Abstract
Background
Left ventricular assist devices (LVADs) provide a bridge to recovery or heart transplantation, but require serial assessment. Echocardiographic approaches may be limited by device artifact and acoustic window. Cardiovascular computed tomography (CCT) provides noninvasive imaging of LVADs, yet no study has evaluated CCT’s impact on clinical care. We evaluated the diagnostic findings and clinical impact of CCT for noninvasive assessment of patients with LVADs.
Methods
CCT examinations performed between 2005 and 2008 in patients with LVADs were identified. Acquisitions were completed on the identical 64 detector-row scanner with intravenous contrast administration; electrocardiographic gating was used in patients with pulsatile devices, while peripheral pulse gating was used in patients with continuous-flow devices. Comparison was made between CCT results and 30-day outcomes, including echocardiographic and intraoperative findings.
Results
Thirty-two CCT examinations from 28 patients were reviewed. Indications included evaluation of low cardiac output symptoms, assessment of cannula position, low flow reading on the LVAD, and surgical planning. CCT identified critical findings in 6 patients including thrombosis and inlet cannula malposition, all confirmed intraoperatively; one case of intra-LVAD thrombus was missed by CCT. Using intraoperative findings as the gold standard, CCT’s sensitivity was 85% and specificity was 100%. Echocardiographic LVAD evaluation did not correlate with findings on CCT (kappa = −0.29, 95% CI −0.73−0.13).
Conclusions
This preliminary observational cohort study indicates that noninvasive imaging using CCT of LVADs is feasible and accurate. CCT warrants consideration in the initial evaluation of symptomatic patients with LVADs.
The number of patients awaiting heart transplantation far exceeds the number of donor hearts available. Left ventricular assists devices (LVADs), first employed in the 1980s, were intended to serve as a bridge to transplantation for patients with end-stage cardiac disease waiting for donor hearts (1). As the technology improved, a broader patient pool has benefited from their use. The Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure trial demonstrated that the use of LVADs resulted in meaningful survival benefit and improvement in quality of life for those patients with class IV heart failure on optimal medical management (2). With further technical advances, use of mechanical circulatory support as destination therapy continues to grow (3).
With increasing use has come greater scrutiny of the overall performance of these devices, and numerous short and long-term complications have been identified. Thromboembolism, infection, and cannula malposition are potentially life-threatening complications (4). Evaluation for these adverse sequelae when clinically suspected has historically relied on echocardiography(2, 5), though computed tomography has also been employed (6). Cardiac computed tomography (CCT) with electrocardiographic gating offers noninvasive, high-resolution imaging of cardiovascular structures, but has not been systematically evaluated for LVAD assessment. CCT has several potential advantages over echocardiography for LVAD imaging, as it is not limited by acoustic window and may better visualize LVAD cannulae without acoustic shadowing. We evaluated CCT’s impact on outcomes in patients with LVADs and compare its utility to echocardiography.
Methods
Study Population
Patients who underwent CCT for LVAD evaluation were identified from a large cardiovascular procedures database. Pertinent clinical history, CCT images, and subsequent management and clinical outcomes were reviewed. In patients undergoing echocardiography for similar indication as CCT, echo images were reviewed; in patients undergoing surgery, intra-operative findings were recorded. Institutional Review Board approval was obtained for this investigation.
Imaging Protocol
All CCT examinations were performed on the identical multidetector scanner (Somatom Sensation64, Siemens Medical Solutions, Inc., Forchheim, Germany). After scout images were obtained, a single axial slice in the ascending aorta distal to the anastamosis of the output cannula was selected. A region of interest in the ascending aorta was selected, and 15–20 mL of iodinated contrast (Visipaque, GE Healthcare, Waukesha, WI, USA) was injected during a test bolus scan to determine optimal timing for study. The helical scan starting above the aortic anastomosis and extending to the diaphragm was then performed with retrospective electrocardiographic gating and peripheral intravenous injection of 80 mL contrast at 4–5 mL/s followed by a 20–30 mL saline flush. In patients with continuous-flow devices, peripheral pulse gating was used instead of the ECG signal. Typical scan parameters included a tube voltage setting of 120 mV and minimal tube current based on scan volume; We used techniques to reduce the effective radiation dose such as electrocardiography-based dose modulation and reduction of tube current and tube voltage based upon body mass index. Patients were instructed to breathhold during helical scan acquisition, which ranged from 10–15 sec in duration.
