Abstract
We compared working memory (WM) for location of social vs. non-social targets in infant siblings of children with Autism Spectrum Disorders (sibs-ASD, n=25) and typically developing children (sibs-TD, n=30) at 6.5 and 9 months of age. There was a significant interaction of risk group and target-type on WM, in which the sibs-ASD had better WM for non-social targets as compared to controls. There was no group by stimulus interaction on two non-memory measures. The results suggest that the increased competency of sibs-ASD in WM (creating, updating, and using transient representations) for non-social stimuli distinguishes them from sibs-TD by 9 months of age. This early emerging strength is discussed as a developmental pathway that may have implications for social attention and learning in children at risk for ASD.
Bridging clinical and genetic understanding of Autism Spectrum Disorders (ASD) will require identifying the prodromal disruptions and subsequent developmental pathways through which these disruptions lead to core symptoms. One strategy for exploring the earliest differences associated with ASD is to study the broader ASD phenotype in infant siblings of children with ASD. The broader ASD phenotype refers to atypical core functions characteristic of children with ASD and some of their school-age siblings and parents. Social-communicative development and responsiveness are disrupted in some siblings of children with ASD (Zwaigenbaum et al., 2007; Constantino et al., 2006). Disrupted social-communication development, especially coordinated social attention, is among the first symptoms of ASD (Mundy, 1995; Stone, Coonrod, Turner & Pozdol, 2004). There is evidence that disrupted coordinated social attention is a component of the broader ASD phenotype affecting infant siblings of children with ASD. In the second year of life, some younger siblings of children with ASD (sibs-ASD) initiate and respond to fewer bids for coordinated social attention (Stone, McMahon, Yoder & Walden, 2007; Presmanes, Walden, Stone & Yoder, 2007). Although 5-10% of sibs-ASD are expected to develop autism, this effect does not seem to be driven by a small subset of poorly-performing infants. Studying sibs-ASD who are younger than one year of age may help us identify the precursors of this disrupted coordinated social attention.
Decreased social memory ability could lead to delays in coordinated social attention. The process of coordinating social attention involves the ability to create, maintain and update representations of a social partner. During infancy, working memory (WM) is an information handling capacity with which the infant actively forms and updates transient representations (Reznick, 2007). Although we have much to learn about infant WM, there is a growing body of evidence supports expectations for how WM develops in the first year of life. Typically, WM emerges in developing infants by the middle of the first year (Reznick, Morrow, Goldman & Snyder, 2004; Schwartz & Reznick, 1999) and improves markedly in subsequent months (Pelphery et al., 2004). Based on current understanding of the emergence of infant representations, we are able to assert that infant working memory performance reflects a capacity that has been shaped by prior experience representing objects (Munakata, 2004).
Some studies have reported poor WM in children with ASD (Bennetto, Pennington & Rogers, 1996; Happé, Hughes, Booth & Charlton, 2006) but others have not (Ozonoff & Strayer, 2001; Dawson et al., 2002). Although some parents of children with autism have been found to have spatial WM deficits (Kaoczat, Rogers, Pennington & Ross, 2002), we are aware of no research assessing WM in children younger than 3 years of age who have received a diagnosis of ASD or are at-risk for ASD. Thus, there is some limited precedence for a global WM decrease seen in infant siblings of children with ASD. However, there is an insufficient basis to consider WM in infants and WM in older children (and adults) a unitary construct. As a result, the suggestion that familial differences in WM might extend down to infants has to be considered exploratory.
An alternative to a global WM difference is a group difference that is specific to the nature of the target being represented. Social stimuli are less likely to draw the attention of young children with ASD than non-social stimuli (e.g., children with ASD pay less attention to a person humming than to a phone ringing; Dawson et al., 2004). In infant sibs-ASD, atypical interaction patterns have been reported as early as 4 months of age (Yirmiya et al., 2006). It should be noted that while like Yirmiya and colleagues, some researchers have found evidence of early differences in sibs-ASD (McCleery, Allman, Carver & Dobkins, 2007; Merin, Young, Ozonoff & Rogers, 2007; Ibanez, Messinger, Newell, Sheskin & Lambert, in press) others studies have found no sibs-ASD differences (Nadig et al., 2007; Zwaigenbaum et al., 2005; Landa & Garrett-Mayer, 2006). Nonetheless, early differences in social engagement in sibs-ASD could contribute to both reduced social WM and disrupted social-communication abilities. Investigating infant WM for different types of targets not only allows us to make direct comparisons of which target an infant represents more readily, but also provides a window into the representational histories that have lead up to their current information handling capacity with those types of targets.
