Abstract
There is a substantial need to develop better influenza virus vaccines that can protect populations that are not adequately protected by the currently licensed vaccines. While live attenuated influenza virus vaccines induce superior immune responses compared to inactivated vaccines, the manufacturing process of both types of influenza virus vaccines is time consuming and may not be adequate during a pandemic. Adjuvants would be particularly useful if they could enhance the immune response to live attenuated influenza virus vaccines so that the amount of vaccine needed for a protective dose could be reduced. The glycolipid, alpha-galactosylceramide (alpha-GalCer), has recently been shown to have adjuvant activity for both inactivated and replicating recombinant vaccines. The goal of these experiments was to determine whether a derivative of alpha-GalCer, alpha-C-galactosylceramide (alpha-C-GalCer) can enhance the immune response elicited by a live-attenuated influenza virus vaccine containing an NS1 protein truncation and reduce the amount of vaccine required to provide protection after challenge. Our results indicated that the adjuvant reduced both morbidity and mortality in BALB/c mice after challenge with wild type influenza virus. The adjuvant also increased the amount of influenza virus specific total IgG, IgG1, and IgG2a antibodies as well as IFN-gamma secreting CD8+ T cells. By using knockout mice that are not able to generate NKT cells, we were able to demonstrate that the mechanism of adjuvant activity is dependent on NKT cells. Thus, our data indicate that stimulators of NKT cells represent a new avenue of adjuvants to pursue for live attenuated virus vaccines.
1. Introduction
Despite the availability of two FDA-Licensed influenza virus vaccines, influenza remains a significant cause of morbidity and mortality in the United States. The inactivated influenza virus vaccine elicits an antibody response but has limited effectiveness in the elderly, the population most at risk of dying after infection with influenza virus [1, 2]. Live replicating vaccines that can be administered intranasally have several advantages over inactivated vaccines. Live vaccines, including live influenza virus vaccines, provide exposure to more antigens and induce production of immunoglobulin A, which is associated with mucosal immunity [3-5]. Live vaccines induce not only humoral immunity to the vaccine strains, but also cellular immunity to other strains since the internal influenza virus proteins that elicit cellular immunity are highly conserved [6]. The currently licensed cold-adapted live attenuated virus vaccine elicits both humoral and cellular immune responses but it is not indicated for use in the elderly [6, 7]. Another disadvantage of the cold-adapted vaccine is that large amounts of the vaccine are required to induce protection [8]. Thus, the need persists for development of live-attenuated influenza virus vaccines that can overcome these limitations.
Rationally designed live attenuated influenza vaccines containing truncations of non-structural (NS1) protein have been demonstrated to be protective in several animal models [3, 9-11]. NS1 protein is an ideal target for attenuation because it is responsible for inhibition of the innate antiviral interferon response and it also inhibits the adaptive immune response by suppressing dendritic cell function [12-16]. While the NS1 protein truncation vaccines are effective in animal models, an adjuvant would be useful to reduce the amount of vaccine required to elicit a protective immune response. The adjuvant alpha-galactosylceramide (alpha-GalCer) functions by stimulating cytokine release of Natural Killer T (NKT) cells that in turn activates the adaptive immune response [17, 18]. Alpha-GalCer enhances the immune response of both live recombinant vaccines and inactivated vaccines, including an inactivated influenza virus vaccine [19-23]. An analogue of alpha-GalCer, alpha-C-galactosylceramide (alpha-C-GalCer) has been recently shown to have enhanced immunostimulatory properties by inducing increased and prolonged production of the Th1 cytokine, interferon gamma (IFN-gamma) [24, 25]. Alpha-C-GalCer has also been reported to have 100 fold increased anti-tumor activity in a melanoma metastases mouse model and 1000 fold increased anti-malaria activity in mice challenged with live sporozoites compared to alpha-GalCer [24]. While alpha-GalCer is well tolerated in humans, alpha-C-GalCer has not yet been tested in humans [26, 27].
The goal of these experiments was to determine whether alpha-C-GalCer can function as an adjuvant of a live-attenuated influenza virus vaccine that has a truncated NS1 protein. Our results indicated that the alpha-C-GalCer reduced both morbidity and mortality in mice after challenge with wild type influenza virus. The adjuvant increased humoral and cellular immune responses to the vaccine and reduced the amount of virus required to protect mice. Our results confirmed that the mechanism of action of alpha-C-GalCer is to stimulate NKT cells. Thus, our data indicate that stimulators of NKT cells can function as adjuvants for live virus vaccines.
2 Materials and Methods
2.1 Viruses
A/PR/8/34, rwt, was generated by reverse genetics as previously described [28]. An A/PR/8/34 mutant virus expressing only the first 73 amino acids in the NS1 gene, rNS1 1-73 virus, was generated by using two sets of primers. For the 3′ end of the NS viral genome, the following primers were used: GCGCTTAATTAATCAAGATCTAGGATTCTTCTTTCAGAATC and GATCGCTCTTCTGGGAGCAAAAGCAGGGTGACAAAGAC. For the 5′ end of the NS viral genome, the following primers were used: GCGCTTAATTAAGAGGGAGCAATTGTTGGCG and CATCGCTCTTCTATTAGTAGAAACAAGGGTGTTTTTTATTATTAAATAA. rwt virus was propagated in 10 day old embryonated chicken eggs (Charles River Laboratories, Inc) and rNS1 1-73 virus was propagated in 8 day old embryonated chicken eggs, since 8 day old eggs have less mature interferon systems compared to 10 day old eggs and permit the growth of viruses that cannot inhibit interferon.
2.2 Mice
Female 6 week old BALB/c (Jackson Laboratories) and male and female 6 week old CD1d-/- mice (Charles River Laboratories) were anesthetized for all procedures. Mice were anesthetized with intraperitoneal (IP) injection of 0.1 ml of ketamine/xylazine (0.15 mg ketamine and 0.03 mg xylazine). alpha-C-galactosylceramide was synthesized as described and also obtained from the NIH tetramer facility (Emory University) [29]. Infectious virus and alpha-C-galactosylceramide were diluted in PBS and administered intranasally (IN) in a volume of 50ul. For weight loss and survival experiments, groups of 5 mice were challenged on day 21 postvaccination, weighed daily, and mice that lost more than 25% of their initial body weight were sacrificed according to institutional guidelines.
2.3 Plaque assay
Lungs were harvested from mice and homogenized in 1ml of PBS. rwt and rNS1 1-73 viruses (Figure 1) and lung homogenates (Figure 5) were serially diluted in PBS containing bovine serum albumin (BSA). Plaque assays were performed on Madin-Darby canine kidney cells in the presence of 1ug/ml TPCK trypsin. Plaques were visualized by immunostaining. Briefly, cells were fixed for 2h with 4% formaldehyde and permeabilized for 5 min with 1% Triton-X-100. Cells were blocked with 5% milk in PBS for 1 h. Cells were incubated with a 1:2000 dilution of primary antibody, rabbit anti-influenza virus NP protein, for 1h, washed and incubated with 1:2000 dilution of the secondary antibody, anti-rabbit-HRP (GE Healthcare). Plaques were visualized by 10 minute treatment with True blue peroxidase substrate (KPL) and washed with dH2O.
Figure 1.

The NS1 truncation vaccine virus induces interferon and generates smaller plaques than wild type virus. Plaque assays of rNS1 1-73 virus and rwt virus were performed on MDCK cells. The overlay was removed, cells were fixed and permeabilized, and plaques were visualized by immunostaining using an antibody to NP protein as described in materials and methods. Representative images of the plaque assay are shown.
