Abstract
Polymer-based, injectable systems that can simultaneously deliver multiple bioactive agents in a controlled manner could significantly enhance the efficacy of next generation therapeutics. For immunotherapies to be effective, both prophylactically or therapeutically, it is not only critical to drive the antigen (Ag) specific immune response strongly towards either T helper type 1 (Th1) or Th2 phenotype, but also to promote recruitment of a high number of antigen-presenting cells (APCs) at the site of immunization. We have recently reported a microparticle-based system capable of simultaneously delivering siRNA and DNA to APCs. Here we present an in situ crosslinkable, injectable formulation containing dendritic cell (DC)-chemoattractants and dual-mode DNA-siRNA loaded microparticles to attract immature DCs and simultaneously deliver, to the migrated cells, immunomodulatory siRNA and plasmid DNA antigens. These low crosslink density hydrogels were designed to degrade within 2–7 days in-vitro and released chemokines in a sustained manner. Chemokine carrying gels attracted 4–6 folds more DCs over a sustained period in vitro, compared to an equivalent bolus dose. Interestingly, migrated DCs were able to infiltrate the hydrogels and efficiently phagocytose the siRNA/DNA carrying microparticles. Hydrogel embedded microparticles co-delivering Interleukin-10 siRNA and plasmid DNA antigens exhibited efficient Interleukin-10 gene knockdown in migrated primary DCs in-vitro.
Keywords: In-situ crosslinking, Hydrogel, Gene transfer, siRNA, Chemotaxis, Plasmid DNA, Microsphere
Introduction
Stimulation of innate immune response results in the expression of pro-inflammatory cytokines and chemokines. This leads to a cascade of events causing migration of professional antigen presenting cells (APCs), including dendritic cells (DCs) and macrophages, to the site of infection or vaccination. These APCs ingest, process and present the antigen and themselves get activated to further transmit specific modulatory signals to T cells. The number of APCs migrating to the site, the efficiency of antigen uptake and presentation, the extent of APC activation and types of cytokines and surface ligands expressed by the activated APCs dictate the strength and type of T cell response (e.g. Th1 or Th2) during immunotherapy [1–3].
Chemokines have been reported to specifically attract immature APCs and mediate their localization and homeostasis. Immature APCs that continuously sample the bloodstream, skin, and tissues are available in relatively fewer numbers (approximately 1% of cells) [1, 4]. Thus, various strategies have been explored to design vaccines that can recruit more APCs at the site of immunization[1, 5–9]. Although some reports have demonstrated APC recruiting using chemokine released from non-degradable implants [10] or microparticles [1], delivery of antigen/adjuvant carrying microparticles and chemokines in a single, injectable formulation, that can attract APCs and at the same time prime and modulate their immune response, has not be shown. Kumamoto et al. reported delivery of MIP3β, a mature Langerhans' cell attractant, using an implanted polymer matrix of ethylene–vinyl–acetate [10]. However, such a system has limited use because of use of a non-degradable polymer implant that requires surgical removal. Zhao et al. encapsulated formyl-Nle-Leu-Phe-Nle-Tyr-Lys (fN'LFN'YK) peptides or MIP3α in biodegradable poly(lactide-co-glycolide) (PLGA) microspheres for chemoattracting DCs [1]. Although both the chemoattractants induced strong, sustained attraction of mouse DCs, human DCs and monocytes, use of formyl peptides, which can themselves be immunogenic, raises safety concerns. In addition, while the microparticles were able to encapsulate up to 89 % of formyl peptide (hydrophobic), the encapsulation with MIP3α (hydrophilic) was only 53 % [1]. Thus, improved delivery systems for co-delivery of chemokines and antigen/adjuvant loaded microparticles are necessary.
We recently reported development of a microparticle based delivery system that can simultaneously deliver IL-10 targeted short interfering RNA (siRNA) along with a plasmid DNA (pDNA)-antigen to the same APCs. The simultaneous silencing of IL-10 in APCs significantly enhanced DC activation and T cell proliferation in vitro while successfully “switching” the in vivo immune response towards a strong Th1 and CTL phenotype [11]. We hypothesize that further enhancement of the immune response can be achieved by co-delivery of DC-attracting chemokines (e.g. MIP3α) and these siRNA/pDNA carrying microparticles in a single, injectable, controlled-release formulation. This would create a synthetic DC priming center at the site of immunization, attract immature DCs in large numbers and allow efficient delivery of immune-modulatory siRNA and pDNA antigens.
We report here, an integrated platform comprising of an in-situ crosslinkable, fast-degradable, polymeric network for combinatorial delivery of DC chemo-attractants (MIP3α) and pDNA/siRNA loaded microparticles. Hydrogels comprising of either (a) Dextran vinyl sulfone (DextranVS) and tetra functional polyethyleneglycol (PEG) thiol (PEG4SH) or (b) PEG diacrylate (PEGDA) and PEG4SH, were synthesized [12, 13]. Both systems were characterized and optimized to crosslink in-situ as well as efficiently encapsulate and deliver high amounts of chemokine and microparticles.
Materials and Methods
Materials
Acid end-capped PLGA RG502H (MW ~ 11,000 Da) was purchased from Boehringer Ingelheim (Petersburg,VA). Poly(vinyl alcohol) (MW ~ 31,000 Da) was obtained from Fluka (Sigma, St. Louis, MO). 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and sulfo N-hydroxysuccinimide (NHS) were purchased from Pierce Biotechnology (Rockford, IL). Branched PEI (MW = ~70,000 Da) was purchased from Polysciences (Warrington, PA). Custom synthesized mouse IL-10 siRNA–targeting the specific sequence 5′-AATAAGCTCCAAGAGAAAGGC-3′, Silencer Negative Control #1 siRNA, and Silencer Select Cy3-GAPDH siRNA (mouse) were gifts from Ambion (Austin, TX). Dextran from Leuconostoc mesenteroides (average MW 15000–20000 Da), divinyl sulfone (DVS; 97 %, MW 118.15 Da) and 3-Mercaptopropionic acid (MW 106.14 Da) were purchased from Sigma-Aldrich (St. Louis, MO). Tetra-functional PEG-thiol (PEG4SH) (82.7% activity) was purchased from Sunbio (Anyang City, South Korea). Dimethyl sulfoxide (DMSO), p-toluenesulfonic acid (pTSA) and tetrahydrofuran (THF, HPLC grade) were from Thermo Fisher Scientific.
Bone marrow derived primary APC isolation and cell lines
Mouse APCs were derived from bone marrow isolated progenitor cells of female Balb/c mice (H-2d, 5–10 weeks old, Jackson laboratories) as described previously [14] [11]. Isolated cells were differentiated to primary APCs in complete RPMI-1640 Glutamax® medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT) and 1% penicillin-streptomycin (Sigma, St.Louis, MO). The culture medium was supplemented with 20 ng/ml mouse GM-CSF and 10 ng/ml IL-4 (eBioscience, San Diego, CA). Loosely adherent APCs were harvested on day 6. We have earlier shown these APCs to be 65–82 % CD11c+. Animal handling and surgical procedures were performed in accordance to protocols approved by the University of Texas at Austin's Institutional Animal Care and Use Committee. Mouse C2C12 myoblast (ATCC, Manassas, VA) and human dermal fibroblast (Cambrex BioScience, Walkersville, MD) cell lines were cultured with DMEM (Sigma, St.Louis, MO) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT) and 1% penicillin-streptomycin (Sigma, St.Louis, MO).
Synthesis of (Dimethylamino)pyridinium 4-toluenesulfonate and dextran vinylsulfone
Synthesis of dextran vinylsulfone (DextranVS) with ethyl spacer was performed as mentioned earlier using N,N'-dicyclohexyl-carbodiimide (DCC, Fisher Scientific) and 4-(Dimethylamino)pyridinium 4-toluenesulfonate (DPTS) catalyst [12]. DPTS was synthesized as reported earlier [15]. Briefly, 5 g of pTSA monohydrate was dissolved in 100 ml THF. 4-(dimethylamino)-pyridine (DMAP, 99%) (Sigma, St Loius, MO) at one molar equivalent to pTSA was added to this mixture and filtered to obtain precipitate which was further dissolved in dichloromethane (DCM, Fisher Scientific) and recrystallized using rotary vacuum evaporator. Dextran vinyl sulfone ester synthesis was performed by adding 2.5 or 5g DVS in 90 ml of inert nitrogen saturated DMSO followed by drop wise addition of 3-MPA to it under continuous stirring. The reaction was continued for 4 hrs in dark. Dextran was dissolved in 30 ml DMSO and solution of DCC and pTSA in 30 ml DMSO was added to it drop-wise and stirred until clear solution was obtained. DPTS is a weekly acidic catalyst and enhances the reaction efficacy of DCC. Finally, the mixture was added to DVS/MPA solution in dark and reaction was allowed to proceed for 24 hrs at room temperature. After the completion of reaction, N,N-dicyclohexylurea (DCU) salt was filtered using a vacuum filter and the product was recovered by precipitation in 1000 ml of ice cold 100% ethanol. The precipitate was separated from residual ethanol through centrifugation at 3000 rpm for 15 min followed by vacuum drying. Precipitate was re-dissolved in at least 100 ml of de-ionized water (pH adjusted to 7.8) and vortexed to obtain a clear solution. Finally, unreacted polymer was removed through ultra filtration using Amicon filter (MWCO 10000 Da, Millipore) and the viscous product was lyophilized to remove water. Vinyl sulfone substitution was confirmed and degree of substitution (DS) was determined using NMR as reported earlier [12].
