Abstract
Aim
Modafinil was tested for efficacy in facilitating abstinence in cocaine-dependent patients, compared to placebo.
Methods
This was a double-blind placebo-controlled study, with 12 weeks of treatment and a 4-week follow-up. Six outpatient substance abuse treatment clinics participated in the study. There were 210 treatment-seekers randomized, having a diagnosis of cocaine dependence; 72 participants were randomized to placebo, 69 to modafinil 200 mg, and 69 to modafinil 400 mg, taken once daily on awakening. Participants came to the clinic three times per week for assessments and urine drug screens, and had one hour of individual psychotherapy weekly. The primary outcome measure was the weekly percentage of cocaine non-use days.
Results
The GEE regression analysis showed that for the total sample, there was no significant difference between either modafinil group and placebo in the change in average weekly percent of cocaine non-use days over the 12-week treatment period (p > 0.79). However, two secondary outcomes showed significant effects by modafinil 200 mg: the maximum number of consecutive non-use days for cocaine (p = 0.02), and a reduction in craving (p = 0.04). Also, a post hoc analysis showed a significant effect of modafinil that increased the weekly percentage of non-use days in the subgroup of those cocaine patients who did not have a history of alcohol dependence (p < 0.02).
Conclusions
These data suggest that modafinil, in combination with individual behavioral therapy, was effective for increasing cocaine non-use days in participants without co-morbid alcohol dependence, and in reducing cocaine craving.
Keywords: Modafinil, Cocaine-related disorders, Alcoholism, Pharmacotherapy, Risk factors
1. Introduction
The chronic and destructive disease of cocaine dependence has no US-approved pharmaceutical treatment, in spite of nearly 20 years of directed, federally funded research. In 2007, the United States had 1.16 million people dependent on cocaine during the past year (SAMHSA, 2008), and the United Nations estimated about 14 million users globally (UNODC, 2007). The epidemic has been large, with a devastating impact on patients, their families, and society. In one 12-year follow-up, those who continued to use cocaine had more criminal justice involvement, and more unemployment and psychiatric problems than those who achieved 5 years of abstinence (Hser et al., 2006). Modafinil is a novel, non-amphetamine psychostimulant, approved by the US Food and Drug Administration (FDA, www.fda.gov/cder/foi/label/2007/020717s020s013s018lbl.pdf [accessed date May 8, 2009]) in 1998 for the treatment of narcolepsy. It is as effective as traditional dopamine-acting stimulants for that indication (Roth and Roehrs, 1996), but its pharmacological profile is notably different from the amphetamines, cocaine, or methylphenidate.
The rationale for the use of modafinil to treat cocaine dependence is several-fold. Briefly, modafinil has stimulant properties, which could be therapeutic for alleviating some stimulant withdrawal symptoms (Malcolm et al., 2002; Jasinski, 2000), and in human laboratory test paradigms it has been found to attenuate subjective responses to cocaine (Dackis et al., 2003) and the priming effect of cocaine for self-administration (Hart et al., 2007). Equally important, modafinil has a lower propensity for euphoria and cocaine-like discriminative-stimulus effects than does methylphenidate (Rush et al., 2002). Modafinil improves cognition and mood (Turner et al., 2004; Taneja et al., 2007), and has shown efficacy in the treatment of child and adult attention deficit hyperactivity disorder (Lindsay et al., 2006). Via its actions on the hypocretin/orexin system (Scammell et al., 2000) and the glutamate/GABA (gamma-Aminobutyric Acid) systems (Ferraro et al., 1997) modafinil could help restore the homeostasis disturbed by chronic cocaine use. The role of glutamate in the chronic effects of cocaine and the development of cocaine conditioned responding is well established (Kalivas et al., 2003), and recent studies suggest that hypocretin may also enhance the reinstatement of cocaine self-administration in rats (Boutrel et al., 2005). Finally, modafinil was safe and well tolerated in Phase I interaction studies with cocaine (Donovan et al., 2005; Dackis et al., 2003), and in a pilot study on cocaine dependence treatment (n = 62) it was superior to placebo at promoting cocaine abstinence (Dackis et al., 2005).
2. Method
The objectives of the study were to evaluate the efficacy and safety of modafinil relative to placebo, for reducing cocaine use in cocaine dependent outpatients, as assessed by self-report confirmed with urine assays for benzoylecgonine (BE). The study had a double-blind, placebo-controlled, parallel-group design in which, after a 3-week screening/baseline period, participants were randomly assigned with equal probability to one of three treatment groups to receive 200 mg modafinil, 400 mg modafinil or matched placebo, daily for 12 weeks, with a follow-up assessment 4 weeks after treatment completion. Adaptive “urn” randomization was used to balance treatment groups (Wei and Lachin, 1988), based on site, gender, and frequency of cocaine use in the last 30 days (≤18 days versus >18 days).
