Abstract
Necrotizing enterocolitis (NEC) is a devastating disease of premature babies. Previously, we have shown that epidermal growth factor (EGF) reduces NEC and that overproduction of hepatic TNF-α is associated with intestinal damage. Leakage of TNF-α may be a consequence of epithelial hepatic cellular junction dysfunction. The aim of this study was to investigate changes in the composition of hepatic tight junctions (TJs) and adherens junctions (AJs). Using an established rat model of NEC, animals were divided into the following groups: dam fed (DF); formula fed (NEC); or fed with formula supplemented with EGF (EGF). Serum EGF and histological localization of major TJ and AJ proteins were evaluated. Distribution patterns of hepatic TJ and AJ proteins were significantly altered in the NEC group compared to DF or EGF groups. Cytoplasmic accumulation of occludin, claudin-2, and ZO-1 with reduction of claudin-3 signal was detected in the liver of NEC rats. Localization of β-catenin was associated with the hepatocyte membrane in EGF and DF groups, but diffused in the NEC group. These data show that hepatic cellular junctions are significantly altered during NEC pathogenesis. EGF-mediated reduction of experimental NEC is associated with protection of hepatic integrity and structure.
Necrotizing enterocolitis (NEC) is the most common intestinal disease of premature babies. The pathogenesis of this disease is still poorly understood despite its morbidity, mortality and increasing occurrence in the United States. Severe NEC is characterized by a hemorrhagic inflammatory necrosis of the distal ileum and proximal colon. One of the best animal models to study this disease is the neonatal rat NEC model (1). To develop this model, formula feeding of prematurely born rats and their exposure to bacterial colonization and intestinal hypoxia are required (2,3). Intestinal damage resulting from this protocol exhibits similar pathological signs to those found in NEC patients (4).
Epidermal growth factor (EGF) is a peptide with trophic, maturational, and healing effects on intestinal mucosa (5). A potential role for EGF in the prevention of NEC in human neonates has been suggested (6-8). In a rat model of NEC, we have previously shown that supplementation of formula with EGF reduces the incidence of disease (2), down regulates production of pro-inflammatory cytokines (9), decreases intestinal apoptosis (10), improves intestinal barrier function (11), and maintains bile acid (BA) homeostasis (12). In the liver, a hepatoprotective effect of EGF has been shown in several experimental injury models. For example, in mice exposed to thioacetamide, EGF treatment significantly reduces mortality, and intestinal and renal injury (13). EGF also protects against severe hepatic necrosis induced by carbon tetrachloride in rats (14).
Severe intestinal damage is often accompanied by multiple organ dysfunction resulting in hepatic, renal and/or pulmonary failure (19). Ischemia-reperfusion (I/R) has been implicated in NEC pathogenesis and multiple organ failure is frequently observed in I/R injury models (20,21). In babies with severe forms of NEC, pathological changes are also observed in hepatic morphology and hepatobiliary functions (22).
In hepatocytes, tight junctions (TJs) seal the canalicular lumen from the sinusoidal space and form a paracellular barrier between bile and blood (16). Studies with murine models of experimental colitis have shown increased permeability and altered TJ structure in liver (17). The pathogenic mechanisms responsible for hepatobiliary changes in human NEC or IBD are not known. However, if hepatic TJ structure is altered, the entrance of inflammatory cytokines and gut-derived bacteria toxins from portal circulation into the hepatobiliary system may occur (17).
Our laboratory was the first to report the role of the liver in the pathogenesis of experimental NEC using the neonatal rat model (23). We have shown that the majority of TNF-α found in the ileal lumen of animals with severe NEC injury originates from Kupffer cells (KCs) (23). Further, treatment of experimental NEC with anti-TNF-α reduced overproduction of hepatic TNF-α and decreased intestinal injury in this model (24). Hepatocytes secrete cytokines into the canaliculi and then into the bile ducts. These secretions can enter the intestinal lumen. KCs are found in the sinusoids of the liver. Blood flows through the sinusoids, empties into the central vein and exits the liver via the vena cava. Thus, cytokines that are KC-derived should not enter the intestinal lumen unless hepatic cellular junctions are altered.
TJs form the intercellular contacts between epithelial cells. The formation of TJs involves the assembly of cytosolic proteins (ZO-1, ZO-2) and integral membrane proteins (occludin, claudins, JAM1) (15). We have previously shown that TJs in ileal epithelium are altered during NEC pathogenesis and normalized with EGF treatment (11).