DNA-siRNA co-loaded PEI modified PLGA microparticle synthesis
PLGA microparticles with or without encapsulated siRNA (IL-10 or Silencer Negative Control #1 siRNA or Silencer Select Cy3-GAPDH) were synthesized with PLGA RG502H using a water-in-oil-in-water double emulsion, solvent evaporation technique as reported earlier by our research group[11]. A modified EDC-sulfo NHS chemistry [11, 16, 17], was performed to conjugate PEI to the surface of siRNA-encapsulated microparticles to obtain cationic PEI-PLGA-siRNA microparticles (Figure 1A). The particles were characterized (both before and after PEI conjugation as well as post DNA loading) for size and zeta potential (data not shown) using Laser Light Scattering (ZetaPlus, Brookhaven Instruments, NY) as reported earlier [11]. pgWizLuciferase plasmid DNA (Aldevron, ND) was loaded on the surface of the PEI-functionalized microparticles as before [11, 16].
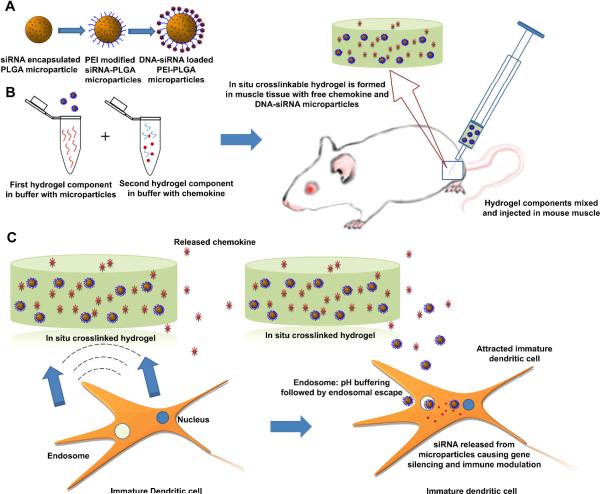
Figure 1. Schematic outline of proposed delivery system.
(A) siRNA encapsulated, biodegradable, polymer microparticles are surface modified to attach a cationic polymer, PEI resulting in a cationic microparticle. Plasmid DNA is electrostatically loaded on these cationic microparticles. (B) DNA and siRNA co-loaded microparticles, along with MIP3α are mixed with a two component polymer solution and injected intramuscularly to form an in situ crosslinked hydrogel with entrapped chemokine and microparticles (C) Release of the chemokine from the degrading hydrogel in the tissue would attract naïve (immature) DCs to the site of administration. These DCs phagocytose the DNA/siRNA carrying particles released from the degrading gel. The availability of secondary and tertiary amines on the particle surface enhances the buffering capacity of the particles and may allow endosomal escape of the particles. Escaping particles release siRNA to induce gene silencing and pDNA is simultaneously delivered to the same cells.
Hydrogel synthesis and gelling time
In situ crosslinkable, degradable depot systems were fabricated using Michael type addition reactions [12, 13, 18]. Briefly a 10, 20 or 30 % w/v hydrogel precursor solution of DextranVS (DS 2 and DS 5) and 10 or 20 % w/v solution of PEG4SH were resuspended in 0.3M triethanolamine (TEA) buffer (total volume 100 μl, final pH 7.8). Individual components of the hydrogel were re-suspended separately in 50 μl of TEA buffer, sonicated briefly and chemokine MIP3α (10 ng, 100 ng, or 1 μg) was re-suspended with the PEG4SH component. pDNA and siRNA loaded PEI-PLGA microparticles (1 mg, 5 mg or 50 mg) were re-suspended with the DextranVS component (Figure 1B). The two component mixtures were added together in a centrifuge tube and incubated at 37°C. Similarly, to prepare PEGDA and PEG4SH based hydrogels, 10, 20 or 30 % w/v of PEGDA and 10 or 20 % w/v solution of tetra-functional PEG4SH were resuspended in triethanolamine (TEA) buffer (total volume 100 μl, final pH 7.8). Hydrogels with various components were formed as described above. The various w/v concentrations were chosen to alter the crosslink density of the polymer which in turn would allow us to control the release rate of the entrapped biomolecules and the degradation of the hydrogel [12, 13]. In separate experiments, FITC-tagged dextran (4 kDa and10 kDa) was encapsulated in similar way to assess the effect of molecular weight on encapsulation inside the hydrogel. Gelation times for various formulations were determined by tilting the vials for at least 45 sec [12].
Hydrogel loading efficiency and SEM
Encapsulation efficiency of chemokine (MIP3α) was determined using a sandwich ELISA (Mouse CCL20/MIP3 alpha Quantikine ELISA Kit (R&D Systems, Minneapolis, MN)) on the residual solution collected from the centrifuge tube after removing the hydrogel (loaded with chemokine and microparticles). Similarly, encapsulation efficiency of microparticles was determined from the residual particle solution. Collected particles were freeze dried in a pre-weighed micro-centrifuge tube for 12 hrs and weight of dried microparticles were obtained. Encapsulation efficiency was calculated as follows:
Scanning electron microscopy for hydrogel morphology
The internal morphology of freeze-dreied hydrogels was observed using a scanning electron microscope (Zeiss Supra 40 VP). Hydrogels (with and without microparticles) were flash-frozen in liquid nitrogen, lyophilized overnight and fractured. Dried hydrogels were mounted on SEM stubs and coated with Pt/Pd layer.
Swelling ratio and degradation time
Hydrogels with or without microparticles were weighed after gelling and allowed to swell at 37°C in PBS (pH 7.4) on a slow speed rocker and at regular time intervals weight of the swelled gels were recorded. Swelling ratio of these hydrogels was determined using following relation:
Hydrogel degradation time was recorded as the time when the hydrogel almost or completely collapsed [12].
In vitro release of chemokines from hydrogels
Hydrogel solutions containing 50 ng MIP3α and 5 mg pDNA-siRNA loaded PEI-PLGA microparticles were added in low protein binding microcentrifuge tubes and cured for 2 hours in a water bath at 37 °C. Hydrogels were rinsed with 1 ml of PBS and transferred to a 12 well plate with 2 ml PBS (pH 7.4). Release studies were conducted at 37 °C on a slow speed rocker. Released MIP3α was collected at different time interval and stored at −20 °C until assayed. Each well was replenished with 2 ml of fresh PBS at each time point. Released medium was analyzed for amount of chemokine using Mouse CCL20/MIP3 alpha Quantikine ELISA Kit (R&D Systems, Minneapolis, MN).
Bioactivity of encapsulated chemokine
Hydrogel precursor solutions (50 μl) with 50 ng MIP3-α and with or without PEI-PLGA microparticles were cured at 37°C for 2 hrs. These gels were then transferred to a 24 well tissue culture plate in 1000 μl of RPMI medium. Encapsulated chemokine (50 ng/gel) was allowed to release from hydrogels in 1 ml of RPMI medium for eight days (or till the hydrogels degraded completely). The whole medium was then transferred to another 24 well plate and chemotaxis assay was performed using Transwell™ inserts (6.5 mm, Costar, Cambridge, MA) with a pore size of 5μm [10]. Primary APC suspension (2.5×105 cells in 100 μl medium) was added to the upper chamber of each insert. After 5 hrs the inserts were removed and CellTiter 96® AQueous One Solution Cell Proliferation assay solution (20 μl reagent/100 μl medium) was added to each well and incubated for 3 hours at 37°C and 5% CO2. In a separate well, 50 ng unprocessed (fresh) chemokine in 1 ml of medium was used as positive control. Viable APC migration was evaluated by measuring the absorbance at 490 nm and comparing with a standard curve of optical density (490 nm) versus number of APCs (R2=0.9948). Hydrogels without chemokines were used as negative control to minimize the background optical density. Chemotactic index (CI), defined as the fold increase in the number of migrating cells in the presence of test factors over the spontaneous cell migration (in the absence of test factors), was calculated for each group. Chemokine bioactivity was calculated as percent retention of bioactivity after normalization with the fresh chemokine control:
Cytotoxicity studies
The cyto-compatability of hydrogel and precursor polymers on mouse C2C12 myoblasts, mouse primary bone marrow derived APCs, and human dermal fibroblast (HDF) was evaluated using CellTiter 96 AQueous One Solution Cell Proliferation Assay (MTS assay) (Promega,Madison, WI) . For studies with C2C12 and HDF, 2×104 cells/ well of 48- well plate were seeded for 24 hours. Thereafter, 1 mm thick hydrogels were prepared (TEA solution at pH 7.8) and placed in the wells for another 3 days. For APC cytotoxicity studies, hydrogels were placed in a 24 well plate in complete RPMI medium and primary APC suspension (1×106 cells/well) was dispensed in the medium and incubated at 37°C. After 3 days of incubation, 20 μl MTS reagent per 100 μl medium was added and further incubated for 2–3 hrs at 37°C. Cell viability was evaluated using a Dynex plate reader at 490 nm. Cell cultured on regular tissue culture treated wells were used as positive control.
