Abstract
Objective
To determine whether C-reactive protein (CRP) measured by a high sensitivity (hs) assay is a surrogate marker of disease activity and damage in systemic lupus erythematosus (SLE).
Methods
Five hundred eighty-eight patients with SLE participating in a multiethnic cohort (Hispanic, African American, and Caucasian) were studied. Disease activity was measured with the Systemic Lupus Activity Measure-Revised (SLAM-R) and damage with the Systemic Lupus International Collaborating Clinics (SLICC) Damage Index (SDI). hs-CRP was measured by immunometric assay. Disease activity and hs-CRP were measured at enrollment and damage accrual at last visit. The association of hs-CRP with the SLAM-R and SDI was examined by univariable (Pearson's correlation) and multivariable (linear regression) analyses. The association of hs-CRP and each individual domain of the SLAM-R and SDI was examined by Spearman's correlation.
Results
hs-CRP was associated with the SLAM-R in the univariable (r = 0.35, p < 0.001) and multivariable (t = 7.11, coefficient ß = 0.27, p < 0.001) analyses. It also correlated with the constitutional, eye, pulmonary, gastrointestinal, neuromotor, and laboratory domains of the SLAM-R. hs-CRP was associated with the SDI (r = 0.12, p = 0.004) in the univariable analysis but not in the multivariable analysis. When the individual domains of the SDI were analyzed, hs-CRP correlated with the renal, pulmonary, cardiovascular, musculoskeletal, and diabetes domains.
Conclusion
hs-CRP was associated with disease activity but not with overall damage accrual; however, it correlated with specific domains of the damage index. hs-CRP may be useful to monitor the course of the disease and predict its intermediate outcome, but longitudinal studies with serial hs-CRP measurements are necessary to define its clinical value.
Keywords: SYSTEMIC LUPUS ERYTHEMATOSUS, DISEASE ACTIVITY, C-REACTIVE PROTEIN, DISEASE DAMAGE
C-reactive protein (CRP) is the prototypical acute-phase reactant in humans. It has been used to detect and monitor treatment response in infections as well as in other inflammatory conditions such as rheumatoid arthritis (RA) and vasculitis1. When assessed by a highly sensitive method, CRP is a measure of low-grade inflammation that has been found to be a risk factor for type II diabetes mellitus and cardiovascular disease. It has also been found to be associated with the metabolic syndrome and with hypertension1. Notably, the applicability of high sensitivity (hs)-CRP in rheumatic autoimmune disorders is limited to the assessment of disease activity in RA2 and of cardiovascular risk3.
The role CRP plays in systemic lupus erythematosus (SLE) is still controversial. While some investigators have found an association between CRP and disease activity4,5 as well as some clinical manifestations such as serositis6, poly-arthritis7, and nephritis8, others failed to demonstrate a significant CRP response in active SLE9,10. It has been postulated that this protein has not only inflammatory, but also antiinflammatory properties. CRP binds to apoptotic cell surfaces, promoting activation of the early classical complement pathway, improving the opsonization and phagocytosis of apoptotic material, therefore inducing an antiinflammatory response, and, maybe, preventing autoimmunity11. This hypothesis has been corroborated in animal lupus models12. Further, in humans, a polymorphism at the CRP locus (CRP 4) has been linked not only with low levels of CRP but also with SLE and antinuclear autoantibody production13.
The majority of studies examining CRP in SLE, however, have measured it by conventional methods; less is known about the significance of CRP measured by a high sensitivity method (hs-CRP) in the course of SLE, other than as a possible marker of cardiovascular disease14. Using data from the LUMINA cohort (Lupus in Minority: NAture vs Nurture), a lupus multiethnic cohort, we investigated whether hs-CRP correlates with disease outcomes. We hypotfiesized that hs-CRP is a marker of disease activity and damage in SLE.
MATERIALS AND METHODS
LUMINA, established in 1994, is a. longitudinal study of outcomes in lupus. It includes patients from 3 ethnic groups, African American, Hispanic (from Texas and Puerto Rico), and Caucasian. The constitution of this cohort, the variables, and the frequency and design of the study visits have been described15. Variables included in these analyses are disease activity assessed using the Systemic Lupus Activity Measure-Revised (SLAM-R)16 and damage accrual measured with the Systemic Lupus Internationa] Collaborating Clinics (SLICC)/American College of Rheumatology (ACR) Damage Index (SDI)17. Serum hs-CRP was measured by immunometric assay (Immulite 2000; Diagnostic Products, Los Angeles, CA,USA) at baseline (T0). Ail variables were measured at enrollment (T0).