Adherens junctions (AJs) are another type of cellular connections anchoring cells one to another and attaching to components of the intracellular matrix. AJs are composed of transmembrane and cytosolic components. Cadherins and catenins are the two major families of proteins involved in AJs structure (18).
The aim of this study was to investigate changes in the formation of hepatic TJs and AJs during NEC pathogenesis using a rat model of NEC. In addition, we evaluated the effect of EGF treatment during development of NEC on hepatic cell junctions. NEC was induced in neonatal rats by formula feeding and exposure to asphyxia/cold stress. Serum EGF levels, histological localization of major TJ proteins (occludin, claudin-1, -2, -3, JAM1, ZO-1, ZO-2) and AJ proteins (E-cadherin, α-catenin, β-catenin) were evaluated.
Materials and Methods
Animal model and diets
The protocol was approved by the Animal Care and Use Committee of the University of Arizona (A-324801-95081). Neonatal Sprague-Dawley rats (Charles River Laboratories, Pontage, MI) were collected by caesarian section 1 day before scheduled birth. Pups were divided into three experimental groups: rats hand-fed with a cow's milk-based formula (25) (NEC, n = 36); rats fed with a formula supplemented with 500ng/ml of rat EGF (Harlan Bioproducts, Indianapolis, IN; EGF, n = 24) and dam fed animals (DF, n = 22). Hand-fed pups were given a total of 850μl of milk every day. All experimental groups were stressed twice a day with asphyxia (breathing 100% nitrogen gas for 1 minute) followed by cold stress (4°C for 10 minutes) (2, 23). After 96 hrs, all surviving animals were terminated via decapitation.
NEC evaluation
Pathological changes in intestinal architecture were evaluated using our previously described NEC scoring system (2). Histological changes in the ileum were scored by a blinded evaluator and graded as follows: 0 (normal), no damage; 1 (mild), slight submucosal and/or lamina propria separation; 2 (moderate), moderate submucosal and/or lamina propria separation and/or edema in the submucosa and muscular layers; 3 (severe), severe submucosal and/or lamina propria separation and/or severe edema in the submucosa and muscular layers with regional villous sloughing; and 4 (necrosis), loss of villi and necrosis. Animals with histological scores of 2 or greater were considered to have developed NEC.
Radioimmunoassay (RIA) analysis of EGF in serum
Radiolabelling of EGF peptide utilized rat EGF (Biomedical Technologies Inc., Stoughton, MA) and Na125I (Amersham Corp., Arlington Heights, IL) in a modification of the chloramine-T method. The protocol for the EGF radioimmunoassay utilized a rabbit anti-rat EGF serum (Peninsula Laboratories) as primary antibody. Goat anti-rabbit IgG secondary antibody (Antibodies, Inc., Davis, CA) and normal rabbit serum (Sigma, St. Louis, MO) were used for EGF RIA. Inter-assay variation was 10% or less.
Immunofluorescence microscopy of TJ and AJ proteins
After deparaffinization and rehydration, sections were blocked in 5% BSA to prevent nonspecific staining and incubated with one of the following rabbit polyclonal antibodies: anti-occludin, anti-claudin-1, anti-claudin-2, anti-claudin-3, anti-ZO-1, anti-ZO-2, anti-JAM1 (Zymed Laboratories, San Francisco, CA), anti-E-cadherin (R&D, Minneapolis, MN), anti-α-catenin, and anti-β-catenin (Zymed Laboratories), followed by incubation with Alexa-conjugated secondary antibody (Molecular Probes, Eugene, OR) and mounted with Vectashield Hard Set Mounting Medium containing DAPI as a nuclear counterstain (Vector Laboratories). Negative control sections were treated with the same procedure in the absence of primary antibody; no immunostaining was observed in the controls (not shown).
Statistics
Statistical analyses between DF, NEC, and EGF groups were performed using ANOVA followed by Fisher PLSD. The Chi-Square (χ2) test was utilized to analyze differences in disease incidence. All statistical analyses were conducted using the statistical program StatView for Macintosh computers (Abacus Concepts, Berkely, CA). All numerical data are expressed as mean ± SE.