We tested WM in infants at 6.5 and 9 months of age in a task that challenged them to remember the location of social and non-social targets, and to update their WM across a sequence of trials involving three different locations sampled with replacement. We used a delayed-response task (Pelphrey et al., 2004; Reznick et al., 2004; Schwartz & Reznick, 1999) that is a modification of the peek-a-boo game that infants typically find intrinsically rewarding. We tested two groups of infants: sibs-ASD and infants whose older sibling(s) were typically developing (sibs-TD).
Each infant participated in two conditions: a social target trial block and a non-social target trial block. If WM is globally compromised in at-risk infants, then WM performance would be lower for the sibs-ASD in both social and non-social conditions. If elevated risk is associated with a relative decrease in social WM, then this liability would emerge in an interaction of target type condition and risk-status. An interaction could also be driven by sibs-ASD having better non-social WM than sibs-TD. An early sibs-ASD advantage in non-social WM would be consistent with the finding that high-functioning individuals with ASD and their parents have relative strengths in reasoning about non-social objects (Baron-Cohen et al., 1997).
In summary, we tested simultaneously for two possibilities: 1) a risk group (sibs-ASD vs. sibs-TD) difference in WM independent of target type, and 2) an interaction of risk group and target type. Further, to investigate the degree to which any group difference was specific to WM, we also compared the groups on their responsiveness to the social and non-social stimuli when WM was not required. There were two measures of non-WM stimuli interest: latency to orient and preferential looking.
Methods
Participants
Twenty-five sibs-ASD and thirty sibs-TD were seen for two visits. At the 6.5-month visit, the 19 sibs-ASD available (73% male) were on average 6 months, 19 days old (SD=7 days), and the 22 sibs-TD (74% male) were on average 6 months, 21 days old (SD=6 days). At the 9-month visit, the 23 sibs-ASD available (52% male) were on average 9 months, 7 days old (SD=11 days) and the 29 sibs-TD (52% male) were on average 9 months, 7 days old (SD=9 days). The groups did not differ by age or gender at either visit. Sibs-TD were recruited from phone contacts to parents of infants in a birth records database maintained by the state. All sibs-TD had at least one typically developing older sibling and no atypical development in any sibling. Recruitment of sibs-ASD was primarily through a university-based service and outreach program specialized for children with ASD. Assignment to the sibs-ASD group was based on having an older sibling with a diagnosis of autism (n=11), Asperger's (n=2), or Pervasive Developmental Disorder-Not Otherwise Specified (PDD-NOS, n=12). Seventeen sibs-ASD and 21 sibs-TD participated in both the 6.5-month and 9-month visits. Some infants were recruited late and only participated in the 9-month visit (sibs-ASD n=6, sibs-TD n=8) and others participated in the 6.5-month visit but were not available for the second visit (sibs-ASD n=2, sibs-TD n=1). Informed consent was obtained from the parent prior to participation following a protocol approved by the University's Institutional Review Board.
Materials
A 30-inch wide black-cloth screen, with a manually operated central distracter, was positioned 26 inches in front of the infant (see Figure 1). On each trial, a target appeared in a target zone defined as a one-foot square extending from the right, left or top edge of the screen. For each infant, the same examiner appeared throughout the social-trial block and the same toy appeared throughout the non-social trial block. Across the course of the study, there were 5 individuals who served as the examiners (4 female, 1 male), and there were two non-social stimuli (an array of multi-faceted reflective bows with rattling beads, and a glass bowl with balls attached). Both non-social stimuli had clear plastic facades covering the colorful objects that moved when the toy was shaken. At each age, the social and non-social stimuli were used with equal rates between the groups.
Figure 1.
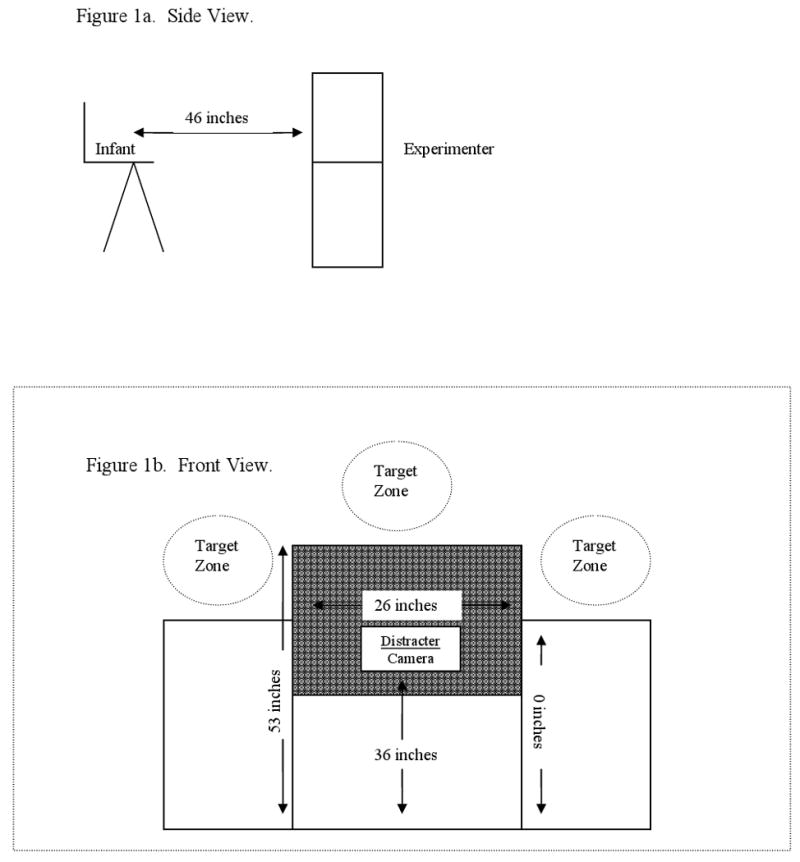

The side view (Figure 1a) demonstrating the distance from the infant to the apparatus and the infants' view (Figure 1b&c).