All CCT studies were interpreted by an experienced cardiologist (7). Normal inflow cannula position was defined by an LV apical location, with the cannula tip not directly abutting the LV wall i.e. without obstruction to inflow. Normal outflow graft position was defined as insertion into the ascending aorta prior to innominate artery origin, with no kinks along its course. CCT images were evaluated in multiple planes including traditional axial, sagittal, and coronal views. Multiplanar reformatted views were constructed in views that best demonstrated the inflow and outflow cannulae along their course in each patient.
A critical finding was defined as presence of thrombus, vegetation or malposition causing cannula obstruction. Echocardiograms were interpreted by cardiologists blinded to the CCT results. Only echocardiograms performed within 48 hours of CCT, and prior to any surgical intervention, were included for comparison.
Statistical Analysis
Sensitivity and specificity of CCT was computed using intraoperative diagnosis as the gold standard where available. The kappa statistic was used to measure agreement between CCT and echocardiography.
RESULTS
Twenty-eight patients with 32 CCT scans were identified (Table 1). These studies were performed between May 2005 and December 2008. Four patients underwent 2 CCT examinations, each during separate hospitalizations. Patient age ranged from 24 to 71 years, averaging 45 years. Twenty-two of the examinations were done in men, 10 in women. Nine of the 28 patients (32%) had undergone LVAD implantation as a bridge to transplantation, the remainder as destination therapy. The average time between implantation and CCT examination was 167 days (range: 111 – 865 days). The most common indications for CCT were evaluation of low cardiac output symptoms, assessment of cannula position, low flow alarms and surgical planning. In 14 instances, echocardiography was available for comparison—all were transthoracic procedures save one transesophageal study. CCT was the sole modality used for LVAD evaluation in 19 patients.
Table 1.
Patient Characteristics and Comparison of CCT, Echo, and Intraoperative Findings (N=32)
| ID | INDICATION FOR CCT |
SEX | AGE | CCT FINDING | ECHO/CCT CORRELATION | CLINICAL OUTCOME |
|---|---|---|---|---|---|---|
| 1a | Low flow | Male | 56 | Thrombus in outflow cannula | TEE failed to identify thrombus | Thrombus confirmed in OR; device and cannulae replaced |
| 5 | Low flow | Male | 28 | Normal LVAD CCT | Echo not obtained | Insulation coming off drive-line connector. Device replaced |
| 4a | Low flow | Female | 31 | Normal LVAD CCT | Echo not obtained | Alive and well at 30 days |
| 15 | Low Flow | Male | 39 | Normal LVAD CCT | Echo not obtained | Alive and well at 30 days |
| 19b | Low Flow | Male | 37 | Inflow cannula abuts apex | Echo not obtained | CCT findings confirmed in OR. Device replaced with improved flows |
| 27 | Low flow | Male | 48 | Normal LVAD CCT | Normal LVAD position/flow | Treated for poor nutritional status. Alive and well at 30 days. |
| 17b | Suspected infection | Female | 36 | Normal LVAD CCT | LVAD noted, no vegetations | Patient given broad spectrum anti-biotics. Alive and well at 30 days |
| 2 | Infection | Male | 55 | Native LV not fully decompressed | Echo shows normal VAD position. No vegetations | Treated for clinical infection. Died unexpectedly |
| 21 | Suspected infection | Male | 47 | Normal LVAD CCT | Echo not obtained | Alive and well at 30 days |
| 6 | Assess Placement | Male | 42 | Normal LVAD CCT | Echo not obtained | Alive and well at 30 days |
| 12a | Assess Placement | Female | 24 | Inflow cannula angled anteriorly toward interventricular septum | Echo failed to identify abnormal LVAD position | Taken to OR; inlet cannula found to be abutting apex. Repositioned |
| 14 | Assess Placement | Male | 41 | Questionable filling defect of outflow cannula | Echo not obtained | Treated for anemia. Alive and well at 30 days |
