Abstract
Our objective was to assess predictors of self-reported opioid use among patients with back pain due to lumbar disc herniation or spinal stenosis. Data was from the Spine Patient Outcomes Research Trial (SPORT), a multi-site observational study and randomized trial. We examined characteristics shown or hypothesized to be associated with opioid use. Using generalized estimating equations, we modeled associations of each potential predictor with opioid use at 12 and 24 months. At baseline, 42% of participants reported opioid use. Of these participants, 25% reported continued use at 12 months and 21% reported use at 24 months. In adjusted models, smoking (RR=1.9, p<0.001 at 12 months; RR=1.5, p=0.043 at 24 months) and non-surgical treatment (RR=1.7, p<0.001 at 12 months; RR=1.8, p=0.003 at 24 months) predicted long-term opioid continuation. Among participants not using opioids at baseline, incident use was reported by 8% at 12 and 7% at 24 months. We found no significant predictors of incident use at 12 or 24 months in the main models. In conclusion, nonsurgical treatment and smoking independently predicted long-term continued opioid use. To our knowledge, this is the first longitudinal study to assess predictors of long-term and incident opioid use among patients with lumbar spine conditions.
INTRODUCTION
Opioid analgesic prescribing for chronic noncancer-related pain, including back pain, has increased in recent years.5;19;22;35 This trend has occurred despite limited evidence for the long-term effectiveness of opioids in chronic back pain.20 Consensus on appropriate prescribing is lacking; some experts advocate greater use of opioids in noncancer pain, whereas others recommend an opioid-sparing approach. As a result, long-term opioid use for noncancer pain remains controversial and prescribing practices vary substantially.19;22;27;35
The published literature provides little evidence about which patients are most likely to benefit from long-term opioid treatment. However, observational studies have identified some patient characteristics that are associated with negative outcomes; for example, patients with mental and substance use disorders appear to be at higher risk for developing problematic opioid use or addiction in the setting of long-term opioid therapy.10;15
Some cross-sectional studies have found that opioid prescribing is associated with greater pain severity and pain-related clinical findings.13;24 In contrast, others have found that opioid prescribing is more strongly associated with mental health disorders, problem drug use, and pain behaviors than with pain severity or clinical findings.4;25;28 A nationally-representative longitudinal survey of the general population (including individuals with and without pain) found that problem drug use and the presence of a mental disorder predicted prescription opioid use, even after controlling for pain interference and the presence of a pain condition.26 No published prospective longitudinal studies have assessed predictors of opioid use in patients with specific pain conditions.
Our objective was to assess predictors of long-term opioid use among patients with back pain related to specific lumbar spine conditions. Using longitudinal data from the Spine Patient Outcomes Research Trial (SPORT), we compared characteristics of opioid users and non-users at baseline and assessed predictors of self-reported continued and incident opioid use over two years of follow-up.
METHODS
We conducted a secondary analysis of data from SPORT, a parallel observational cohort and randomized trial comparing surgical with non-surgical treatment for patients with back pain and associated leg symptoms due to three lumbar spine conditions: intervertebral disc herniation (IDH), spinal stenosis (SpS), and spinal stenosis with degenerative spondylolisthesis (DS). SPORT methods have been described in detail elsewhere and are summarized briefly in the following sections.3
Participants and procedures
Participants were recruited from 13 spine specialty centers in 11 different US states. Eligible patients were adults with IDH, SpS, or DS, as determined by the presence of an appropriate clinical presentation, examination findings, and imaging results. Exclusion criteria included prior spine surgery; inadequate trial of nonsurgical therapy (< 6 weeks for IDH and <12 weeks for SpS/DS); presence of a contraindication to surgery or indication for urgent surgery; active cancer; and fracture, infection, or significant deformity of the spine. Institutional review boards at each participating site approved the protocol and all participants gave informed consent.
Eligible patients who agreed to randomization enrolled in the randomized controlled trial; those who agreed to participate but declined randomization enrolled in the parallel observational cohort study. Other than randomization to surgical vs. nonsurgical therapy in the randomized trial, all study procedures were the same for participants in the randomized and observational studies. Participants received treatment guided by protocols for surgical or nonsurgical care. Those in the nonsurgical arms started with an initial set of treatments that included at least active physical therapy, education/counseling with home exercise instruction, and a non-steroidal anti-inflammatory drug if appropriate. For those nonsurgical participants with inadequate initial response, participating clinicians chose additional physical, psychological, and pharmacologic therapies from an extensive menu. Physicians had the discretion to choose specific therapies, including opioids, based on individual patients’ needs. For this analysis, we included data from participants in both the randomized and observational studies.
Measures
At the time of enrollment, participants provided medical history, demographic, and lifestyle information by interview and questionnaire. They reported the history of their spine condition (including current and prior treatments), employment status, level of education achieved, cigarette smoking, and any current or pending disability or legal action related to their spine condition. Participants indicated whether they had ever received a diagnosis or were currently receiving treatment for a list of medical problems. We derived a medical comorbidity count using a list of 10 chronic conditions from this checklist: joint problems, coronary artery disease, cancer, congestive heart failure, lung problem, diabetes, hypertension, liver problem, kidney problem, and stroke.23 The checklist also assessed participants’ mental health, including single-item queries about personal history of depression, anxiety or panic attacks, post-traumatic stress (PTSD), alcoholism, or drug dependency.