Figure 5.
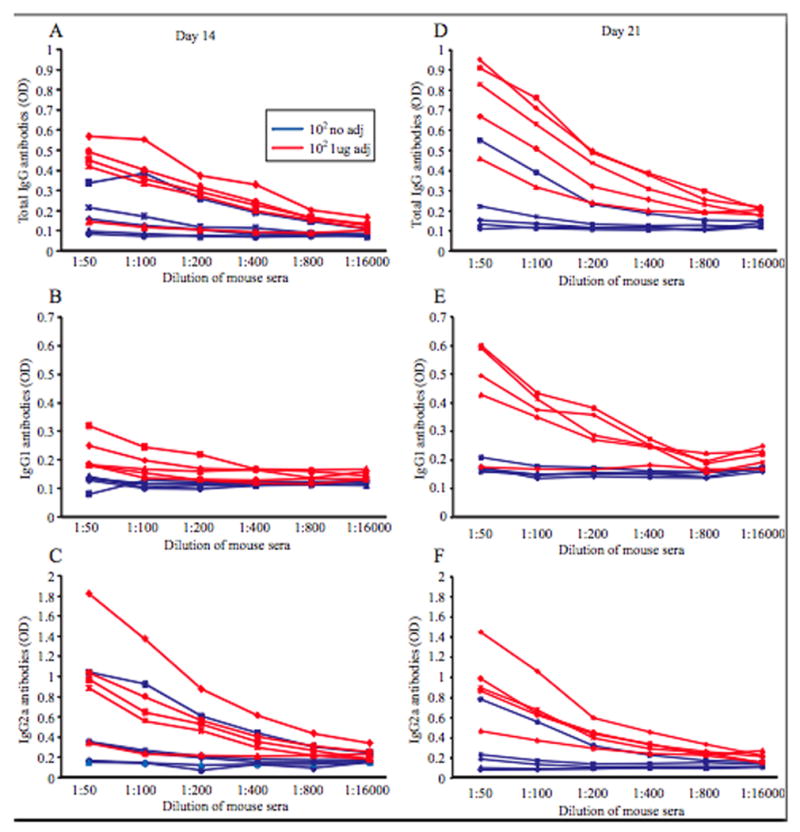
Mice inoculated with vaccine plus adjuvant (alpha-C-GalCer) generated more influenza virus specific antibodies compared with mice inoculated with vaccine alone. A-C. Mice were inoculated with PBS or 102 PFU of the rNS1 1-73 virus with or without 1ug adjuvant for 14 days and sera was harvested. An ELISA for influenza virus specific antibodies was performed on dilutions of the sera using a secondary antibody to total IgG (A), IgG1 (B), or IgG2a (C). The results for each individual mouse are graphed. D-E. Mice were inoculated with PBS or 102 PFU of the rNS1 1-73 virus with or without 1ug adjuvant for 21 days and sera was harvested. An ELISA for influenza virus specific antibodies was performed on dilutions of the sera using a secondary antibody to total IgG (D), IgG1 (E), or IgG2a (F).
2.4 ELISAs
Mice were bled on the indicated days after vaccination, and serum was separated from red blood cells by a 15 minute spin at 13,000G in a microcentrifuge. Sera were stored at -20° C until the ELISA was performed. For the IFN-γ ELISAs, the R&D Systems kit for mouse IFN-γ was used and followed according to the manufacture directions. For the total IgG, IgG1, and IgG2a ELISAs, wells of ELISA plates were coated with 50ul of purified rwt virus at a concentration of 5ug per ml and incubated overnight at 4°C. Virus was removed and plates were incubated with blocking buffer (1% BSA in PBS) for 1 hour at room temperature. Plates were washed with an auto plate washer and incubated with 50ul of the indicated dilutions of sera. Plates were washed and incubated with 50ul of 1:500 dilutions of alkaline phoshatase linked secondary antibodies, anti-IgG total, anti-IgG1, and anti-IgG2a (all Zymed) and incubated at room temperature for 1 hour. Plates were washed and incubated with substrate (PNPP (1×)-substrate for alk. phosphatase, Zymed) for 30 minutes at room temperature. The reaction was stopped with the addition of 50ul of 0.75N NaOH and the plates were read at 405nm in an ELISA plate reader.
2.5 ELISpot
Spleens were harvested from mice and put through a cell strainer (BD Falcon). Red blood cells were lysed using buffers supplied with the mouse erythrocyte lysing kit (R&D Systems). Splenocytes from naïve mice (including antigen presenting cells) were infected with rwt MOI=10 for 2h in a volume of 100ul, treated with 6ul of 0.5mg/ml mitomycin C (Sigma) to stop cell growth, and used to stimulate the CD8+ T cells from vaccinated mice as described [30, 31]. The splenocytes from vaccinated mice were counted and incubated with CD8 beads (Miltenyi Biotec) in buffer (PBS, pH 7.2, 0.5% BSA, and 2mM EDTA, degassed for 15min) according to the manufacturer's instructions. CD8+ T cells were purified by adherence and elution from LS MACS columns (Miltenyi Biotec) and then counted. The assay was performed using an ELISpot kit (R&D Systems) and 2 × 105 infected splenocytes from naïve mice were added to each well. The purified CD8+ T cells from vaccinated mice were added to wells in amounts of 106, 5 × 105, and 2.5 × 105 in triplicate. The manufacturer's instructions for the ELISpot kit were followed and the plate was read using an ELISpot plate reader (Cellular Technology Ltd.).
3. Results
3.1 alpha-C-galactosylceramide co-administered with live attenuated influenza virus vaccine reduces morbidity and mortality after challenge
We have previously demonstrated that live attenuated influenza virus vaccine candidates can be generated by truncating the NS1 protein [9-11, 32]. The viruses containing NS1 truncations are attenuated because they have a reduced ability to inhibit a key component of the innate immune response, interferon. Since interferon is induced by the NS1 truncation viruses, their ability to spread from cell to cell is limited, leading to a smaller plaque morphology compared to wild type influenza virus. Figure 1A shows the plaque morphology of wild type A/PR8 influenza virus, rwt, and Figure 1B shows the plaque morphology of the A/PR8 recombinant NS1 protein truncation virus, rNS1 1-73, which contains only the first 73 out of 230 amino acids of NS1 protein. In addition to generating smaller plaques, the rNS1 1-73 virus grows to titers of approximately two logs less than rwt virus when rNS1 1-73 virus was grown in 8-day old embryonated chicken eggs and rwt virus was grown in 10-day old embryonated chicken eggs.
While NS1 truncation mutant viruses have previously been shown to protect mice from wild type influenza virus challenge, the goal of the experiments is to determine whether an adjuvant can enhance the immunogenicity of the vaccine and allow lower amounts of vaccine to confer protection from wild type challenge in mice. To determine whether the adjuvant increases protection of the vaccine, BALB/c mice were vaccinated with either 102, 103, or 104 PFU of the rNS1 1-73 virus with 0, 1, 2, or 4 ug of the adjuvant alpha-C-galactosylceramide. There was no weight loss following vaccination of PBS or rNS1 1-73 virus with or without adjuvant prior to challenge (Figure 2). While all of the mice vaccinated with 103 PFU of the rNS1 1-73 virus without adjuvant survived challenge with wild type influenza virus (data not shown), all of the mice vaccinated with 102 PFU of the rNS1 1-73 virus without adjuvant died after challenge (Figure 3A). Eighty percent of mice vaccinated with 102 PFU of the rNS1 1-73 virus with 1ug adjuvant survived, indicating that the adjuvant can increase the protection of the vaccine and reduce mortality due to influenza virus challenge. Increasing the amount of adjuvant did not increase the protection of the vaccine. Mice vaccinated with 102 PFU of the rNS1 1-73 virus and 2ug of adjuvant had a 40% survival rate after challenge while mice vaccinated with 102 PFU of the rNS1 1-73 virus with 4ug of adjuvant did not survive challenge.