The association of hs-CRP with the SLAM-R at T0 and SDI at last visit (TL) was examined by univariable (Pearson's correlation) and multivariable (linear regression) analyses. In the latter, variables previously found to be independently associated with disease activity (ethnicity, having health insurance, acute disease-onset, illness-related behaviors, helplessness, the presence of anti-Ro antibodies and HLA-DRBl*030118 and damage accrual (age, ethnicity, number of ACR criteria at diagnosis, acute disease-onset, SLAM-R, and cumulative doie of glucocorticoids)19,20 were added to those models as adjustment variables. Finally, the association between hs-CRP and each individual domain of the SLAM-R and SDI was examined by Spearman's correlation. A p value ≤ 0.05 was considered significant. All statistical analyses were performed using SPSS software, version 11.0 (SPSS, Chicago, IL, USA).
RESULTS
Five hundred eighty-eight patients were included in these analyses. Four patients were excluded from the analyses because the value of their hs-CRP was an outlier in the right bound of the distribution. Patients were predominantly women (89.3%) and of middle age [mean 36.7 (SD 12.5) yrs]. All ethnic groups were represented; 103 (17.5%) were Hispanics from Texas, 102 (17.3%) Hispanics from Puerto Rico, 213 (36.2%) were African Americans, and 170 (28.9%) Caucasians. Disease duration from diagnosis to enrollment was 17.5 (SD 16.3) months. The mean SLAM-R score at T0 was 9.2 (SD 5.6) and the mean SDI scores at T0 and TL were 0.7 (SD 1.2) and 1.8 (SD 2.2), respectively. Hispanics from Puerto Rico had lower disease activity at T0 compared to Hispanics from Texas, African Americans, and Caucasians [mean SLAM-R scores: 6.7 (SD 3.7), 10.7 (SD 6.4), 10.9 (SD 6.2), and 7.7 (SD 4.2), respectively; p < 0.001].
The mean hs-CRP concentration for the entire cohort was 12.3 (SD 21.7) mg/l; the median was 4.9 mg/l [range (25th–75th percentiles) 1.8–13.3 mg/l]. hs-CRP levels were significantly lower in Hispanics from Puerto Rico compared to other ethnic groups [7.3 (SD 9.5) vs 13.3 (SD 23.4) mg/l; p = 0.036]. Specifically, mean hs-CRP levels for Hispanics from Texas, Hispanics from Puerto Rico, African Americans, and Caucasians were 16.0 (SD 28.1), 7.3 (SD 9.5), 13.0 (SD 22.3), and 12.0 (SD 21.5) mg/l, respectively. Male patients had higher mean hs-CRP levels than female patients [20.7 (SD 33.2) vs 11.3 (SD 19.7) mg/l; p = 0.03], Among women, the mean hs-CRP level was higher among Hispanics from Texas compared to Hispanic from Puerto Rico, African, Americans, and Caucasians [16.4 (28.8), 7.4 (9.7), 10.3 (16.6), and 11.7 (20.6) mg/l;p = 0.013].
Other factors that may influence hs-CRP levels were also studied. Body mass index and the use of glucoconicoids, estrogens, and statins at T0 were not associated with hs-CRP levels (data not shown).
Disease activity and hs-CRP
Using a cutoff value of 6, taken to represent a meaningful change in disease activity for the SLAM-21, we found that patients with SLAM ≥ 6 (n = 429) had a median hs-CRP of 5.8 mg/l (25th–75th percentiles 1.9–15.6 mg/l) and those with SLAM < 6 (n = 159) had a median hs-CRP of 3.2 mg/l (13–7.7 mg/l).
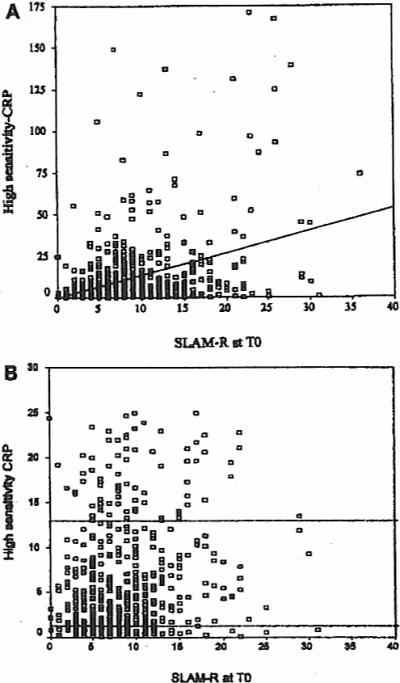
High-sensitivity CRP was associated with the SLAM-R at T0 in both univariable (r = 0.35, p < 0.001) and multivariable (t = 7.11, coefficient ß = 0.27, p < 0.001) analyses. The data points for the univariable associations are shown in Figure 1. When the individual domains were examined, hs-CRP was modestly associated with the constitutional, eye, pulmonary, gastrointestinal, and neuromotor domains as well as with the laboratory domain, including the hematologic and renal measures. High-sensitivity CRP also correlated with the erythrocyte sedimentation rate (ESR, Westergren method) when measured by the SLAM-R categories (Table 1).