2.1. Study population
Six outpatient drug treatment clinic sites received IRB approval to recruit about 35 participants each, using print and radio ads, and to pay participants for coming to clinic three times weekly to provide a urine sample and other research data. A total of 210 participants who met criteria for cocaine dependence, determined by the Structured Clinical Interview (SCID) of the Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV), were randomized into the three treatment groups (~70 participants per group). The number of 70 in each arm was selected based on previous NIDA studies on cocaine abuse (Kampman et al., 2005) as well as the estimated effect size of the earlier pilot trial which reported a significant effect of modafinil treatment on cocaine abuse (Dackis et al., 2005). Participants with the ability to understand and provide written informed consent were at least 18 years old, and provided at least one benzoylecgonine (BE)-positive urine within the 3-week screening/baseline period prior to randomization. Urines were tested three times per week, and inclusion required at least 6 baseline urines. Exclusion criteria included dependence on a drug other than cocaine, marijuana or nicotine, or physiological dependence on alcohol requiring medical detoxification. Some other exclusions were: serious medical illness including AIDS, psychiatric or neurological disorder requiring (pharmacologic) treatment, pregnancy or lactation, and court-mandated treatment for cocaine dependence.
2.2. Treatments
During the 12 weeks of outpatient treatment, participants took a 200 or 400 mg tablet of modafinil, or the matched placebo, once daily on awakening. All participants received individual standardized psychosocial therapy, which consisted of manual-guided Cognitive Behavioral Therapy (Longabaugh et al., 2005) once per week during the 12-week treatment period. All were offered (referral for) HIV (Human Immunodeficiency Virus) counseling and testing. One session of Motivational Enhancement Therapy was provided in the third week of screening/baseline.
2.3. Safety assessments
Candidates for study enrollment had a medical history and physical examination, a 12-lead electrocardiogram(ECG), clinical laboratory studies (blood chemistry, hematology, urinalysis, and pregnancy test if female), and Hamilton Depression Rating Scale (HAM-D) during screening or baseline. Vital signs, concomitant medication use, and a urine screen for other substances of abuse were assessed weekly, and pregnancy tests for females were assessed every two weeks during treatment. Repeat HAM-D and clinical laboratory studies were assessed at Weeks-4, -8, and -12. Adverse Events (AEs) were assessed at each study visit and reviewed weekly by the site physician. At Treatment-Week-12 or study discontinuation, all participants had a repeat physical examination and ECG.
2.4. Efficacy assessments
Success in reduction of cocaine use was measured by comparing treatment groups on the change in average weekly percent of cocaine non-use days, over the treatment period. An individual’s weekly percent was derived by taking the number of non-use days in a week (determined by self-report at each study visit, confirmed or disproved by urine BE level), and dividing by the total number of non-missing self-report days that week. A cocaine-positive self-report day was always accepted as such, but a self-report day of cocaine non-use was confirmed by a negative or decreasing urine BE level, according to the Preston Rules (Preston et al., 1997). If the urine BE level indicated “new use,” that day’s self-report of use was set to positive. Secondary outcomes included treatment group comparisons of: the proportion of ‘successful’ participants (e.g., cocaine use-days decreased to 50% of baseline); the average maximum number of consecutive non-use days; the quantitative decrease in the average weekly median of the log10 of urine BE concentration; and the average reduction in scores on the HIV Risk-Taking Behavior Scale (HRBS). Treatment compliance (medication or counseling) was evaluated based on the percent of days medication was taken or counseling sessions (1×/week) were attended out of (a) the total scheduled for 12 weeks and (b) the total scheduled up to the point of study discontinuation. For the medication compliance assessment, self-report of use was aided by pill count. Study retention was calculated based on date of last study visit, until completion of Week-12 (note: after 6 consecutive missed visits, participants were discontinued from the study). Severity of cocaine dependence was evaluated by comparing groups on the average change in scores of the Addiction Severity Index (ASI-Lite), Brief Substance Craving Scale (BSCS), Cocaine Craving Questionnaire (CCQ), and Clinical Global Impression, as assessed by participant’s self-report (CGI-S) and observer report (CGI-O). The ASI-Lite and CCQ were performed at baseline and Week-12. The BSCS, CGI-S, CGI-O, and the Cocaine Selective Severity Assessment (CSSA) were performed weekly during the baseline and treatment periods. The HRBS was given at baseline, Week-12, and at follow-up (Week-16).
2.5. Analysis
For evaluation of medication treatment effect on cocaine abuse, the primary outcome analysis used Generalized Estimating Equations (GEE) to compare treatment groups’ change in average weekly percent of cocaine non-use days. GEE is a model-based regression method, appropriate for the analysis of correlated data that results from repeated measures in a longitudinal study. It assumes that missing data is missing completely at random. The SAS GENMOD procedure (SAS Institute, Inc. Cary, NC) was used to estimate and test the models.
Each primary and secondary outcome variable was analyzed using appropriate statistical methods for the intent-to-treat population, defined as the participants who were randomized to treatment and received the first dose of study agent. The primary outcome, and secondary outcomes that had repeat measures, were analyzed using GEE, adjusted for factors of treatment group (3 levels of medication dose: 0, 200 and 400 mg), and study week (continuous over 12 weeks). Various models were tested, including with covariates for gender, race, or baseline cocaine use during the screening period (average weekly% non-use days in Weeks-1 and -2). It was hypothesized that modafinil treatment, compared to placebo, would be associated with a decrease in cocaine use, as assessed by self-report confirmed by urine assays for benzoylecgonine (BE). Statistical tests were two-sided at a 5% Type I error rate.