Participants reported current medications for their spine condition and the frequency of use for each medication. They also completed a battery of self-report measures, including the SF-36 Health Survey21;29 and the modified Oswestry Disability Index (American Academy of Orthopaedic Surgeons/MODEMS version)8;12. Follow-up data were collected by questionnaire and interview at 6 weeks and 3, 6, 12, and 24 months. At each time point, study nurses assessed opioid and other medication use and patients completed the self-report measures.
Pain was assessed with the SF-36 bodily pain subscale, which includes two items assessing pain severity and interference. Mental health symptoms were assessed with the 5-item SF-36 mental health subscale. For SF-36 scales, responses are transformed into 0–100 subscale scores, with lower scores indicating worse health.21;29 Back pain-related disability was assessed with the modified Oswestry Disability Index, which assesses pain-related functional limitations in 9 domains: getting dressed, lifting, walking/running, sitting, standing, sleeping, social/recreational activities, traveling, and sexual activity.8;12 Impairment with each activity is rated on a 6-point scale (from limitless/pain-free activity to complete limitation due to pain). A total 0–100 score is calculated, with higher scores indicating worse disability.
Statistical analysis
We compared characteristics of participants with baseline self-reported opioid use with those who reported no use at baseline using chi-square and t-tests for categorical and continuous variables, respectively. We then used longitudinal models to evaluate potential predictors of opioid use at follow-up. We refer to opioid use at follow-up as “continued” if participants also reported opioid use at baseline and “incident” if they didn’t report use at baseline; however, these terms may not be completely accurate because participants may have started or stopped opioids before or between assessments. For all analyses, dichotomous opioid use indicators at follow-up were the dependent variables.
We examined characteristics shown or hypothesized to be associated with opioid use as potential predictors: pain severity (SF-36 bodily pain subscore); pain-related disability (Oswestry Disability Index score); mental health (SF-36 mental health subscore); spine-related disability claim or legal action (current or pending); current smoking; and receipt of spine surgery. We analyzed the effect of non-operative or surgical treatment on an “as treated” basis. Surgery status was a time-varying variable, meaning the value at each time point reflects the subject’s surgery status at that follow-up assessment. We used baseline values for all other potential predictor variables. Receipt of spine surgery, smoking status, and legal action were modeled as dichotomous variables, with surgery, non-smoking and no legal action, respectively, as the reference groups. SF-36 subscales and Oswestry scores were divided into tertiles and modeled as categorical variables. We designated the “healthiest” tertile (i.e., higher values for SF-36 subscales, lower values for Oswestry) as the reference group.
We used generalized estimating equations (GEE)9 with a logit link to model opioid use longitudinally measured during follow-up. Separate models were fit for users of opioids at baseline to address “continued” long-term opioid use; and for non-users at baseline to address incident opioid use. All models included adjustment for baseline variables representing possible confounders and variables associated with missing data. This basic set of variables included sociodemographic factors (age, sex, race, education, employment), medical comorbidity count, study site, and spine diagnosis. First, we examined associations between each potential predictor and opioid use, adjusted for the basic set of variables. Second, to identify independent predictors of opioid use, we examined associations between each potential predictor and follow-up opioid use in fully-adjusted models including all other potential predictors. To confirm that it was appropriate to combine data across SPORT cohorts, we examined analyses stratified in two ways: 1) by study enrollment (randomized trial and observational cohort study) and 2) by diagnostic subgroups (IDH and SpS/DS). Analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC).
RESULTS
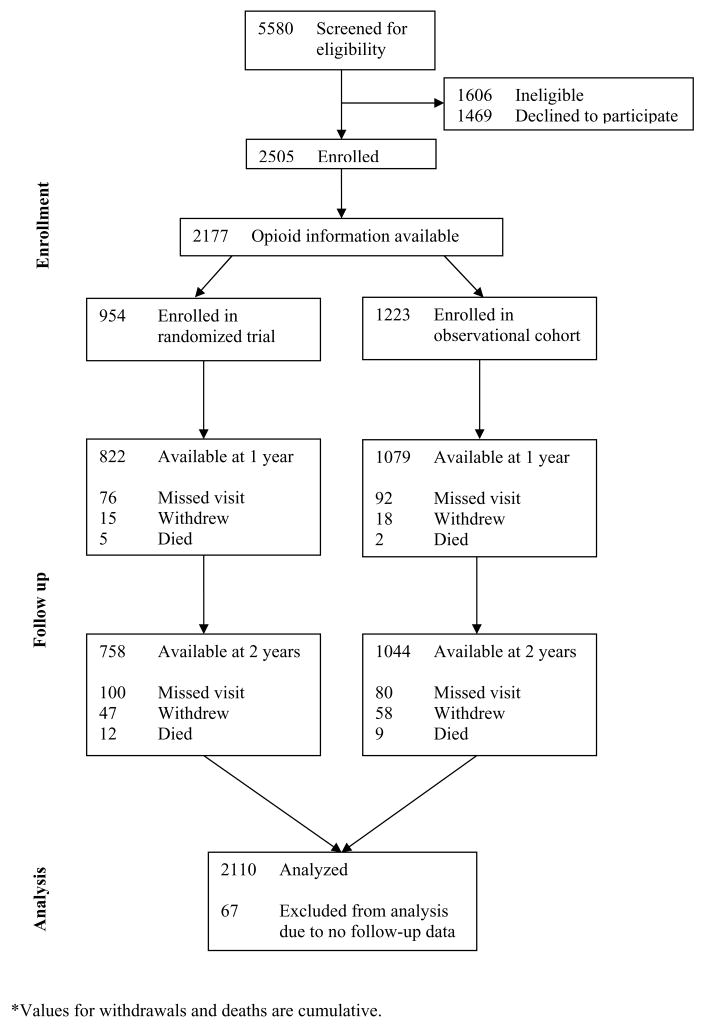
We included 2110 participants for whom data from at least one follow-up visit were available (Figure 1). At baseline, 892 (42.3%) participants reported that they currently used opioids for their spine condition, including 697 (33.0%) who reported daily opioid use and 195 (9.2%) who reported less than daily use.
