias et al., 2001; Pritchard AL et al., 2006; Rocchi et al., 2003), and with depressive symptoms in AD (Holmes et al., 2003), found the rs6313 (C) allele to be associated with increased risk of psychosis or depression. In contrast, Assal et al (2004) reported a significant association of the rs6313 (T) allele with delusions. In the first study to examine whether genetic variation associates with liability to both depression and psychosis in AD, we found that variation at rs6313 moderates the effect of depression severity to decrease time to psychosis in TT homozygotes. Though we observed a modest increase in frequency of the rs6313 (T) allele in AD+P subjects, this did not reach significance, perhaps reflecting limited power in our sample. In contrast, there was no evidence of an association of rs6313 alleles with depressive symptom severity. Both our study and that of Assal et al (2004) were conducted in the US, while the other studies were based in Europe. Thus there may be population differences in linkage disequilibrium between the tested locus and the variations contributing to a causal alteration in HTR2A expression or function. Though conflicting findings of either independent (T) or (C) allele associations with depression or psychosis may be reflective of population variation, we cannot confidently exclude the possibility that our result is a false positive, and that no true association exists. Comprehensive evaluation of SNPs tagging all HTR2A haplotypes in larger samples characterized for AD+P is needed.
Several limitations to the current study deserve comment. The subjects were enrolled from the Alzheimer Disease Research Center, and are unlikely to be representative of the general population of elderly subjects with AD. Because this was a two-year prospective study, some subjects classified as without psychosis at study onset may develop psychosis later in the course of their illness, a result that could impact our current findings. Our analysis of subjects with MMSE<15 to some extent mitigates the concern that following people longer would have made a difference. Our analyses were also restricted to Caucasian subjects and may not generalize to subjects with differing socioeconomic or ethnic backgrounds.
We present evidence in a prospective study that depression symptom severity is strongly associated with increased risk for psychosis in AD patients. The HTR2A genotype moderated the effect of depression symptom severity on psychosis risk such that rs6313 TT homozygous individuals with increased depression symptom severity were at the highest risk for the development of psychosis during follow-up. Additional studies must be done to determine whether a joint depression–psychosis phenotype in AD is associated with polymorphic variations in other genes, such as COMT. Determining whether depressive symptoms occurring prior to the onset of AD (Chen et al., 1999) are also predictive of subsequent psychosis would be informative regarding whether liability genes exert their effects prior to the onset of neurodegeneration, and potentially provide a clinical marker for future interventions to prevent the cognitive and functional morbidity associated with AD+P.
Acknowledgement
Supported in part by USPHS grant AG05133
Reference List
- Washington, D.C: American Psychiatric Association; DSM-IV: Diagnostic and Statistical Manual of Mental Health Disorders. 1994
- Abdolmaleky HM, Faraone SV, Glatt SJ, Tsuang MT. Meta-analysis of association between the T102C polymorphism of the 5HT2a receptor gene and schizophrenia. Schizophr Res. 2004;67:53–62. doi: 10.1016/s0920-9964(03)00183-x. [DOI] [PubMed] [Google Scholar]
