Abstract
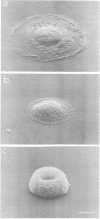
Cultures of human epidermal keratinocytes provide a useful experimental model with which to study the factors that regulate cell proliferation and terminal differentiation. One situation that is known to trigger premature terminal differentiation is suspension culture, when keratinocytes are deprived of substratum and intercellular contact. We have now investigated whether area of substratum contact, and hence cell shape, can regulate terminal differentiation. Keratinocytes were grown on circular adhesive islands that prevented cell-cell contact. By varying island area we could vary cell shape from fully spread to almost spherical. We found that when substratum contact was restricted, DNA synthesis was inhibited and expression of involucrin, a marker of terminal differentiation, was stimulated. Inhibition of proliferation was not a sufficient stimulus for involucrin synthesis in fully spread cells. When DNA synthesis and involucrin expression were plotted against contact area, classic dose-response curves were obtained. Thus cell shape acts as a signal for the terminal differentiation of keratinocytes in culture.
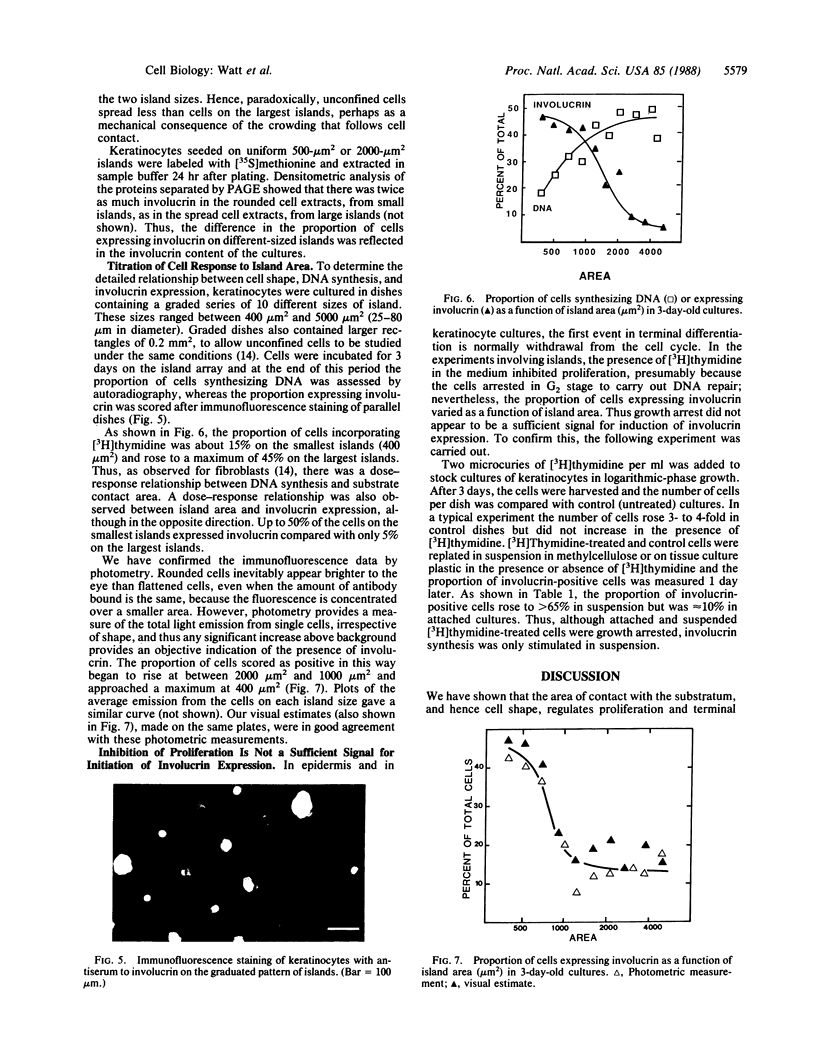
Full text
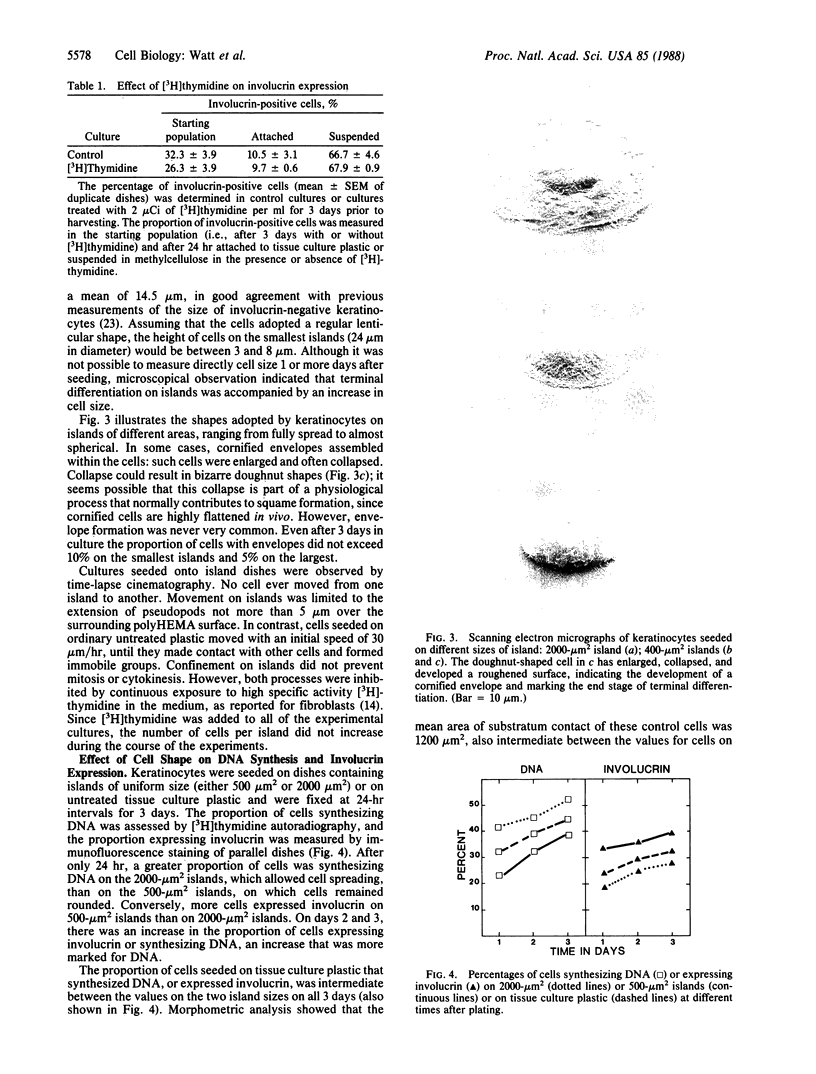
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan M., Harrison P. Co-expression of differentiation markers in hybrids between Friend cells and lymphoid cells and the influence of the cell shape. Cell. 1980 Feb;19(2):437–447. doi: 10.1016/0092-8674(80)90518-8. [DOI] [PubMed] [Google Scholar]
- Barrandon Y., Green H. Cell size as a determinant of the clone-forming ability of human keratinocytes. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5390–5394. doi: 10.1073/pnas.82.16.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A. Cell shape, the complex cellular networks, and gene expression. Cytoskeletal protein genes as a model system. Cell Muscle Motil. 1985;6:23–53. doi: 10.1007/978-1-4757-4723-2_2. [DOI] [PubMed] [Google Scholar]
- Bissell M. J., Hall H. G., Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982 Nov 7;99(1):31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brunette D. M. Mechanical stretching increases the number of epithelial cells synthesizing DNA in culture. J Cell Sci. 1984 Jul;69:35–45. doi: 10.1242/jcs.69.1.35. [DOI] [PubMed] [Google Scholar]