Figure 1.
Enrollment and follow-up of participants
Associations with baseline opioid use
Table 1 shows characteristics of participants with and without self-reported opioid use at baseline. Those reporting opioid use at baseline were more numerous in the IDH group, younger, less likely to have any college education, less likely to be employed, more likely to have applied for disability or pursued legal action, more likely to smoke, and more likely to report a history of mental disorders compared with non-users. Baseline scores for all SF-36 scales and the Oswestry Disability Index were worse for opioid users than non-users.
Table 1.
Characteristics of participants with and without opioid analgesic use at baseline
| Characteristic | Baseline opioid use (n = 892) | No baseline opioid use (n = 1218) | P value* |
|---|---|---|---|
| Age, mean years (SD) | 50.6 (15.6) | 55.5 (16.1) | <0.001 |
| Female sex, n (%) | 466 (52%) | 590 (48%) | 0.09 |
| Race, n (%) | |||
| White | 762 (85%) | 1037 (85%) | 0.97 |
| Black | 71 (8%) | 97 (8%) | |
| Other | 59 (7%) | 84 (7%) | |
| Education—at least some college, n (%) | 583 (66%) | 875 (73%) | 0.003 |
| Employment, n (%) | |||
| Working full or part time | 389 (44%) | 580 (48%) | <0.001 |
| Disabled | 155 (17%) | 95 (8%) | |
| Retired or other | 347 (39%) | 543 (45%) | |
| Disability application or payments, n (%)‡ | 187 (21%) | 126 (10%) | <0.001 |
| Legal action related to spine problem, n (%)† | 60 (7%) | 49 (4%) | 0.007 |
| Current smoking, n (%) | 196 (22%) | 164 (14%) | <0.001 |
| Number of medical comorbidities, mean (SD)§ | 0.93 (1.1) | 1.0 (1.1) | 0.09 |
| Depression history, n (%) | 136 (15%) | 140 (12%) | 0.01 |
| Anxiety or PTSD history, n (%) | 83 (9%) | 58 (5%) | <0.001 |
| Alcohol or drug dependence history, n(%) | 20 (2.2%) | 17 (1.4%) | 0.20 |
| Spine Diagnosis | |||
| IDH, n (%) | 540 (61%) | 520 (43%) | <0.001 |
| SS/DS, n (%) | 352 (39%) | 698 (57%) | |
| Duration of spine problem > 6 months, n (%) | |||
| Acute (≤ 6 weeks) | 80 (9%) | 80 (7%) | <0.001 |
| Sub-acute (> 6 weeks to 6 months) | 496 (56% | 607 (50%) | |
| Chronic (> 6 months) | 316 (35%) | 531 (44%) | |
| SF-36 | |||
| Bodily Pain subscale, mean (SD) | 21.1 (14.1) | 32.4 (17.7) | <0.001 |
| Physical Function subscale, mean (SD) | 27.8 (22.0) | 40.5 (23.8) | <0.001 |
| Mental Health subscale, mean (SD) | 61.0 (20.3) | 69.3 (19.0) | <0.001 |
| Physical Component Score, mean (SD) | 27.2 (7.2) | 31.2 (8.2) | <0.001 |
| Mental Component Score, mean (SD) | 44.3 (11.8) | 49.0 (11.6) | <0.001 |
| Oswestry Disability Index, mean (SD) | 55.5 (18.4) | 40.8 (18.6) | <0.001 |
P value for unadjusted comparison between participants reporting any opioid use and those reporting no opioid use
Affirmative response to the following: “Have you brought any legal action related to your spine-related problem?”
Affirmative response to the following: “Have you applied to or are you now receiving payments from either Worker’s Compensation, Social Security Disability, or any other disability insurance programs for your spine-related problem?”
Number of medical comorbidities is the count of problems from the following list: hypertension, diabetes, joint problems, coronary artery disease, cancer, congestive heart failure, lung problem, diabetes, liver problem, kidney problem, stroke (possible range 0–10).
We also examined baseline opioid use by diagnostic and study enrollment subgroups. Among patients with IDH, those who chose to enroll in the observational study were more likely to use opioids than those who agreed to be randomized (55% vs. 45%, p=0.001). Baseline opioid use did not differ between observational and randomized patients for DS or SpS.