In vitro APC functionality assay
Hydrogel precursor solutions (100 μl) containing 50 ng MIP3α and 5 mg of pDNA-IL10 siRNA loaded microparticles were prepared in TEA buffer at pH 7.8. Hydrogels were allowed to cure for 2 hrs and rinsed briefly with 1 ml sterile PBS (pH 7.4). Immature APCs (3×105 cells/well) were co-incubated with the hydrogels for 48 hours followed by further pulsing with Lipolysachcharide (LPS, 100 ng/ml) for 24 hours. pgWizLuciferase was used as the model pDNA (900 ng/100 μl gel) in the study and LPS (100 ng/ml culture medium) was used as a positive control for APC activation. Hydrogels and the wells were rinsed three times to harvest cells for flow cytometry staining. Cells were washed twice with FACS buffer (1% bovine serum albumin and 0.05% sodium azide (Sigma-Aldrich, St. Louis, MO) in PBS) and blocked with anti-mouse CD16/CD32 Fc Block (BD Pharmingen, San Diego, CA) at 4°C for 10 min. Cells were analyzed for surface expression of co-stimulatory markers using FACScalibur (BD Biosciences, San Jose, CA) by staining the cells with monoclonal antibodies against PerCP-Cy5.5 anti-mouse CD11c, FITC anti-mouse CD40, and FITC anti-mouse CD86 (B7-2) (eBioscience, San Diego, CA). Untreated cells stained for same surface markers were used as negative controls.
In vitro chemotaxis assay
Chemotaxis assays were performed as detailed by Kumamoto et al. [10], with modifications. Briefly, thin hydrogels were prepared (TEA solution at pH 7.8) and placed in a 24 well plate and 1000 μl of complete RPMI medium was added to each well containing hydrogels. Hydrogels (with and without microparticles) contained various amounts of MIP3α (0, 10, 50, or 100 ng per gel). Transwell™ inserts (6.5 mm, Costar, Cambridge, MA) with a pore size of 5μm were placed in each well. Inserts were carefully balanced on a sterile external support to increase the distance of Transwell™ membrane from the surface of hydrogel (thus maintaining sufficient distance between swollen hydrogel and membrane). Primary APC suspension (3×105 cells in 100 μl medium) was dispensed in the upper chamber of each insert and allowed to migrate under the effect of chemokine for 28 hrs. At various time points after incubation (4h, 7h, 20h), CellTiter 96® AQueous One Solution Cell Proliferation assay solution (20 μl reagent/100 μl medium) was added to each well and incubated for 3 hours at 37°C and 5% CO2. Viable APC migration was evaluated using a standard curve of optical density (490 nm) versus number of APCs (R2 = 0.998).
In vitro APC migration through collagen tissue phantom
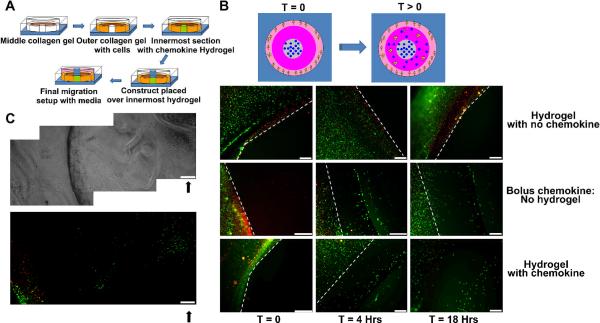
Purified Rat tail type I collagen (BD Biosciences, USA) was diluted to desired concentration by adding 0.2% acetic acid. Ice-cold collagen solution was mixed with 10X DMEM and HEPES solution in the ratio of 8:1:1 (collagen gel precursor solution). The precursor solution was then casted in a 12 well tissue culture treated plates. The study design was a multi-step process where each well was first sectioned into 3 compartments using circular inserts made of polydimethylsiloxane (PDMS) molds (thus making three concentric circular boundaries). The middle compartment was first filled with collagen precursor solution and allowed to gel for 30 minutes. Following this, the outermost compartment was filled with primary APC encapsulated collagen solution prepared by suspending 1×106 cells/ ml complete collagen solution and allowed to gel for another 30 min. To visualize initial boundaries the outermost collagen gels were also loaded with 1 μm sized red polystyrene microspheres. Finally the innermost compartment was filled with DextranVS-PEG4SH solutions or PEGDA-PEG4SH solutions (carrying microparticles and 50 ng of chemokine) or controls and allowed to gel for 10 minutes. Bolus chemokine suspended in 0.3M TEA buffer was used as positive control. To prevent the diffusion of chemokines from top surface of the hydrogel, a sterile construct was positioned over the innermost circle thus preventing the media from covering the top of innermost hydrogel. The wells were filled with complete RPMI medium and incubated at 37°C, 5% CO2 for migration. To aid in cell tracing, cells were pre-stained by adding 2 μM calcein at the start of experiment. Calcein was again added after 18 hours to improve the fading signal. Cell migration was observed using fluorescence microscopy (IX-70, Olympus).
IL-10 gene knockdown in migrated, bone marrow-derived primary APCs
Hydrogel precursor solutions containing 50 ng MIP3α and 5 mg of pDNA-IL10 siRNA loaded microparticles were prepared in TEA buffer at pH 7.8. Thin hydrogels (100 ul) covering the base of a well in 24 well plates were formed to maximize cell-hydrogel contacts. Hydrogels were allowed to cure for 2 hrs and rinsed briefly with 1 ml sterile PBS (pH 7.4). Immature APCs (2×106 cells/well) were loaded into the upper migratory chamber of Transwell™ inserts (6.5 mm, Costar, Cambridge, MA) with a pore size of 5μm. Cells were allowed to migrate in response to the released MIP3a from the hydrogel in the 24 well plates as detailed earlier in the manuscript. After 6 hrs of migration, inserts were removed and cells were incubated with hydrogels for 5 days in complete RPMI-Glutamax® medium. pDNA loaded PEI-PLGA microparticles with scrambled siRNA were used as negative control. APCs transfected with pDNA-IL10 siRNA loaded microparticles were used for comparison. To examine gene silencing efficacy of the microparticles, Trizol reagent (Invitrogen, Carlsbad, CA) was added to each well and all samples were pestle homogenized and total RNA was extracted as reported earlier [11, 19]. Total RNA was then treated with TURBO DNA-free (Ambion, Tx) to remove genomic DNA and cDNA was synthesized using the SuperScript III First Strand Synthesis System for RT-PCR (Invitrogen). Real time RT-PCR amplification was performed using RT2 SYBR® Green/ ROX (SuperArray, MD) and an ABI 7200. Specific primers were used for IL-10 and house keeping gene β-Actin (SuperArray, MD). Relative gene knockdown levels were obtained using the ΔΔCT method [11] using mouse β-Actin as the reference gene. IL-10 gene expression in untreated cells (100%) was used for normalization.
APC infiltration studies
To investigate whether the APCs migrated to the surface of hydrogels were able to migrate inside and interact with microparticles, hydrogels containing 50 ng MIP3α and 5 mg of pDNA-Cy3GAPDH siRNA loaded microparticles were prepared and placed in a 24 well tissue culture plate. Transwell inserts with 5×105 cells/well were incubated with hydrogels as mentioned earlier. After 3 hours inserts were removed and migrated cells were allowed to interact with hydrogels for another 5 hours at 37°C, 5% CO2. Hydrogels were then fixed with 4 % paraformaldehyde, treated with 0.1% TritonX-100 and 3% bovine serum albumin and stained with Alexa Fluor® 488 phalloidin. Hydrogels were then imaged using a Leica SP2 AOBS confocal microscope and Z-sections were taken upto 340 μm depth in 1–5 μm z-steps, as far as clear image could be resolved. Volumetric three-dimensional (3D) reconstruction and projections were processed using Imaris software (Bitplane Inc., St.Paul, MN).
Statistical analysis
Student's t-test was used to evaluate significance between pairs of groups and a p<0.05 was considered to be significant. All in vitro experiments were repeated three times (with at least n=3 for each sample) to ensure reproducibility of results except APC migration through collagen gels, which was performed two times.
Results
Figure 1 represents a schematic of the overall rational of our combinatorial delivery system. The in situ crosslinked hydrogel carrying chemokines and pDNA-siRNA carrying microparticles would attract immature DCs to the injection site. These DCs would efficiently internalize the microparticles (either by migrating inside the porous gel or by taking up released particles), get activated and process the pDNA and siRNA molecules resulting in a controlled immune response. The following sections present results on synthesis and in-vitro characterization of this multi-component system.