Baseline characteristics of the participants in each treatment group were summarized to demonstrate the results of randomization. Chi-square or Fisher’s Exact tests were used for comparisons. A summary was prepared of dropouts, i.e., study retention over time, by treatment group, and for a priori defined subgroups. The number of missing observations was compared between treatment groups, and for a priori defined subgroups. All AEs were MedDRA-coded and reported in tabular form, indicating the frequency of each ‘preferred term’ type of AE by treatment group. AE’s leading to discontinuation of study medication were also tabulated.
3. Results
3.1. Baseline demographics
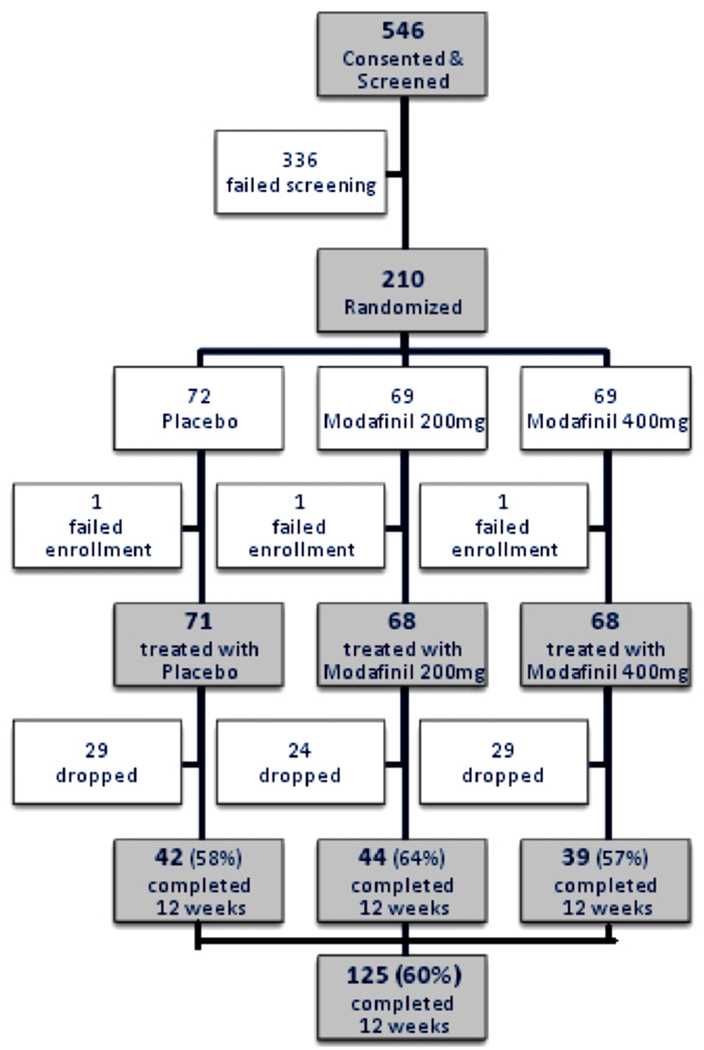
Fig. 1 is a flow diagram of the numbers of study participants, from consent through randomization and on to completion of the study. One participant in each treatment group did not begin the study medication, and was considered to have failed enrollment. In addition, one participant dropped out in the first week of treatment. The screen failure rate (336/546 = 62%) is not unusual for studies in this population. The most common reasons for failure in screening were: (1) did not return to clinic (41%); (2) had a serious medical illness, such as uncontrolled hypertension, significant heart, renal, or hepatic disease or potentially life-threatening or progressive illness (19%); (3) did not provide a cocaine-positive urine during the 3-week screening period (18%); (4) had a psychiatric or neurologic disorder requiring ongoing treatment that would make study participation unsafe, or compliance difficult (9.5%); and (5) had an abnormal lab value that was clinically significant (8%).
Fig. 1.
Flow chart of participants in the clinical trial of modafinil for cocaine dependence. One pt. in each treatment group dropped out before the first dose of medication, i.e., ‘failed enrollment.’.
Demographics and baseline characteristics of study participants are summarized in Table 1. The distribution of race among the treatment groups was not equivalent. The group that received modafinil 200 mg had more African–Americans and fewer Whites than the other two groups (p = 0.01). Also of possible relevance is the nearly significant difference among groups in the number of years using cocaine. Again, the 200 mg modafinil group had used cocaine, on average, about 2.5–3 years longer than the other two groups (p = 0.07).
Table 1.
Baseline characteristics of participants in the modafinil cocaine study.