Predictors of long-term opioid use
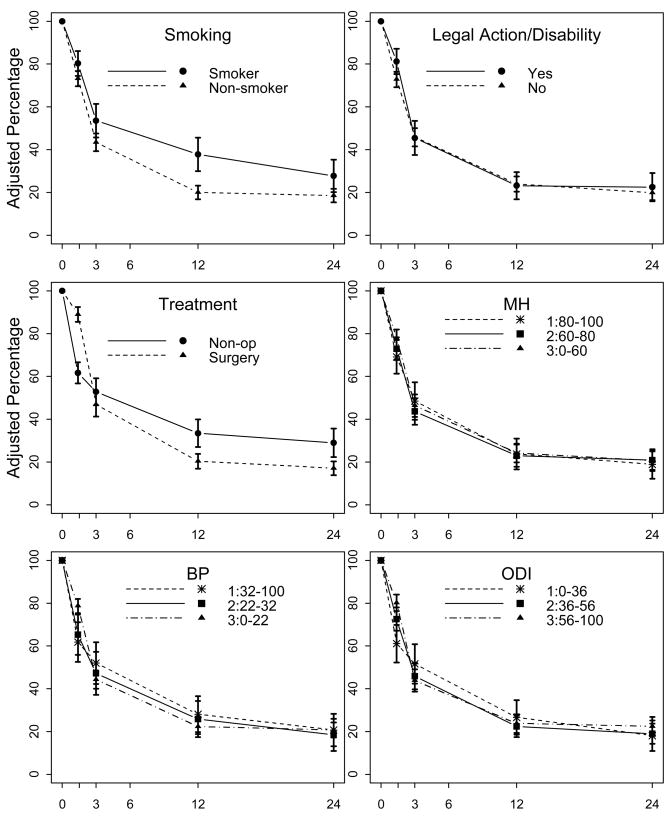
Among participants who reported using opioids at baseline, 25% also reported opioid use at 12 months and 21% reported use at 24 months. Figure 2 shows the relationship between each potential predictor and continued opioid use at follow-up, adjusted for the basic set of baseline variables. Table 2 shows basic adjusted and fully-adjusted risk ratios (RR) for this long-term continued opioid use at 12 and 24 months according to potential predictor variables. After adjustment for the basic set of variables, nonsurgical treatment and smoking predicted opioid use at both 12 and 24 months. In fully-adjusted models including all potential predictors, both smoking (RR=1.9, p<0.001 at 12 months; RR=1.5, p=0.043 at 24 months) and non-surgical treatment (RR=1.7, p=0.001 at 12 months; RR=1.8, p=0.003 at 24 months) predicted opioid continuation. Disability/legal action, mental health, pain, and disability were not significant predictors of continued opioid use at either follow-up time point.
Figure 2. Percentage of participants with baseline opioid use reporting use at each follow-up time-point (n=892).
MH = SF-36 mental health subscale, BP = SF-36 bodily pain subscale, ODI = Oswestry Disability Index. Figures show percentages with 95% confidence intervals, adjusted for age, sex, race, education, employment, baseline duration of spine problem, medical comorbidity count, study site, and spine diagnosis. SF-36 subscale scores and Oswestry scores are divided into tertiles.
Table 2.
Risk of continued opioid use at 12 and 24 months among participants with opioid use at baseline (n = 892)
| Basic covariable-adjusted models† | Fully-adjusted models‡ | |||||||
|---|---|---|---|---|---|---|---|---|
| 12 months | 24 months | 12 months | 24 months | |||||
| RR (95% CI) | p | RR (95% CI) | p | RR (95% CI) | p | RR (95% CI) | p | |
| Nonsurgical treatment | 1.6 (1.2, 2.1) | 0.002 | 1.7 (1.2, 2.2) | 0.006 | 1.7 (1.3, 2.2) | 0.001 | 1.8 (1.3, 2.3) | 0.003 |
| Current smoking | 1.9 (1.4, 2.4) | <0.001 | 1.5 (1.0, 2.0) | 0.045 | 1.9 (1.4, 2.4) | <0.001 | 1.5 (1.0, 2.0) | 0.043 |
| Legal/disability action | 1.0 (0.7, 1.3) | 0.84 | 1.1 (0.7, 1.5) | 0.50 | 1.0 (0.6, 1.3) | 0.76 | 1.2 (0.8, 1.6) | 0.45 |
| SF-36 Mental Health* | ||||||||
| Tertile 1 (≥80) | 1.0 | 0.94 | 1.0 | 0.90 | 1.0 | 0.94 | 1.0 | 0.87 |
| Tertile 2 (60–79) | 1.0 (0.6, 1.3) | 1.1 (0.6, 1.6) | 0.9 (0.6, 1.3) | 1.1 (0.6, 1.6) | ||||
| Tertile 3 (<60) | 1.0 (0.7, 1.4) | 1.1 (0.6, 1.5) | 1.1 (0.6, 1.3) | 1.1 (0.6, 1.6) | ||||
| SF-36 Bodily Pain* | ||||||||
| Tertile 1 (≥32) | 1.0 | 0.27 | 1.0 | 0.86 | 1.0 | 0.23 | 1.0 | 0.81 |
| Tertile 2 (22–31) | 0.9 (0.5, 1.3) | 0.9 (0.4, 1.4) | 0.9 (0.5, 1.3) | 0.9 (0.4, 1.3) | ||||
| Tertile 3 (<22) | 0.8 (0.5, 1.1) | 1.0 (0.6, 1.4) | 0.8 (0.5, 1.0) | 1.0 (0.6, 1.4) | ||||
| Oswestry Disability* | ||||||||
| Tertile 1 (<36) | 1.0 | 0.62 | 1.0 | 0.55 | 1.0 | 0.83 | 1.0 | 0.53 |
| Tertile 2 (36–55) | 0.8 (0.5, 1.2) | 1.1 (0.6, 1.5) | 0.9 (0.5, 1.2) | 1.1 (0.6, 1.1) | ||||
| Tertile 3 (≥56) | 0.9 (0.6, 1.2) | 1.2 (0.7, 1.8) | 0.9 (0.6, 1.3) | 1.3 (0.7, 1.9) | ||||
P-values are for trend with tertile 1 (the “healthiest” group) as the reference group.