Procedure
Paired comparison screening
Prior to the WM task, each child saw the social and non-social stimuli presented simultaneously in the left and right target zones for 30 seconds. During this paired comparison, each stimulus produced a call (see “call” details below) that was repeated until the infant oriented to the stimulus. Presentation location and call order were counterbalanced across infants.
Working memory task
Each infant participated in two blocks of WM trials: one with a social target and one with a non-social target. Within each block of 17 potential trials, the number of trials contributing to the percent correct score (see Analysis below) varied and was vulnerable to infant fussiness. For the sib-TD infants the mean number of valid trials in the social trial block was 10.9 (SD = 4.4) and in the non-social trial block was 11.6 (SD = 4.6). For the sib-ASD infants the social mean was 9.8 (SD = 4.3) and non-social mean was 11.6 (SD = 3.9). The number of valid trails did not differ by risk group, nor was there a group by condition interaction.
WM was assessed using a modification of the peek-a-boo game, involving 3 steps: a target appeared at either the top, right, or left of the screen; a mechanical distracter reoriented the infant to the center of the apparatus for 1 second; and infant's gaze toward the three locations was then monitored for 3 seconds with no target present. An initial gaze toward the location where the target had most recently appeared was deemed a correct response. The order of the two trial blocks was counterbalanced and not different between the two risk groups at either age.
Each trial began with the target appearing in one of the three target zones and producing an audible call. For the social stimulus “call,” the examiner delivered a contextually appropriate phrase (“Hi, ______ (baby's name)!”; “Peekaboo.”). For the non-social stimulus “call,” the toy was rotated, producing a sound and movement. The call continued until the infant oriented to the target. In the social condition, orientating was rewarded with praise from the examiner. In the non-social condition, orientating was rewarded with the opportunity to watch the toy continue rattling.
After the infant oriented on each trial, the target was withdrawn and a distracter was activated, drawing the infant's attention to the center of the apparatus. The distracter was a pair of lights that spotlighted six jingle bells suspended from elastic strings. The examiner was able to simultaneously engage the lights (with a foot-pedal) and jostle the jingle bells (by moving the elastic strings through a finger access port). Within each age group, individuals from the two risk groups were assigned with equal likelihood to three counterbalanced target-zone appearance sequences (e.g., left, right, center). The target appeared in the same order on both trial blocks of a visit. Successful WM was defined as a first look toward the location where the examiner or toy had appeared prior to the delay (Reznick et al., 2004; Schwartz & Reznick, 1999).
Measures and analysis
The videotapes were digitized and then coded using ProcoderDV software (Tapp, 2003), which allowed frame-by-frame marking of the critical events during the task sequences. The coders were undergraduate research assistants unaware of the risk status of the infants.
WM score
The score for each block of 17 trials was the number of trials with a “correct” first look, defined as a look to the location where the target had most recently appeared, divided by the number of trials in which the infant made one or more looks to any target zone during the response window. In other words, the WM memory score was based on a percentage: # of trials with a first look to the correct target zone divided by the # of trials with looks to any target zone. When the denominator was less than 3 for any trial block (n=5), the WM score for that trial block was considered invalid because there were too few trials to calculate a robust percentage correct score. The sensitivity of this score to the task's memory demands has been empirically verified (Schwartz & Reznick, 1999). Both trial blocks for 9 test sessions (10% of test sessions) were coded by two assistants and there was substantial agreement as to whether or not there was a correct first look (Kappa=.75).