Figure 2.
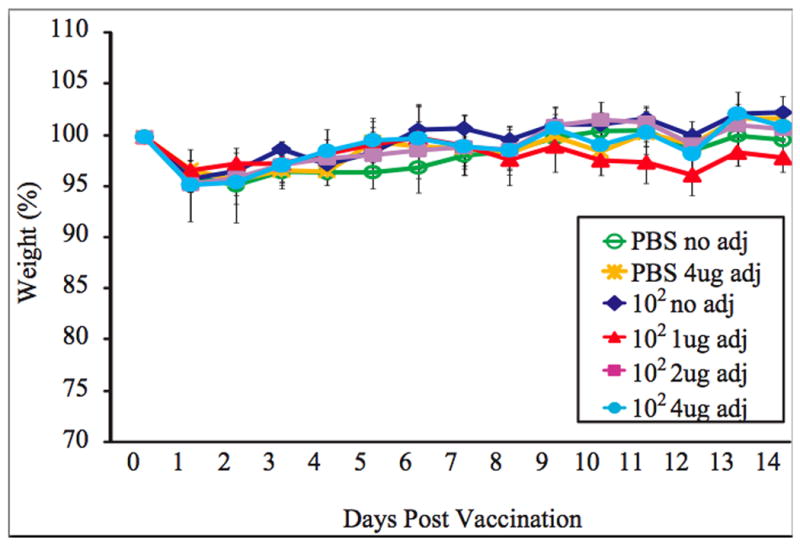
Mice inoculated with vaccine plus adjuvant (alpha-C-GalCer) do not have morbidity after vaccination. Five mice in each group were inoculated intranasally with PBS or 102 PFU of the rNS1 1-73 virus with the indicated amount of adjuvant. The average weight of the mice after vaccination as a percent of the starting weight is graphed +/- S.D.
Figure 3.
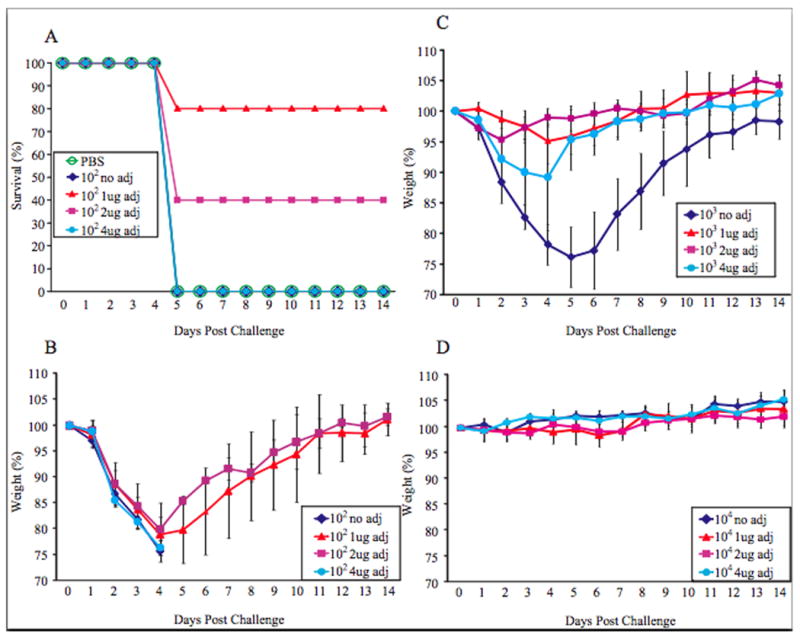
Mice inoculated with vaccine plus adjuvant (alpha-C-GalCer) show reduced mortality and morbidity after challenge compared to mice inoculated with vaccine alone. A. Five mice in each group were inoculated intranasally with PBS or 102 PFU of the rNS1 1-73 virus with the indicated amount of adjuvant. Three weeks post vaccination, mice were challenged intranasally with 100 LD50 rwt virus. Survival of the mice is plotted on the graph for each group. B. The mice in A. were weighed daily after challenge. The average weight of the mice as a percent of the starting weight is graphed +/- S.D. C. Mice were vaccinated with 103 PFU of the rNS1 1-73 virus with the indicated amount of adjuvant and challenged as in part A. The mice were weighed daily after challenge and graphed as average +/- S.D.
Mice vaccinated with 102 PFU of the rNS1 1-73 virus lost a substantial amount of weight whether or not they were also given adjuvant following challenge (Figure 3B). However, the surviving 80% of the mice vaccinated with 102 PFU of the rNS1 1-73 virus in the presence of 1ug adjuvant and the surviving 40% of the 102 PFU of the rNS1 1-73 virus in the presence of 2ug adjuvant regained weight and made a complete recovery within two weeks of challenge. Mice vaccinated with 103 PFU of the rNS1 1-73 virus without adjuvant all survived, but they lost a substantial amount of weight before making a full recovery (Figure 3C). Mice vaccinated with 103 PFU of the rNS1 1-73 virus with 1 or 2ug of adjuvant all survived and lost little weight after challenge with rwt virus. Thus, at these amounts the adjuvant also reduces morbidity caused by challenge with rwt virus. At 4 ug of adjuvant, there was less protection from weight loss and one out of five mice died indicating that this dose of adjuvant did not protect against morbidity. Mice vaccinated with 104 PFU of the rNS1 1-73 virus with or without adjuvant did not lose weight after challenge (Figure 3D).
After demonstrating that 1ug of adjuvant administered with influenza virus vaccine reduces mortality and morbidity associated with wild type influenza virus challenge, but that larger doses of adjuvant are less effective, it was determined whether reducing the amount of adjuvant to less than 1ug would also be effective. Mice were vaccinated with 2.5 × 101 rNS1 1-73 virus and either 0, 3, 1, 0.33, or 0.11ug of adjuvant. Mice vaccinated with 2.5 × 101 rNS1 1-73 virus without adjuvant all died while 3/5 or 4/5 mice co-administered 1ug and 0.33ug adjuvant respectively survived (Table 1). There was a reduction in survival when the amount of adjuvant was lowered to 0.11ug indicating that the maximum effective range of the adjuvant is between 0.1 and 1ug per mouse. The weights of the mice after challenge demonstrate that mice vaccinated with 2.5 × 101 rNS1 1-73 virus and 0.33 or 1ug of adjuvant have reduced morbidity (Figure 4).
Table 1.
Titration of adjuvant in vaccinated micea.
| Vaccine amount | Adjuvant amount | Survivors |
|---|---|---|
| PBS | 0 | 0/5 |
| 2.5 × 101 | 0 | 0/5 |
| 2.5 × 101 | 3ug | 1/5 |
| 2.5 × 101 | 1ug | 3/5 |
| 2.5 × 101 | 0.33ug | 4/5 |
| 2.5 × 101 | 0.11ug | 1/5 |
Groups of 5 mice were vaccinated with the indicated PFU of rNS1 1-73 virus vaccine and the indicated amount of adjuvant. Three weeks post vaccination, mice were challenged with 100 LD50 rwt virus. Survivors of the challenge are shown.