Results
EGF reduces the incidence and severity of NEC
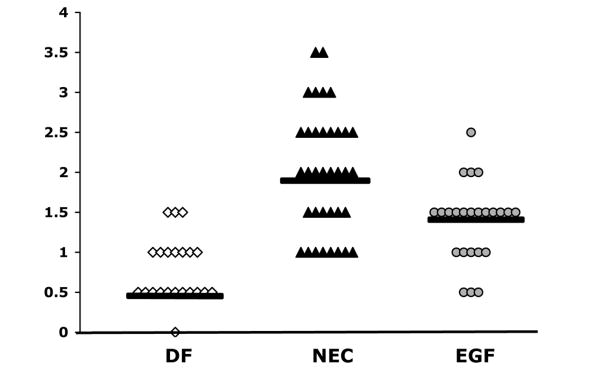
As shown in our previous work, oral administration of EGF reduces ileal damage in the rat model (2,9,11). Ileal damage was reduced in rats receiving EGF from a median histological score of 2.0 in the NEC group to 1.5 in the EGF group (Fig. 1). Histological changes in the ileum were scored by a blinded evaluator. The incidence of NEC was reduced from 62% in the NEC group (n = 36) to 14% in the EGF group (P ≤ 0.01, χ2 analysis; n = 24). In the DF group (n = 22), the incidence of NEC was 0%. The survival rates for these studies were as follows: DF, 100%; NEC 81.8%; and EGF 85.7%.
Figure 1.
Histological scores of NEC from ileal tissue in animals from DF (n = 22), NEC (n = 36) and EGF (n = 24) groups. Ileal tissue was scored using our previously published NEC scoring scale from 0 to 4, in which a score of 0 indicates normal, undamaged tissues and 4 indicates complete necrosis. Bars indicate median.
Levels of EGF in serum
EGF is a potent mitogen and a key regulator of rat liver growth and development. Interestingly, liver metabolism regulates EGF levels in systemic circulation. In order to determine if enterally administered EGF can survive digestive and hepatic catabolism and enter systemic blood, we evaluated serum EGF levels. There was no statistically significant difference in serum EGF concentrations between DF and NEC groups. As expected, serum EGF concentrations were significantly higher in animals orally administered with EGF compared to DF and NEC rats (Table 1).
Table 1.
Levels of EGF in rat serum
| DF (n=8) | NEC (n=15) | EGF (n=14) | |
|---|---|---|---|
| EGF (pg/ml) | 103.0 ± 20.8 | 90.5 ± 13.0 | 225.4 ± 35.3 * |
Values are expressed as means ± SE.
P ≤ 0.01 vs. NEC or DF
Changes in Distribution of Hepatic TJ Proteins during NEC and after EGF treatment
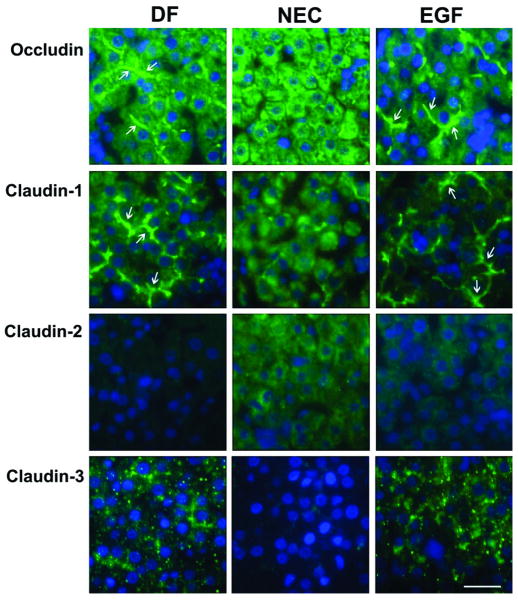
Occludin, the major integral membrane protein of TJs, was uniformly distributed in the cells of the liver lobule of DF and EGF animals and its expression was associated with the hepatocyte membrane (Fig. 2, arrows). In contrast, staining in the NEC group revealed dissociation of occludin within the cytoplasm of hepatocytes. Similar changes were observed for claudin-1, another major integral protein.
Figure 2.
Effect of EGF treatment on localization of integral TJ proteins (occludin, claudin-1, claudin-2 and claudin-3) evaluated by immunofluorescent histochemistry. Representative slides from DF, NEC, and EGF groups are shown (n = 5 animals/experimental group). Magnification: × 600. Scale bar: 20 μm.