Adjusted for age, sex, race, education, employment, baseline duration of spine problem, medical comorbidity count, study site, and spine diagnosis
Adjusted for Model 1 covariables and all other predictor variables: surgical treatment (time-varying), smoking, spine-related legal or disability action, and baseline SF-36 Mental Health, SF-36 Bodily Pain, and Oswestry Disability Index
Results were similar when we examined models stratified by spine diagnosis (IDH and DS/SpS) and study enrollment (randomized trial and observational study). Smoking and non-surgical treatment were similarly predictive of continued opioid use at follow-up in stratified analyses, but confidence intervals were wider and crossed 1.0 in some cases.
Predictors of incident opioid use
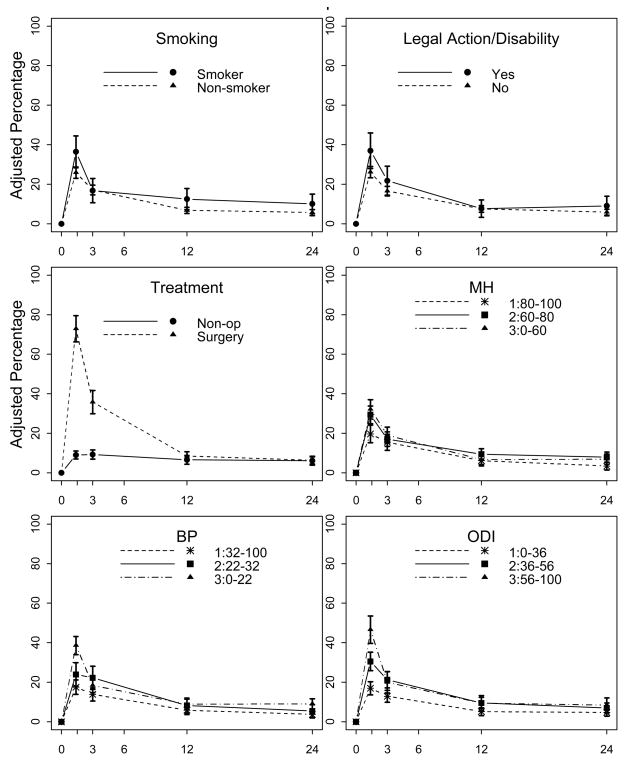
Among participants who reported no opioid use at baseline, 8% reported opioid use at 12 months and 7% at 24 months. Figure 3 shows the relationship between each potential predictor and incident opioid use at all follow-up time-points, after adjustment for the basic set of baseline variables. In both basic and fully-adjusted models, we found no significant predictors of incident opioid use at 12 and 24 months.
Figure 3. Percentage of participants with no baseline opioid use reporting use at each follow-up time-point (n=1218).
MH = SF-36 mental health subscale, BP = SF-36 bodily pain subscale, ODI = Oswestry Disability Index. Figures show percentages with 95% confidence intervals, adjusted for age, sex, race, education, employment, baseline duration of spine problem, medical comorbidity count, study site, and spine diagnosis. SF-36 subscale scores and Oswestry scores are divided into tertiles.
In models stratified by study enrollment, we found no significant associations between predictor variables and incident opioid use among randomized trial participants. Among participants in the observational study, choosing nonsurgical treatment predicted significantly less incident opioid use only at 24 months (fully adjusted RR=0.7, p=0.10 at 12 months; RR=0.5, p=0.025 at 24 months).
In models stratified by spine diagnosis, we found no significant associations between predictor variables and incident opioid use among participants with DS/SpS. Among those with IDH, nonsurgical treatment predicted less incident opioid use at 12, but not 24, months (fully adjusted RR=0.3, p=0.004 at 12 months; RR=0.8, p=0.59 at 24 months).
DISCUSSION
To our knowledge, this is the first longitudinal study to prospectively assess predictors of opioid use in a population of patients with pain. We found that nonsurgical treatment and smoking independently predicted continued long-term opioid use among patients reporting opioid use at baseline, whereas pain severity, pain-related disability, mental health, and spine-related legal/disability status did not.