- Dover R., Watt F. M. Measurement of the rate of epidermal terminal differentiation: expression of involucrin by S-phase keratinocytes in culture and in psoriatic plaques. J Invest Dermatol. 1987 Oct;89(4):349–352. doi: 10.1111/1523-1747.ep12471751. [DOI] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Green H. The keratinocyte as differentiated cell type. Harvey Lect. 1980;74:101–139. [PubMed] [Google Scholar]
- Ireland G. W., Dopping-Hepenstal P., Jordan P., O'Neill C. Effect of patterned surfaces of adhesive islands on the shape, cytoskeleton, adhesion and behaviour of Swiss mouse 3T3 fibroblasts. J Cell Sci Suppl. 1987;8:19–33. doi: 10.1242/jcs.1987.supplement_8.2. [DOI] [PubMed] [Google Scholar]
- Kam E., Watt F. M., Pitts J. D. Patterns of junctional communication in skin: studies on cultured keratinocytes. Exp Cell Res. 1987 Dec;173(2):431–438. doi: 10.1016/0014-4827(87)90283-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- O'Neill C., Jordan P., Ireland G. Evidence for two distinct mechanisms of anchorage stimulation in freshly explanted and 3T3 Swiss mouse fibroblasts. Cell. 1986 Feb 14;44(3):489–496. doi: 10.1016/0092-8674(86)90470-8. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975 Nov;6(3):331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G. The role of terminal differentiation in the finite culture lifetime of the human epidermal keratinocyte. Int Rev Cytol Suppl. 1979;(10):25–33. doi: 10.1016/s0074-7696(08)60610-5. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Green H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: activation of the cross-linking by calcium ions. Cell. 1979 Nov;18(3):681–694. doi: 10.1016/0092-8674(79)90123-5. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Green H. Relation of protein synthesis and transglutaminase activity to formation of the cross-linked envelope during terminal differentiation of the cultured human epidermal keratinocyte. J Cell Biol. 1978 Mar;76(3):705–711. doi: 10.1083/jcb.76.