SAS PROC MIXED was used to examine four possible time points for WM scores (i.e., the first and second trial block from the 6.5 month visit, and the first and second trial block from the 9-month visit) for each infant. Performance was expected to improve from the 6.5-month to the 9-month visit (Pelphrey et al., 2004). We included the age group variable in the model to control for variability due to age Thus, the model included 4 terms: 3 main effects (target type, risk group, age group) and one 2-way interaction (risk group × target type). The mixed model analysis is similar to a repeated-measures general linear model, but the mixed model permitted the use of data from all infants, including the 17 infants who were only available for one visit.
Latency to orient
For each trial, the target re-emergence point was defined as the frame at which the target was first visible as it emerged from behind the screen. The infants' orientation to the target was defined as the frame in which the infant completed his or her gaze shift to the re-emerged target. Latency to orient was the time that elapsed between re-emergence and orientation (to the 1/300 second). The marked times for the 10% of trials coded by two individuals were substantially correlated (Pearson r =.94, p.001). This value was averaged across the 17 trials for each target type, and the mixed model was used to evaluate the main effects of risk group, target type, and a target-type by risk-group interaction. As with the WM analysis, developmental variability was controlled by including the age-group variable in the model.
Preferential looking (during paired comparison screening, that occurred before the WM task)
Duration of gaze at the social and non-social stimuli in the paired-comparison screening was coded during the 30-sec period when both stimuli were presented simultaneously. Video frames during this 30-sec paired comparison screening were classified as either: looking at the social stimulus, looking at the non-social stimulus, or not looking at either stimulus. Coders were in agreement as to whether or not a look was occurring (Kappa= 0.82). A social-stimuli preference score was created by subtracting the total duration of non-social looking from the total duration of social looking time. The risk groups were compared on this preference score with separate t-tests for the 6.5- and 9- month data.
Results
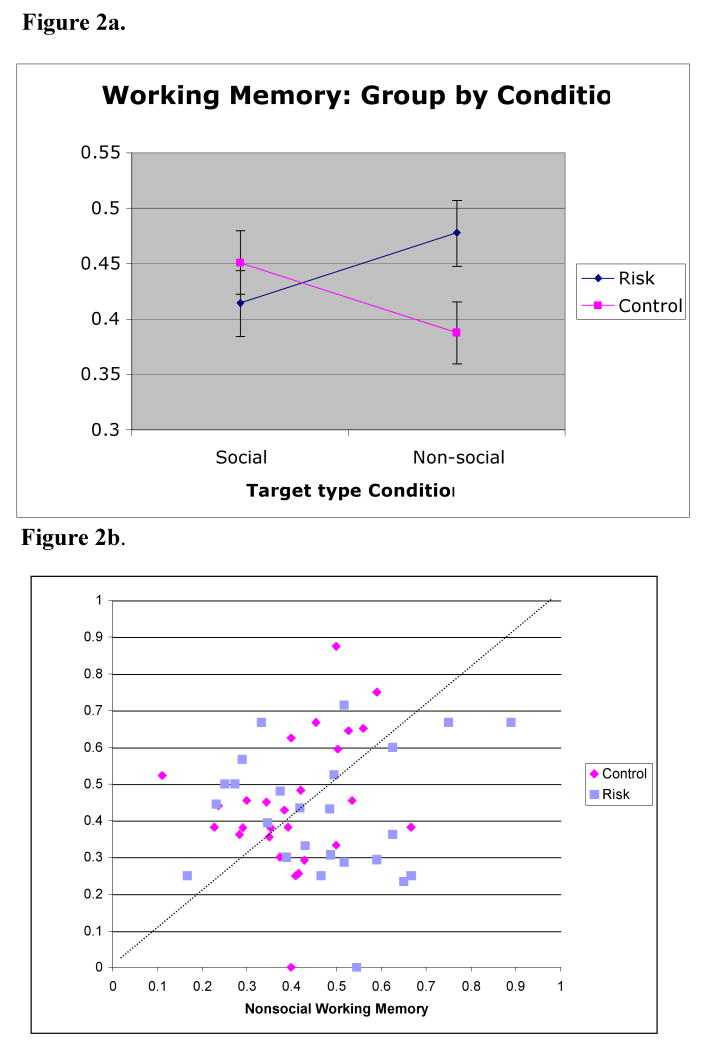
The WM data were normally distributed and reflected a wide range of performance (see Table 1). The main effects were not statistically significant: age group, F(1,37) = 3.4, p<.07; risk group, F(1,53) = .8, p<.38; and target type, F(1,51) = 0, p<.9. The trend for a main effect of age group suggests that working memory improves with the development in the direction suggested by the literature reviewed in the introduction, even across this relatively restricted age span. The interaction of risk group and target type was significant, F(1,51) = 5.3, p< .025. As can be seen in Figure 2, the sibs-ASD had higher WM scores than the sibs-TD for the non-social condition and there was no group difference for the social condition. This pattern was confirmed in a planned follow-up analysis with separate mixed models calculated for performance in the social and non-social conditions. There was a significant effect of risk group, F(1, 53) = 4.8, p<.03, in the non-social condition. In the social condition, there was no effect of risk group, F(1, 51) = .69, p<.4. The effect of age was not significant in either single-condition model.