Figure 4.
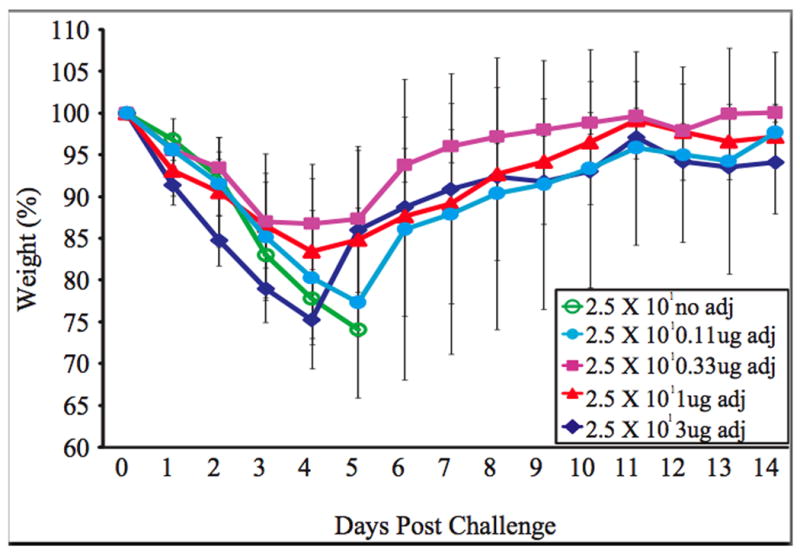
Mice inoculated with vaccine plus 0.33ug or 1ug adjuvant (alpha-C-GalCer) show reduced morbidity after challenge compared to mice inoculated with vaccine alone. Five mice in each group were inoculated intranasally with PBS or 102 PFU of the rNS1 1-73 virus with the indicated amount of adjuvant. Three weeks post vaccination, mice were challenged intranasally with 100 LD50 rwt virus. The average weight of the mice as a percent of the starting weight is graphed +/- S.D.
3.2 alpha-C-galactosylceramide enhances the immunogenicity of a live attenuated influenza virus vaccine
To determine the mechanism by which alpha-C-GalCer enhances protection of influenza virus vaccines, mice were bled on days 14 and 21 post vaccination. Sera were analyzed for influenza virus specific antibodies using an ELISA to total IgG antibodies (Figure 5A and D) and also subtypes IgG1 (Figure 5B and E) and IgG2a (Figure 5C and F). IgG1 antibodies are associated with a Th2 response while IgG2a antibodies are associated with a Th1 response. One advantage of live attenuated vaccines is that they elicit a long-lasting immune response involving both antibodies and CD8+ T cells, while inactivated vaccines in general elicit a more short-lived antibody response. Most of the mice vaccinated with 102 PFU of the rNS1 1-73 virus with adjuvant had more total IgG antibodies to influenza virus on both days 14 and 21 compared to mice vaccinated with 102 PFU of the rNS1 1-73 virus alone (p<0.05 for both day 14 and 21). Most of the mice vaccinated with 102 PFU of the rNS1 1-73 virus with adjuvant had more of both subtypes of IgG, IgG1 and IgG2a antibodies, indicating that the adjuvant broadly stimulates the adaptive immune response (p<0.05 on day 21).
To determine whether the adjuvant enhances the CD8+ T cell response induced by the live attenuated influenza virus vaccine, mice were vaccinated with and without adjuvant for 21 days. CD8+ T cells were purified from the splenocytes of vaccinated mice and stimulated by splenocytes from naïve mice that were infected with influenza virus for 2h. An ELISpot was performed to quantify the number of influenza virus specific CD8+ T cells harvested from the vaccinated mice (Figure 6). The adjuvant increased the number of CD8+ T cells that recognize influenza virus peptides in mice vaccinated with both the 102 and 103 PFU of the rNS1 1-73 viruses (P<0.05). Thus, the adjuvant enhances both the humoral and cellular immune response to live attenuated influenza virus vaccine.
Figure 6.
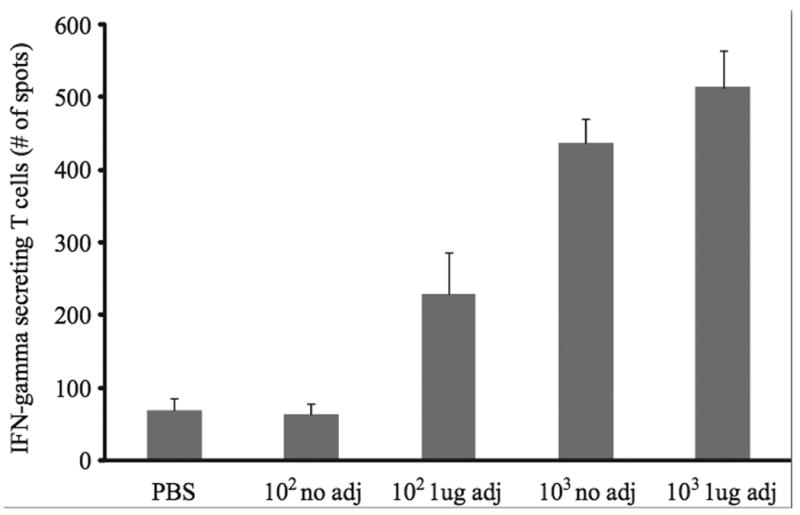
Mice inoculated with vaccine plus adjuvant (alpha-C-GalCer) generated more influenza virus specific CD8+ T cells compared with mice inoculated with vaccine alone. Mice were vaccinated with PBS the indicated amount of rNS1 1-73 virus with or without 1ug adjuvant for 21 days. Spleens were harvested, the red blood cells were lysed, and the CD8+ T cells were isolated from the splenocytes. Splenocytes from naïve mice were infected with influenza virus and used as antigen presenting cells in an ELISpot assay with the CD8+ T cells from the vaccinated mice. 5 × 105 CD8+T cells and 2 × 105 influenza virus infected splenocytes were used for the ELIspot. The experiment was performed in triplicate and average number of spots per well +/- S.D. is graphed.
To determine whether the adjuvant expedites viral clearance in mouse lungs after challenge, mice were vaccinated with 102 or 103 PFU of the rNS1 1-73 virus with and without adjuvant and challenged 21 days after vaccination. Lungs were harvested and homogenized 5 days post challenge and plaque assays were performed to determine the viral titer in the lungs (Figure 7). Mice vaccinated with PBS or 102 PFU of the rNS1 1-73 virus with and without adjuvant all had lung titers of approximately 105 PFU/ml. The increased survival observed in the 102 PFU plus adjuvant vaccinated mice may be due to the increased influenza virus specific CD8+ T cells observed in Figure 6. Forty percent of mice vaccinated with 103 PFU of the rNS1 1-73 virus without adjuvant had viral titers in their lungs, though their titers were approximately two logs lower than those of the 102 PFU of the rNS1 1-73 virus vaccinated mice. The mice vaccinated with 103 PFU of the rNS1 1-73 virus with adjuvant did not have detectable virus in their lungs on day 5 post challenge. This indicates that the adjuvant accelerates clearance of virus in the lungs after challenge with wild type influenza virus in mice vaccinated with 103 PFU of the rNS1 1-73 virus.