| 18 | Assess Placement | Male | 43 | Inflow cannula angulated toward septum | Echo without evidence of device mis-alignment |
Taken to OR with multiple attempts at repositioning without success. Continued low flows. Subsequent heart transplantation |
| 20 | Assess placement | Male | 28 | Normal LVAD CCT | Echo not obtained | Alive and well at 30 days |
| 22 | Assess Placement | Male | 45 | Inflow cannula abuts the anterolateral LV myocardium | Echo not obtained |
Patient developed cardiogenic shock; CCT findings confirmed in OR. Subsequent heart transplantation |
| 7 | Anemia | Female | 69 | Normal LVAD CCT | Echo not obtained | Treated for Coombs-positive anemia. Alive and well at 30 days |
| 8 | Syncope | Male | 56 | Normal LVAD CCT | Air microbubbles thought to be seen entering LA via pulmonary veins | Alive and well at 30 days |
| 26 | Syncope | Female | 21 | Normal LVAD CCT. Outflow cannula not well opacified due to late bolus timing | Echo failed to identify VAD | Alive and well at 30 days |
| 9 | Shock | Female | 52 | Normal LVAD CCT | Normal inflow cannula; outflow cannula not visualized | Taken to OR. Thrombus on the arterial valve. Device replaced |
| 10 | Shock | Male | 58 | Normal LVAD CCT | Poor echo windows. No comment on LVAD | Treated for pseudomonas sepsis. Alive at 30 days |
| 25 | Shock | Female | 42 | Normal LVAD CCT | Echo not obtained | MRSA identified in the drive line. Developed shock, care withdrawn. |
| 11 | Suspect thrombus | Male | 62 | Normal LVAD CCT | Echo not obtained | Treated for systemic infection. Alive at 30 days |
| 12b | Arrhythmia | Female | 24 | Normal LVAD CCT | Echo not obtained | Alive and well at 30 days |
| 19a | Arrhythmia | Male | 37 | Inflow cannula abuts LV apex | Inflow cannula noted; no evidence of mis-alignment |
Taken to OR and confirmed findings. Device repositioned with improved flows. Alive at 30 days. |
| 13 | Surgical Planning | Male | 62 | Normal LVAD CCT | Echo not obtained | Taken to OR; LVAD battery at end-of-life. Normal positioning found |
| 23 | Surgical Planning | Male | 37 | Normal LVAD CCT | Echo showed normal inflow position of LVAD | CCT findings confirmed in OR. Explant due to MRSA infection. Alive at 30 days |
| 24 | Surgical Planning | Male | 61 | Normal LVAD CCT | Echo not obtained | CCT findings confirmed in OR. Device replaced at end of life. Alive at 30 days |
| 28 | Surgical Planning | Male | 58 | Normal LVAD CCT | Echo not obtained | CCT findings confirmed in OR. Device replaced due to end-of-life. |
| 16 | Cardiac Arrest | Male | 71 | Normal LVAD CCT | Echo not obtained | Battery malfunction/low power found to be cause of arrest. Withdrawal of care |
| 17a | Chest Pain | Female | 36 | Normal LVAD CCT | Echo not obtained | No changes made. Alive and well at 30 days |
| 4b | Worsened heart failure | Female | 31 | Normal LVAD CCT | Poor echo windows; thrombus in inflow cannula | No changes made. Alive and well at 30 days. Heart transplant at 65 days; normal intraoperative LVAD findings |
| 1b | Dyspnea | Male | 56 | Normal LVAD CCT | Echo windows poor; unable to comment on device | Alive and well at 30 days |
NOTES: Patients shown in bold had critical findings on CCT.
In all 32 cases, outflow cannula position and aortic anastomosis appeared intact. Six patients had a critical finding on CCT. These included 1 patient with outflow cannula thrombosis and 5 patients with significant inflow cannula malposition. One patient with inflow cannula malposition who did not undergo immediate operative intervention developed acute decompensated heart failure and subsequently underwent successful heart transplantation. Four of these 6 patients also underwent echocardiography; none of these echo studies identified the critical findings demonstrated by CCT.
There were three patient deaths at 30 days within the overall study group. One was related to methycillin-resistant staphylococcus aureus (MRSA) sepsis, another from loss of battery power leading to cardiac arrest as an outpatient, and the third from an infection whose source was not identified (Table 1).