Immunofluorescent signal for claudin-2 (Fig. 2) and JAM1 (not shown) was very weak or absent in the liver of DF and EGF rats. However, in the liver sections from the NEC group, the signal was stronger and diffused throughout the cytoplasm of hepatocytes. In contrast, subcellular localization of claudin-3 showed very weak signal in the NEC group compared to the DF and EGF groups. In general, neonatal rats treated with EGF exhibited distribution patterns of occludin and claudins similar to those seen in the DF animals (Fig. 2).
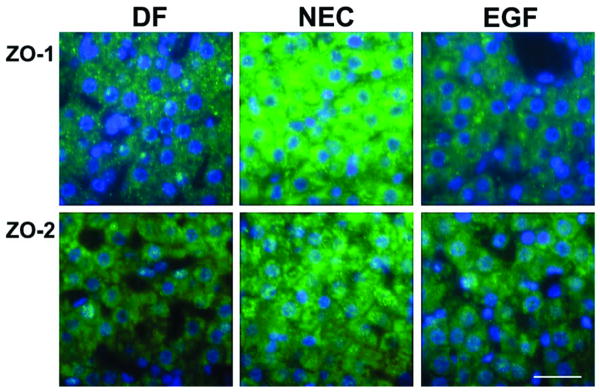
Changes in the distribution of the cytosolic TJ protein ZO-1 in the NEC group were obvious as well (Fig. 3). Immunofluorescent signal for ZO-1 was significantly stronger and dispersed within the cytoplasm of hepatocytes in the NEC group compared to both the DF and EGF groups. There were no significant differences in the distribution pattern of ZO-2 protein. In general, the distribution patterns of hepatic TJ proteins were similar in neonatal rats treated with EGF and DF rats.
Figure 3.
Localization of cytosolic TJ proteins (ZO-1 and ZO-2) in representative slides of liver sections from NEC, DF and EGF groups evaluated by immunofluorescent histochemistry (n = 5 animals/experimental group). Magnification: × 600. Scale bar: 20 μm.
Changes in Distribution of Hepatic AJ Proteins during NEC and after EGF treatment
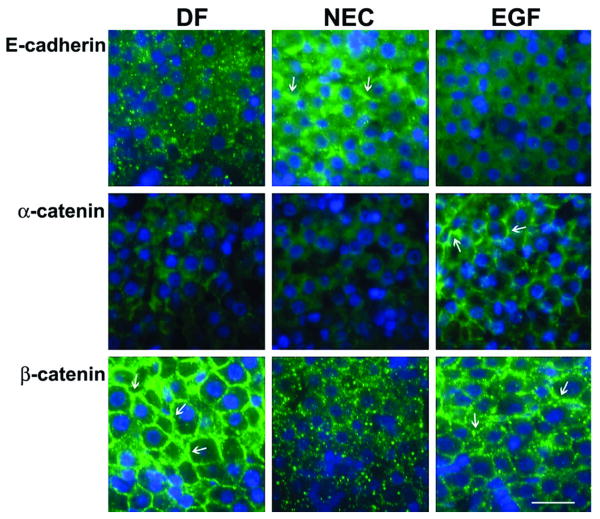
Staining patterns for E-cadherin were different in all groups. In the DF group, weak E-cadherin signal was scattered throughout the tissue. In the NEC group, E-cadherin was increased and associated with both the membrane (arrows) and cytoplasm of hepatocytes. EGF treatment reduced the intensity of E-cadherin staining, but did not affect histological distribution in the liver tissue (Fig. 4).
Figure 4.
Localization of AJ proteins (E-cadherin, α-catenin and β-catenin) was evaluated by immunofluorescent histochemistry. Representative slides from DF, NEC, and EGF groups are shown (n = 5 animals/experimental group). Magnification: × 600. Scale bar: 20 μm.
Staining for α–catenin showed diffused, weak signal in the NEC group, whereas in the DF group, this protein was associated with the membrane of hepatocytes. In the EGF group, the staining pattern was similar to that seen in DF rats, but with higher signal intensity (Fig. 4).
Striking differences in the histological localization of β–catenin were observed between groups. In the NEC group, β–catenin signal was reduced and scattered in the cytoplasm (Fig. 4). In the DF and EGF animals, β–catenin staining was strong and clearly associated with the hepatocyte membrane (Fig. 4, arrows).