Synthesis and characterization of DextranVS and DNA-siRNA loaded microparticles
DextranVS with degree of substitution two and five (DS2 and DS5) were successfully synthesized. DS, defined as the number of vinyl sulfone groups per 100 anhydroglucosidic rings of dextran, was confirmed using 1H NMR by comparing the peak areas corresponding to the dextran glucosidic protons (δ 3.4–4.1, 5.2, and 5.4) and the protons of the vinyl sulfone group (δ 6.5 and 6.9) as reported earlier [12] (data not shown). We have earlier reported synthesis and extensive characterization of pDNA-siRNA loaded PEI-PLGA microparticles [11]. These microparticles retain bioactivity of encapsulated siRNA and surface-loaded pDNA and induce efficient simultaneous gene silencing and transgene expression in primary bone marrow derived APCs [11]. The average IL10 siRNA encapsulation efficiency after PEI modification of microparticles used in the following studies was ~59 ± 6.0 %, and average pDNA loading was ~86 ± 4.7 %.
Hydrogel synthesis and gelling time measurements
DextranVS based hydrogels and PEGDA based hydrogels were formed in situ via Michael type addition with PEG4SH at pH 7.8 and 37 °C. As shown in Table 1, DextranVS-PEG4SH hydrogels (with encapsulated microparticles) at 10 % w/v PEG4SH and 10, 20 or 30 % w/v DextranVS (DS 2) gelled in 56 ± 4.5 sec, 43 ± 5 sec, and 13 ± 6 sec, respectively. Without microparticles, these gels gelled in 64 ± 6 Sec, 50 ± 5 sec, and 24 ± 3 sec, respectively. With increase in DS, the gelation time (with microparticles) reduced significantly. Specifically, the 10 % w/v DextranVS (DS 5) gel gelled in 18 ± 2 sec and the 20 % w/v ones gelled in 22 ± 1.5 sec. For simplicity, henceforth these DextranVS based hydrogels are referred to as 10X10Y, 20X10Y and 30X10Y where X represents w/v % DextranVS and Y represents w/v % PEG4SH, respectively. Hydrogels with 20% and 30% w/v DextranVS (DS5) did not gel completely as un-crosslinked polymer solution was observed in the gelation tube even after 2 hours of curing. On the other hand, 10 % w/v PEG4SH when combined with 10, 20 or 30 % w/v PEGDA (with microparticles) gelled in 553 ± 110 sec, 1411.0 ± 57.93 sec, and 1944.0 ± 442.31 sec, respectively. In case of PEGDA based hydrogels, X represents w/v % PEGDA and Y represents w/v % PEG4SH. However, considerable amount of un-reacted polymer solution was recovered with 20 % and 30 % PEGDA hydrogels after 2 hours of curing. In subsequent studies, hydrogels that gelled completely (no remaining fluid) were used. Further gelation time studies were performed with 20 % w/v PEG4SH and 10, 20 or 30 % w/v DextranVS (DS 2 or 5) or PEGDA. All characterization data are summarized in Table 1. It should be noted that no gelling was observed on mixing the hydrogel precursor solutions at room temperature for at least 60 min.
Table 1.
In vitro gelation time of various in situ crosslinakble hydrogels
| Formulation | DS | Composition | Gelling Time (sec) |
Uncrosslinked polymer Recovered | |
|---|---|---|---|---|---|
| (Without MPs) | (Without MPs) | ||||
| DextranVS-PEG4SH | 2 | 10X10Y | 64.0 ± 6.24 | 56.7 ± 4.50 | No |
| 2 | 20X10Y | 50.0 ± 5.00 | 43.0 ± 5.29 | No | |
| 2 | 30X10Y | 24.3 ± 3.05 | 12.7 ± 2.08 | No | |
| 2 | 10X20Y | 130.4 ± 24.02 | 143.0 ± 31.43 | Yes | |
| 2 | 20X20Y | 69.3 ± 8.62 | 63.7 ± 5.03 | No | |
| 2 | 30X20Y | 37.3 ± 6.65 | 37.7 ± 5.68 | No | |
| 5 | 10X10Y | 18.3 ± 2.08 | 18.3 ± 4.90 | No | |
| 5 | 20X10Y | 22.6 ± 1.52 | 16.3 ± 2.08 | No | |
| 5 | 30X10Y | 20.3 ± 1.52 | 19.0 ± 2.00 | No | |
| 5 | 10X20Y | 53.6 ± 11.84 | 38.7 ± 5.51 | Yes | |
| 5 | 20X20Y | 14.3 ± 5.13 | 11.3 ± 1.54 | No | |
| 5 | 30X20Y | 22.3 ± 12.34 | 14.3 ± 0.57 | No | |
| PEGDA-PEG4SH | - | 10X10Y | 608.0 ± 74.90 | 553.0 ± 110.87 | No |
| - | 20X10Y | 1505.7 ± 57.10 | 1411.0 ± 57.93 | Yes | |
| - | 30X10Y | 1810.0 ± 124.30 | 1944.0 ± 442.31 | Yes | |
| - | 10X20Y | 647.0 ± 145.50 | 800.67 ± 97.63 | Yes | |
| - | 20X20Y | 256.7 ± 72.41 | 209.7 ± 74.90 | No | |
| - | 30X20Y | 376.7 ± 48.0 | 529.0 ± 209.58 | No | |
Values represent Mean ± S.D of at least three independent samples
Hydrogel loading efficiency and SEM
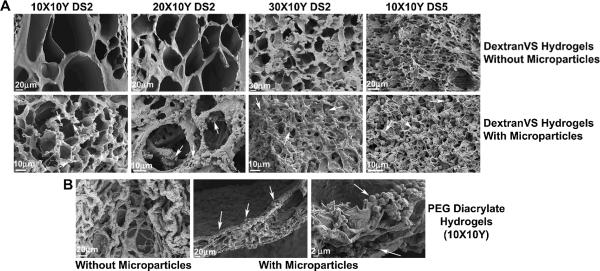
Encapsulation efficiency of MIP3α in DextranVS or PEGDA-based hydrogels was calculated by performing ELISA on any residual suspension obtained from the tubes used for hydrogel synthesis. Different initial chemokine payloads were used to examine the capacity of hydrogels to encapsulate chemokines without significant loss. As shown in Table 2, successful encapsulation of MIP3α was obtained with retention up to 95–99 % with 10, 20 or 30 % w/v DextranVS (DS 2) based gels, thus successfully loading chemokines from a low dose (10 ng/gel) to as high as 1000 ng/gel. Similar results were obtained with 10 % w/v DextranVS (DS 5) based hydrogels as well as 10 % w/v PEGDA based hydrogels. However, 20 % w/v PEGDA gels showed significantly low encapsulation efficacy of 34–41%. All data are summarized in Table 2. We also calculated the loading efficiency of microparticles in the hydrogels and were successfully able to load as low as 1 mg to 50 mg microparticles per 100 μl gel suspension. The opacity of hydrogel increased with increase in microparticles. Subsequent in vitro studies were performed with 5 mg microparticles per 100 μl gel suspension in order to keep the formulation injectable (low viscosity) through an insulin needle. SEM was performed to look at the interior morphology of hydrogels with and without microparticles. As shown in Figure 2A, DextranVS (DS2) based hydrogels were highly porous while DS5 showed somewhat lower pore size. The pore size also decreased with increasing DextranVS percentage. Microparticles were evenly distributed throughout these hydrogels (arrows). PEGDA based hydrogels also showed macroporous morphologies however unlike DextranVS based gels, microparticles in PEGDA gels were entrapped in layers and not thoroughly distributed (Figure 2B).
Table 2.
Encapsulation Efficiency of chemokines in in situ crosslinakble hydrogels
| Formulation | DS | Composition | Theoretical MIP3α loading (ng) | Encapsulation Efficiency % |
|---|---|---|---|---|
| DextranVS-PEG4SH | 2 | 10X10Y | 10 | 95.15 ± 2.15 |
| 100 | 98.85 ± 0.81 | |||
| 1000 | 98.40 ± 0.84 | |||
| 2 | 20X10Y | 10 | 97.57 ± 0.35 | |
| 100 | 99.55 ± 0.07 | |||
| 1000 | 99.79 ± 0.09 | |||
| 2 | 30X10Y | 10 | 97.20 ± 1.13 | |
| 100 | 98.96 ± 1.05 | |||
| 1000 | 99.81 ± 0.02 | |||
| 5 | 10X10Y | 10 | 96.40 ± 3.02 | |
| 100 | 98.98 ± 0.32 | |||
| 1000 | 99.76 ± 0.01 | |||
| PEGDA-PEG4SH | - | 10X10Y | 10 | 96.88 ± 0.84 |
| 100 | 97.87 ± 0.75 | |||
| 1000 | 99.74 ± 0.03 | |||
| - | 20X10Y | 10 | 34.84 ± 18.92 | |
| 100 | 34.39 ± 7.10 | |||
| 1000 | 41.69 ± 7.30 |
Values represent Mean ± S.D of at least three independent samples
Figure 2. Structural morphologies of hydrogels with or without microparticles.
(A) SEM images of DextranVS-PEG4SH hydrogels with different degree of substitutions (DS) and w/v percent of DextranVS and PEG4SH. Top panel represents hydrogel without embedded microparticles and bottom panel represents hydrogels with microparticles (white arrows). (B) SEM images of PEGDA-PEG4SH hydrogels with 10X10Y composition. Microparticles deposited as layers in the hydrogel matrix as indicated by solid arrows.