Thirteen patients underwent surgery after CCT examination. In addition to the 6 patients with critical CT findings as described as above, 7 patients with normal CCT were also taken to surgery for various indications. CCT diagnosis was confirmed intra-operatively in 12 of the 13 cases (Table 1). All 6 patients with critical findings discovered on CCT had these findings confirmed intra-operatively. Patient 9 had a CCT which demonstrated normal LVAD cannula position and no evidence of thrombus within the cannula. However, continued hemodynamic instability prompted return to the operating room where thrombus on the arterial valve was discovered. Thus, for detection of critical findings in this cohort, the sensitivity of CCT was 85% and specificity was 100%.
Fourteen patients underwent both CCT and echocardiography within a 48-hour period. There were 4 observed agreements between the two imaging modalities. Expected agreement by chance was 6, giving a kappa value of −0.29 (95% CI −0.73 −0.13) i.e. no agreement between echocardiography and CCT. Thus, neither a normal nor an abnormal echocardiogram for LVAD evaluation correlated with findings on CCT. A representative example is Patient 1A who was evaluated for low cardiac output (Figure 1). Transesophageal echocardiography did not identify evidence of LVAD dysfunction. CCT on the same day as echocardiography showed thrombosis of the outflow graft. Echocardiography in another patient suggested a critical finding – thrombus in the inflow cannula – but CCT showed normal LVAD position and no evidence of thrombus. No changes were made to the patient’s management based on CCT findings. Heart transplantation was successfully performed 65 days after imaging without any interval adverse events. Intraoperative evaluation revealed normal position and function of the LVAD; no thrombus was identified. Patient 8’s echocardiogram described ‘air bubbles entering the aorta’ from the outflow graft; CCT showed no air in the aorta. No changes were made to the patient’s clinical care, and no evidence of stroke or TIA was subsequently observed.
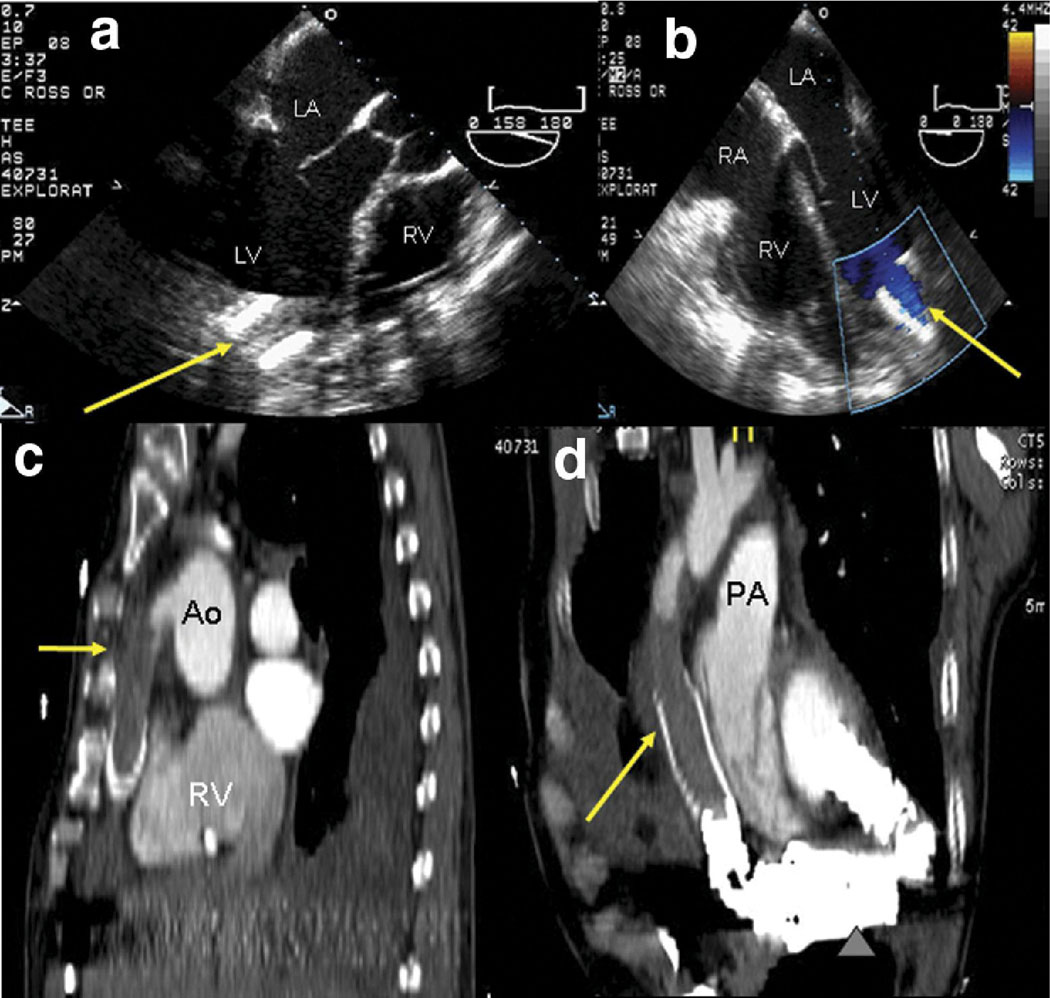
Figure 1.