The rate of opioid use at baseline was at the high end of prevalence rates reported in the literature;20 long-term continuation rates were also impressive, although opioid use declined steadily over the two year study period. The prevalence of opioid use at baseline was much higher in SPORT (42.3%) than in a cross-sectional study by Fanciullo et al. (3.4%) that also evaluated patients seen at spine specialty centers.13 Enrollment of spine center patients with sufficient pain to be candidates for surgery may explain the greater prevalence of opioid use in SPORT. Additionally, opioid prescribing for non-cancer pain became generally more common in the years between the Fanciullo et al. study (1995–1998) and SPORT baseline assessments (2000–2003).18;22;35 Our findings that baseline opioid use was associated with sociodemographic factors and worse pain, function, and quality of life are consistent with the literature. Unlike some previous studies,6;16 we did not find evidence of racial disparities in opioid use.
We found a striking association between smoking and self-reported opioid use, in that baseline cigarette smoking independently predicted continued long-term opioid use. Prior cross-sectional studies have found an association between smoking and greater opioid use in spine center patients13 and workers with back injury.24 Longitudinal studies have found that smokers may be at risk for worse outcomes14 and adherence17 in multidisciplinary pain treatment programs. Worse nonsurgical pain treatment response or adherence among smokers is one potential explanation for our finding. Another possible explanation is confounding by substance use disorders. Most studies of smoking and opioid use have not controlled for drug or alcohol abuse, which are more prevalent in smokers; therefore, smoking may be a marker of substance use disorders in these populations. We were unable to control for substance use disorders because participants were not assessed with adequately sensitive measures of drug or alcohol use.
In this study, patients who received surgical treatment were less likely to continue opioid use on a long-term basis than those who didn’t receive surgery. This finding supports SPORT’s primary results,31–34 which showed a symptomatic benefit for surgery, and suggests a potential additional benefit for patients considering surgery. Risks of opioid use for longer than 1–2 years are not yet adequately understood, but may include hypogonadism, increased fracture risk, opioid-induced hyperalgesia, substance use disorders, and central sleep apnea, in addition to immediate side effects such as constipation and sedation.1;2;7;11;30 Given these potential risks and the practical difficulties of long-term opioid prescribing experienced by both prescribers and patients, information about decreased opioid use after surgery could potentially be useful in surgical decision-making. Although the balance of risks and benefits associated with long-term opioids is not clear, for some patients with well-defined spine conditions, the risk of continued pain management with opioids may outweigh the risk of surgery. The likelihood of requiring long-term opioid use and the attendant potential for adverse consequences of this use could be provided to patients considering spine surgery to help them make better informed decisions.
The relationship between surgery and incident opioid use is less clear. A peak in incident opioid use was evident in the initial months of study, representing expected new use in the acute postoperative period; however, results for incident use at 12 and 24 months were conflicting. We found no significant predictors of incident use among participants who were in the randomized trial, but among those who declined randomization and enrolled in the observational study, nonsurgical treatment predicted less incident opioid use. Selection bias is a likely explanation for this finding (i.e., participants in the observational study who had less pain or were improving with conservative therapy were less likely to choose to undergo surgery and to start opioids). We also found a difference in incident use patterns between diagnostic strata. We found no predictors of incident opioid use among participants with DS/SpS, but among those with IDH, non-surgical treatment was associated with less incident use at 12 (not 24) months. Overall, results suggest that patients who received incident post-operative opioids discontinued them as expected.
We were surprised that, although mental health symptoms were strongly associated with opioid use at baseline, they did not predict future opioid use (continued or incident) in our models. These results differ from those of a prior longitudinal study by Sullivan et al., which found that problem drug use and mental disorders independently predicted opioid use in the general population.26 Differences between the studies may explain the discrepant results. SPORT enrolled a clinically well-defined population of patients with confirmed spinal pathology who had been referred to spine specialty centers, whereas Sullivan et al. used data from the Healthcare for Communities (HCC) survey, which enrolled members of the general population. The majority of HCC participants did not have pain, and those who did had a diverse collection of painful conditions. It may be that mental health is a stronger predictor of opioid use in a diverse population than in a population of patients with well-defined spine pathology. Another possibility is that the difference in results reflects a difference in measurement: we used tertiles of the SF mental health subscale as our measure of mental health symptoms, whereas the HCC survey used the presence of specific mental health disorders derived from a diagnostic interview. Less likely, the association of mental health with opioid use in Sullivan et al. may be due to confounding by pain severity.
Strengths of this study include our use of prospective longitudinal data from a large, well-defined population of patients with back pain due to common spine conditions. Opioid use was common in this study population and we had detailed demographic and clinical data, allowing us to control for multiple potentially confounding factors in our analysis. To our knowledge, this is the first longitudinal study to assess predictors of opioid use among patients with specific lumbar spine disorders.