Swelling ratio and degradation time
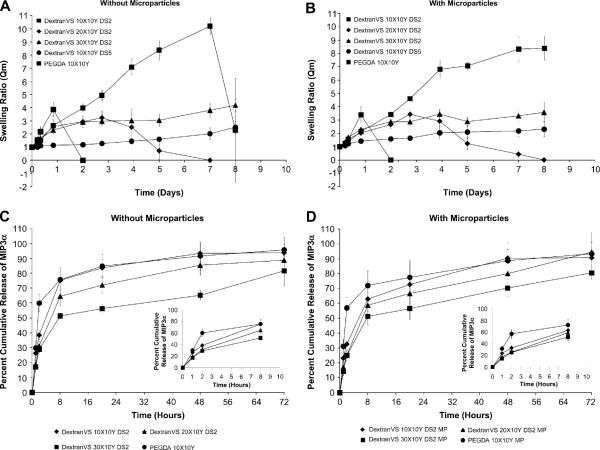
Swelling and degradation behavior of microparticle-encapsulated hydrogels were evaluated at 37 °C, in PBS (pH 7.4). As shown in Figure 3A and B a gradual increase in swelling ratio with time was observed with all hydrogels until the hydrogels started physically disintegrating. DextranVS DS2 10X10Y and 20X10Y and PEGDA 10X10Y hydrogels showed a faster rate of swelling as compared to DextranVS DS2 30X10Y and DS5 (10X10Y) hydrogels. Similar trends were observed between empty hydrogels and microparticle-loaded hydrogels in some formulations. DextranVS DS2 10X10Y hydrogels degraded in ~2 days after an initial, rapid, 3–4 fold increase in mass swelling ratio. DextranVS DS2 20X10Y hydrogels degraded in ~6–8 days. Swelling ratio of PEGDA 10X10Y hydrogels was significantly higher than other hydrogels beyond day 2 and gels without microparticles exhibited a steep rise until day 7 followed by physical disintegration (either complete degradation or intact gels cannot be recovered). PEGDA hydrogels with microparticles continued to swell even after day 8. Dextran gels with DS2 30X10Y and DS5 10X10Y compositions did not degrade until day 15.
Figure 3.
Swelling and degradation studies of various in situ crosslinking network formulations with (A) or without (B) co-encapsulated microparticles. In vitro release of chemokines from hydrogels. (C) Cumulative release of MIP3α protein from DextranVS-PEG4SH hydrogels and PEGDA-PEG4SH hydrogels (n=6). (D) Cumulative release of MIP3α protein from microparticle-encapsulated DextranVS-PEG4SH hydrogels and PEGDA-PEG4SH hydrogels (n=6).
In vitro release of chemokines from hydrogels
The cumulative release profiles of MIP3α from hydrogels (with and without microparticles) are shown in Figure 3C and D. As shown in Figure 3C, a rapid 70–75 % release of MIP3α was observed with PEGDA (10X10Y) based and DextranVS DS2 (10X10Y) based hydrogels without microparticles (see figure in inset for initial release profile). DextranVS DS2 (20X10Y) showed slower release than PEGDA (10X10Y) based and DextranVS DS2 (10X10Y) hydrogels however there was no significant differences between the percentage released. DextranVS DS2 (30X10Y) also showed a significantly slower release of chemokine with 50% of encapsulant released in initial 8 hours and ~ 60 % released in 48 hours (Figure 3C). Similar chemokine release profiles were obtained with hydrogels containing microparticles (Figure 3D) including an initial burst release, followed by 70–90 % encapsulated chemokine released in 72 hours.
Bioactivity of released chemokines and hydrogel cyto-compatibility
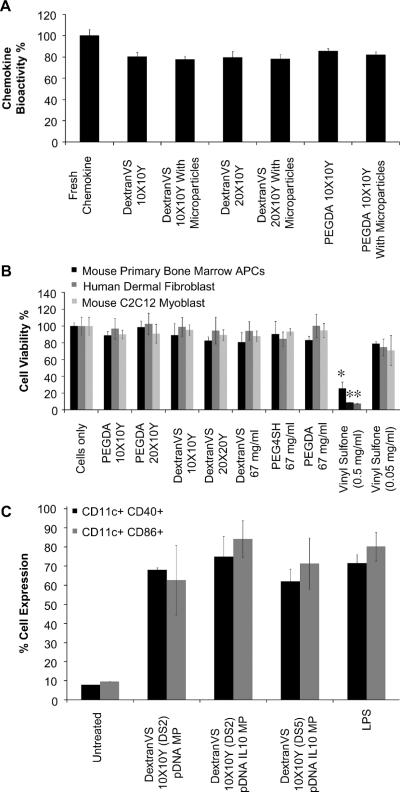
The bioactivity of released MIP3α was tested using a chemotaxis assay measuring the ability of the released chemokine to attract primary APCs. As shown in Figure 4A, all formulations retained 80–85 % bioactivity as compared to fresh (unprocessed) chemokine (deemed 100%). There was no significant difference in the bioactivity of MIP3α released from the various formulations. Further, cell viability studies showed that all hydrogel formulations were essentially non-toxic (Figure 4B) to murine bone marrow derived primary APCs, murine C2C12 myoblasts, and human dermal fibroblasts, even though the vinyl sulfone monomer at high doses is significantly cytotoxic (p<0.05).
Figure 4. In vitro chemokine bioactivity, hydrogel cytotoxicity, and cellular functionality.
(A) Chemotaxis of primary bone marrow derived APCs induced by hydrogel-encapsulated chemokine or un-processed (fresh) chemokine. Percent bioactivity calculated as chemotactic index (CI). (B) In vitro cell viability of murine primary bone marrow derived APCs, murine C2C12 myoblasts, human dermal fibroblasts incubated with various formulations, constituent polymers or monomers (n=3). *P < 0.05 compared to all other formulations. (C) In vitro cell functionality of murine primary bone marrow derived APCs post incubation with hydrogel formulations. Cells were pulsed with LPS and analyzed for expression levels of phenotypic surface markers; CD11c, CD40, and CD86 (n = 9).
Primary bone marrow APC functionality assay
Efficient maturation and antigen presentation by APCs is necessary for the generation of effective immune response in vivo. We have earlier shown that simultaneous delivery of IL-10 siRNA and immunogenic pDNA results in synergistic up regulation of phenotypic surface markers (CD40 and CD86) in exposed primary bone marrow derived APCs. We confirm in present studies that the ability of primary APCs to maturate in presence of immunogenic antigen is retained even after incubation with microparticles loaded hydrogels by further exposing the cells with LPS for maturation. As shown in Figure 4C, CD11c+ APCs exposed the DextranVS (DS2) 10X10Y hydrogels containing pDNA-IL-10 siRNA co-delivery system induced high expression of CD40 (74.91 ± 10.73 %) and CD86 (84.17 ± 9.71 %) which were equivalent to levels induced by LPS (CD40; 71.57 ± 4.44 %, and CD86; 80.26 ± 7.5 %). Similar CD11c+CD40+ and CD11c+CD86+ expression levels were observed in APCs exposed to DextranVS (DS5) 10X10Y hydrogels pDNA/IL-10 siRNA loaded microparticles and DextranVS (DS2) 10X10Y hydrogels with only pDNA loaded microparticles. There was no significant difference in expression levels between cells first exposed to formulation and then exposed to LPS as compared to cells only exposed to LPS.
Chemokine dose optimization for chemotactic migration
A Transwell™ migration assay was used to quantify the efficacy of MIP3α, to initiate chemotactic migration of the primary APCs. In this setup, a chemokine-loaded (or blank) gel was placed on the bottom of cell culture wells and primary APCs were loaded onto Transwel™ inserts. The data (Figure 5) is reported as a Chemotactic Index (CI), a ratio defined as the number of cells migrating in response to MIP 3α to the number of cells undergoing spontaneous migration. The number of cells migrating to the surface of a hydrogel was in the range of 1.1 × 105 to 1.6 × 105. It should be noted that APCs have 60–80 % CD11c+ dendritic cells and chemokine used in the study only attracts immature dendritic cells thus leaving cells other than immature DCs in the upper chamber. Average number of cells spontaneously migrating in absence of chemokine was 0.25 × 105.
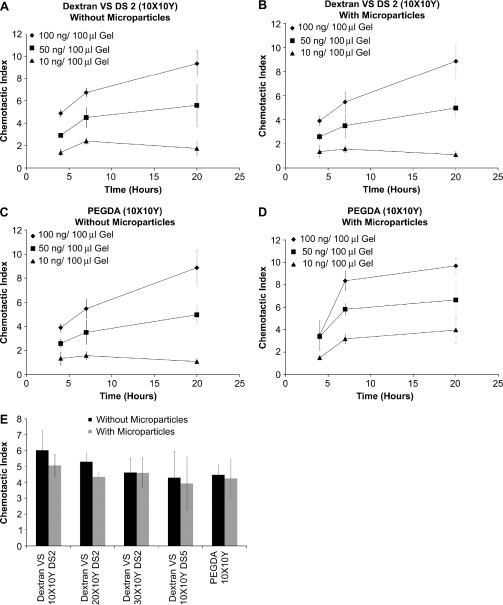
Figure 5. In vitro chemotaxis using Transwell™ migration.