A 56 year-old patient with LVAD had low cardiac output signaling of the device. Initial transesophageal echocardiography shows the proximal inflow cannula at the LV apex (a, arrow). The outflow cannula could not be visualized. Normal inflow cannula flow is suggested by color Doppler (b, arrow); velocities (not shown) were less than 1.5 m/s. However, CCT revealed extensive thrombus in the outflow cannula (c, sagittal plane reformat). Isotropic spatial resolution of CCT images allows reformatting in an oblique sagittal plane (d) to demonstrate the entire outflow graft, further illuminating the extensive thrombus burden. The device itself and portions of the inflow cannula are also seen.
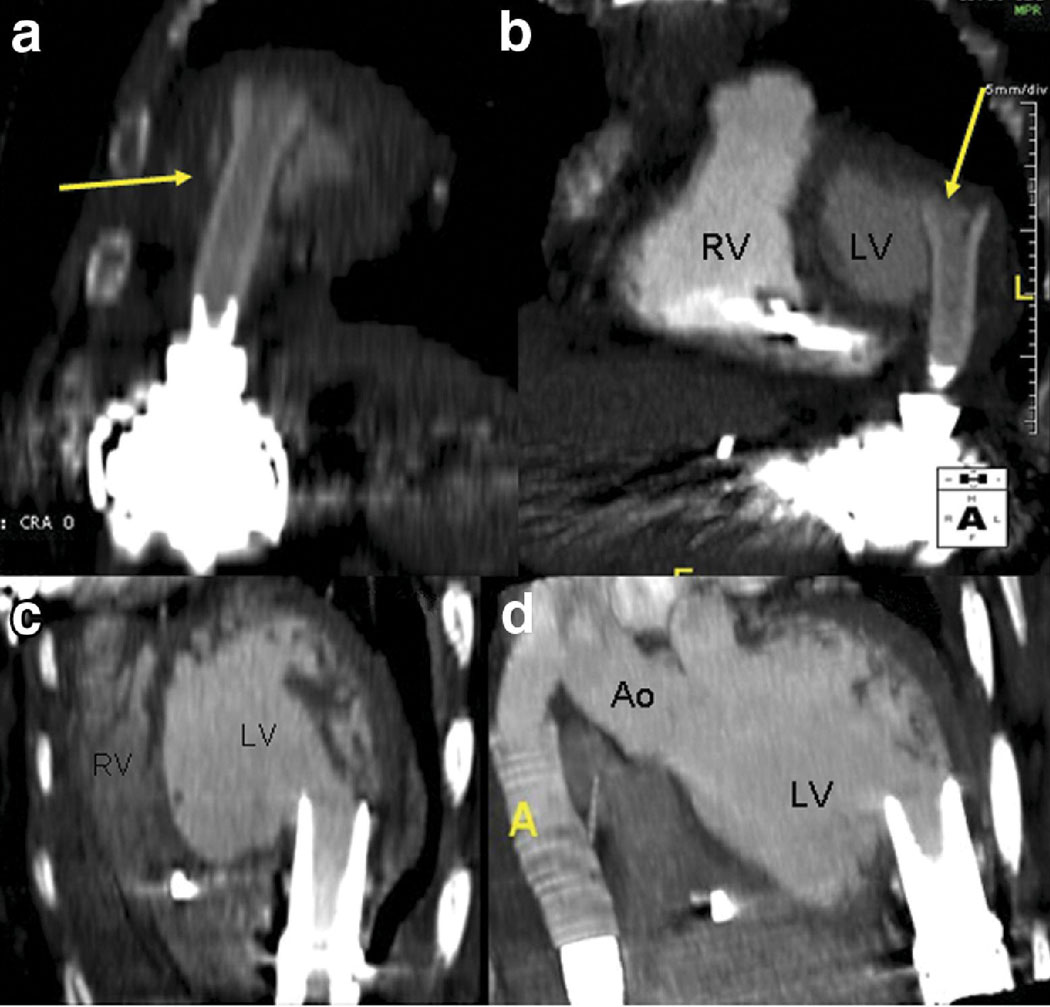
Of 19 patients who underwent CCT evaluation as their only non-invasive imaging, 9 patients with normal CCT scans were alive and well at 30-day follow-up without surgical intervention. Two studies had critical findings of cannula malposition (Figure 2), both confirmed intraoperatively. In both of these cases, cardiac output improved after cannula reposition. One CCT-only patient with an abnormal finding – ‘questionable filling defect of the outflow cannula’ – had no changes made in management, and was alive and well at 30 days.