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M., Green H. Participation of membrane-associated proteins in the formation of the cross-linked envelope of the keratinocyte. Cell. 1984 Apr;36(4):827–834. doi: 10.1016/0092-8674(84)90032-1. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Ginty C. A. Fibronectin modulation of cell shape and lipogenic gene expression in 3T3-adipocytes. Cell. 1983 Dec;35(3 Pt 2):657–666. doi: 10.1016/0092-8674(83)90098-3. [DOI] [PubMed] [Google Scholar]
- Stoker M. Abortive transformation by polyoma virus. Nature. 1968 Apr 20;218(5138):234–238. doi: 10.1038/218234a0. [DOI] [PubMed] [Google Scholar]
- Sun T. T., Green H. Differentiation of the epidermal keratinocyte in cell culture: formation of the cornified envelope. Cell. 1976 Dec;9(4 Pt 1):511–521. doi: 10.1016/0092-8674(76)90033-7. [DOI] [PubMed] [Google Scholar]
- Watt F. M., Boukamp P., Hornung J., Fusenig N. E. Effect of growth environment on spatial expression of involucrin by human epidermal keratinocytes. Arch Dermatol Res. 1987;279(5):335–340. doi: 10.1007/BF00431227. [DOI] [PubMed] [Google Scholar]
- Watt F. M., Green H. Involucrin synthesis is correlated with cell size in human epidermal cultures. J Cell Biol. 1981 Sep;90(3):738–742. doi: 10.1083/jcb.90.3.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt F. M., Green H. Stratification and terminal differentiation of cultured epidermal cells. Nature. 1982 Feb 4;295(5848):434–436. doi: 10.1038/295434a0. [DOI] [PubMed] [Google Scholar]
- Watt F. M. Influence of cell shape and adhesiveness on stratification and terminal differentiation of human keratinocytes in culture. J Cell Sci Suppl. 1987;8:313–326. doi: 10.1242/jcs.1987.supplement_8.17. [DOI] [PubMed] [Google Scholar]
- Watt F. M. Selective migration of terminally differentiating cells from the basal layer of cultured human epidermis. J Cell Biol. 1984 Jan;98(1):16–21. doi: 10.1083/jcb.98.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. J., Parker L. M., Binder N. E., Beckett M. A., Sinard J. H., Griffiths C. T., Rheinwald J. G. The mesothelial keratins: a new family of cytoskeletal proteins identified in cultured mesothelial cells and nonkeratinizing epithelia. Cell. 1982 Dec;31(3 Pt 2):693–703. doi: 10.1016/0092-8674(82)90324-5. [DOI] [PubMed] [Google Scholar]