(A) Chemotaxis of APCs in response to DextranVS DS2 (10X10Y) hydrogels loaded with different chemokine doses (n=3) (B) Chemotaxis of APCs in response to microparticle-embedded DextranVS DS2 (10X10Y) hydrogels loaded with different chemokine doses (n=3) (C) Chemotaxis of APCs in response to PEGDA (10X10Y) hydrogels loaded with different chemokine doses (n=3) (D) Chemotaxis of APCs in response to microparticle-embedded PEGDA (10X10Y) hydrogels loaded with different chemokine doses (n=3) (E) Chemotaxis of APCs in response to microparticles embedded various hydrogels loaded with 50 ng chemokine dose per 100 μl gel (n=3)
In the dose response versus time study, Dextran VS (DS2) 10X10Y and PEGDA 10X10Y gels (with and without encapsulated microparticles) were loaded with varying concentrations of MIP 3α at 100ng/100μl gel, 50ng/100μl gel, and 10ng/100ul gel, respectively. Both the Dextran VS (DS2) and PEGDA formulations demonstrated significant increase in CI with increasing chemokine payload concentration (Figures 5a–d). Additionally, the presence of microparticles did not significantly affect the chemotactic migratory response of the primary APCs in any of the gel-payload formulations. In Figures 5c and 5d, though, it was noted that no significant difference in CI was observed at the initial 4 hour time point between the chemokine concentrations of 50ng/100μl gel and 100ng/100μl gel. These dose-response studies were terminated at 20 hours because of the gels' high degree of swelling and subsequent likelihood for direct contact with the Transwell membrane which could compromise the integrity of the experimental setup. As shown in Figure 5e when the % w/v combinations of polymers is increased, while keeping the MIP 3α concentration (50ng/100μl gel) and the time interval constant, hydrogels without microparticles showed an average 4–6 fold enhancement of chemokine guided APC migration whereas hydrogels with microparticles showed a 4–5 fold enhancement; however, this differences in CI, between various gels (with and without microparticles) were not statistically significant. In this specific experiment, bolus chemokine doses were not investigated because the amount of chemokine “available” for migration would be more compared to chemokine getting released from hydrogels.
In vitro APC migration through a collagen tissue phantom
To study the migration of DCs in response to the gradient of chemokine released from hydrogels, a collagen based migration set up was created, as shown in Figure 6A. A light metallic construct was kept over the innermost hydrogel to minimize flow of media through hydrogel top surface and primarily favor lateral diffusion. At various time points, stained migrating cells were imaged using a fluorescence microscope. For visualizing a defined boundary at the start of migration experiment, red colored polystyrene microparticles were co-encapsulated with primary APCs in the outermost collagen layer. As indicated in Figure 6B, control wells with hydrogels containing no MIP3α showed no observable migration of cells towards the central chemokine-containing hydrogel source (towards right of white broken boundary line) even after 18 hours of incubation. With bolus dose (i.e. MIP3α in TEA buffer) limited migration towards the chemokine source was observed. Specifically, significant movement of cells was observed in the initial 4 hours. However, beyond 4 hrs (up to 18 hrs), cells failed to move probably due to rapid diffusion of bolus chemokine and a loss of gradient. On the other hand, sustained migration was observed with MIP3 loaded hydrogels even after initial 4 hours and continued until 18 hours (end of experiment). Interestingly, after 18 hours, unlike all other samples, these cells had migrated the farthest distance towards the chemokine source. The initial gel boundary could not be captured in the field of view under this magnification. Therefore, we imaged continuous frames of the whole migration set up and as shown in Figure 6C, the cells migrated to the centre of the chemokine loaded hydrogel. Similar frames taken with bolus and no MIP3α samples failed to show such migration (data not shown). These findings strongly suggest that primary APCs can migrate inside the porous hydrogels (even before complete degradation) and thus could directly interact and phagocytose the pDNA-siRNA loaded microparticles.
Figure 6. Primary APC migration studies through 3D collagen tissue phantom model.
(A) Schematic, illustrating the migration setup. Each well of a six well plate is sectioned into three concentric zones using PDMS molds. Middle zone is filled with collagen solution and allowed to gel for 30 minutes. Outermost zone is then filled with primary APCs and red polystyrene microparticle containing collagen solution and allowed to gel for 30 minutes. Innermost zone is filled with hydrogel of interest (or bolus chemokine) and allowed to gel for 30 min. Metallic construct is placed on top of innermost zone and culture media is added to outer zones. (B) Primary APCs migrating in response to chemokine released from control hydrogels (top panel), bolus dose (middle panel), MIP3α loaded hydrogels (bottom panel) at different time points (C) Primary APCs migrating to the center of hydrogels in response to released chemokine. Top panel represents phase contrast image of sequential frames attached together to form single image. Bottom panel represent fluorescence image of sequential frames attached together to form single image. Solid black arrows represent location of migrated APCs in the inner gel. Scale bar = 230 microns. Red: Polystyrene particles. Green: Calcein stained primary APCs. White broken lines: Initial cell-collagen boundary at t=0
Primary APCs infiltration inside hydrogels
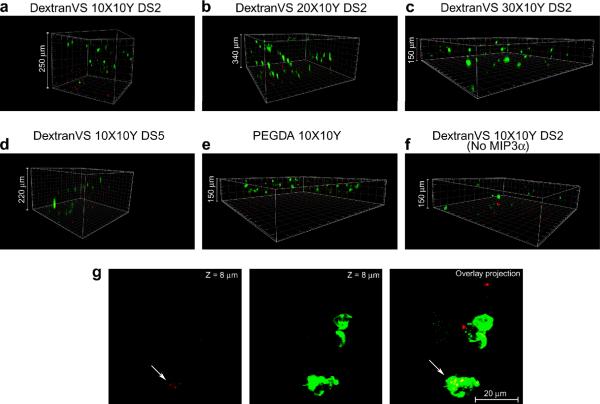
We anticipated that the high porosity of hydrogels may allow migrated cells to infiltrate inside. In order to test this hypothesis we performed migration experiment and allowed the migrated cell to interact with hydrogels for ~9 hours. Confocal images reveal that the attracted bone marrow derived APCs that migrated to the hydrogel infiltrated within the gels. Interestingly, the depth to which they migrated was dependent on the gel pore size. As shown in Figures 7a, b, with highly porous DextranVS 10X10Y and 20X10Y (both DS 2), more number of cells were able to infiltrate deeper (up to 340 μm) as compared to hydrogels with comparatively smaller pores (Figure 7c,d). More importantly, more cells infiltrated in dextran-based gels (achieved greater depth within same time) as compared to PEGDA based gels (Figure 7e). We however did see small number of cells migrating inside dextran hydrogels even in the absence of chemokine (spontaneous migration). It should be mentioned that in all the groups examined (except hydrogels with no chemokine) we observed a high number of cells thoroughly distributed throughout the hydrogel surface suggesting strong chemokine-guided migration. However, in case of control gels with no chemokine we did not observe cells on the surface other that at few small areas including the one shown in Figure 7f. Interestingly, migrated cells were able to phagocytose encapsulated microparticles inside the hydrogels (Figure 7g).
Figure 7. Primary APC infiltration through 3D hydrogels.
(a) Migration through DextranVS DS2 10X10Y hydrogels (APCs stained with calcien (green) and microparticles loaded with pDNA-Cy3 GPADH siRNA (Red), (b) migration through DextranVS DS2 20X10Y hydrogels, (c) migration through DextranVS DS2 30X10Y hydrogels, (d) migration through DextranVS DS5 10X10Y hydrogels, (e) migration through PEGDA 10X10Y hydrogels, (f) migration through DextranVS DS2 10X10Y hydrogels without chemokine, (g) Primary APC migrate inside hydrogels and phagocytose microparticles (arrows in overlay projection)
IL-10 gene knockdown in bone marrow-derived primary APCs
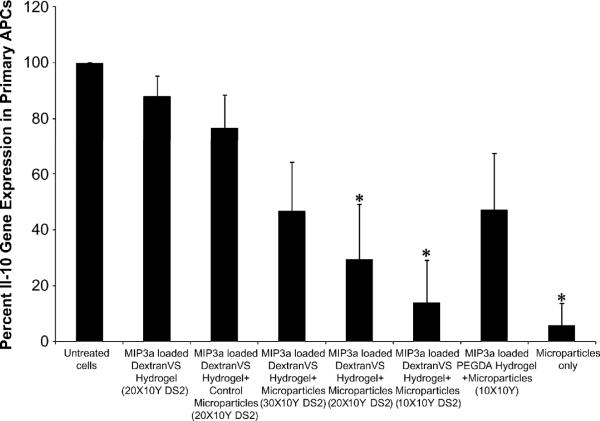
Efficacy of hydrogel-encapsulated siRNA-pDNA loaded microparticles to carry out IL-10 gene knockdown in primary APCs was analyzed using real time RT-PCR. As shown in Figure 8, primary APCs migrating into DextranVS DS2 10X10Y hydrogels showed almost 80% (13.9 ± 15.2 % residual expression) IL-10 gene silencing, as compared to untreated cells. Similarly, APCs transfected with microparticles embedded in DextranVS DS2 20X10Y resulted in lowering the IL-10 gene expression levels to 29.5 ± 19.8 %. The silencing effect was sequence specific since a scrambled siRNA sequence (Silencer® Negative control #1 siRNA, Ambion, TX) failed to induce significant IL-10 gene silencing (control microparticles group). In addition, although there was some decrease in average gene expression levels with DextranVS DS2 30X10Y and PEGDA 10X10Y, the difference was not statistically significant when compared to the scrambled siRNA control treated samples. These finding suggest that siRNA encapsulated microparticles embedded in DextranVS DS2 10X10Y and 20X10Y are able to induce significant level of gene silencing similar to those observed with siRNA microparticles without hydrogels.