Figure 2.
The inflow cannula abuts the anteroapical wall of the left ventricle (a, b), obstructing inflow. Clinically, this patient had poor cardiac output and borderline shock; flow improved and symptoms resolved with intra-operative repositioning of the inflow cannula. In a different patient, proper positioning of the inflow cannula in relation to the walls of left ventricle is illustrated (c, d).
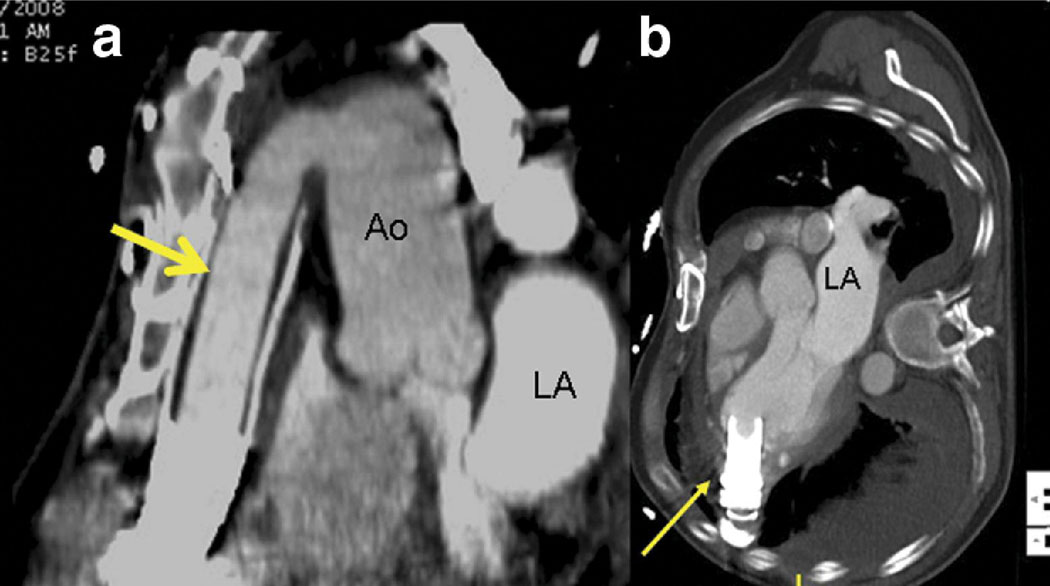
Due to severe acoustic shadowing, echocardiography failed to visualize both inflow and outflow cannula position in all but one of the 14 studies. Positions of both cannulae were easily demonstrated in all 32 CCT examinations (Figure 3). Four echocardiograms were described as being technically limited studies due to poor acoustic windows. One CCT was considered technically difficult due to mis-timing of the contrast bolus.
Figure 3.
After thrombus removal in patient 1A, the outflow cannula is unobstructed (a, arrow). LA = left atrium. The inflow cannula (b), while angled slightly toward the LVOT, is otherwise unobstructed.
Discussion
In a large cohort of patients with left ventricular assist devices, CCT was accurate and instrumental in identifying both normal device configurations as well as critical findings that would prompt change in management. Our study indicates that CCT performs well in detecting etiologies such as cannula thrombosis and malposition. Other causes of LVAD malfunction such as battery failure or natural device wear and tear are readily identified via device readouts and routine clinical follow-up. Poor LVAD output may not be reliably detected from device data; prior work from our laboratory indicates that CCT may be helpful in providing hemodynamic assessment of LVAD output as well (8).