| Placebo (N = 71) | 200 mg (N = 68) | 400 mg (N = 68) | Fisher’s Exact or χ2p-value | ||
|---|---|---|---|---|---|
| Age | Mean # of years (SD) | 41.46 (7.48) | 42.89 (7.94) | 42.84 (9.43) | 0.33 |
| Gender | Male | 50(70.4%) | 49(72.1%) | 49(72.1%) | 0.98 |
| Female | 21(29.6%) | 19(27.9%) | 19(27.9%) | ||
| White | 32(45.1%) | 19(27.9%) | 30(44.1%) | 0.01 | |
| African American | 38(53.5%) | 46(67.6%) | 32(47.1%) | ||
| Major race | Asian or Pacific Islander | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Amer. Indian or Alaska Native | 0(0.0%) | 0 (0.0%) | 2(2.9%) | ||
| Other | 0 (0.0%) | 3(4.4%) | 4(5.9%) | ||
| Ethnicity | Hispanic or Latino | 9(12.7%) | 9(13.2%) | 11(16.2%) | 0.92 |
| Not Hispanic or Latino | 62(87.3%) | 59(86.8%) | 57(83.8%) | ||
| Education | Mean # of years (SD) | 12.85 (1.64) | 13.28 (2.24) | 13.09 (2.12) | 0.46 |
| Cocaine use, last 30 days | Mean # of days (SD) | 16.48 (8.93) | 16.82 (8.66) | 16.49 (9.02) | 0.97 |
| Lifetime cocaine use | Mean # of years (SD) | 13.38 (7.24) | 16.24 (8.32) | 13.94 (6.89) | 0.07 |
| Alcohol dependence (current or lifetime) | # with SCID diagnosis | 30(42.3%) | 25(36.8%) | 30(44.8%) | 0.63 |
| Depression symptoms | HAM-D> 12 | 21(29.6%) | 15(22.1%) | 13(19.4%) | 0.34 |
| HAM-D≤12 | 50(70.4%) | 53(77.9%) | 54(80.6%) |
Not shown in Table 1 are the frequencies of other illicit drug use. At screening, by self-report about the past 30 days, overall frequency of any alcohol use was 82%, alcohol used to intoxication 49%, nicotine 80%, cannabis 42%, sedative/benzodiazepine 7%, heroin 2%, other opiate/analgesic 6%, methamphetamine 2%, other amphetamines 2%. The treatment groups were not significantly different on these rates.
3.2. Treatment retention and compliance
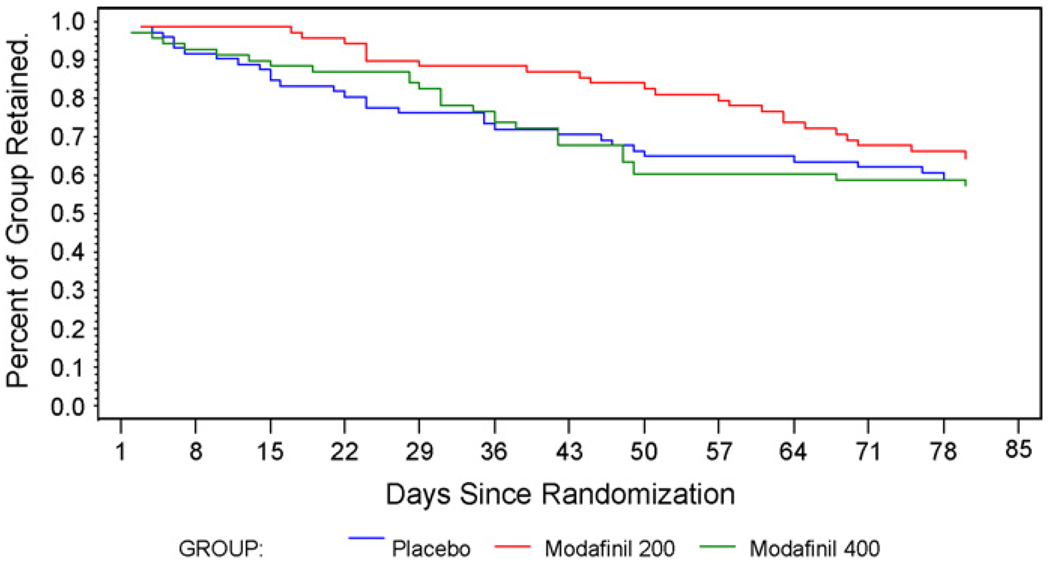
A total of 125 participants (60%) completed the 12 weeks of treatment, with no significant differences in retention between groups (p = 0.48, log-rank test) (Fig. 2). Retention also did not differ significantly between those who were and were not alcohol dependent [current or lifetime] (p = 0.85, log-rank test). Counseling attendance was similar across all groups, averaging 88% until the participant’s point of study discontinuation, and 72% for the full 12-week treatment period. Medication compliance was also similar across all treatment groups, averaging 93% (range 36–100%) up until the point of discontinuation, and 72% (range 2.4–100%) for the full 12-week treatment period.
Fig. 2.
Study retention for participants in the placebo, 200 and 400 mg/day modafinil treatment groups. Shown are the percentage remaining from randomization to the day of the last study visit.