Discussion
While severe NEC is associated with multiple organ dysfunctions, including changes in the liver and hepatobiliary functions, the role of hepatic TJs and AJs during NEC pathogenesis has not been studied. Previously we have shown that KCs are the major source of TNF-α in the intestinal lumen of the NEC model (23). The present study demonstrates that significant changes occur in the distribution of both TJ and AJ proteins in the liver during NEC pathogenesis. We speculate that disturbance of liver cellular junctions leads to leakage of KC-derived TNF-α into the intestinal lumen, which further exacerbates intestinal injury. Importantly, EGF treatment of NEC normalizes the changes in cellular junction's structure to what is seen in the liver of healthy control animals.
During the early postnatal period, maternal milk is the major source of EGF in the lumen of the developing gut, whereas liver metabolism regulates EGF levels in the systemic circulation (26). Under normal physiological conditions, EGF binds to its receptor and is internalized within liver cells where the majority of EGF and its receptor are destroyed in lysosomes. In our study, serum EGF levels in the EGF treated rats were significantly higher compared to the NEC and healthy controls (DF). These results indicate that orally administered EGF was able to pass through the digestive system in a biologically active form and that EGF delivered to the liver via portal blood was not completely cleared by the liver from systemic circulation.
Increased systemic inflammation is associated with hepatobiliary epithelial barrier dysfunction (17,27). We have previously shown that production of pro-inflammatory TNF-α and IL-18 is markedly increased in the liver of animals with NEC and EGF treatment reduces hepatic overproduction of inflammatory cytokines (24). We have also shown that EGF treatment in NEC has significant effects not only on intestinal structure and integrity (11), but also on the gut/liver axis (24). We speculate that increased production of pro-inflammatory cytokines in the liver of animals with NEC enter the small intestine via biliary circulation and further exacerbates intestinal injury. This tendency is reversed in animals treated with EGF (24).
Liver functions such as bile formation and secretion are maintained by apical/basal cellular polarity, which determines the localization of BAs transporters in hepatocytes (28). Liver TJs and AJs form a boundary between apical and basolateral plasma membrane domains and serve as the major paracellular barrier between bile and blood (29). In rodents, functional hepatic TJs and AJs are not formed before term birth and full assembly of intercellular junctions occurs during the suckling period (30). In the rat NEC model, premature pups are exposed to asphyxia/cold stress and formula feeding immediately after delivery. This may accelerate the maturational processes in the liver, but also compromise proper development and assembly of hepatic cellular junctions. Thus, we speculate that these alternations in TJs and AJs formation may be responsible for the leakage of proinflammatory cytokines from the liver into the intestinal lumen.
Occludin, claudins, and JAM1 are the major transmembrane TJ proteins creating the seal between cells and regulating paracellular permeability (31). Cytosolic proteins (such as ZO-1 or ZO-2) interact with the cytoplasmatic tail of occludin and claudins, which then interact with various actin-binding proteins, linking the TJs to the cytoskeleton (32). Thus, ZO-1 and ZO-2 play a central role in the assembly of mature TJs (33) and junctional integrity (16). In a rat liver injury model, common bile duct ligation (CBDL) leads to changes in expression and localization of ZO-1, followed by alteration in hepatic occludin (16). Recently, Maly and Landmann (34) have shown critical changes not only in the distribution of ZO-1 and occludin, but also hepatic claudin-1 and claudin-2. Our present results also indicate significant changes in the distribution pattern of both TJ transmembrane proteins (mainly occludin, claudin-1 and -2) as well as a cytosolic TJ component (ZO-1) in NEC animals. Moreover, increased expression of occludin, claudin-2, and ZO-1 in NEC liver is associated with the distribution of these molecules in the cytoplasm rather then at the plasma membrane. In addition, expression of claudin-3 - a key regulator of TJ permeability and selectivity – is almost absent in the NEC group. Thus, we conclude that markedly disturbed patterns of major TJ proteins in NEC animals indicate disassemblage of these junctions, leading to increased permeability in this tissue.
A study using an in vitro model has shown that EGF prevents reorganization of TJs and AJs proteins from cellular junctions to intracellular compartments (35). In our study, EGF treatment of NEC normalized expression of occludin, claudin-1, -2, -3, and ZO-1 to that found in DF animals. Therefore, we conclude that EGF treatment inhibits disassembly of hepatic TJs and prevents the leakage of pro-inflammatory cytokines from the liver into intestinal lumen.