- Arias B, Gasto C, Catalan R, Gutierrez B, Pintor L, Fananas L. THe 5-HT(2A) receptor gene 102T/C polymorphism is associated with suicidal behavior in depressed patients. Am J Med Genetics. 2001a;105:801–804. doi: 10.1002/ajmg.10099. [DOI] [PubMed] [Google Scholar]
- Arias B, Gutierrez B, Pintor L, Gasto C, Fananas L. Variability in the 5-HT(2A) receptor gene is associated with seasonal pattern in major depression. Mol Psychiatry. 2001b;6:239–242. doi: 10.1038/sj.mp.4000818. [DOI] [PubMed] [Google Scholar]
- Arranz B, Blennow K, Eriksson A, Månsson JE, Marcusson J. Serotonergic, noradrenergic, and dopaminergic measures in suicide brains. Biol Psychiatry. 1997;41:1000–1009. doi: 10.1016/s0006-3223(96)00239-9. [DOI] [PubMed] [Google Scholar]
- Assal F, Alarcon M, Solomon EC, Masterman D, Geschwind DH, Cummings JL. Association of the serotonin transporter and receptor gene polymorphisms in neuropsychiatric symptoms in Alzheimer disease. Arch Neurol. 2004;61:1249–1253. doi: 10.1001/archneur.61.8.1249. [DOI] [PubMed] [Google Scholar]
- Bacanu SA, Devlin B, Chowdari KV, DeKosky ST, Nimgaonkar VL, Sweet RA. Linkage analysis of Alzheimer disease with psychosis. Neurology. 2002;59:118–120. doi: 10.1212/wnl.59.1.118. [DOI] [PubMed] [Google Scholar]
- Bacanu SA, Devlin B, Chowdari KV, DeKosky ST, Nimgaonkar VL, Sweet RA. Heritability of psychosis in Alzheimer disease. Am J Geriatr Psychiatry. 2005;13:624–627. doi: 10.1176/appi.ajgp.13.7.624. [DOI] [PubMed] [Google Scholar]
- Badner JA, Gershon ES. Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry. 2002;7:405–411. doi: 10.1038/sj.mp.4001012. [DOI] [PubMed] [Google Scholar]
- Ballard CG, O'Brien JT, Coope B, Wilcock G. Psychotic symptoms in dementia and the rate of cognitive decline. J Am Geriatr Soc. 1997;45:1031–1032. doi: 10.1111/j.1532-5415.1997.tb02980.x. [DOI] [PubMed] [Google Scholar]
- Bassiony MM, Rosenblatt A, Baker A, Steinberg M, Steele CD, Sheppard J, Lyketsos CG. The relationship between delusions and depression in Alzheimer's disease. Int J Geriatr Psychiatry. 2002;17:549–556. doi: 10.1002/gps.641. [DOI] [PubMed] [Google Scholar]
- Becker JT, Boller F, Lopez OL, Saxton J, McGonigle KL. The natural history of Alzheimer's disease: description of study cohort and accuracy of diagnosis. Arch Neurol. 1994;51:585–594. doi: 10.1001/archneur.1994.00540180063015. [DOI] [PubMed] [Google Scholar]
- Bird TD, Wijsman EM, NOchlin D, Leehey M, Sumi SM, Payami H, Poorkaj P, Nemens E, Rafkind M, Schellenberg GD. Chromosome 17 and hereditary dementia: linkage studies in three non-Alzheimer families and kindreds with late-onset FAD. Neurology. 1997;48:950–955. doi: 10.1212/wnl.48.4.949. [DOI] [PubMed] [Google Scholar]
- Blackwood DH, Fordyce A, Walker MT, St Clair DM, Porteous DJ, Muir WJ. Schizophrenia and affective disorders--cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am J Hum Genet. 2001;69:428–433. doi: 10.1086/321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroni B, Agosti C, Archetti S, Costanzi C, Bonomi S, Ghianda D, Lenzi GL, Caimi L, Di Luca M, Padovani A. Catechol-O-methyltransferase gene polymorphism is associated with risk of psychosis in Alzheimer Disease. Neurosci Lett. 2004;370:127–129. doi: 10.1016/j.neulet.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Borroni B, Grassi M, Agosti C, Archetti S, Costanzi C, Cornali C, Caltagirone C, Caimi L, Di Luca M, Padovani A. Cumulative effect of COMT and 5-HTTLPR polymorphisms and their interaction with disease severity and comorbidities on the risk of psychosis in Alzheimer disease. Am J Geriatr Psychiatry. 2006;14:343–351. doi: 10.1097/01.JGP.0000192491.50802.c3. [DOI] [PubMed] [Google Scholar]