Figure 7.

Mice inoculated with vaccine plus adjuvant (alpha-C-GalCer) cleared virus from the lungs faster than mice inoculated with vaccine alone. Groups of five mice were vaccinated with PBS or the indicated amount of rNS1 1-73 virus with or without 1ug adjuvant for 21 days and challenged with 100 LD50 rwt virus. Five days after challenge, lungs were harvested from the mice and homogenized in 1 ml PBS. Plaque assays were performed to determine viral titers in the lungs. The average +/- S.D. is graphed.
3.3 NKT cells are required for adjuvant activity of alpha-C-GalCer
Alpha-C-GalCer stimulates the immune response by activating NKT cells leading to a rapid burst of cytokines including interferon-gamma (IFN-γ). To determine whether the increased protection observed in mice vaccinated with live attenuated influenza virus vaccine and the adjuvant is due to activation of NKT cells, wild type BALB/c mice were compared to knockout BALB/c mice lacking NKT cells. These mice are not able to produce NKT cells because they lack the CD1d molecule (CD1d-/-) that is expressed on cortical thymocytes, as well as on antigen presenting cells and is required for NKT cell development and activation. Alpha-GalCer and alpha-C-GalCer interact directly with CD1d on antigen presenting cells and the CD1d/adjuvant complex is recognized by NKT cells and leads to the activation of NKT cells and the subsequent cytokine burst [33, 34]. It has previously been shown that the adjuvant activity of alpha-GalCer and alpha-C-GalCer is exclusively mediated by its interaction with NKT cells in several different vaccine systems [19-21, 24]. The goal of our experiments was to determine whether NKT cells are required for the adjuvant activity of alpha-C-GalCer in mice vaccinated with our live attenuated influenza virus vaccine. To determine whether the adjuvant induces an NKT cell dependent cytokine burst after vaccination, wild type 6 week old BALB/c mice (Figure 8A) or 6 week old CD1d-/- mice (Figure 8B) were vaccinated with PBS or 5 × 101 PFU of the rNS1 1-73 virus with and without adjuvant. Mice were bled 24h post vaccination and sera were analyzed for the presence of IFN-γ using an ELISA. BALB/c and CD1d-/- mice vaccinated with either PBS or 5 × 101 PFU of the rNS1 1-73 virus without adjuvant did not have detectable levels of IFN-γ in their sera 24h post vaccination. However, all 5 BALB/c mice vaccinated with 5 × 101 PFU of the rNS1 1-73 virus with adjuvant did have detectable levels of IFN-γ in their sera, though there was some variation of IFN-γ among the individual mice. The increase of IFN-gamma in BALB/c mice vaccinated with 5 × 101 PFU with 1ug adjuvant compared to 5 × 101 PFU without adjuvant was statistically significant (P<0.05). The CD1d-/- mice vaccinated with 5 × 101 PFU of the rNS1 1-73 virus with adjuvant did not have detectable levels of IFN-γ in their sera, consistent with the lack of NKT cells in these mice and therefore lack of the cytokine burst of NKT cells.
Figure 8.

The adjuvant (alpha-C-GalCer) stimulates the release of IFN-gamma from NKT cells. A. BALB/c mice or B. CD1d knockout mice in the BALB/c background were inoculated with PBS or 50 PFU of the rNS1 1-73 virus with or without 1ug adjuvant. Sera were harvested 24h post vaccination. An IFN-gamma ELISA was performed and the result for each mouse is shown.
The CD1d-/- mice were challenged with wild type influenza virus 21 days post vaccination to determine whether the protection observed in vaccinated mice also administered adjuvant is solely due to NKT cells (Figure 9). PBS vaccinated mice quickly lost weight and needed to be sacrificed several days after challenge with influenza virus. The CD1d-/- mice vaccinated with 5 × 101 PFU of the rNS1 1-73 virus with and without adjuvant lost similar amounts of weight after challenge, indicating that the adjuvant did not increase protection of the vaccine in mice lacking NKT cells. While the mice vaccinated with 5 × 101 PFU without adjuvant appeared to lose slightly more weight between days 3 and 5 post challenge than the adjuvant mice, this difference was not statistically significant (P>0.05). In addition, survival after challenge was similar in the mice vaccinated with and without adjuvant (3/5 mice and 2/5 mice respectively). Thus, we can conclude that the adjuvant increases immunogenicity and enhances protection of the live attenuated influenza virus vaccine only in wild type mice in which NKT cells can be stimulated.
Figure 9.
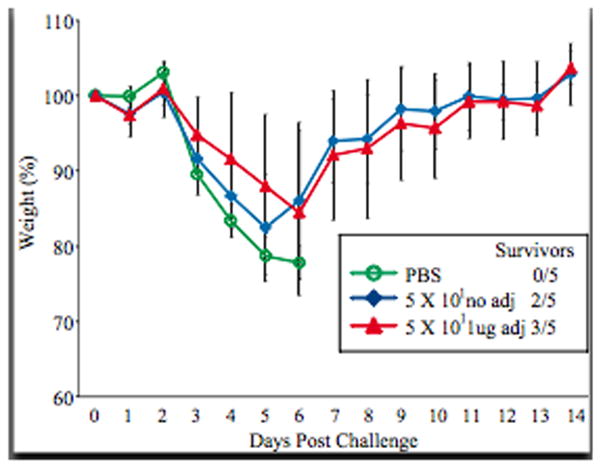
NKT cells are required for the adjuvant (alpha-C-GalCer) to enhance protection after challenge. Groups of five CD1d knockout mice were vaccinated with PBS or 50 PFU of the rNS1 1-73 virus with or without 1ug adjuvant. Twenty one days post vaccination, mice were challenged with 100 LD50 rwt virus. Mice were weighed daily after challenge and average weight +/- S.D. is graphed.
4. Discussion
While a live attenuated influenza virus vaccine, the cold-adapted vaccine, is currently licensed for children and healthy adults up to age 49, this vaccine has several disadvantages that could be overcome with a new influenza virus vaccine. One major short-coming of the cold-adapted vaccine is that large amounts of the vaccine are required to elicit a protective immune response. The cold-adapted vaccine administered to humans comprises approximately 107 TCID50 of three reassortants containing the 6 internal genes of the cold-adapted strain A/Ann Arbor/6/60 or the cold-adapted strain B/Ann Arbor/1/66 and HA and NA genes from three different pathogenic influenza viruses currently circulating throughout the population. In order to induce an adequate immune response and provide protection in mice after challenge, between 105 and 106 TCID50 of the cold adapted vaccine needs to be administered [35-37]. In contrast, the truncated NS1 vaccine used for these experiments is protective at much lower doses in mice. Without adjuvant, 10000 PFU is adequate to protect against weight loss and death in mice after challenge with wild type virus, while 1000 PFU of the truncated NS1 vaccine plus alpha-C-GalCer can protect against weight loss and death in mice after challenge (Figure 3). The lower the amount of vaccine required to confer protection after challenge, the faster large quantities of the vaccine can be produced. Vaccine production costs would also be dramatically diminished. It should be noted that while alpha-C-GalCer has adjuvant activity in inbred mice, the effective range of the adjuvant is narrow and this would likely pose problems in determining an effective dose for the heterogenous human population. However, our data suggests that other stimulators of NKT cells with wider effective ranges may be useful adjuvants for humans.