This study does have several limitations. First, as with all observational studies, our results may be affected by unmeasured differences between opioid users and non-users. For example, drug and alcohol use were not fully assessed, so we were unable to evaluate substance use problems as predictors of opioid use. Patients were asked at baseline to indicate whether they had been diagnosed or treated for “alcoholism” or “drug dependency,” but were not formally assessed for current or past substance use disorders. Fewer than 2% of participants endorsed a history of alcoholism or drug dependency, which is likely to be a substantial underestimation of the true prevalence. Second, we were unable to assess use of specific opioid medications and doses used by SPORT participants. Medications used for the spine condition were assessed at each visit, but only the frequency of use for each medication class was recorded. Finally, results may not be generalizable to all patients with back pain or other chronic pain conditions. The SPORT study population included patients who had one of three well-defined spine conditions; patients with nonspecific back pain and those with pain due to other spine conditions were not included.
In conclusion, in this prospective study of self-reported opioid use among patients with common spine conditions, we found that nonsurgical treatment and smoking predicted long-term continuation of opioid use. The greater use of long-term opioids among patients who received nonsurgical therapy may be a factor worth considering in surgical decision-making for patients with disc herniation or spinal stenosis. To better understand the causes and consequences of long-term opioid use, future research should focus on untangling the complex longitudinal relationship between opioid use and mental health and substance use disorders, including smoking, among people with chronic pain.
Acknowledgments
We thank Tamara Morgan for her contributions to this manuscript.
-
The authors acknowledge the following potential conflicts of interest:
Dr. Lurie: grant support from St. Francis Medical Technologies and the American Board of Orthopaedic Surgery and consulting fees from Merck, Ortho-McNeil, Pfizer, Centocor, Myexpertdoctor.com, Pacific Business Group on Health, and the Foundation for Informed Medical Decision Making
SPORT was funded by grant U01-AR45444-01A1 from NIAMS and by the NIH Office of Research on Women’s Health and NIOSH, Centers for Disease Control and Prevention.
Dr. Krebs is supported by a VA HSR&D Research Career Development Award
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Angst MSM, Clark JD. Opioid-induced Hyperalgesia: A Qualitative Systematic Review. Anesthesiology. 2006;104:570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349:1943–53. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- 3.Birkmeyer NJ, Weinstein JN, Tosteson AN, Tosteson TD, Skinner JS, Lurie JD, Deyo R, Wennberg JE. Design of the Spine Patient outcomes Research Trial (SPORT) Spine. 2002;27:1361–1372. doi: 10.1097/00007632-200206150-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breckenridge J, Clark JD. Patient characteristics associated with opioid versus nonsteroidal anti-inflammatory drug management of chronic low back pain. J Pain. 2003;4:344–50. doi: 10.1016/s1526-5900(03)00638-2. [DOI] [PubMed] [Google Scholar]
- 5.Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109:514–9. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Chen I, Kurz J, Pasanen M, Faselis C, Panda M, Staton LJ, O’Rorke J, Menon M, Genao I, Wood J, Mechaber AJ, Rosenberg E, Carey T, Calleson D, Cykert S. Racial differences in opioid use for chronic nonmalignant pain. J Gen Intern Med. 2005;20:593–8. doi: 10.1111/j.1525-1497.2005.0106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniell HW. Opioid endocrinopathy in women consuming prescribed sustained-action opioids for control of nonmalignant pain. J Pain. 2008;9:28–36. doi: 10.1016/j.jpain.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Deyo RA, Battie M, Beurskens AJ, Bombardier C, Croft P, Koes B, Malmivaara A, Roland M, Von KM, Waddell G. Outcome measures for low back pain research. A proposal for standardized use. Spine. 1998;23:2003–2013. doi: 10.1097/00007632-199809150-00018. [DOI] [PubMed] [Google Scholar]
- 9.Diggle PJ, Heagerty P, Kung-Lee L, Zeger S. The Analysis of Longitudinal Data. New York: Oxford University Press; 2002. [Google Scholar]
- 10.Edlund MJ, Steffick D, Hudson T, Harris KM, Sullivan M. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129:355–362. doi: 10.1016/j.pain.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Ensrud KE, Blackwell T, Mangione CM, Bowman PJ, Bauer DC, Schwartz A, Hanlon JT, Nevitt MC, Whooley MA. Central nervous system active medications and risk for fractures in older women. Arch Intern Med. 2003;163:949–957. doi: 10.1001/archinte.163.8.949. [DOI] [PubMed] [Google Scholar]
- 12.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25:2940–2952. doi: 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 13.Fanciullo GJ, Ball PA, Girault G, Rose RJ, Hanscom B, Weinstein JN. An observational study on the prevalence and pattern of opioid use in 25,479 patients with spine and radicular pain. Spine. 2002;27:201–5. doi: 10.1097/00007632-200201150-00016. [DOI] [PubMed] [Google Scholar]