Figure 8. In vitro gene silencing efficacy of microparticles encapsulated in hydrogels.
IL-10 gene silencing in bone marrow-derived primary APCs after 5 days post transfection with hydrogel embedded pgWizLuciferase IL-10 siRNA–PEI–PLGA microparticles (quantified using real time RT-PCR). All groups contained hydrogel based formulation except untreated cells and microparticles only formulations. Control microparticles refer to microparticles carrying scrambled siRNA sequence. *P < 0.05 compared to negative controls.
Discussion
Naked DNA vaccines and DNA antigen loaded microparticles, administered intramuscularly, often fail to induce a strong Th1 response. This could be attributed to low number of APCs available at the site of injection and high production of IL-10, a Th1 suppressor, by the antigen-loaded DCs. We recently demonstrated that combinatorial delivery of IL-10 targeted siRNA along with pDNA-based antigens using a dual-mode siRNA-DNA loaded microparticle formulation can significantly enhance APC activation and T cell proliferation in vitro while successfully “switching” the in vivo immune response towards a strong Th1 and CTL type response [11]. However, it is evident that the strength of immune response following immunotherapy also depends on efficient migration of APCs to the site of immunization [1]. Here we present an integrated delivery platform comprising of an in situ crosslinkable, biodegradable, hydrogel network as an injectable delivery system for both DC chemo-attractants (MIP3α) and siRNA-DNA loaded PLGA microparticles that enhances recruitment and migration of DCs, allows efficient IL-10 silencing in the migrated DCs and can therefore promote stronger divergence towards a Th1 response.
Hydrogels carrying microparticles has been recently reported for controlled release application in tissue engineering [20–24]. However, limited effort has been made in using in-situ crosslinking hydrogels for immunotherapeutic purposes, Roy et al. previously reported an in situ injectable, biodegradable hydrogels for nucleic acid delivery that remains liquid outside the body and undergoes gelation once injected [25]. These hydrogels gelled in vitro in ~18 minutes and the encapsulated DNA retained its bioactivity. The hydrogel however degraded completely in ~ 31days. Recently, Hori et al. also reported an alginate-based system for delivery of CpG oligonucleotides [26, 27]. We report here for the first time, a multimodal delivery system that can deliver chemokine, DNA and siRNA together in a single injection and create a depot-type DC priming center at the site of immunization. The design criteria require that the hydrogel network is injectable, rapidly gels at 37°C, is porous (allowing APC migration) and degrades within a few days (2–7 days). These are to ensure that (a) the chemo-attractants are released over a 1–5 day period thereby attracting large number of immature DCs to the site of injection, and (b) these DCs can effectively ingest the pDNA/siRNA carrying microparticles either inside the hydrogels or as they are released during gel degradation.
Crosslinked polymer networks of DextranVS and PEG4SH with chemokine and DNA-siRNA co-loaded microparticles were formed within two minutes of mixing at pH 7.8 and 37 °C. It should be noted that hydrogel precursor solutions when mixed and kept at room temperature did not form hydrogel even in 60 minutes (data not shown). Separate preliminary studies evaluating the ability to inject formulations at room temperature in mouse quad muscles (data not shown) have also been attempted and hydrogels did not gel during injection process. Hydrogels attempted at pH 8.2 however gelled instantaneously at room temperature upon vortexing and took approximately five minutes to gel at pH 7.4 (data not shown). Similarly, PEGDA crosslinked networks with PEG4SH with chemokine and microparticles were formed however the gelling process was relatively slower and resulted in settling down of microparticles as observed in SEM. We anticipate that fast gelling dextran hydrogels would be more advantageous since there will be less chances of losing (immediate diffusion away from injection site) chemokine or delivered biomolecules. Both microparticles and chemokines were successfully encapsulated (~ 95–99%) except for formulations that did not gel completely (PEGDA-PEG4SH 20X10Y). Although we were successfully able to entrap up to 50 mg microparticles per 100 μl volume hydrogel, it was practically difficult to load and eject out hydrogel suspension with 50 mg microparticles through an insulin needle (27½ G), typically used for intramuscular injection in mice. We have previously used up to 5 mg microparticle dose for DNA (with or without siRNA) immunizations per flank, in vivo in mice, and thus for practical purpose we used 5 mg microparticle per 100 μl hydrogel in all our subsequent studies.
The degree of VS substitution was altered to tune the network degradation time as described by Hiemstra et al. [12, 13]. Degree of substitution appear to play an important role in swelling and degradation of hydrogels and as demonstrated here, microparticles containing hydrogels degraded in two to seven days at DS2 while degradation took more than 30 days at DS5 (at same polymer % w/v). The swelling ratio initially increased with time probably because of more water penetration with reduction in internal crosslink density [18], and finally, the hydrogel either dissolved rapidly or fell apart into small pieces after hydrolytic cleavage of the thioether-ester bonds [18], [12, 13]. Further, choice of polymer affected the swelling ratio (Figure 3). Swelling ratios for dextran based gels were significantly less as compared to PEGDA based hydrogels used in the present study. The high swelling ratio of PEGDA based gels could be attributed to possible formation of non-ideal networks as discussed by Metters and Hubbell [18]. Earlier studies have shown that Michael-addition based hydrogels with acrylates as reacting moieties swell as well as degrade rapidly as compared to gels formed using conventional photo polymerization techniques [28]. We hypothesize that an overall low swelling gel that also degrades faster would offer a better choice over gels that degrade slowly or swell to a great extent. PEGDA-PEG4SH gels swelled nine folds from the initial gel mass while DextranVS-PEG4SH combination at DS2 and 10X10Y composition swelled only three fold and degraded in two days. Similarly, dextran gels with DS2 and 20X10Y composition swelled less than two fold and degraded in 6–7 days.
The encapsulation of chemokines and DNA-siRNA loaded microparticles within the hydrogels did not adversely affect their bioactivity. Encapsulated chemokines retained more than 85 % bioactivity as compared to fresh (non-encapsulated) chemokine as demonstrated by their capacity to induce chemotaxis-guided migration of bone marrow derived APCs. Gene silencing efficacy of the IL10 siRNA was also not altered and we were successfully able to knockdown up to 80 % IL10 gene expression level in primary bone marrow derived APCs. This gene silencing efficacy would be further dependant on the ability of the hydrogel to degrade fast or allow uptake of microparticles based on infiltration inside the macroporous structure. We are now investigating the DNA transfection efficacy of these hydrogel embedded microparticles. However Roy et al. have earlier shown that naked DNA delivered using in situ crosslinkable systems do not alter the transfection efficacy of the DNA [25]. Hori et al. recently demonstrated that CpG attached on surface of alginate microspheres and delivered in an alginate suspension retained its bioactivity after in situ gelation [26].
We earlier hypothesized that controlled release of chemokine using such hydrogel based delivery system can attract more DCs at the site of injection. In fact, our in vitro chemotaxis assay (Figure 5) shows five to six fold more number of cells migrated towards the chemokine source in initial eight hours as compared to migration in absence of a chemokine source. We further evaluated movement of DCs in a tissue phantom model (to mimic migration in muscle tissues) and observed considerably higher number of cells moving towards the chemokine source and migrating to a longer distance as compared to bolus chemokine or no chemokine systems. We did not observe considerable movement of cells with bolus samples (no hydrogel) after the initial 4 hours and cells migrated relatively smaller distance than cells migrating in response to hydrogels with chemokine, probably because of rapid diffusion of chemokine in bolus dose. In fact, with hydrogel-chemokine samples, cells migrated to the center of hydrogels after migrating through the collagen field. No such movement was observed without chemokine or bolus chemokine samples (data not shown). Finally, it was interesting to observe that cells migrating to the surface of hydrogel (in a Transwell migration set up) infiltrated hydrogels and were able to migrate up to 340 μm depth with DextranVS DS (20X10Y) hydrogels. The ability to infiltrate appears to depend on the pore size of the hydrogels and cells migrated to greater depth in dextran based gels as compared to PEGDA based gels. We however did see a few cells on surface and migrating inside the control hydrogel without chemokine (DextranVS DS2, 10X10Y). In addition, the migrated cells exhibited phagocytic characteristics (Figure 6) indicating that attracted immature DCs can ingest antigen and siRNA carrying microparticles both inside the porous hydrogels as well as by taking up particles released from the degrading gels. This should allow for enhanced activation and antigen processing by the APCs. We hypothesize that this process might improve the immune response however any concrete comment can only be made after performing elaborate in vivo studies. Based on these observations the optimal material intended for in vivo use would be DextranVS based hydrogels and not PEGDA based hydrogels. PEGDA based hydrogels containing microparticles swell to a greater extent and degrade extremely slowly as compared to DextranVS based hydrogels (10X10Y DS2 and 20X10Y DS2). Further, our observations as reported in paper show ability of cells to migrate inside hydrogels and thus it would be of interest to evaluate and compare in vivo immune response from gels that allow cell infiltration and degrade faster (like DextranVS 10X10Y DS2 and DextranVS 20X10Y DS2) as compared to gels that allow cell infiltration but degrade slower (like DextranVS 30X10Y DS2 and DextranVS 10X10Y DS 5). Since DextranVS (10X10Y) DS 5 hydrogels gel faster (but degrade significantly slower) than DextranVS (10X10Y) DS 2 hydrogels , in future, we plan to evaluate both slow gelling and fast gelling hydrogels for in vivo immune response.