AJs play an important role in organogenesis of epithelial tissues, including the liver. Molecular analysis of hepatocyte AJs show that E-cadherin is directly bound to β-catenin (36). Importantly, β-catenin anchors E-cadherin to actin filaments by binding to its intracellular domain (36). The development of functional hepatic AJs during the early postnatal period is still not fully understood (37). However, results from the present study clearly show that β-catenin is localized at the junctional zone of hepatocytes of the DF group. This distribution pattern of β-catenin is completely altered in NEC animals. Interestingly, E-cadherin expression is increased in the liver of NEC animals and localization of this protein is associated with both the membrane and cytoplasm of hepatocytes. We speculate that higher levels of E-cadherin found in the NEC animals might be the result of early maturation as they experience additional stress compared to DF animals. Abnormal histological localization of β-catenin in the liver of NEC animals suggests additional irregularity in the formation of hepatic intracellular structures during NEC pathogenesis.
The structural integrity of the E-cadherin/β-catenin complex is determined by the phosphorylation status of β-catenin. One of the factors responsible for phosphorylation of specific tyrosine residues is EGF receptor (EGF-R). Activation of the EGF-R leads to changes in the E-cadherin/β-catenin complex (38). Our results show that altered β-catenin distribution in the NEC group is normalized in EGF treated animals. Although histological localization of E-cadherin in the EGF group remains different from the pattern seen in DF rats, the consequences of this change are still not fully understood.
In summary, this study shows for the first time that the composition and structure of hepatic cellular junctions is significantly altered during NEC pathogenesis. EGF treatment normalizes the expression and localization of the majority of TJ and AJ proteins. Severe forms of NEC cause not only dramatic intestinal damage, but also changes in other organs including the liver. Results from this study indicate that EGF-mediated reduction of experimental NEC is associated with protection of hepatic TJ and AJ structure. Better understanding of the complexity of EGF-mediated protection in NEC provides an important base for the development of therapeutic strategies to cure this devastating disease.
Acknowledgments
Financial Support: National Institute of Child Health and Human Development Grant HD039657 (to B. Dvorak).
Abbreviations
- AJ
adherens junction
- DF
dam fed
- KC
Kupffer cells
- NEC
necrotizing enterocolitis
- TJ
tight junction
References
- 1.Crissinger KD. Animal models of necrotizing enterocolitis. J Pediatr Gastroenterol Nutr. 1995;20:17–22. [PubMed] [Google Scholar]
- 2.Dvorak B, Halpern MD, Holubec H, Williams CS, McWilliam DL, Dominguez JA, Stepankova R, Payne CM, McCuskey RS. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol Gastrointest Liver Physiol. 2002;282:G156–G164. doi: 10.1152/ajpgi.00196.2001. [DOI] [PubMed] [Google Scholar]
- 3.Caplan MS, Hedlund E, Adler L, Hsueh W. Role of asphyxia and feeding in a neonatal rat model of necrotizing enterocolitis. Pediatr Pathol. 1994;14:1017–1028. doi: 10.3109/15513819409037698. [DOI] [PubMed] [Google Scholar]
- 4.Barlow B, Santulli TV. Importance of multiple episodes of hypoxia or cold stress on the development of enterocolitis in an animal model. Surgery. 1975;77:687–690. [PubMed] [Google Scholar]
- 5.Dvorak B, Philipps A, Koldovsky O. Milk-born growth factors and gut development. Williams & Wilkins; Philadelphia, PA: 1999. pp. 245–255. [Google Scholar]