It has yet to be determined whether NS1 truncation vaccines are also able to overcome another limitation of the cold-adapted vaccine, efficacy in the elderly. The vast majority of influenza virus-related deaths occur in people over the age of 65 years [38]. The cold-adapted vaccine is not currently licensed for the elderly and the inactivated virus vaccine has limited effectiveness in this age group. Thus, it is imperative to develop novel influenza virus vaccines that are safe, immunogenic, and protective in the elderly. We are currently analyzing the efficacy of NS1 truncation vaccines in elderly mice.
Another method to increase immunogenicity of vaccines in the elderly and increase their protection is through the use of adjuvants. Aluminum based adjuvants have been used with many vaccines including vaccines against tetanus, diphtheria, pertussis, poliomyelitis, hepatitis A and hepatitis B virus [39]. It appears that there are multiple mechanisms that facilitate the adjuvant activity of aluminum. In preparation of vaccines containing aluminum adjuvants, the soluble antigen is absorbed to the aluminum. This converts the soluble antigen to a particulate matter that increases uptake by macrophages. Also, macrophages are activated by aluminum increasing antigen presentation [40]. As aluminum adjuvants stimulate a Th2 response, they do not significantly increase protection of inactivated influenza virus vaccines in humans [41].
One adjuvant that does appear to increase protection of inactivated influenza virus vaccines in humans is MF59. MF59 is an oil-in-water emulsion, with the oil phase consisting of squalene oil and the aqueous phase consisting of citrate buffer. The emulsion is stabilized by the surfactants Tween 80 and Span 85 [42, 43]. Inactivated influenza virus vaccine adjuvanted with MF59 is available in the marketplace in Europe and has been administered to an estimated 30 million people [42]. MF59 is believed to function by inducing a local pro-inflammatory response at the injection site. MF59 targets antigen presenting cells, including dendritic cells, and also enhances antigen uptake [44]. Interestingly, when the adjuvant effects of MF59 were directly compared to alpha-GalCer in young mice vaccinated with purified HA and NA proteins, alpha-GalCer increased influenza virus specific antibody titers and survival after challenge to a similar extent as MF59 [22].
The alpha-GalCer adjuvant and its derivatives stimulate the immune system by a distinct mechanism. Alpha-GalCer [(2S,3S,4R)-1-O-(alpha-D-galactopyranosyl)-2-(N-hexacosanoylamino)-1,3,4-octadecanetriol] is a galactose carbohydrate attached by an α-linkage to a ceramide lipid that has both acyl and sphingosine chains. It binds to the CD1d molecule on antigen presenting cells and is recognized by the semi-invariant T cell receptor on NKT cells [45]. Activation of NKT cells leads to a short but potent cytokine release that aids the formation of an adaptive immune response [18]. The cytokines that have been associated with NKT stimulation by alpha-GalCer include IFN-γ, TNF, GM-CSF, IL-2, IL-4, IL-10, and IL-13 [18]. The release of these cytokines results in the subsequent stimulation of NK, B, CD4+, and CD8+ T cells. Alpha-GalCer was originally identified as an anti-tumor agent and is also being evaluated as treatment against autoimmune diseases since it was discovered that NKT cells are reduced and sometimes defective in mouse strains that are predisposed to develop autoimmunity [46-48]. The potent stimulation of the immune response by alpha-GalCer led investigators to evaluate its potential as an adjuvant for vaccines.
Alpha-GalCer has been demonstrated to enhance the immunogenicity of a wide variety of vaccines. Alpha-GalCer administered with a malaria vaccine consisting of irradiated malaria sporozoites provided more protection from challenge with live malaria sporozoites compared to vaccine alone [19]. Alpha-GalCer increased immunogenicity of adenovirus and Sindbis virus recombinant vectors expressing malaria antigens indicating that alpha-GalCer is an effective adjuvant for both live and inactivated vaccines [19]. Alpha-GalCer is also able to enhance protection by a new generation of vaccines. A protein vaccine consisting of the B subunit Shiga toxin (dendritic cell-targeting protein) conjugated to an antigen elicited antigen specific CD8+ T cells when administered with alpha-GalCer while no antigen specific CD8+ T cells were detected when the vaccine was administered alone [49]. DNA vaccines including an HIV vaccine as well as a Leishmania vaccine consisting of both a DNA prime and a vaccinia virus boost elicited enhanced immune responses to the pathogens when given with alpha-GalCer [20, 50].
Alpha-GalCer has also been tested for adjuvant activity with inactivated and subunit influenza virus vaccines. The intranasal administration of alpha-GalCer with inactivated PR8 vaccine in mice resulted in a dramatic increase in both IgG and IgA virus specific antibodies compared to vaccine alone [23]. Alpha-GalCer had similar adjuvant activity when co-administered with purified PR8 HA vaccine [21]. Alpha-GalCer has also been reported to increase cross-protection of a purified HA vaccine after heterologous challenge [51]. Further studies on the effects of alpha-GalCer on the immune system have helped to understand the reason that it is a potent adjuvant when administered intranasally. While it had been established that alpha-GalCer functions as an adjuvant by stimulating NKT cells, it was unclear how NKT cells were able to stimulate such a potent immune response after intranasal vaccination since there are not many NKT cells in the nasal mucosa. It was recently determined that alpha-GalCer induces a local proliferation of NKT cells in the nasal passageway and facilitates a cytokine burst that activates immunity [51]. Interestingly, alpha-GalCer reduces influenza virus growth when administered intraperitoneally but alpha-GalCer increases influenza virus growth when administered intranasally [52]. Thus, alpha-GalCer may increase replication of live attenuated and live recombinant vaccines that are inoculated intranasally and thereby increase the amount of antigen presented to the immune system. On the other hand, too much adjuvant may reduce viral growth after vaccination and may explain why 4ug of alpha-C-GalCer was not protective in our system. Our data that the alpha-GalCer derivative, alpha-C-GalCer, functions as an adjuvant for a live attenuated influenza virus vaccine demonstrate that new vaccine and adjuvant combinations can improve vaccine efficacy.
Acknowledgments
This work was partially supported by the Bill and Melinda Gates Foundation (grant 38648; David Ho, PI), NIH grant (1 U01 AI070469), the NIH training grant T32 A1007645 (S.A.K-B and K.F.), the Northeast Biodefense Center (U54AI057158), and by NIH grant RO1 AI 41111 (T.M). We thank Lily Ngai for excellent technical assistance.