Table 1.
Working Memory Performance by Target Type Condition
| Working memory score, proportion of first looks correct | ||||||
|---|---|---|---|---|---|---|
| Social | Non-social | |||||
| Age and Risk Group | n | Mean | SD | n | Mean | SD |
| 6.5 months | ||||||
| SIB-ASD | 19 | .39 | .17 | 19 | .44 | .17 |
| SIB-TD | 22 | .42 | .15 | 21 | .37 | .14 |
| 9 months | ||||||
| SIB-ASD | 21 | .43 | .22 | 23 | .51 | .21 |
| SIB-TD | 27 | .47 | .23 | 29 | .42 | .20 |
Figure 2.
Figure 2a. Working memory performance by Target Condition for sibs-TD (n=30) and sibs-ASD (n=25). Least square means estimates and corresponding standard errors.
Figure 2b. The individual distribution of social vs. nonsocial working memory scores contributing to the interaction depicted in Figure 2a. The dashed line indicates equal success between social and non-social conditions. Where data is complete, average of the 6.5 and 9-months visit is included. Otherwise, the data is from the single available visit.
As an exploratory follow-up, we repeated the final model and added a three-way interaction term: age group*risk group*target type. We found that this interaction was not significant but that the risk group*target type interaction term remained significant (F(1,51) = 5.2, p< .03). Thus, the interaction between risk group and target type is not isolated to performance within either test age but is independent of age, within the limited age range considered.
Non-WM variables (Latency to orient, preferential looking, Table 2)
Table 2.
Non-memory responses to social and non-social stimuli: Duration of looking during prescreening and latency to orient during WM task.
| Duration looking for prescreening paired-comparison in seconds | ||||
|---|---|---|---|---|
| Social | Non-social | |||
| Age and Risk Groups | Mean | SD | Mean | SD |
| 6.5 months | ||||
| SIB-ASD | 12.4 | 6.1 | 12.8 | 6.4 |
| SIB-TD | 11.2 | 5.6 | 14.0 | 6.3 |
| 9 months | ||||
| SIB-ASD | 7.8 | 4.4 | 16.6 | 5.2 |
| SIB-TD | 7.2 | 4.6 | 17.7 | 7.1 |
| Average latency to orient during WM task in seconds | ||||
| Social | Non-social | |||
| Age and Risk Groups | Mean | SD | Mean | SD |
| 6.5 months | ||||
| SIB-ASD | 1.43 | .71 | 1.07 | .42 |
| SIB-TD | 1.15 | .54 | 1.08 | .32 |
| 9 months | ||||
| SIB-ASD | 1.2 | .54 | 1.11 | .51 |
| SIB-TD | 1.23 | .42 | 1.04 | .46 |
For Latency to orient, results indicated a main effect for target type, with shorter response latencies for the non-social target as compared with the social target, F(53) = 8.21, p<.01. There were no other significant main effects, nor a significant interaction of target type and group. For the paired comparison screening, t-tests revealed no group differences on the social stimulus preference score at either 6.5 or 9- months of age.
Discussion
The target-type by group interaction reported for the WM score was driven by the better performance of the sibs-ASD in the non-social target condition, suggesting that they are more successful in forming and using transitory non-social representations of toy location. Below we review the indicators suggesting that the non-social WM advantage for sibs-ASD generalizes beyond this specific testing situation and reflects a distinct development pathway.
Two factors support the conclusion that the sibs-ASD were not merely more interested in the particular non-social objects used in the tasks but were actually more accurate in representing the trial-by-trial location of the non-social objects in WM. The first factor is that the WM score is best interpreted as the proportion of first looks for the target correct (i.e., proportion of first looks that were directed toward the target's most recent location). Therefore, the sibs-ASD do not score higher because they produce more attempts to locate the non-social stimuli, but rather because the attempts they make are more accurate. The second factor is that both groups showed shorter latency to orient to the non-social target relative to the social target. This faster orientation in the non-social trial block suggests that both groups of infants found the non-social targets more compelling than the social targets. This contrasts with the significant advantage that only the sibs-ASD demonstrated for remembering the most recent location of the preferred target. The sibs-ASD were distinguished by non-social WM accuracy, i.e. success in keeping track of the location where the non-social objects previously appeared. These two factors, the nature of the WM score and the specificity of the finding to WM, increase our confidence that performance of the sibs-ASD reflects a non-social WM advantage that generalizes beyond this specific task.