While ventricular assist devices vary in their engineering, they share many important design elements. Blood from the left ventricle is removed via an apical inflow cannula. The blood moves through the device by either pulsatile or continuous flow to the outflow graft that typically returns blood via an anastomosis to the mid-ascending aorta. Regardless of the type of device or flow mechanisms, this cannulation remains fairly standard. If the inflow cannula is obstructed in the left ventricle, which may result from technical error at the time of implantation, migration, or reverse ventricular remodeling, the risk of device malfunction increases. In our series, inflow cannula obstruction most often resulted from displacement of the cannula due to migration, such that it abutted the anteroapical wall or anteroseptum. There were no abnormalities of outflow graft position seen in our series, likely because of greater control and visualization of the aortic anastomosis at the time of implantation as well minimal ascending aorta remodeling over time compared to LV remodeling. There were no kinks seen in the outflow grafts, even when the devices had migrated to produce compromise of inflow. This results from surgical philosophy that a shorter outflow graft is desirable to prevent kinking.
CCT identified critical findings in the evaluation of LVADs. All critical findings led to changes in patient management, and were confirmed intraoperatively. The clinical utility of a normal CCT exam was just as important, as it largely avoided further LVAD manipulation and allowed subsequent evaluation to be directed toward other causes of any symptoms. We believe CCT performed well in this series for several reasons. First, three-dimensional manipulation of the imaging data afforded by isotropic spatial resolution allowed the interpreting physician to interrogate the device from a multitude of views. Second, the volume of coverage provided direct visualization of the entire outflow and inflow cannula, allowing recognition of cannula thrombus or deformation as well as placement. In contrast, conventional transthoracic echocardiography leaves the majority of the device unviewed due to limitations imposed by the acoustic window and acoustic shadowing. As a result, critical findings detected by CCT were often missed by echocardiography, precluding appropriate clinical management based on TTE alone. Transesophageal echo, less affected by acoustic window, does not provide adequate visualization of the entire inflow and outflow cannulae due to limited depth of imaging and volume of coverage.
While direct visualization of cannulae may not be technically feasible with echocardiography, this modality does offer indirect assessment of device integrity via Doppler measurement of cannula flow, mitral regurgitation, aortic valve function, and evaluation of right and left ventricular function. Again, adequate acoustic window comes into play – should the angle of incidence between Doppler and cannula flow exceed 20 degrees, the resulting velocity profile becomes unreliable. Additionally, peak filling velocities vary depending on preload, limiting the utility of a single Doppler assessment. While outflow cannula flow velocity greater than 2–2.5 m/s has high specificity for obstruction (5, 8), sensitivity is limited by acoustic window, as was the case in many of our patients. While transesophageal echocardiography (TEE) may provide better visualization of the intracardiac portions of the device, the majority of the device is extracardiac and may not be adequately imaged with TEE. Further, this modality requires sedation and carries attendant risk of respiratory distress as well as risks associated with esophageal intubation.
We have previously demonstrated that CCT can provide hemodynamic assessment of LVAD output that is more reliable than that provided by device readings(9). The combination of hemodynamic information and reliable anatomic information that we have shown in the present study provides in one imaging modality a powerful tool for noninvasive LVAD assessment.
Limitations of CCT in patients with LVADs include risk of nephrotoxicity from iodinated contrast administration, which may be contraindicated in this group of patients who often have significant renal dysfunction. Allergy represents an additional risk that may be minimized but not necessarily eliminated with appropriate pretreatment of patients with known allergy to iodinated contrast. CCT involves ionizing radiation on an order of magnitude comparable to that found in x-ray angiography and nuclear imaging – diagnostic modalities frequently used in patients with heart failure. We used techniques to minimize the effective radiation dose, while recognizing that the potential long-term risks of radiation exposure must be balanced with the immediate life-threatening risk of mis-diagnosing a fatal complication in these patients. Ongoing technical advances that to further reduce dose for CCT are welcome in this respect. Finally, future prospective studies that randomize diagnostic strategy in clinical decision-making could further strengthen the results of this retrospective analysis that support the potential utility of CCT in the management of patients with LVAD.