Figure 1.
A. SLAM-R scores (x axis) and hs-CRP (mg/l, y axis) were determined in 588 SLE patients at enrollment (T0). A positive correlation was foond between these variables (r = 035, p < 0.001). B. SLAM-R scores and hs-CRP levels shown in greater detail for patients with hs-CRP levels ≤ 25 mg/l. Broken lines represent 25th–75th percentiles.
Table 1.
Association between high-sensitivity CRP and domains of the Systemic Lupus Activity Measured-Revised (SLAM-R) at baseline.
| SLAM-R Domains | r | p* |
|---|---|---|
| Constitutional | 0.18 | < 0.001 |
| Integument | −0.04 | |
| Eye | 0.10 | 0.024 |
| Reticuloendothelial | 0.01 | |
| Pulmonary | 0.13 | 0.001 |
| Cardiovascular | 0.08 | |
| Gastrointestinal | 0.14 | 0.001 |
| Neuromotor | 0.08 | 0.043 |
| Joints | 0.08 | |
| Laboratory | ||
| Hematologic + ESR | 0.24 | < 0.001 |
| ESR alone | 0.29 | < 0.001 |
| Renal | 0.17 | < 0.001 |
Only p values ≤ 0.05 are noted.
ESR: erythrocyte sedimentation rate. Westergren method.
Damage accrual and hs-CRP
High-sensitivity CRP was associated with SDI score at TL in the univariable analysis (r = 0.12, p = 0.004); however, hs-CPR did not retain significance in the multivariable analysis (t = −0.48, coefficient ß = −0.02, p = 0.635). When the individual domains of the SDI were examined, however, hs-CRP was modestly associated with the renal, pulmonary, cardiovascular, musculoskeletal, and diabetes domains (Table 2).
Table 2.
Association between baseline high-sensitivity CRP and domains of the Systemic Lupus International Collaborating Clinics Damage Index (SDI) at last visit.
| SDI Domains | r | p* |
|---|---|---|
| Ocular | 0.06 | |
| Neuropsychiatric | 0.03 | |
| Renal | 0.09 | 0.039 |
| Pulmonary | 0 11 | 0.006 |
| Cardiovascular | 0.19 | < 0.001 |
| Peripheral vascular | 0.07 | |
| Gastrointestinal | 0.05 | |
| Musculoskeletal | 0.12 | 0.003 |
| Skin | 0.03 | |
| Gonadal | 0.03 | |
| Diabetes | 0.17 | < 0.001 |
| Malignancy | −0.03 |
Ouly p values ≤ 0.05 are noted.
DISCUSSION
As an acute-phase reactant, the synthesis of CRP is upregulated by cytokines such as interleukin 6 (IL-6), IL-1, and tumor necrosis factor-α22. Although these cytokines increase during periods of active lupus, this is not the same for CRP, as it has not been found to be consistently associated with disease activity9,10,23. A plausible explanation could be the presence of antibodies against CRP24, but such a hypothesis requires confirmation. Further, in most studies addressing this issue standard methods to assess CRP concentrations were used9,10,23, rather than high-sensitivity assays; this may have precluded Finding an association between CRP and disease outcomes such as disease activity. In contrast, we found a significant association between CRP (measured by a high-sensitivity method) and disease activity. Moreover, hs-CRP was found to be associated with the laboratory measures of the SLAM-R. Of particular interest is the correlation between hs-CRP and the ESR, also found to be associated with disease activity in the LUMINA cohort15.
Our data, however, contrast with the results from Barnes, et al25, who failed to find an association between hs-CRP and disease activity in 213 SLE patients. As well, none of the individual measures of the instrument used to measure disease activity, the SLE Disease Activity Index (SLEDAI) was found to be associated with hs-CRP. However, it should be noted that the patients studied by Barnes, et al25 had much longer disease duration than our patients and, not unexpectedly, they also had lower degrees of disease activity.
Although we failed to Find an association between hs-CRP and overall damage accrual. hs-CRP did correlate with certain domains of the SDI. Of interest, we corroborated our previous report regarding the association between hs-CRP levels and vascular arterial events in SLE14. Also notable was the association found in this study between diabetes and hs-CRP, as the latter has been described as a risk factor for development of type II diabetes.