- 14.Fishbain DA, Lewis JE, Cutler R, Cole B, Steele RR, Rosomoff HL. Does smoking status affect multidisciplinary pain facility treatment outcome? Pain Med. 2008;9:1081–1090. doi: 10.1111/j.1526-4637.2007.00306.x. [DOI] [PubMed] [Google Scholar]
- 15.Fleming MF, Balousek SL, Klessig CL, Mundt MP, Brown DD. Substance use disorders in a primary care sample receiving daily opioid therapy. J Pain. 2007;8:573–582. doi: 10.1016/j.jpain.2007.02.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green CR, Anderson KO, Baker TA, Campbell LC, Decker S, Fillingim RB, Kalauokalani DA, Lasch KE, Myers C, Tait RC, Todd KH, Vallerand AH. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Med. 2003;4:277–294. doi: 10.1046/j.1526-4637.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 17.Hooten WMM, Townsend COP, Bruce BKP, Warner DOM. The Effects of Smoking Status on Opioid Tapering Among Patients with Chronic Pain. Anesthesia & Analgesia. 2009;108:308–315. doi: 10.1213/ane.0b013e31818c7b99. [DOI] [PubMed] [Google Scholar]
- 18.Kuehn BM. Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA. 2007;297:249–251. doi: 10.1001/jama.297.3.249. [DOI] [PubMed] [Google Scholar]
- 19.Luo X, Pietrobon R, Hey L. Patterns and trends in opioid use among individuals with back pain in the United States. Spine. 2004;29:884–90. doi: 10.1097/00007632-200404150-00012. [DOI] [PubMed] [Google Scholar]
- 20.Martell BA, O’Connor PG, Kerns RD, Becker WC, Morales KH, Kosten TR, Fiellin DA. Systematic review: opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146:116–127. doi: 10.7326/0003-4819-146-2-200701160-00006. [DOI] [PubMed] [Google Scholar]
- 21.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Olsen Y, Daumit GL, Ford DE. Opioid prescriptions by US primary care physicians from 1992 to 2001. J Pain. 2006;7:225–235. doi: 10.1016/j.jpain.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Perkins AJ, Kroenke K, Unutzer J, Katon W, Williams JW, Hope C, Callahan CM. Common comorbidity scales were similar in their ability to predict health care costs and mortality. J Clin Epidemiol. 2004;57:1040–1048. doi: 10.1016/j.jclinepi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Stover BD, Turner JA, Franklin G, Gluck JV, Fulton-Kehoe D, Sheppard L, Wickizer TM, Kaufman J, Egan K. Factors associated with early opioid prescription among workers with low back injuries. J Pain. 2006;7:718–725. doi: 10.1016/j.jpain.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan MD, Edlund MJ, Steffick D, Unutzer J. Regular use of prescribed opioids: association with common psychiatric disorders. Pain. 2005;119:95–103. doi: 10.1016/j.pain.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan MD, Edlund MJ, Zhang L, Unutzer J, Wells KB. Association Between Mental Health Disorders, Problem Drug Use, and Regular Prescription Opioid Use. Arch Intern Med. 2006;166:2087–2093. doi: 10.1001/archinte.166.19.2087. [DOI] [PubMed] [Google Scholar]
- 27.Turk DC, Brody MC, Okifuji EA. Physicians’ attitudes and practices regarding the long-term prescribing of opioids for non-cancer pain. Pain. 1994;59:201–208. doi: 10.1016/0304-3959(94)90072-8. [DOI] [PubMed] [Google Scholar]
- 28.Turk DCP, Okifuji AP. What factors affect physicians’ decisions to prescribe opioids for chronic noncancer pain patients? Clinical Journal of Pain. 1997;13:330–336. doi: 10.1097/00002508-199712000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 30.Webster LRM, Choi YM, Desai HM, Webster LR, Grant BJBM. Sleep-Disordered Breathing and Chronic Opioid Therapy. Pain Medicine. 2008;9:425–432. doi: 10.1111/j.1526-4637.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- 31.Weinstein JN, Lurie JD, Tosteson TD, Skinner JS, Hanscom B, Tosteson AN, Herkowitz H, Fischgrund J, Cammisa FP, Albert T, Deyo RA. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT) observational cohort. JAMA. 2006;296:2451–2459. doi: 10.1001/jama.296.20.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinstein JN, Tosteson TD, Lurie JD, Tosteson AN, Hanscom B, Skinner JS, Abdu WA, Hilibrand AS, Boden SD, Deyo RA. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT): a randomized trial. JAMA. 2006;296:2441–2450. doi: 10.1001/jama.296.20.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinstein JN, Lurie JD, Tosteson TD, Hanscom B, Tosteson AN, Blood EA, Birkmeyer NJO, Hilibrand AS, Herkowitz H, Cammisa FP, Albert TJ, Emery SE, Lenke LG, Abdu WA, Longley M, Errico TJ, Hu SS. Surgical versus Nonsurgical Treatment for Lumbar Degenerative Spondylolisthesis. N Engl J Med. 2007;356:2257–2270. doi: 10.1056/NEJMoa070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinstein JN, Tosteson TD, Lurie JD, Tosteson AN, Blood E, Hanscom B, Herkowitz H, Cammisa F, Albert T, Boden SD, Hilibrand A, Goldberg H, Berven S, An H, The SI. Surgical versus Nonsurgical Therapy for Lumbar Spinal Stenosis. N Engl J Med. 2008;358:794–810. doi: 10.1056/NEJMoa0707136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zerzan JT, Morden NE, Soumerai S, Ross-Degnan D, Roughead E, Zhang F, Simoni-Wastila L, Sullivan S. Trends and geographic variation of opiate medication use in state medicaid fee-for-service programs, 1996 to 2002. Medical Care. 2006;44:1005–1010. doi: 10.1097/01.mlr.0000228025.04535.25. [DOI] [PubMed] [Google Scholar]