- Borroni B, Grassi M, Agosti C, Costanzi C, Archetti S, Franzoni S, Caltagirone C, Di Luca M, Caimi L, Padovani A. Genetic correlates of behavioral endophenotypes in Alzheimer disease: Role of COMT, 5-HTTLPR and APOE polymorphisms. Neurobiol Aging. 2005 doi: 10.1016/j.neurobiolaging.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Chen P, Ganguli M, Mulsant BH, DeKosky ST. The temporal relationship between depressive symptoms and dementia. Arch Gen Psychiatry. 1999;56:261–266. doi: 10.1001/archpsyc.56.3.261. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang X, O'Neill AF, Walsh D, Kendler KS. Variants in the catechol-o-methyltransferase (COMT) gene are associated with schizophrenia in Irish high-density families. Mol Psychiatry. 2004;9:962–967. doi: 10.1038/sj.mp.4001519. [DOI] [PubMed] [Google Scholar]
- Craddock N, O'Donovan MC, Owen MJ. Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophr Bull. 2006;32:9–16. doi: 10.1093/schbul/sbj033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig D, Hart DJ, Carson R, McIlroy SP, Passmore AP. Psychotic symptoms in Alzheimer's disease are not influenced by polymorphic variation at the dopamine receptor DRD3 gene. Neurosci Lett. 2004;368:33–36. doi: 10.1016/j.neulet.2004.06.052. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Rubin EH. Psychotic symptoms and the longitudinal course of senile dementia of the Alzheimer type. Biol Psychiatry. 1989;25:39–48. doi: 10.1016/0006-3223(89)90145-5. [DOI] [PubMed] [Google Scholar]
- Go RC, Perry RT, Wiener H, Bassett SS, Blacker D, Devlin B, Sweet RA. Neuregulin-1 polymorphism in late onset Alzheimer's disease families with psychoses. Am J Med Genet B Neuropsychiatr Genet. 2005;139B:28–32. doi: 10.1002/ajmg.b.30219. [DOI] [PubMed] [Google Scholar]
- Gormley N, Rizwan MR. Prevalence and clinical correlates of psychotic symptoms in Alzheimer's disease. Int J Geriatr Psychiatry. 1998;13:410–414. doi: 10.1002/(sici)1099-1166(199806)13:6<410::aid-gps787>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Hamshere ML, Bennett P, Williams N, Segurado R, Cardno A, Norton N, Lambert D, Williams H, Kirov G, Corvin A, Holmans P, Jones L, Jones I, Gill M, O'Donovan MC, Owen MJ, Craddock N. Genomewide linkage scan in schizoaffective disorder: significant evidence for linkage at 1q42 close to DISC1, and suggestive evidence at 22q11 and 19p13. Arch Gen Psychiatry. 2005;62:1081–1088. doi: 10.1001/archpsyc.62.10.1081. [DOI] [PubMed] [Google Scholar]
- Handoko HY, Nyholt DR, Hayward NK, Nertney DA, Hannah DE, Windus LC, McCormack CM, Smith HJ, Filippich C, James MR, Mowry BJ. Separate and interacting effects within the catechol-O-methyltransferase (COMT) are associated with schizophrenia. Mol Psychiatry. 2004 doi: 10.1038/sj.mp.4001606. [DOI] [PubMed] [Google Scholar]
- Harvey RJ, Ellison D, Hardy J, Hutton M, Roques PK, Collinge J, Fox NC, Rossor MN. Chromosome 14 familial Alzheimer's disease: the clinical and neuropathological characteristics of a family with a leucine-->serine (L250S) substitution at codon 250 of the presenilin 1 gene. J Neurol Neurosurg Psychiatry. 1998;64:44–49. doi: 10.1136/jnnp.64.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth P, Hamshere M, Holmans P, Jones L, O'Donovan M, Myers A, Hardy J, Goate A, Lovestone S, Owen M, Williams J. Familiality and Linkage Analysis of Behavioral Symptoms and Age at Disease Onset in Late-Onset Alzheimer's Disease. Alzheimer's & Dementia. 2006;2:S194. [Google Scholar]