- 6.Dvorak B. Epidermal growth factor and necrotizing enterocolitis. Clin Perinatol. 2004;31:183–192. doi: 10.1016/j.clp.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Nair RR, Warner BB, Warner BW. Role of epidermal growth factor and other growth factors in the prevention of necrotizing enterocolitis. Semin Perinatol. 2008;32:107–113. doi: 10.1053/j.semperi.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Warner BB, Ryan AL, Seeger K, Leonard AC, Erwin CR, Warner BW. Ontogeny of salivary epidermal growth factor and necrotizing enterocolitis. J Pediatr. 2007;150:358–363. doi: 10.1016/j.jpeds.2006.11.059. [DOI] [PubMed] [Google Scholar]
- 9.Halpern MD, Dominguez JA, Dvorakova K, Holubec H, Williams CS, Meza YG, Ruth MC, Dvorak B. Ileal cytokine dysregulation in experimental necrotizing enterocolitis is reduced by epidermal growth factor. J Pediatr Gastroenterol Nutr. 2003;36:126–133. doi: 10.1097/00005176-200301000-00024. [DOI] [PubMed] [Google Scholar]
- 10.Clark JA, Lane RH, Maclennan NK, Holubec H, Dvorakova K, Halpern MD, Williams CS, Payne CM, Dvorak B. Epidermal growth factor reduces intestinal apoptosis in an experimental model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G755–G762. doi: 10.1152/ajpgi.00172.2004. [DOI] [PubMed] [Google Scholar]
- 11.Clark JA, Doelle SM, Halpern MD, Saunders TA, Holubec H, Dvorak K, Boitano SA, Dvorak B. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol. 2006;291:G938–G949. doi: 10.1152/ajpgi.00090.2006. [DOI] [PubMed] [Google Scholar]
- 12.Halpern MD, Holubec H, Saunders TA, Dvorak K, Clark JA, Doelle SM, Ballatori N, Dvorak B. Bile acids induce ileal damage during experimental necrotizing enterocolitis. Gastroenterology. 2006;130:359–372. doi: 10.1053/j.gastro.2005.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caballero ME, Berlanga J, Ramirez D, Lopez-Saura P, Gozalez R, Floyd DN, Marchbank T, Playford RJ. Epidermal growth factor reduces multiorgan failure induced by thioacetamide. Gut. 2001;48:34–40. doi: 10.1136/gut.48.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berlanga J, Caballero ME, Ramirez D, Torres A, Valenzuela C, Lodos J, Playford RJ. Epidermal growth factor protects against carbon tetrachloride-induced hepatic injury. Clin Sci (Lond) 1998;94:219–223. doi: 10.1042/cs0940219. [DOI] [PubMed] [Google Scholar]
- 15.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 16.Fallon MB, Brecher AR, Balda MS, Matter K, Anderson JM. Altered hepatic localization and expression of occludin after common bile duct ligation. Am J Physiol. 1995;269:C1057–C1062. doi: 10.1152/ajpcell.1995.269.4.C1057. [DOI] [PubMed] [Google Scholar]
- 17.Lora L, Mazzon E, Martines D, Fries W, Muraca M, Martin A, d'Odorico A, Naccarato R, Citi S. Hepatocyte tight-junctional permeability is increased in rat experimental colitis. Gastroenterology. 1997;113:1347–1354. doi: 10.1053/gast.1997.v113.pm9322530. [DOI] [PubMed] [Google Scholar]
- 18.Blaschuk OW, Rowlands TM. Plasma membrane components of adherens junctions. Mol Membr Biol. 2002;19:75–80. doi: 10.1080/09687680210132467. [DOI] [PubMed] [Google Scholar]
- 19.Morecroft JA, Spitz L, Hamilton PA, Holmes SJ. Necrotizing enterocolitis--multisystem organ failure of the newborn? Acta Paediatr Suppl. 1994;396:21–23. [PubMed] [Google Scholar]
- 20.Beeby PJ, Jeffery H. Risk factors for necrotising enterocolitis: the influence of gestational age. Arch Dis Child. 1992;67:432–435. doi: 10.1136/adc.67.4_spec_no.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horie Y, Wolf R, Anderson DC, Granger DN. Nitric oxide modulates gut ischemia-reperfusion-induced P-selectin expression in murine liver. Am J Physiol. 1998;275:H520–H526. doi: 10.1152/ajpheart.1998.275.2.H520. [DOI] [PubMed] [Google Scholar]
- 22.Moss RL, Das JB, Raffensperger JG. Necrotizing enterocolitis and total parenteral nutrition-associated cholestasis. Nutrition. 1996;12:340–343. doi: 10.1016/s0899-9007(96)00062-7. [DOI] [PubMed] [Google Scholar]