3.3. Primary outcome—efficacy by self-report and urine analysis
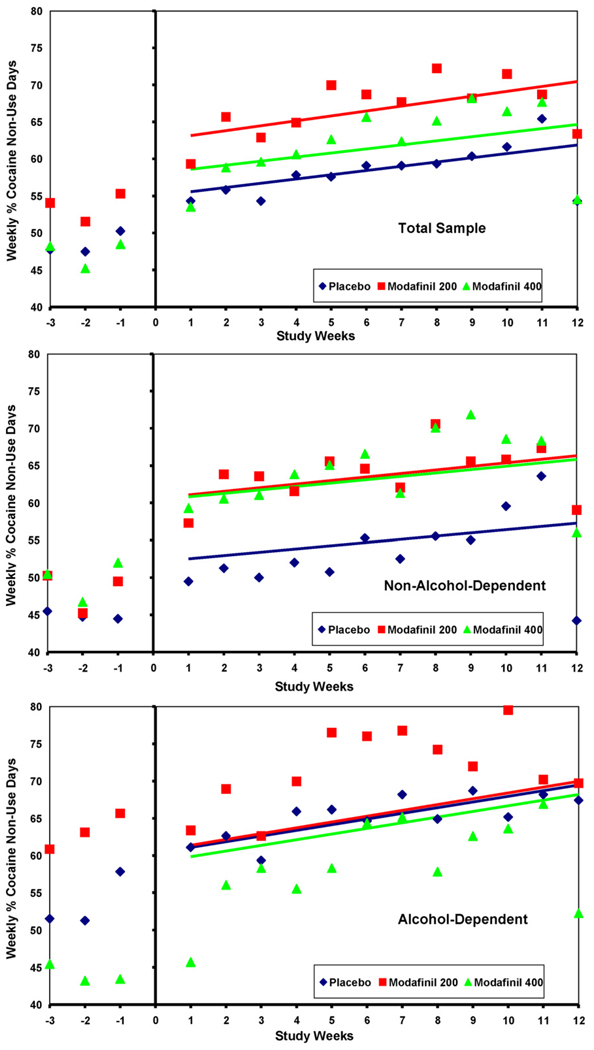
Our simple GEE model contained only factors for treatment Group (3 levels: placebo, modafinil 200 mg, modafinil 400 mg), Study Week (continuous over 12 weeks), and their interaction, Week × Group (3 levels, which represented the medication effect). The response variable was our primary outcome: average weekly percent of cocaine non-use days, which was based on self-report confirmed by urine BE. This model showed no significant difference between either modafinil group and placebo in the rate of change (slope) in the primary outcome over the 12-week treatment period (see Fig. 3, top panel, p = 0.92 for Week × Group/Modaf 400, and p = 0.79 for Week × Group/Modaf 200, GEE). In the simple model the factor of Study Week, or time in treatment, had a significant effect (p < .0001), while treatment Group did not (p = 0.19). A second model showed significant effects of baseline cocaine use (mean of weekly% non-use days from Study Weeks-1 and -2, p < .0001), and of Study Week (p < .0001) on the primary outcome, but again, non-significant effects of Group (p = 0.24) and Week × Group (p = 0.65 overall). We also tested a secondary outcome ‘maximum number of consecutive cocaine non-use days’ (by self-report and urine BE), and found a significant difference between modafinil groups and placebo (p = 0.05, overall Kruskal–Wallis test). The average maximum number of consecutive cocaine non-use days was 12.6 for modafinil 200 mg, compared to 8.8 days for placebo (p = 0.02, Wilcoxon 2-sample), and 12.0 days for modafinil 400 mg (p = 0.46, Wilcoxon 2-sample).
Fig. 3.
Treatment group’s average weekly% of cocaine non-use days (self-report confirmed by urine benzoylecgonine) in: (A) the total sample; and (B) non-alcohol-dependent, and (C) alcohol-dependent (current or lifetime per DSM-IV), by dose of modafinil 400 mg/day, 200 mg/day, or placebo, over 12 weeks. [Plots show observed Group means and Least Square Means fitted lines.]
3.4. Alcohol dependence, current and lifetime
In a post hoc exploratory analysis, each participant’s alcohol dependence, either current (within the past month) or lifetime (ever present), was determined from the SCID interview done at screening. Participants with current or lifetime alcohol dependence were combined, and groups with and without alcohol dependence were tested for an independent association with the primary outcome and/or an interaction with the effect of modafinil treatment. The subgroup that was never alcohol-dependent (i.e., neither current nor lifetime) N = 125, demonstrated a significant difference in average percent of cocaine non-use days between the modafinil groups and placebo (p < 0.02, GEE), where the 200 mg and 400 mg doses of modafinil treatment resulted in an average of 8.9% and 8.5% more cocaine non-use days, respectively, than placebo (see Fig. 3,middle panel). This subgroup, “non-alcohol-dependent,” also had more consecutive cocaine non-use days in the modafinil treatment groups than in placebo (mean: 15.2 days for 400 mg, 13.5 for 200 mg, 6.6 for placebo; p = 0.01, Kruskal–Wallis). By contrast, in the subgroup with either current or lifetime alcohol dependence, N = 82, the modafinil 400 mg treatment group had an average of 1.3% fewer cocaine non-use days than placebo, although this difference was not significant (see Fig. 3, lower panel, p = 0.92, GEE), and the modafinil 200 mg treatment group had 0.2% more non-use days than placebo. This alcohol-dependent subgroup had baseline differences in weekly percent of non-use days among the treatment groups, which remained roughly parallel throughout the treatment period (i.e., no differential treatment effect) (Fig. 3).
3.5. Major race categories
Because only 9 participants described themselves as a race other than White or African American, we used only the two large categories to test for an interaction of Race with the primary outcome. We found that our GEE model showed a significant effect of race (p = 0.0003), in addition to significant effects of the baseline level of cocaine use (from Study Weeks-1 and -2, p < .0001), and the Study Week (time in treatment, p < .0001). The GEE parameter estimate for African American race, with White as reference, was −11.9 (SE 3.7), i.e., the weekly percent of cocaine non-use days was 12% less for African Americans. The factor of medication dose continued to be non-significant (interaction of Group × Study Week, p = 0.65). Interactions of race with treatment Group and with Study Week were also non-significant (p > 0.9).