References
- 1.Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann Intern Med. 1995;123(7):518–27. doi: 10.7326/0003-4819-123-7-199510010-00008. [DOI] [PubMed] [Google Scholar]
- 2.Nichol KL, Wuorenma J, von Sternberg T. Benefits of influenza vaccination for low-, intermediate-, and high-risk senior citizens. Arch Intern Med. 1998;158(16):1769–76. doi: 10.1001/archinte.158.16.1769. [DOI] [PubMed] [Google Scholar]
- 3.Richt JA, Lekcharoensuk P, Lager KM, Vincent AL, Loiacono CM, Janke BH, Wu WH, Yoon KJ, Webby RJ, Solorzano A, Garcia-Sastre A. Vaccination of pigs against swine influenza viruses by using an NS1-truncated modified live-virus vaccine. J Virol. 2006;80(22):11009–18. doi: 10.1128/JVI.00787-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muster T, Ferko B, Klima A, Purtscher M, Trkola A, Schulz P, Grassauer A, Engelhardt OG, Garcia-Sastre A, Palese P, et al. Mucosal model of immunization against human immunodeficiency virus type 1 with a chimeric influenza virus. J Virol. 1995;69(11):6678–86. doi: 10.1128/jvi.69.11.6678-6686.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desheva JA, Lu XH, Rekstin AR, Rudenko LG, Swayne DE, Cox NJ, Katz JM, Klimov AI. Characterization of an influenza A H5N2 reassortant as a candidate for live-attenuated and inactivated vaccines against highly pathogenic H5N1 viruses with pandemic potential. Vaccine. 2006;24(4748):6859–66. doi: 10.1016/j.vaccine.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 6.Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol. 2004;59(1):1–15. doi: 10.1111/j.0300-9475.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- 7.Clements ML, Murphy BR. Development and persistence of local and systemic antibody responses in adults given live attenuated or inactivated influenza A virus vaccine. J Clin Microbiol. 1986;23(1):66–72. doi: 10.1128/jcm.23.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendelman PM, Cordova J, Cho I. Safety, efficacy and effectiveness of the influenza virus vaccine, trivalent, types A and B, live, cold-adapted (CAIV-T) in healthy children and healthy adults. Vaccine. 2001;19(1719):2221–6. doi: 10.1016/s0264-410x(00)00449-7. [DOI] [PubMed] [Google Scholar]
- 9.Talon J, Salvatore M, O'Neill RE, Nakaya Y, Zheng H, Muster T, Garcia-Sastre A, Palese P. Influenza A and B viruses expressing altered NS1 proteins: A vaccine approach. Proc Natl Acad Sci U S A. 2000;97(8):4309–14. doi: 10.1073/pnas.070525997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baskin CR, Bielefeldt-Ohmann H, Garcia-Sastre A, Tumpey TM, Van Hoeven N, Carter VS, Thomas MJ, Proll S, Solorzano A, Billharz R, Fornek JL, Thomas S, Chen CH, Clark EA, Murali-Krishna K, Katze MG. Functional genomic and serological analysis of the protective immune response resulting from vaccination of macaques with an NS1-truncated influenza virus. J Virol. 2007;81(21):11817–27. doi: 10.1128/JVI.00590-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vincent AL, Ma W, Lager KM, Janke BH, Webby RJ, Garcia-Sastre A, Richt JA. Efficacy of intranasal administration of a truncated NS1 modified live influenza virus vaccine in swine. Vaccine. 2007;25(47):7999–8009. doi: 10.1016/j.vaccine.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geiss GK, Salvatore M, Tumpey TM, Carter VS, Wang X, Basler CF, Taubenberger JK, Bumgarner RE, Palese P, Katze MG, Garcia-Sastre A. Cellular transcriptional profiling in influenza A virus-infected lung epithelial cells: the role of the nonstructural NS1 protein in the evasion of the host innate defense and its potential contribution to pandemic influenza. Proc Natl Acad Sci U S A. 2002;99(16):10736–41. doi: 10.1073/pnas.112338099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mibayashi M, Martinez-Sobrido L, Loo YM, Cardenas WB, Gale M, Jr, Garcia-Sastre A. Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus. J Virol. 2007;81(2):514–24. doi: 10.1128/JVI.01265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talon J, Horvath CM, Polley R, Basler CF, Muster T, Palese P, Garcia-Sastre A. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J Virol. 2000;74(17):7989–96. doi: 10.1128/jvi.74.17.7989-7996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Sastre A, Egorov A, Matassov D, Brandt S, Levy DE, Durbin JE, Palese P, Muster T. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology. 1998;252(2):324–30. doi: 10.1006/viro.1998.9508. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Sesma A, Marukian S, Ebersole BJ, Kaminski D, Park MS, Yuen T, Sealfon SC, Garcia-Sastre A, Moran TM. Influenza virus evades innate and adaptive immunity via the NS1 protein. J Virol. 2006;80(13):6295–304. doi: 10.1128/JVI.02381-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujii S, Shimizu K, Hemmi H, Steinman RM. Innate Valpha14(+) natural killer T cells mature dendritic cells, leading to strong adaptive immunity. Immunol Rev. 2007;220:183–98. doi: 10.1111/j.1600-065X.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 18.Van Kaer L. alpha-Galactosylceramide therapy for autoimmune diseases: prospects and obstacles. Nat Rev Immunol. 2005;5(1):31–42. doi: 10.1038/nri1531. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Aseguinolaza G, Van Kaer L, Bergmann CC, Wilson JM, Schmieg J, Kronenberg M, Nakayama T, Taniguchi M, Koezuka Y, Tsuji M. Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J Exp Med. 2002;195(5):617–24. doi: 10.1084/jem.20011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Y, Chen A, Li X, Chen Z, Zhang W, Song Y, Gurner D, Gardiner D, Basu S, Ho DD, Tsuji M. Enhancement of HIV DNA vaccine immunogenicity by the NKT cell ligand, alpha-galactosylceramide. Vaccine. 2008;26(15):1807–16. doi: 10.1016/j.vaccine.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Ko SY, Ko HJ, Chang WS, Park SH, Kweon MN, Kang CY. alpha-Galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J Immunol. 2005;175(5):3309–17. doi: 10.4049/jimmunol.175.5.3309. [DOI] [PubMed] [Google Scholar]
- 22.Galli G, Pittoni P, Tonti E, Malzone C, Uematsu Y, Tortoli M, Maione D, Volpini G, Finco O, Nuti S, Tavarini S, Dellabona P, Rappuoli R, Casorati G, Abrignani S. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci U S A. 2007;104(10):3984–9. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Youn HJ, Ko SY, Lee KA, Ko HJ, Lee YS, Fujihashi K, Boyaka PN, Kim SH, Horimoto T, Kweon MN, Kang CY. A single intranasal immunization with inactivated influenza virus and alpha-galactosylceramide induces long-term protective immunity without redirecting antigen to the central nervous system. Vaccine. 2007;25(28):5189–98. doi: 10.1016/j.vaccine.2007.04.081. [DOI] [PubMed] [Google Scholar]
- 24.Schmieg J, Yang G, Franck RW, Tsuji M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand alpha-Galactosylceramide. J Exp Med. 2003;198(11):1631–41. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujii S, Shimizu K, Hemmi H, Fukui M, Bonito AJ, Chen G, Franck RW, Tsuji M, Steinman RM. Glycolipid alpha-C-galactosylceramide is a distinct inducer of dendritic cell function during innate and adaptive immune responses of mice. Proc Natl Acad Sci U S A. 2006;103(30):11252–7. doi: 10.1073/pnas.0604812103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giaccone G, Punt CJ, Ando Y, Ruijter R, Nishi N, Peters M, von Blomberg BM, Scheper RJ, van der Vliet HJ, van den Eertwegh AJ, Roelvink M, Beijnen J, Zwierzina H, Pinedo HM. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. 2002;8(12):3702–9. [PubMed] [Google Scholar]