Conclusion
We have developed an injectable, in-situ crosslinking polymer formulation that allows simultaneous delivery of chemokines, siRNA and pDNA. DextranVS-PEG4SH 10X10Y and 20X10Y hydrogels demonstrated more controllable gelation kinetics, pore size and microparticle distribution compared to PEGDA-PEG4SH gels and other highly crosslinked DextranVS gels. Primary APCs migrated efficiently in response to gel-released chemokines, migrated inside the porous gels and were able to internalize pDNA-siRNA carrying microparticles leading to efficient gene silencing. These formulations could be used in vivo to create an in situ DC priming center during immunotherapy by attracting immature APCs at the site of injection and allowing efficient delivery of immune-modulating siRNA and pDNA antigens.
Acknowledgement
The authors would like to acknowledge financial support from the Wallace H. Coulter Foundation (Roy) and the National Institutes of Health, R21 AI064179 (Roy). The authors would also like to acknowledge Prof. Christine E. Schmidt and The Institute for Cell and Molecular Biology at the University of Texas at Austin for access to equipments as well as Dr. Nitin Puri (Ambion, TX) for providing siRNAs.
References
- 1.Zhao X, Jain S, Benjamin Larman H, Gonzalez S, Irvine DJ. Directed cell migration via chemoattractants released from degradable microspheres. Biomaterials. 2005;26(24):5048–5063. doi: 10.1016/j.biomaterials.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278(5342):1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 3.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146(10):3444–3451. [PubMed] [Google Scholar]
- 4.Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90(9):3245–3287. [PubMed] [Google Scholar]
- 5.Song R, Liu S, Leong KW. Effects of MIP-1 alpha, MIP-3 alpha, and MIP-3 beta on the induction of HIV Gag-specific immune response with DNA vaccines. Mol Ther. 2007;15(5):1007–1015. doi: 10.1038/mt.sj.6300129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biragyn A, Ruffini PA, Coscia M, Harvey LK, Neelapu SS, Baskar S, et al. Chemokine receptor-mediated delivery directs self-tumor antigen efficiently into the class II processing pathway in vitro and induces protective immunity in vivo. Blood. 2004;104(7):1961–1969. doi: 10.1182/blood-2004-02-0637. [DOI] [PubMed] [Google Scholar]
- 7.Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298(5595):1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 8.Haddad D, Ramprakash J, Sedegah M, Charoenvit Y, Baumgartner R, Kumar S, et al. Plasmid vaccine expressing granulocyte-macrophage colony-stimulating factor attracts infiltrates including immature dendritic cells into injected muscles. J Immunol. 2000;165(7):3772–3781. doi: 10.4049/jimmunol.165.7.3772. [DOI] [PubMed] [Google Scholar]
- 9.Sedegah M, Weiss W, Sacci JB, Jr., Charoenvit Y, Hedstrom R, Gowda K, et al. Improving protective immunity induced by DNA-based immunization: priming with antigen and GM-CSF-encoding plasmid DNA and boosting with antigen-expressing recombinant poxvirus. J Immunol. 2000;164(11):5905–5912. doi: 10.4049/jimmunol.164.11.5905. [DOI] [PubMed] [Google Scholar]
- 10.Kumamoto T, Huang EK, Paek HJ, Morita A, Matsue H, Valentini RF, et al. Induction of tumor-specific protective immunity by in situ Langerhans cell vaccine. Nat Biotechnol. 2002;20(1):64–69. doi: 10.1038/nbt0102-64. [DOI] [PubMed] [Google Scholar]
- 11.Singh A, Nie H, Ghosn B, Qin H, Kwak LW, Roy K. Efficient modulation of T-cell response by dual-mode, single-carrier delivery of cytokine-targeted siRNA and DNA vaccine to antigen-presenting cells. Mol Ther. 2008;16(12):2011–2021. doi: 10.1038/mt.2008.206. [DOI] [PubMed] [Google Scholar]
- 12.Hiemstra C, Aa LJ, Zhong Z, Dijkstra PJ, Feijen J. Rapidly in situ-forming degradable hydrogels from dextran thiols through Michael addition. Biomacromolecules. 2007;8(5):1548–1556. doi: 10.1021/bm061191m. [DOI] [PubMed] [Google Scholar]
- 13.Hiemstra C, Zhong Z, van Steenbergen MJ, Hennink WE, Feijen J. Release of model proteins and basic fibroblast growth factor from in situ forming degradable dextran hydrogels. J Control Release. 2007;122(1):71–78. doi: 10.1016/j.jconrel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176(6):1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teng J, Zubarev ER. Synthesis and self-assembly of a heteroarm star amphiphile with 12 alternating arms and a well-defined core. J Am Chem Soc. 2003;125(39):11840–11841. doi: 10.1021/ja036705t. [DOI] [PubMed] [Google Scholar]
- 16.Kasturi SP, Sachaphibulkij K, Roy K. Covalent conjugation of polyethyleneimine on biodegradable microparticles for delivery of plasmid DNA vaccines. Biomaterials. 2005;26(32):6375–6385. doi: 10.1016/j.biomaterials.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 17.Pai Kasturi S, Qin H, Thomson KS, El-Bereir S, Cha SC, Neelapu S, et al. Prophylactic anti-tumor effects in a B cell lymphoma model with DNA vaccines delivered on polyethylenimine (PEI) functionalized PLGA microparticles. J Control Release. 2006;113(3):261–270. doi: 10.1016/j.jconrel.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Metters A, Hubbell J. Network formation and degradation behavior of hydrogels formed by Michael-type addition reactions. Biomacromolecules. 2005;6(1):290–301. doi: 10.1021/bm049607o. [DOI] [PubMed] [Google Scholar]
- 19.Underhill GH, Chen AA, Albrecht DR, Bhatia SN. Assessment of hepatocellular function within PEG hydrogels. Biomaterials. 2007;28(2):256–270. doi: 10.1016/j.biomaterials.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 20.Ferreira LS, Gerecht S, Fuller J, Shieh HF, Vunjak-Novakovic G, Langer R. Bioactive hydrogel scaffolds for controllable vascular differentiation of human embryonic stem cells. Biomaterials. 2007;28(17):2706–2717. doi: 10.1016/j.biomaterials.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holland TA, Tabata Y, Mikos AG. In vitro release of transforming growth factor-beta 1 from gelatin microparticles encapsulated in biodegradable, injectable oligo(poly(ethylene glycol) fumarate) hydrogels. J Control Release. 2003;91(3):299–313. doi: 10.1016/s0168-3659(03)00258-x. [DOI] [PubMed] [Google Scholar]
- 22.Holland TA, Tabata Y, Mikos AG. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J Control Release. 2005;101(1–3):111–125. doi: 10.1016/j.jconrel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Jin R, Moreira Teixeira LS, Dijkstra PJ, Karperien M, van Blitterswijk CA, Zhong ZY, et al. Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials. 2009;30(13):2544–2551. doi: 10.1016/j.biomaterials.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 24.Tan H, Chu CR, Payne KA, Marra KG. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials. 2009;30(13):2499–2506. doi: 10.1016/j.biomaterials.2008.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy K, Wang D, Hedley ML, Barman SP. Gene delivery with in-situ crosslinking polymer networks generates long-term systemic protein expression. Mol Ther. 2003;7(3):401–408. doi: 10.1016/s1525-0016(03)00008-x. [DOI] [PubMed] [Google Scholar]
- 26.Hori Y, Winans AM, Irvine DJ. Modular injectable matrices based on alginate solution/microsphere mixtures that gel in situ and co-deliver immunomodulatory factors. Acta Biomater. 2009;5(4):969–982. doi: 10.1016/j.actbio.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hori Y, Winans AM, Huang CC, Horrigan EM, Irvine DJ. Injectable dendritic cell-carrying alginate gels for immunization and immunotherapy. Biomaterials. 2008;29(27):3671–3682. doi: 10.1016/j.biomaterials.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 28.van de Wetering P, Metters AT, Schoenmakers RG, Hubbell JA. Poly(ethylene glycol) hydrogels formed by conjugate addition with controllable swelling, degradation, and release of pharmaceutically active proteins. J Control Release. 2005;102(3):619–627. doi: 10.1016/j.jconrel.2004.10.029. [DOI] [PubMed] [Google Scholar]