3.6. Secondary outcomes
There were no significant differences overall between modafinil and placebo groups in many secondary outcome measures, including:
For quantitative cocaine use, the (slope) decrease in average weekly log10 median urine benzoylecgonine over the 12-week treatment period (p = 0.34, GEE).
Any of seven Addiction Severity Index (ASI)-Lite domains, examining the difference in means from baseline to last observation using a two-sample t-test or a Mann–Whitney–Wilcoxon two-sample rank sum test (all p > 0.10).
Either of the two outcomes (improvement or experience) from the Clinical Global Impression scales, separately regressed on treatment Group and the Group × Week interaction using GEE, for the-Observer (CGI-O, p > 0.35) or for the-Self (CGI-S, p > 0.11).
For the sexual risk domain of the HIV Risk Behavior Scale (HRBS), examining change from baseline to last visit for each participant (two sample t-test contrasts between each active group and placebo, all p > 0.30). [96% of the changes in injection risk domain scores were zero, therefore, analysis was restricted to sexual risk.]
3.6.1. Cocaine craving
Total scores for the Brief Substance Craving Scale (BSCS) for cocaine (items 1–8) were ordinal interval transformed to reduce skewness prior to regression analysis (Kyomen et al., 1999). The BSCS total score was tested with factors of treatment Group, Week, and the Group × Week interaction, using GEE. The slope over the treatment period was compared between modafinil 200 or modafinil 400 and placebo. Relative to placebo, we estimated that craving declined on average within each active arm, and this difference from placebo was nominally significant for 200 mg (p = 0.04) but not for 400 mg (p = 0.90). However, the attained significance level of p = 0.04 was not significant after correction for multiple comparisons. Total scores for the Cocaine Craving Questionnaire (CCQ) were examined using means at baseline and end of treatment (Week-12) via a two-sample t-test. No significant effect on total score was noted, however, the sub-domains “Anticipation” (p = 0.04) and “Relief” (p = 0.03) were significant with treatment of 200 mg modafinil.
3.7. Safety
There were 16 Serious Adverse Events in the entire study, none of which met the criteria for expedited reporting, i.e., serious, unexpected, and possibly related to the study medication. Our non-serious Adverse Events totaled 1507 events among 200 participants. Among the events that had a greater incidence with 400 mg of modafinil than with 200 mg or placebo (p < 0.10), and occurred in participants at a >5% rate, were nausea (26%, p = 0.085), insomnia (20%, p = 0.02), dizziness (11%, p = 0.07), pain in extremity (7%, p = 0.02), irritability (6%, p = 0.04), and the total events from the Psychiatric Disorders Body System (43%, p = 0.04). Decreased appetite was equally more frequent with 200 or 400 mg modafinil than with placebo (p < 0.001). There were 17 participants with at least one AE leading to permanent discontinuation of study drug (placebo: 6, modafinil 200 mg: 1, modafinil 400 mg: 10). The most common category of such AEs was gastrointestinal disorders, with the modafinil 400 mg group having four instances of nausea and two of dry mouth, while the placebo group had one of each. The modafinil 200 mg group had one abnormal liver function test. The modafinil 400 mg group also had one instance each of abnormal electrocardiogram, hypertension, chest discomfort, ‘bitter taste’, irritability, agitation, anxiety, tension, and two instances of insomnia, and in the skin disorders one ‘sweaty palms’ and one ‘upper lip swelling’, with none of these events in the other groups. Aside from the one instance of hypertension requiring discontinuation, average blood pressures remained stable throughout the 12 weeks of treatment. The weekly average change from baseline had no greater increase in any treatment group than 2.6 mmHg systolic and 2.8 mmHg diastolic, with similar size average decreases in some weeks.
4. Discussion
In this study, the overall main effect of modafinil on the planned primary outcome (weekly% of cocaine non-use days) was not significant. However, among the secondary outcome measures, a greater number of consecutive days of cocaine abstinence and a trend toward reduction in cocaine craving were associated with modafinil 200 mg/day. In addition, a post hoc analysis using the same primary outcome measure demonstrated an effect we had not predicted, suggesting that modafinil was superior to placebo in a subgroup of cocaine patients without co-dependence on alcohol. We carried out this subgroup analysis because we observed that our trial results differed from the positive results of an earlier trial, in which participants with co-dependence on alcohol had been excluded (Dackis et al., 2005). Our results suggest that there may be an interaction among modafinil, cocaine, and alcohol, which is observed (possibly only) after the neuro-adaptations of chronic use.
Our primary analysis included observations from participants who dropped out early, and whose remaining urine data was missing. GEE generally treats missing as missing; however, the Dackis 2005 analysis assumed that missing urines were cocaine-positive. We re-analyzed our data using Dackis’ primary outcome (mean proportion of cocaine-negative urines, calculated for each patient as % of the 24 urines expected over 8 weeks), with the assumption that ‘missing is dirty’, and still found that in our study there was not a significant modafinil treatment effect. We also did a retrospective power analysis, based on the average response for each participant over the study period. Group means of these per-person averages were 58% non-use days for placebo and 66% non-use days for modafinil 400 mg. Our study sample, with 70 participants per arm and a pooled within-group standard deviation of 20%, provided statistical power of 60% to detect an effect of this size. For purposes of planning future studies, this suggests the need for larger sample sizes or amore homogeneous population. However, if the observed effect size (8% difference in weekly non-use days) is not considered large enough to be clinically meaningful, then the sample size may be adequate but a more effective treatment is needed.