- 27.Nieda M, Okai M, Tazbirkova A, Lin H, Yamaura A, Ide K, Abraham R, Juji T, Macfarlane DJ, Nicol AJ. Therapeutic activation of Valpha24+Vbeta11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103(2):383–9. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- 28.He Q, Martinez-Sobrido L, Eko FO, Palese P, Garcia-Sastre A, Lyn D, Okenu D, Bandea C, Ananaba GA, Black CM, Igietseme JU. Live-attenuated influenza viruses as delivery vectors for Chlamydia vaccines. Immunology. 2007;122(1):28–37. doi: 10.1111/j.1365-2567.2007.02608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen G, Chien M, Tsuji M, Franck RW. E and Z alpha-C-galactosylceramides by Julia-Lythgoe-Kocienski chemistry: a test of the receptor-binding model for glycolipid immunostimulants. Chembiochem. 2006;7(7):1017–22. doi: 10.1002/cbic.200500386. [DOI] [PubMed] [Google Scholar]
- 30.Russmann H, Shams H, Poblete F, Fu Y, Galan JE, Donis RO. Delivery of epitopes by the Salmonella type III secretion system for vaccine development. Science. 1998;281(5376):565–8. doi: 10.1126/science.281.5376.565. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen HH, Boyaka PN, Moldoveanu Z, Novak MJ, Kiyono H, McGhee JR, Mestecky J. Influenza virus-infected epithelial cells present viral antigens to antigen-specific CD8+ cytotoxic T lymphocytes. J Virol. 1998;72(5):4534–6. doi: 10.1128/jvi.72.5.4534-4536.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quinlivan M, Zamarin D, Garcia-Sastre A, Cullinane A, Chambers T, Palese P. Attenuation of equine influenza viruses through truncations of the NS1 protein. J Virol. 2005;79(13):8431–9. doi: 10.1128/JVI.79.13.8431-8439.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borg NA, Wun KS, Kjer-Nielsen L, Wilce MC, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, Rossjohn J. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448(7149):44–9. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 34.Lee A, Farrand KJ, Dickgreber N, Hayman CM, Jurs S, Hermans IF, Painter GF. Novel synthesis of alpha-galactosyl-ceramides and confirmation of their powerful NKT cell agonist activity. Carbohydr Res. 2006;341(17):2785–98. doi: 10.1016/j.carres.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Romanova JR, Tannock GA, Alexandrova GI. Protective responses in mice to vaccination with multiply administered cold-adapted influenza vaccine reassortants and wild-type viruses. Vaccine. 1997;15(67):653–8. doi: 10.1016/s0264-410x(96)00245-9. [DOI] [PubMed] [Google Scholar]
- 36.Tannock GA, Paul JA, Barry RD. Relative immunogenicity of the cold-adapted influenza virus A/Ann Arbor/6/60 (A/AA/6/60-ca), recombinants of A/AA/6/60-ca, and parental strains with similar surface antigens. Infect Immun. 1984;43(2):457–62. doi: 10.1128/iai.43.2.457-462.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mak NK, Zhang YH, Ada GL, Tannock GA. Humoral and cellular responses of mice to infection with a cold-adapted influenza A virus variant. Infect Immun. 1982;38(1):218–25. doi: 10.1128/iai.38.1.218-225.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barker WH, Mullooly JP. Impact of epidemic type A influenza in a defined adult population. Am J Epidemiol. 1980;112(6):798–811. doi: 10.1093/oxfordjournals.aje.a113052. [DOI] [PubMed] [Google Scholar]
- 39.Lindblad EB. Aluminium compounds for use in vaccines. Immunol Cell Biol. 2004;82(5):497–505. doi: 10.1111/j.0818-9641.2004.01286.x. [DOI] [PubMed] [Google Scholar]
- 40.Rimaniol AC, Gras G, Verdier F, Capel F, Grigoriev VB, Porcheray F, Sauzeat E, Fournier JG, Clayette P, Siegrist CA, Dormont D. Aluminum hydroxide adjuvant induces macrophage differentiation towards a specialized antigen-presenting cell type. Vaccine. 2004;22(2324):3127–35. doi: 10.1016/j.vaccine.2004.01.061. [DOI] [PubMed] [Google Scholar]
- 41.Davenport FM, Hennessy AV, Askin FB. Lack of adjuvant effect of A1PO4 on purified influenza virus hemagglutinins in man. J Immunol. 1968;100(5):1139–40. [PubMed] [Google Scholar]
- 42.O'Hagan DT, Wack A, Podda A. MF59 is a safe and potent vaccine adjuvant for flu vaccines in humans: what did we learn during its development? Clin Pharmacol Ther. 2007;82(6):740–4. doi: 10.1038/sj.clpt.6100402. [DOI] [PubMed] [Google Scholar]
- 43.Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007;5(7):505–17. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]
- 44.Dupuis M, Murphy TJ, Higgins D, Ugozzoli M, van Nest G, Ott G, McDonald DM. Dendritic cells internalize vaccine adjuvant after intramuscular injection. Cell Immunol. 1998;186(1):18–27. doi: 10.1006/cimm.1998.1283. [DOI] [PubMed] [Google Scholar]
- 45.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 46.Mieza MA, Itoh T, Cui JQ, Makino Y, Kawano T, Tsuchida K, Koike T, Shirai T, Yagita H, Matsuzawa A, Koseki H, Taniguchi M. Selective reduction of V alpha 14+ NK T cells associated with disease development in autoimmune-prone mice. J Immunol. 1996;156(10):4035–40. [PubMed] [Google Scholar]
- 47.Esteban LM, Tsoutsman T, Jordan MA, Roach D, Poulton LD, Brooks A, Naidenko OV, Sidobre S, Godfrey DI, Baxter AG. Genetic control of NKT cell numbers maps to major diabetes and lupus loci. J Immunol. 2003;171(6):2873–8. doi: 10.4049/jimmunol.171.6.2873. [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi E, Motoki K, Uchida T, Fukushima H, Koezuka Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol Res. 1995;7(1011):529–34. [PubMed] [Google Scholar]
- 49.Adotevi O, Vingert B, Freyburger L, Shrikant P, Lone YC, Quintin-Colonna F, Haicheur N, Amessou M, Herbelin A, Langlade-Demoyen P, Fridman WH, Lemonnier F, Johannes L, Tartour E. B subunit of Shiga toxin-based vaccines synergize with alpha-galactosylceramide to break tolerance against self antigen and elicit antiviral immunity. J Immunol. 2007;179(5):3371–9. doi: 10.4049/jimmunol.179.5.3371. [DOI] [PubMed] [Google Scholar]
- 50.Dondji B, Deak E, Goldsmith-Pestana K, Perez-Jimenez E, Esteban M, Miyake S, Yamamura T, McMahon-Pratt D. Intradermal NKT cell activation during DNA priming in heterologous prime-boost vaccination enhances T cell responses and protection against Leishmania. Eur J Immunol. 2008;38(3):706–19. doi: 10.1002/eji.200737660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamijuku H, Nagata Y, Jiang X, Ichinohe T, Tashiro T, Mori K, Taniguchi M, Hase K, Ohno H, Shimaoka T, Yonehara S, Odagiri T, Tashiro M, Sata T, Hasegawa H, Seino K. Mechanism of NKT cell activation by intranasal coadministration of alpha -galactosylceramide, which can induce cross-protection against influenza viruses. Mucosal Immunol. 2008;1:208–218. doi: 10.1038/mi.2008.2. [DOI] [PubMed] [Google Scholar]
- 52.Ho LP, Denney L, Luhn K, Teoh D, Clelland C, McMichael AJ. Activation of invariant NKT cells enhances the innate immune response and improves the disease course in influenza A virus infection. Eur J Immunol. 2008 doi: 10.1002/eji.200738017. [DOI] [PubMed] [Google Scholar]