Our study has some limitations. First, it was a cross-sectional design, therefore further inferences about the usefulness of hs-CRP on the longterm course and outcome of SLE cannot be made. Second, it is possible that the elevated hs-CRP concentrations reflect a continuing subclinical infection rather than disease activity; although infections were not specifically ascertained at the baseline visit, it is highly unlikely infections are the sole explanation for our findings. Third, a cutoff value for hs-CRP other than as a risk factor for cardiovascular risk has not been established; thus, we examined hs-CRP as a continuous variable. Finally, although the association between hs-CRP levels and disease activity and certain domains of the SDI were found to be statistically significant, they were not very robust.
Despite these limitations, the associations found suggest that hs-CRP may be a suitable biomarker of disease severity in SLE. Notably, higher hs-CRP concentrations correlated not only with disease activity but also with damage in major organs/systems. However, longitudinal studies with serial hs-CRP measurements are necessary to determine the applicability of hs-CRP to the management of patients with lupus.
ACKNOWLEDGMENT
The authors acknowledge all LUMINA patients, without whom this study would have not been possible; and our supporting staff (Martha L. Sanchez. MD, MPH, Jigna M. Liu, MPH, Mandar Apte, MD, MPH, and Ellen Sowell, University of Alabama at Birmingham; Carmine Pinilla-Diaz, MT, University of Puerto Rico: and Robert Sandoval, BA. University of Texas Houston) for their efforts in securing patients' followup and other LUMINA-related tasks.
Supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases P01-AR49084. General Clinical Research Centers M01-RR02558 (UTH-HSC)and M01-RR00032 (UAB), the National Center for Research Resources (NCRR/NIH) RCMI Clinical Research Infrastructure Initiative (RCRII) award JP20 RR11126 (UPR-MSC). and by an unrestricted educational grant from Bristol-Myers Squibb Company (UPR-MSC).
REFERENCES
- 1.Kushner I, Rzewnicki D, Samols D. What does minor elevation of C-reactive protein signify? Am J Med. 2006;119:166–28. doi: 10.1016/j.amjmed.2005.06.057. [DOI] [PubMed] [Google Scholar]
- 2.Dessein PH, Joffe BI, Stanwix AE. High sensitivity C-reactive protein as a disease activity marker in rheumatoid arthritis. J Rheumatol. 2004;31:1095–7. [PubMed] [Google Scholar]
- 3.Abou-Raya S, Abou-Raya A, Naim A, Abuelkheir H. Chronic inflammatory autoimmune disorders and atherosclerosis. Ann NY Acad Sci. 2007;1107:56–67. doi: 10.1196/annals.1381.007. [DOI] [PubMed] [Google Scholar]
- 4.Williams RC, Jr, Harmon ME, Burlingame R, Du Clos TW. Studies of serum C-reactive protein in systemic lupus erythematosus. J Rheumatol. 2005;32:454–61. [PubMed] [Google Scholar]
- 5.Zein N, Ganuza C, Kushner I. Significance of serum C-reactive protein elevation in patients with systemic lupus erythematosus. Arthritis Rheum. 1979;22:7–12. doi: 10.1002/art.1780220102. [DOI] [PubMed] [Google Scholar]
- 6.Suh CH, Jeong YS, Park HC, et al. Risk factors for infection and role of C-reactive protein in Korean patients with systemic lupus erythematosus. Clin Exp Rheumatol. 2001;19:191–4. [PubMed] [Google Scholar]
- 7.Moutsopoulos HM, Mavridis AK. Acritidis NC, Avgerinos PC. High C-reactive protein response in lupus polyarthritis. Clin Exp Rheumatol. 1983;1:53–5. [PubMed] [Google Scholar]
- 8.Zuniga R, Markowitz GS, Arkachaisri T, Imperatore EA, D'Agati VD, Salmon JE. Identification of IgG subclasses and C-reactive protein in lupus nephritis: She relationship between the composition of immune deposits and FC-gamma receptor type IIA alleles. Arthritis Rheum. 2003;48:460–70. doi: 10.1002/art.10930. [DOI] [PubMed] [Google Scholar]
- 9.Suh CH, Chun HY, Ye YM, Park HS. Unresponsiveness of C-reactive protein in the non-infectious inflammation of systemic lupus erythematosus is associated with interleukin 6. Clin Immunol. 2006;119:291–6. doi: 10.1016/j.clim.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Linares LF, Gomez-Reino JJ, Carreira PE, Morillas L, Ibero I. C-reactive protein levels in systemic lupus erythematosus. Clin Rheumatol. 1986;5:66–9. doi: 10.1007/BF02030970. [DOI] [PubMed] [Google Scholar]