- 23.Halpern MD, Holubec H, Dominguez JA, Meza YG, Williams CS, Ruth MC, McCuskey RS, Dvorak B. Hepatic inflammatory mediators contribute to intestinal damage in necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G695–G702. doi: 10.1152/ajpgi.00353.2002. [DOI] [PubMed] [Google Scholar]
- 24.Halpern MD, Holubec H, Clark JA, Saunders TA, Williams CS, Dvorak K, Dvorak B. Epidermal growth factor reduces hepatic sequelae in experimental necrotizing enterocolitis. Biol Neonate. 2006;89:227–235. doi: 10.1159/000090015. [DOI] [PubMed] [Google Scholar]
- 25.Dvorak B, McWilliam DL, Williams CS, Dominguez JA, Machen NW, McCuskey RS, Philipps AF. Artificial formula induces precocious maturation of the small intestine of artificially reared suckling rats. J Pediatr Gastroenterol Nutr. 2000;31:162–169. doi: 10.1097/00005176-200008000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Marti U, Burwen SJ, Jones AL. Biological effects of epidermal growth factor, with emphasis on the gastrointestinal tract and liver: an update. Hepatology. 1989;9:126–138. doi: 10.1002/hep.1840090122. [DOI] [PubMed] [Google Scholar]
- 27.Han X, Fink MP, Uchiyama T, Yang R, Delude RL. Increased iNOS activity is essential for hepatic epithelial tight junction dysfunction in endotoxemic mice. Am J Physiol Gastrointest Liver Physiol. 2004;286:G126–G136. doi: 10.1152/ajpgi.00231.2003. [DOI] [PubMed] [Google Scholar]
- 28.Gerloff T, Geier A, Stieger B, Hagenbuch B, Meier PJ, Matern S, Gartung C. Differential expression of basolateral and canalicular organic anion transporters during regeneration of rat liver. Gastroenterology. 1999;117:1408–1415. doi: 10.1016/s0016-5085(99)70291-x. [DOI] [PubMed] [Google Scholar]
- 29.Balda MS, Matter K. Tight junctions. J Cell Sci. 1998;111:541–547. doi: 10.1242/jcs.111.5.541. [DOI] [PubMed] [Google Scholar]
- 30.Stamatoglou SC, Enrich C, Manson MM, Hughes RC. Temporal changes in the expression and distribution of adhesion molecules during liver development and regeneration. J Cell Biol. 1992;116:1507–1515. doi: 10.1083/jcb.116.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fanning AS, Mitic LL, Anderson JM. Transmembrane proteins in the tight junction barrier. J Am Soc Nephrol. 1999;10:1337–1345. doi: 10.1681/ASN.V1061337. [DOI] [PubMed] [Google Scholar]
- 32.Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol. 1997;138:181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheth B, Moran B, Anderson JM, Fleming TP. Post-translational control of occludin membrane assembly in mouse trophectoderm: a mechanism to regulate timing of tight junction biogenesis and blastocyst formation. Development. 2000;127:831–840. doi: 10.1242/dev.127.4.831. [DOI] [PubMed] [Google Scholar]
- 34.Maly IP, Landmann L. Bile duct ligation in the rat causes upregulation of ZO-2 and decreased colocalization of claudins with ZO-1 and occludin. Histochem Cell Biol. 2008;129:289–299. doi: 10.1007/s00418-007-0374-7. [DOI] [PubMed] [Google Scholar]
- 35.Sheth P, Seth A, Thangavel M, Basuroy S, Rao RK. Epidermal growth factor prevents acetaldehyde-induced paracellular permeability in Caco-2 cell monolayer. Alcohol Clin Exp Res. 2004;28:797–804. doi: 10.1097/01.alc.0000125358.92335.90. [DOI] [PubMed] [Google Scholar]
- 36.Higashi N, Kojima N, Miura M, Imai K, Sato M, Senoo H. Cell-cell junctions between mammalian (human and rat) hepatic stellate cells. Cell Tissue Res. 2004;317:35–43. doi: 10.1007/s00441-004-0891-9. [DOI] [PubMed] [Google Scholar]
- 37.Matsui T, Kinoshita T, Morikawa Y, Tohya K, Katsuki M, Ito Y, Kamiya A, Miyajima A. K-Ras mediates cytokine-induced formation of E-cadherin-based adherens junctions during liver development. EMBO J. 2002;21:1021–1030. doi: 10.1093/emboj/21.5.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin. Curr Opin Cell Biol. 2005;17:459–465. doi: 10.1016/j.ceb.2005.08.009. [DOI] [PubMed] [Google Scholar]