As imaging options for cardiovascular diagnosis expand, one may ask if there is a single best test for a specific clinical indication or if multiple tests provide needed complementary information to improve patient care. The strength of CCT to evaluate the LVAD patient is in its ability to visualize the entire device, which makes it superior to echocardiography for anatomic LVAD assessment. However a strength of echocardiography, particularly real-time three-dimensional acquisition, is the ability to evaluate right ventricular function at the bedside(10). When RV function is known to be normal or unchanged, simultaneously obtaining both tests to assess the LVAD patient may be unnecessary. Another concern regarding advanced imaging modalities is the cost differential compared to traditional imaging methods. Given the high cost of LVAD therapy, $225,000 US dollars in the first year alone (11), use of strategies to maximize the long-term success of these procedures suggest value in timely imaging using the most effective modality for the clinical questions at hand. Despite higher cost of CT, missing potentially life-threatening or overstating abnormalities using less expensive modalities that may be inherently limited by acoustic artifact may ultimately incur vastly higher costs and worse outcomes. Ultimately, individual patient factors remain central in selecting the appropriate diagnostic approach.
In conclusion, we have shown that noninvasive imaging of left ventricular assist devices using CCT is feasible and accurate, and can identify important findings that impact patient management. CCT warrants further consideration as a first-line modality for imaging in patients with LVADs.
Acknowledgments
Dr. Raman is supported in part by NIH R01 HL095563. Dr. Raman receives research support from Siemens.
Footnotes
Funding and Conflicts of Interest
Drs. Sahu, Merchant, Louis IV, Firstenberg, and Sun have no conflicts.
References
- 1.Pennock JL, Pierce WS, Campbell DB, et al. Mechanical support of the circulation followed by cardiac transplantation. J Thorac Cardiovasc Surg. 1986;92:994–1004. [PubMed] [Google Scholar]
- 2.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 3.Boyle A. Current status of cardiac transplantation and mechanical circulatory support. Curr Heart Fail Rep. 2009;6:28–33. doi: 10.1007/s11897-009-0006-8. [DOI] [PubMed] [Google Scholar]
- 4.Piccione W., Jr Left ventricular assist device implantation: short and long-term surgical complications. J Heart Lung Transplant. 2000;19:S89–S94. doi: 10.1016/s1053-2498(99)00110-2. [DOI] [PubMed] [Google Scholar]
- 5.Catena E, Milazzo F. Echocardiography and cardiac assist devices. Minerva Cardioangiol. 2007;55:247–265. [PubMed] [Google Scholar]
- 6.Jain VR, White CS, Pierson RN, 3rd, Griffith BP, Sorensen EN. Imaging of left ventricular assist devices. J Thorac Imaging. 2005;20:32–40. doi: 10.1097/01.rti.0000146623.32209.36. [DOI] [PubMed] [Google Scholar]
- 7.Budoff MJ, Cohen MC, Garcia MJ, et al. ACCF/AHA clinical competence statement on cardiac imaging with computed tomography and magnetic resonance: a report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training. J Am Coll Cardiol. 2005;46:383–402. doi: 10.1016/j.jacc.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 8.Chumnanvej S, Wood MJ, MacGillivray TE, Melo MF. Perioperative echocardiographic examination for ventricular assist device implantation. Anesth Analg. 2007;105:583–601. doi: 10.1213/01.ane.0000278088.22952.82. [DOI] [PubMed] [Google Scholar]
- 9.Raman SV, Tran T, Simonetti OP, Sun B. Dynamic computed tomography to determine cardiac output in patients with left ventricular assist devices. J Thorac Cardiovasc Surg. 2009;137:1213–1217. doi: 10.1016/j.jtcvs.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 10.Nesser HJ, Tkalec W, Patel AR, et al. Quantitation of right ventricular volumes and ejection fraction by three-dimensional echocardiography in patients: comparison with magnetic resonance imaging and radionuclide ventriculography. Echocardiography. 2006;23:666–680. doi: 10.1111/j.1540-8175.2006.00286.x. [DOI] [PubMed] [Google Scholar]
- 11.Moskowitz AJ, Rose EA, Gelijns AC. The cost of long-term LVAD implantation. Ann Thorac Surg. 2001;71:S195–S198. doi: 10.1016/s0003-4975(00)02621-7. discussion S203-4. [DOI] [PubMed] [Google Scholar]