A possibility raised by the current finding is sibs-ASD found these specific non-social exemplars particularly engaging and were more accurate as a result. However, there is not a clear mechanism for increased sibs-ASD engagement with the non-social exemplars during the task cause their better non-social WM accuracy. Indeed, the reported dissociation between the interest in the target (higher non-social stimuli interest from the entire cohort of infants, irrespective of risk group) and accuracy in tracking the targets' changing location (higher for the nonsocial target in the risk group only) is not easily reconciled which such a mechanism. Nonetheless, the possibility exists that the current findings are tied to the particular exemplars. Follow-up studies employing different exemplars and, indeed different paradigms, are warranted, and are the only way to fully address this possibility.
It would be premature to conclude that the early non-social WM advantage in sibs-ASD seen at 6.5 to 9 months is independent of earlier differences in the at-risk group. Rather, better non-social WM in the sibs-ASD at the tested ages may be the product of an advantage in an earlier emerging component of WM. Some evidence suggests that the ability to form and update representations required by the WM task a first emerges at 5-6 months (Reznick et al., 2004). However, there is also evidence that typically developing 4-month-old infants are representing the locations of non-social objects (Mareschal & Johnson, 2003). In accordance with the rich-get-richer experientially-driven developmental models (Munatkata, 2004), a small inherited bias that increases the frequency of non-social location representation al experiences in the sibs-ASD could be expected to become a broader advantage (i.e. WM accuracy) by 6.5 months. Indeed, there is now empirical support for this model's prediction that uneven representation strength for different categories of objects could emerge based on experience (Shinskey & Munakata, 2005). Could there be a categorical distinction between social and non-social objects in the emergence of representational capacity in infancy? Affirming this possibility is the evidence that typically developing infants successfully represent the location of tangible non-social objects before social objects (Mareschal & Johnson, 2003). The implication for the current finding is that a relatively small advantage in experience representing the location of non-social objects could lead to a subsequent advantage in non-social WM.
If further studies confirm better non-social WM accuracy in sib-ASD infants, questions will arise as to the possibly mechanisms for better non-social WM to have developed in the sib-ASD infants. We briefly review the other sibs-ASD findings that may prove relevant for studies that attempt to address these mechanistic questions. What inheritance could lead to the acquisition of more experience representing the location of non-social objects? Enhancement of the motion processing system is a plausible mechanism. Beginning in the first months of life, young infants are sensitive to motion cues that are useful for predicting the future location of inanimate objects but not animate objects like people (Spelke & Kinzler, 2007). For infants, motion is the predominant cue to the behavior of inanimate objects as they move, and attending to motion cues allows infants to predict the future location of inanimate objects (Von Hofsten, Vishton, Spelke, Feng, & Rosander, 1998). There is new evidence that the sibs-ASD might be more sensitive to these motion cues. The magnocellular visual pathway is responsible for carrying information about motion to the visual association areas of the cortex information.
It has recently been reported that sibs-ASD, tested at 6 months, have a two-fold enhancement of sensitivity in their magnocellular pathway (McCleery, Allman, Carver & Dobkins, 2007). Therefore, an enhanced sensitivity to motion cues may draw sibs-ASD to attend to the emergence/re-emergence pattern of inanimate objects. Put another way, the cues to the future location of an inanimate object may be more salient to sibs-ASD infants than sibs-TD. This difference in cue salience could give sibs-ASD more experience representing the location of non-social objects. This possibility is consistent with the processing advantages reported in children with ASD (Baron-Cohen, Wheelwright, Scahill, Lawson & Spong, 2001) and their parents (Baron-Cohen & Hammer, 1997) regarding the laws governing changes in inanimate objects in relation to one another (Baron-Cohen, 2006).
An alternative possibility is that sibs-ASD acquired more experience representing non-social objects as a default because of an inherited tendency that reduces their engagement with other people. From birth, typically developing infants are deeply and consistently involved in learning about their social worlds. If these strong social engagement patterns are disrupted, sibs-ASD might have surplus time to attend to non-social objects. A recent study of eye gaze in sib-ASD and sib-TD 6-month-old infants during face-to-face interactions with their parents supports the hypothesis that disrupted social engagement could lead to more non-social attention (and visa versa). The sibs-ASD engaged in fewer shifts of attention to and from their parent and longer duration looks to elements of the non-social environment (Ibanez, Messinger, Newell, Sheskin & Lambert, in press). Furthermore, the production of to-and-from-parent gaze shifts was negatively correlated with the average duration of non-social gazes. Thus, we see a sibs-ASD disruption in social engagement that is correlated with more attention to non-social objects. The causal direction of that correlation is not clear, but it is possible that atypical social engagement is the initial disruption. If a difference in social engagement is the initial difference, one might have expected the current study to corroborate this hypothesis with a complementary social WM competency in the sibs-TD.