The FDA-approved product labeling for modafinil states, “Patients should be advised that it is prudent to avoid alcohol while taking Provigil (Cephalon, 2007).” One possible concern about drinking alcohol with this medication is based on a theory that modafinil may worsen chronic drinking because of its pro-glutamate action (Ferraro et al., 1999). However, in this study we saw no evidence of increased alcohol drinking. On the contrary, all groups reduced their (self-reported) drinking throughout the treatment period.
Our participants’ drug-use histories showed that, at baseline, the 200 mg modafinil group had a greater number of years using cocaine, which might indicate a higher severity of illness. If all other things were equal, an imbalance in disease severity across treatment groups would be expected to obscure any medication effect, and cause a bias toward a null result.
Our randomization process did not attempt to balance treatment groups on race, and the imbalance we found probably occurred by chance. African Americans comprised 56% of our total sample, and were observed to accumulate a modestly higher weekly percent of cocaine use-days than did Whites. This result was independent of significant effects of baseline level of cocaine use, and time in treatment. We hope to conduct a meta-analysis of our cocaine treatment trials to better discern the relative salience for the outcome of treatment among multiple predictive factors, such as level of recent drug use (month prior to consent, or 2 weeks at baseline), number of years of drug use, co-occurring alcoholism, race, etc.
In our study, the 400 mg dose of modafinil was associated with a greater Adverse Events profile, and more frequent discontinuation of the medication. The FDA label for modafinil notes a dose–dependence for two adverse events—headache and anxiety (Cephalon, Inc., 2007). In our trial, in non-alcohol-dependent patients, the higher dose of modafinil did not have a better effect, on either increasing the weekly percent of cocaine non-use days, or increasing the maximum number of consecutive non-use days. This suggests that higher doses of modafinil may be unnecessary for the treatment of cocaine dependence.
Our results are in line with those from other recent clinical trials for treatment of stimulant dependence (Kampman et al., 2004; Elkashef et al., 2008), where no single medication has been shown to help most patients, but rather, some subgroups seem to respond to a specific medication. Our post hoc finding of an interaction with alcohol dependence generates new hypotheses, and is important in an illness for which there is no approved medication. Further studies with modafinil in stimulant-addicted patients should either systematically examine how alcohol comorbidity mediates treatment effects, or carefully limit participation in regards to alcohol dependence.
Acknowledgments
Role of funding source
Funding for this study was provided by NIDA Contract NO1DA-3-8838. The National Institute on Drug Abuse-NIH had a major role in study design, in the analysis and interpretation of data, and in the writing and submission of this manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Preliminary report on this study was presented at the 2007 annual meeting of the College on the Problems of Drug Dependence.
Contributors
Authors Ahmed Elkashef and Ann Anderson wrote the first draft of the manuscript. Malcolm Reid contributed edits and responses to reviewer comments. Shou-Hua Li, Tyson Holmes, Lynn Shemanski and April Slee did the statistical analyses. Roberta Kahn summarized the adverse events data. Edwina Smith managed literature searches and described the study population and treatments. Nora Chiang, Frank Vocci and Charles Dackis contributed summaries of previous related work. Domenic Ciraulo, Malcolm Reid, John Roache, Ihsan Salloum, Eugene Somoza and Harold Urschel were site Principle Investigators, and edited both the protocol and the manuscript. All authors have approved the final manuscript.
Conflict of interest
Charles Dackis has served as a consultant to Cephalon. Eugene Somoza has served as a consultant to Catalyst Pharmaceutical Partners. Domenic Ciraulo has a clinical trials contract with Catalyst Pharmaceuticals. All other authors declare that they have no conflicts of interest, i.e., no actual or potential conflict of interest including any financial, personal or other relationships with other people or organizations within three (3) years of beginning the work submitted that could inappropriately influence, or be perceived to influence, this work.