- Holmes C, Arranz M, Collier D, Powell J, Lovestone S. Depression in Alzheimer's Disease: The Effect of Serotonin Receptor Gene Variation. American Journal of Medical Genetics Part B (Neuropsyciatric Genetics) 2003;119B:40–43. doi: 10.1002/ajmg.b.10068. [DOI] [PubMed] [Google Scholar]
- Holmes C, Arranz MJ, Powell JF, Collier D, Lovestone S. 5-HT2A and 5-HT2C receptor polymorphisms and psychopathology in late onset Alzheimer's disease. Hum Mol Genetics. 1998;7:1507–1509. doi: 10.1093/hmg/7.9.1507. [DOI] [PubMed] [Google Scholar]
- Holmes C, Smith H, Ganderton R, Arranz M, Collier D, Powell J, Lovestone S. Psychosis and aggression in Alzheimer's disease: the effect of dopamine receptor gene variation. J Neurol Neurosurg Psychiatry. 2001;71:777–779. doi: 10.1136/jnnp.71.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste DV, Wragg RE, Salmon DP, Harris MJ, Thal LJ. Cognitive deficits of patients with Alzheimer's disease with and without delusions. Am J Psychiatry. 1992;149:184–189. doi: 10.1176/ajp.149.2.184. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. Am J Psychiatry. 2001;158:848–856. doi: 10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Walsh D. The structure of psychosis: latent class analysis of probands from the Roscommon Family Study. Arch Gen Psychiatry. 1998;55:492–499. doi: 10.1001/archpsyc.55.6.492. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Mcguire M, Gruenberg AM, Walsh D. Schizotypal Symptoms and Signs in the Roscommon Family Study - Their Factor Structure and Familial Relationship with Psychotic and Affective-Disorders. Arch Gen Psychiatry. 1995;52:296–303. doi: 10.1001/archpsyc.1995.03950160046009. [DOI] [PubMed] [Google Scholar]
- Levy ML, Cummings J, Fairbanks LA, Bravi D, Calvani M, Carta A. Longitudinal assessment of symptoms of depression, agitation, and psychosis in 181 patients with Alzheimer's disease. Am J Psychiatry. 1996;153:1438–1443. doi: 10.1176/ajp.153.11.1438. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I, Williams NM, Schwab SG, Pulver AE, Faraone SV, Brzustowicz LM, Kaufmann CA, Garver DL, Gurling HM, Lindholm E, Coon H, Moises HW, Byerley W, Shaw SH, Mesen A, Sherrington R, O'Neill FA, Walsh D, Kendler KS, Ekelund J, Paunio T, Lonnqvist J, Peltonen L, O'Donovan MC, Owen MJ, Wildenauer DB, Maier W, Nestadt G, Blouin JL, Antonarakis SE, Mowry BJ, Silverman JM, Crowe RR, Cloninger CR, Tsuang MT, Malaspina D, Harkavy-Friedman JM, Svrakic DM, Bassett AS, Holcomb J, Kalsi G, McQuillin A, Brynjolfson J, Sigmundsson T, Petursson H, Jazin E, Zoega T, Helgason T. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. Am J Hum Genet. 2003;73:34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Brenner RP, Rosen J, Bajulaiye OI, Reynolds CF. Alzheimer's disease with delusions and hallucinations: neuropsychological and electroencephalographic correlates. Neurology. 1991;41:906–912. doi: 10.1212/wnl.41.6.906. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Klunk WE, Saxton J, Hamilton RL, Kaufer D, Sweet RA, Cidis Meltzer C, Wisniewski SR, Kamboh MI, DeKosky ST. Research evaluation and diagnosis of possible Alzheimer's disease over the last two decades. II. Neurology. 2000a;55:1863–1869. doi: 10.1212/wnl.55.12.1863. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Klunk WE, Saxton J, Hamilton RL, Kaufer DI, Sweet RA, Cidis Meltzer C, Wisniewski SR, Kamboh MI, DeKosky ST. Research evaluation and diagnosis of probable Alzheimer's disease over the last two decades. I. Neurology. 2000b;55:1854–1862. doi: 10.1212/wnl.55.12.1854. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Jagust WJ, DeKosky ST, Becker JT, Fitzpatrick A, Dulberg C, Breitner J, Lyketsos C, Jones B, Kawas C, Carlson M, Kuller LH. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Kamboh MI, Becker JT, Kaufer DI, DeKosky ST. The apolipoprotein E ε4 allele is not associated with psychiatric symptoms or extrapyramidal signs in probable Alzheimer's disease. Neurology. 1997;49:794–797. doi: 10.1212/wnl.49.3.794. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JCS. Mental and behavioral disturbances in dementia: findings from the Cache County study on memory in aging. Am J Psychiatry. 2000;157:708–714. doi: 10.1176/appi.ajp.157.5.708. [DOI] [PubMed] [Google Scholar]
- Mack JL, Patterson MB, Tariot PN. Behavior Rating Scale for Dementia: development of test scales and presentation of data for 555 individuals with Alzheimer's disease. J Geriatr Psychiatry Neurol. 1999;12:211–223. doi: 10.1177/089198879901200408. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McShane R, Keene J, Gedling J, Fairburn C, Jacoby R, Hope T. Do neuroleptic drugs hasten cognitive decline in dementia? Prospective study with necropsy follow up. BMJ. 1997;314:266–270. doi: 10.1136/bmj.314.7076.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi R, Starkstein SE, Jorge R, Robinson RG. Phenomenology and Clinical Correlates of Delusions in Alzheimer Disease. Am J Geriatr Psychiatry. 2006;14:573–581. doi: 10.1097/01.JGP.0000214559.61700.1c. [DOI] [PubMed] [Google Scholar]
- Nacmias B, Tedde A, Forleo P, Piacentini S, Guarnieri BM, Bartoli A, Ortenzi L, Petruzzi C, Serio A, Marcon G, Sorbi S. Association between 5-HT(2A) receptor polymorphism and psychotic symptoms in Alzheimer's disease. Biol Psychiatry. 2001;50:472–475. doi: 10.1016/s0006-3223(01)01114-3. [DOI] [PubMed] [Google Scholar]
- Nimgaonkar VL. Novel, replicated associations between dopamine D3 receptor gene polymorphisms and schizophrenia in two independent samples. Biol Psychiatry. 2006;60:570–577. doi: 10.1016/j.biopsych.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Owen MJ, Craddock N, O'Donovan MC. Schizophrenia: genes at last? Trends Genet. 2005;21:518–525. doi: 10.1016/j.tig.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Ready RE, Stout JC, Salmon DP, Thal LJ, Grant I, Jeste DV. Neurobehaviors and psychotic symptoms in Alzheimer's disease. Journal of the International Neuropsychological Society. 2000a;6:815–820. doi: 10.1017/s1355617700677081. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Salmon DP, Thal L, Romero R, Weisstein-Jenkins C, Galasko D, Hofstetter CR, Thomas R, Grant I, Jeste DV. Incidence of and risk factors for hallucinations and delusions in patients with probable Alzheimer's disease. Neurology. 2000b;54:1965–1971. doi: 10.1212/wnl.54.10.1965. [DOI] [PubMed] [Google Scholar]
- Polesskaya O, Sokolov B. Differential Expression of the "C" and "T" Alleles of the 5-HTR2A Receptor Gene in the Temporal Cortex of Normal Individuals and Schizophrenics. J Neurosci Res. 2002;67:812–822. doi: 10.1002/jnr.10173. [DOI] [PubMed] [Google Scholar]
- Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol. 1998;43:815–825. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- Pope HGJ, Yurgelun-Todd D. Schizophrenic individuals with bipolar first-degree relatives: analyses of two pedigrees. J Clin Psychiatry. 1990;51:97–101. [PubMed] [Google Scholar]
- Potash JB, Zandi PP, Willour VL. Suggestive linkage to chromosomal regions 13q31 and 22q12 in families with psychotic bipolar disorder. Am J Psychiatry. 2003;160:680–686. doi: 10.1176/appi.ajp.160.4.680. [DOI] [PubMed] [Google Scholar]