Thus there are at least two possibilities for how sibs-ASD may acquire more experience at representing the location of non-social objects: they may have inherited either an increased propensity to attend to non-social objects, or a disrupted pattern of engagement with social beings. The present study does not allow us to distinguish between these possible mechanisms or exclude the possibility that: 1) the cause is an inherited advantage in other aspects of WM favoring non-social stimuli; 2) the current results are tied to the particular exemplars/paradigm employed.
In summary, the present study documents a WM competency with non-social objects that distinguishes the sibs-ASD from sibs-TD. There is currently inadequate understanding of the relation between early and later WM to speculate on a development time-course from infancy to adulthood. However, an infant WM advantage such as the one reported here may be the product of increased experience representing non-social objects. If so, one might expect this advantage to disappear over time as sib-TD infants accumulate more experience representing non-social objects. Although the non-social WM differences revealed by the current findings may be limited to infancy, it may effect the way WM (social and non-social) develops in the months that follow. As infants approach their first birthday, social-WM is a crucial stepping stone for subsequent coordinated social communication (Mundy et al., 2007). In order to engage in triadic communication (communication with a partner about objects or events), infants must be able to represent their social partner richly and flexibly as well as the object of communication. Future research will allow for the investigation of the possibility that sib-ASD children who show differences in social-communicative skills may have had more uneven patterns of infant WM competency, favoring non-social targets.
Acknowledgments
Research supported from Autism Speaks grant #787 to J. Noland, NICHD # P30HD15052 to the Vanderbilt Kennedy Center.
References
- Baron-Cohen S. Two new theories of autism: hyper-systemizing and assortative mating. Archives of Diseases in Childhood. 2006;91:2–5. doi: 10.1136/adc.2005.075846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Scahill V, Lawson J, Spong A. Are intuitive physics and intuitive psychology independent? Journal of Developmental and Learning Disorders. 2001;5:47–78. [Google Scholar]
- Baron-Cohen S, Hammer J. Parents of children with Asperger Syndrome: what is the cognitive phenotype? Journal of Cognitive Neuroscience. 1997;9:548–554. doi: 10.1162/jocn.1997.9.4.548. [DOI] [PubMed] [Google Scholar]
- Baron-Cohon S, Wheelwright S, Stott C, Bolton P, Goodyer I. Is there a link between engineering and autism? Autism: An International Journal of Research and Practice. 1997;1:153–163. [Google Scholar]
- Bennetto L, Pennington B, Rogers S. Intact and impaired memory functions in Autism. Child Development. 1996;67:1816–1835. [PubMed] [Google Scholar]
- Constantino JN, Lajonchere C, Lutz M, Gray T, Abbacchi A, McKenna K, Singh D, Todd RD. Autistic social impairment in the siblings of children with Pervasive Developmental Disorders. American Journal of Psychiatry. 2006;163(2):294–296. doi: 10.1176/appi.ajp.163.2.294. [DOI] [PubMed] [Google Scholar]
- Dawson G, Munson J, Estes A, Osteling J, McPartlan J, Toth K, Carver L, Abbott R. Neurocognitive function and joint attention ability in young children with Autism Spectrum Disorder versus developmental delay. Child Development. 2002;73(2):345–358. doi: 10.1111/1467-8624.00411. [DOI] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J. Defining the early social attention impairments in autism: Social orienting, joint attention, and responses to emotions. Developmental Psychology. 2004;40(2):271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- Happé FGE, Hughes C, Booth R, Charlton R. Executive dysfunction in Autism Spectrum Disorders and Attention Deficit / Hyperactivity Disorder: Developmental profiles. Brain and Cognition. 2006;61:25–39. doi: 10.1016/j.bandc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Ibanez L, Messinger D, Newell L, Sheskin M, Lambert B. Visual disengagement in the infant siblings of children with Autism Spectrum Disorder (ASD) Autism: International Journal of Research and Practice. doi: 10.1177/1362361308094504. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koczat DL, Rogers SJ, Pennington BF, Ross RG. Eye movement abnormality suggestive of a spatial WM deficit is present in parents of autistic probands. Journal of Autism and Developmental Disorders. 2002;32:513–518. doi: 10.1023/a:1021246712459. [DOI] [PubMed] [Google Scholar]
- Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: a prospective study. Journal of Child Psychology and Psychiatry. 2006;47(6):629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Mareschal D, Johnson M. The “what” and “where” of infant object representations. Cognition. 2003;88:259–276. doi: 10.1016/s0010-0277(03)00039-8. [DOI] [PubMed] [Google Scholar]
- McCleery JP, Allman E, Carver LJ, Dobkins KR. Abnormal magnocellular pathway visual processing in infants at risk for autism. Biological Psychiatry. 2007;62(9):1007–1014. doi: 10.1016/j.biopsych.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Merin N, Young GS, Ozonoff S, Rogers SJ. Visual fixation patterns during reciprocal social interaction distinguish a subgroup of 6-month-old infants at-risk for autism from comparison infants. Journal of Autism and Developmental Disorders. 2007;37(1):108–121. doi: 10.1007/s10803-006-0342-4. [DOI] [PubMed] [Google Scholar]
- Munakata Y. Computational cognitive neuroscience of early memory development. Developmental Review. 2004;24:133–153. [Google Scholar]
- Mundy P. Joint attention and social-emotional approach behavior in children with autism. Development and Psychopathology. 1995;7:63–82. [Google Scholar]
- Mundy P, Block J, Delgado C, Pomares Y, VanHecke AV, Parlade MV. Individual differences and the development of joint attention in infancy. Child Development. 2007;78(3):938. doi: 10.1111/j.1467-8624.2007.01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadig AS, Ozonoff S, Young GS, Rozga A, Sigman M, Rogers SJ. A prospective study of response to name in infants at risk for autism. Archives of Pediatrics & Adolescent Medicine. 2007;161(4):378–383. doi: 10.1001/archpedi.161.4.378. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Strayer DL. Further evidence of intact working memory in autism. Journal of Autism and Developmental Disorders. 2001;31:257–263. doi: 10.1023/a:1010794902139. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Reznick JS, Goldman BD, Sasson N, Morrow J, Donahoe A, Hodgson K. Development of visuospatial short-term memory in the second half of the first year. Developmental Psychology. 2004;40:836–851. doi: 10.1037/0012-1649.40.5.836. [DOI] [PubMed] [Google Scholar]
- Presmanes AG, Walden TA, Stone WL, Yoder PJ. Effects of different attentional cues on responding to joint attention in younger siblings of children with Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2007;37(1):133–144. doi: 10.1007/s10803-006-0338-0. [DOI] [PubMed] [Google Scholar]
- Reznick JS. Working memory in infants and toddlers. In: Oakes LM, Bauer PJ, editors. Short- and long-term memory in infancy and early childhood: Taking the first steps toward remembering. Oxford, UK: Oxford University Press; 2007. [Google Scholar]
- Reznick JS, Morrow JD, Goldman BD, Snyder J. The onset of working memory in infants. Infancy. 2004;6 [Google Scholar]
- Shinskey JL, Munakata Y. Familiarity breeds searching: Infants reverse their novelty preferences when reaching for hidden objects. Psychological Science. 2005;16:596–600. doi: 10.1111/j.1467-9280.2005.01581.x. [DOI] [PubMed] [Google Scholar]
- Spelke ES, Kinzler KD. Core knowledge. Developmental Science. 2007;10:89–96. doi: 10.1111/j.1467-7687.2007.00569.x. [DOI] [PubMed] [Google Scholar]
- Schwartz BB, Reznick JS. Measuring infant spatial working memory using a modified delayed-response procedure. Memory. 1999;7:1–17. doi: 10.1080/741943714. [DOI] [PubMed] [Google Scholar]
- Stone WL, Coonrod EE, Turner LM, Pozdol SL. Psychometric properties of the STAT for early autism screening. Journal of Autism and Developmental Disorders. 2004;34:691–701. doi: 10.1007/s10803-004-5289-8. [DOI] [PubMed] [Google Scholar]
- Stone WL, McMahon CR, Yoder PJ, Walden TA. Early social-communicative and cognitive development of younger siblings of children with Autism Spectrum Disorders. Arch Pediatr Adolesc Med. 2007;161:384–390. doi: 10.1001/archpedi.161.4.384. [DOI] [PubMed] [Google Scholar]
- Tapp J. Procoder for Digital Video (Computer Software) Nashville, TN: Vanderbilt Kennedy Center; 2003. [Google Scholar]
- Yirmiya N, Gamliel I, Pilowsky T, Baron-Cohen S, Feldman R, Sigman M. The development of siblings of children with autism at 4 and 14 months: Social engagement, communication and cognition. Journal of Child Psychology and Psychiatry. 2006;47:511–523. doi: 10.1111/j.1469-7610.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- Von Hofsten C, Vishton PM, Spelke ES, Feng Q, Rosander K. Predictive action in infancy: Tracking and reaching for moving objects. Cognition. 1998;67:255–285. doi: 10.1016/s0010-0277(98)00029-8. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23(2):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, et al. Studying the emergence of Autism Spectrum Disorders in high risk infants: Methodological and practical issues. Journal of Autism and Developmental Disorders. 2007;37(3):466–480. doi: 10.1007/s10803-006-0179-x. [DOI] [PubMed] [Google Scholar]