- 11.Marnell L, Mold C, Du Clos TW. C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol. 2005;117:104–11. doi: 10.1016/j.clim.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Du Clos TW, Zlock LT, Hicks PS, Mold C. Decreased autoantibody levels and enhanced survival of (NZB × NZW) F1 mice treated with C-reactive protein. Clin Immunol Immunopathol. 1994;70:22–7. doi: 10.1006/clin.1994.1005. [DOI] [PubMed] [Google Scholar]
- 13.Russell AI, Cunninghame Graham DS, Shepherd C, et al. Polymorphism at the C-reactive protein locus influences gene expression and predisposes to systemic lupus erythematosus. Hum Mol Genet. 2004;13:137–47. doi: 10.1093/hmg/ddh021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toloza SM, Uribe AG, McGwin G, Jr, et al. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA). XXIII. Baseline predictors of vascular events. Arthritis Rheum. 2004;50:3947–57. doi: 10.1002/art.20622. [DOI] [PubMed] [Google Scholar]
- 15.Vila LM, Alarcon GS, McGwin G, Jr, Bastian HM, Fessler BJ, Reveille JD. for the LUMINA Study Group. Systemic lupus erythematosus in a multiethnic cohort (LUMINA): XXIX. Elevation of erythrocyte sedimentation rate is associated with disease activity and damage accrual. J Rheumatol. 2005;32:2150–5. [PubMed] [Google Scholar]
- 16.Liang MH, Socher SA, Larson MG, Schur PH. Reliability and validity of sit systems for the clinical assessment of disease activity in systemic lupus erythematosus. Arthritis Rheum. 1989;32:1107–18. doi: 10.1002/anr.1780320909. [DOI] [PubMed] [Google Scholar]
- 17.Gladman DD, Goldsmith CH, Utowitz MB, et al. for the Systemic Lupus International Collaborating Clinics. The Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) Damage Index for Systemic Lupus Erythematosus international comparison. J Rheumatol. 2000;27:373–6. [PubMed] [Google Scholar]
- 18.Alarcon GS, Roseman J, Bartolucci AA, et al. Systemic lupus erythematosus in three ethnic groups: II. Features predictive of disease activity early in its course. LUMINA Study Group. Lupus in minority populations, nature versus nurture. Arthritis Rheum. 1998;41:1173–80. doi: 10.1002/1529-0131(199807)41:7<1173::AID-ART5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 19.Alarcon GS, McGwin G, Jr, Bartolucci AA, et al. Systemic lupus erythematosus in three ethnic groups. IX. Differences in damage accrual. Arthritis Rheum. 2001;44:2797–806. doi: 10.1002/1529-0131(200112)44:12<2797::aid-art467>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 20.Alarcon GS, Roseman JM, McGwin G, Jr, et al. Systemic lupus erythematosus in three ethnic groups. XX. Damage as a predictor of further damage. Rheumatology Oxford. 2004;43:202–5. doi: 10.1093/rheumatology/keg481. [DOI] [PubMed] [Google Scholar]
- 21.American College of Rheumatology Ad Hoc Committee on SLE Response Criteria The American College of Rheumatology response criteria for systemic lupus erythematosus clinical trials: measures of overall disease activity. Arthritis Rheum. 2004;50:3418–26. doi: 10.1002/art.20628. [DOI] [PubMed] [Google Scholar]
- 22.Weinhold B, Ruther U. Interleukin-6-dependent and -independent regulation of the human C-reactive ptotein gene. Biochem J. 1997;327:425–9. doi: 10.1042/bj3270425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabay C, Roux-Lombard P, de Moerloose P, Dayer JM, Vischer T, Gueme PA. Absence of correlation between interleukin 6 and C-reactive protein blood levels in systemic lupus erythematosus compared with rheumatoid arthritis. J Rheumatol. 1993;20:815–21. [PubMed] [Google Scholar]
- 24.Sjowall C, Bengtsson AA, Sturfelt G, Skogh T. Anli-CRP autoantibody levels correlate with disease activity in systemic lupus erythematosus. Semin Arthritis Rheum. 2005;35:65. doi: 10.1016/j.semarthrit.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Bames EV, Narain S, Naranjo A, et al. High sensitivity C-reactive protein in systemic lupus erythematosus: relation to disease activity, clinical presentation and implications for cardiovascular risk. Lupus. 2005;14:576–82. doi: 10.1191/0961203305lu2157oa. [DOI] [PubMed] [Google Scholar]