References
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc. Natl. Acad. Sci. U. S. A. 2005;102(52):19168–19173. doi: 10.1073/pnas.0507480102. (Epub 2005 December 15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cephalon, Inc. PROVIGIL (modafinil) Tablets [C-IV], FDA Approved Labeling dated August 17, 2007. Frazer, PA: Cephalon, Inc.; 2007. [Google Scholar]
- Dackis CA, Lynch KG, Yu E, Samaha FF, Kampman KM, Cornish JW, Rowan A, Poole S, White L, O’Brien CP. Modafinil and cocaine: a double-blind, placebo-controlled drug interaction study. Drug Alcohol Depend. 2003;70:29–37. doi: 10.1016/s0376-8716(02)00335-6. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O’Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30:205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- Donovan JL, DeVane CL, Malcolm RJ, Mojsiak J, Chiang CN, Elkashef A, Taylor RM. Modafinil influences the pharmacokinetics of intravenous cocaine in healthy cocaine-dependent volunteers. Clin. Pharmacokinet. 2005;44:753–765. doi: 10.2165/00003088-200544070-00006. [DOI] [PubMed] [Google Scholar]
- Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, Chiang N, Kahn R, Vocci F, Ling W, Pearce VJ, McCann M, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Weis D. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33:1162–1170. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, Tanganelli S, O’Connor WT, Perez de la Mora M, Mendez-Franco J, Rambert FA, Fuxe K. The vigilance promoting drug modafinil increases extracellular glutamate levels in the medial preoptic area and the posterior hypothalamus of the conscious rat: prevention by local GABA-A receptor blockade. Neuropsychopharmacology. 1999;20:346–356. doi: 10.1016/S0893-133X(98)00085-2. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, O’Connor WT, Tanganelli S, Rambert F, Fuxe K. The antinarcoleptic drug modafinil increases glutamate release in thalamic areas and hippocampus. Neuroreport. 1997;8:2883–2887. doi: 10.1097/00001756-199709080-00016. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology. 2007;33:761–768. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- Hser YI, Stark ME, Paredes A, Huang D, Anglin MD, Rawson R. A 12-year follow-up of a treated cocaine-dependent sample. J. Subst. Abuse Treat. 2006;30:219–226. doi: 10.1016/j.jsat.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Jasinski DR. An evaluation of the abuse potential of modafinil using methylphenidate as a reference. J. Psychopharmacol. 2000;14:53–60. doi: 10.1177/026988110001400107. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K, Bowers S, Szumlinski K, Xi ZX, Baker D. Glutamate transmission and addiction to cocaine. Ann. N. Y. Acad. Sci. 2003;1003:169–175. doi: 10.1196/annals.1300.009. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Pettinati H, Lynch Kg, Dackis C, Sparkman T, Weigley C, O’Brein CP. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend. 2004;75:233–240. doi: 10.1016/j.drugalcdep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Leiderman D, Holmes T, LoCastro J, Bloch DA, Reid MS, Shoptaw S, Montgomery MA, Winhusen TM, Somoza EC, Ciraulo DA, Elkashef A, Vocci F. Cocaine Rapid Efficacy Screening Trials (CREST): lessons learned. Addiction. 2005;100 Suppl. 1:102–110. doi: 10.1111/j.1360-0443.2005.00987.x. [DOI] [PubMed] [Google Scholar]
- Kyomen HH, Satlin A, Hennen J, Wei JY. Estrogen therapy and aggressive behavior in elderly patients with moderate-to-severe dementia: results from a short-term, randomized, double-blind trial. Am. J. Geriatr. Psychiatry. 1999;7:339–348. [PubMed] [Google Scholar]
- Lindsay SE, Gudelsky GA, Heaton PC. Use of modafinil for the treatment of attention deficit/hyperactivity disorder. Ann. Pharmacother. 2006;40:1829–1833. doi: 10.1345/aph.1H024. [DOI] [PubMed] [Google Scholar]
- Longabaugh R, Zweben A, Locastro JS, Miller WR. Origins, issues and options in the development of the combined behavioral intervention. J. Stud. Alcohol Suppl. 2005;15:179–187. doi: 10.15288/jsas.2005.s15.179. discussion 168-9. [DOI] [PubMed] [Google Scholar]
- Malcolm R, Book SW, Moak D, DeVane L, Czepowicz V. Clinical applications of modafinil in stimulant abusers: low abuse potential. Am. J. Addict. 2002;11:247–249. doi: 10.1080/10550490290088027. [DOI] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Schuster CR, Cone EJ. Assessment of cocaine use with quantitative urinalysis and estimation of new uses. Addiction. 1997;92:717–727. [PubMed] [Google Scholar]
- Roth T, Roehrs TA. Etiologies and sequelae of excessive daytime sleepiness. Clin. Ther. 1996;18:562–576. doi: 10.1016/s0149-2918(96)80207-4. [DOI] [PubMed] [Google Scholar]
- Rush CR, Kelly TH, Hays LR, Wooten AF. Discriminative-stimulus effects of modafinil in cocaine-trained humans. Drug Alcohol Depend. 2002;67:311–322. doi: 10.1016/s0376-8716(02)00082-0. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Estabrooke IV, McCarthy MT, Chemelli RM, Yanagisawa M, Miller MS, Saper CB. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J. Neurosci. 2000;20:8620–8628. doi: 10.1523/JNEUROSCI.20-22-08620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2007 National Survey on Drug Use and Health: National Findings. Office of Applied Studies, NSDUH Series H-34. Rockville, MD: 2008 DHHS Publication No. SMA 08-4343.
- Taneja I, Haman K, Shelton RC, Robertson D. A randomized, double-blind, crossover trial of modafinil on mood. J. Clin. Psychopharmacol. 2007;1:76–79. doi: 10.1097/jcp.0b013e31802eb7ea. [DOI] [PubMed] [Google Scholar]
- Turner DC, Clark L, Dowson J, Robbins TW, Sahakian BJ. Modafinil improves cognition and response inhibition in adult attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2004;55:1031–1040. doi: 10.1016/j.biopsych.2004.02.008. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime. World Drug Report 2007. New York, NY: United Nations; 2007:82f.
- Wei LJ, Lachin JM. Properties of the urn randomization in clinical trials. Control Clin. Trials. 1988;9:345–364. doi: 10.1016/0197-2456(88)90048-7. [DOI] [PubMed] [Google Scholar]