- Pritchard AL, Harris J, Pritchard CW, Coates J, Haque S, Holder R, Bentham P. Role of 5HT(2A) and 5HT(2C) polymorphisms in behavioral and psychological symptoms of Alzheimer's Disease. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Rice J, Reich T, Andreasen NC. The familial transmission of bipolar illness. Arch Gen Psychiatry. 1987;44:441–447. doi: 10.1001/archpsyc.1987.01800170063009. [DOI] [PubMed] [Google Scholar]
- Rippon GA, Crook R, Baker M, Halvorsen E, Chin S, Hutton M, Houlden H, Hardy J, Lynch T. Presenilin 1 mutation in an african american family presenting with atypical Alzheimer dementia. Arch Neurol. 2003;60:884–888. doi: 10.1001/archneur.60.6.884. [DOI] [PubMed] [Google Scholar]
- Rocchi A, Micheli D, Ceravolo R, Manca ML, Tognoni G, Siciliano G, Murri L. Serotoninergic polymorphisms (5-HTTLPR and 5-HT2A): Association studies with psychosis in Alzheimer disease. Genetic Testing. 2003;7:309–314. doi: 10.1089/109065703322783662. [DOI] [PubMed] [Google Scholar]
- Rockwell E, Jackson E, Vilke G, Jeste DV. A study of delusions in a large cohort of Alzheimer's disease patients. Am J Geriatr Psychiatry. 1994;2:157–164. doi: 10.1097/00019442-199405000-00009. [DOI] [PubMed] [Google Scholar]
- Schumacher J, Jamra RA, Becker T. Evidence for a relationship between genetic variants at the brain-derived neurotrophic factor (BNDF) locus and major depression. Biol Psychiatry. 2005;58:307–314. doi: 10.1016/j.biopsych.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Shifman S, Bronstein MSM, Pisante-Shalom A, Lev-Lehman E, Weizman A, Reznik I, Spivak B, Grisaru N, Karp L, Schiffer R, Kotler M, Strous RD, Swartz-Vanetik M, Knobler HY, Shinar E, Beckmann JS, Yakir B, Risch N, Zak NB, Darvasi A. A highly significant association between a COMT haplotype and schizophrenia. Am J Hum Genet. 2002;71:1296–1302. doi: 10.1086/344514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Sarginson J, Kong A, Yates P, Steinthorsdottir V, Gudfinnsson E, Gunnarsdottir S, Walker N, Petursson H, Crombie C, Ingason A, Gulcher JR, Stefansson K, St Clair D. Association of neuregulin 1 with schizophrenia confirmed in a Scottish population. Am J Hum Genet. 2003;72:83–87. doi: 10.1086/345442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, Harvey RP, Lai D, Zhou MD, Brunner D, Mutel V, Gonzalo A, Lemke G, Sainz J, Johannesson G, Andresson T, Gudbjartsson D, Manolescu A, Frigge ML, Gurney ME, Kong A, Gulcher JR, Petursson H, Stefansson K. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Mayeux R, Sano M, Hauser WA, Bush T. Predictors of disease course in patients with probable Alzheimer's disease. Neurology. 1987;37:1649–1653. doi: 10.1212/wnl.37.10.1649. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Devlin B, Pollock BG, Sukonick DL, Kastango KB, Bacanu S-A, Chowdari KV, DeKosky ST, Ferrell RE. Catechol-O-methyltransferase haplotypes are associated with psychosis in Alzheimer disease. Mol Psychiatry. 2005;10:1026–1036. doi: 10.1038/sj.mp.4001709. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Hamilton RL, Healy MT, Wisniewski SR, Henteleff R, Pollock BG, Lewis DA, DeKosky ST. Alterations of striatal dopamine receptor binding in Alzheimer's disease are associated with lewy body pathology and with antemortem psychosis. Arch Neurol. 2001;58:466–472. doi: 10.1001/archneur.58.3.466. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Nimgaonkar VL, Devlin B, Lopez OL, DeKosky ST. Increased familial risk of the psychotic phenotype of Alzheimer disease. Neurology. 2002a;58:907–911. doi: 10.1212/wnl.58.6.907. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Nimgaonkar VL, Kamboh MI, Lopez OL, Zhang F, DeKosky ST. Dopamine receptor genetic variation, psychosis, and aggression in Alzheimer's disease. Arch Neurol. 1998;55:1335–1340. doi: 10.1001/archneur.55.10.1335. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Panchalingam K, Pettegrew JW, McClure RJ, Hamilton RL, Lopez OL, Kaufer DI, DeKosky ST, Klunk WE. Psychosis in Alzheimer disease: postmortem magnetic resonance spectroscopy evidence of excess neuronal and membrane phospholipid pathology. Neurobiol Aging. 2002b;23:547–553. doi: 10.1016/s0197-4580(02)00009-x. [DOI] [PubMed] [Google Scholar]
- Talkowski ME, Mansour H, Chowdari KV, Wood J, Butler A, Varma PG, Prasad S, Semwal P, Bhatia T, Deshpande S, Devlin B, Thelma BK, Nimgaonkar V. Novel, replicated associations between dopamine D3 receptor gene polymorphisms and schizophrenia in two independent samples. Biol Psychiatry. 2006;60:570–577. doi: 10.1016/j.biopsych.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Tariot PN, Mack JL, Patterson MB, Edland SD, Weiner MF, Fillenbaum G, Blazina L, Teri L, Rubin E, Mortimer JA, Stern Y Behavioral Pathology Committee of the Consortium to Establish a Registry for Alzheimer's Disease. The behavior rating scale for dementia of the Consortium to Establish a Registry for Alzheimer's Disease. Am J Psychiatry. 1995;152:1349–1357. doi: 10.1176/ajp.152.9.1349. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Faraone SV, Lyons MJ. Identification of the phenotype in psychiatric genetics. European Archives of Psychiatry and Clinical Neuroscience. 1993;243:131–142. doi: 10.1007/BF02190719. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Winokur G, Crowe RR. Morbidity risks of schizophrenia and affective disorders among first degree relatives of patients with schizophrenia, mania, depression, and surgical conditions. Br J Psychiatry. 1980;137:497–504. doi: 10.1192/bjp.137.6.497. [DOI] [PubMed] [Google Scholar]
- Tunstall N, Fraser L, Lovestone S, Owen MJ, Williams J, Rice F, Carty S, Lillystone S, Kehoe P, Rudrasingham V. Familial influence on variation in age of onset and behavioural phenotype in Alzheimer's disease. Br J Psychiatry. 2000;176:156–159. doi: 10.1192/bjp.176.2.156. [DOI] [PubMed] [Google Scholar]
- Warren JT, Peacock ML, Rodriguez LC, Fink JK. An Mspi Polymorphism in the Hyman Serotonin Receptor Gene (Htr2) - Detection by Dgge and Rflp Analysis. Hum Mol Genetics. 1993;2:338–338. doi: 10.1093/hmg/2.3.338. [DOI] [PubMed] [Google Scholar]
- Wilkosz PA, Miyahara S, Lopez OL, DeKosky ST, Sweet RA. Prediction of Psychosis Onset in Alzheimer Disease: The Role of Cognitive Impairment, Depressive Symptoms, and Further Evidence for Psychosis Subtypes. Am J Geriatr Psychiatry. 2006;14:352–356. doi: 10.1097/01.JGP.0000192500.25940.1b. [DOI] [PubMed] [Google Scholar]
- Williams NM, Norton N, Williams H. A systematic genomewide linkage study in 353 sib pairs with schizophrenia. Am J Hum Genet. 2003a;73:1355–1367. doi: 10.1086/380206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NM, Preece A, Spurlock G, Norton N, Williams HJ, Zammit S, O'Donovan MC, Owen MJ. Support for genetic variation in neuregulin 1 and susceptibility to schizophrenia. Mol Psychiatry. 2003b;8:485–487. doi: 10.1038/sj.mp.4001348. [DOI] [PubMed] [Google Scholar]
- Yang JZ, Si TM, Ruan Y, Ling YS, Han YH, Wang XL, Zhou M, Zhang HY, Kong QM, Liu C, Zhang DR, Yu YQ, Liu SZ, Ju GZ, Shu L, Ma DL, Zhang D. Association study of neuregulin 1 gene with schizophrenia. Mol Psychiatry. 2003;8:706–709. doi: 10.1038/sj.mp.4001377. [DOI] [PubMed] [Google Scholar]


