Abstract
Novel molecular pathways obligatory for bone health are being rapidly identified. One pathway recently revealed involves gut-derived 5-hydroxytryptamine (5-HT) mediation of the complete skeletal effects of low-density lipoprotein receptor-related protein 5 (LRP5). Mounting evidence supports 5-HT as an important regulatory compound in bone with previous evidence demonstrating that bone cells possess functional pathways for responding to 5-HT. In addition, there is growing evidence that potentiation of 5-HT signaling via inhibition of the 5-HT transporter (5-HTT) has significant skeletal effects. The later is clinically significant as the 5-HTT is a popular target of pharmaceutical agents, such as selective serotonin reuptake inhibitors (SSRIs), used for the management of major depressive disorder and other affective conditions. The observation that 5-HT mediates the complete skeletal effects of LRP5 represents a significant paradigm shift from the traditional view that LRP5 located on the cell surface membrane of osteoblasts exerts direct skeletal effects via Wnt/beta-catenin signaling. This paper discusses the mounting evidence for skeletal effects of 5-HT and the ability of gut-derived 5-HT to satisfactorily explain the skeletal effects of LRP5.
Keywords: antidepressants, enterochromaffin cells, hormones, osteoporosis, serotonin
Introduction
Novel molecular pathways obligatory for bone health are being rapidly identified, due in part to the use of advanced research techniques such as the investigation of genetically manipulated animal models and the cataloguing of genetic mutations in clinical populations. One pathway that has gained recent attention involves 5-hydroxytryptamine (5-HT), a monoamine compound classically referred to as ‘serotonin’ due to early observations identifying it as a serum agent (sero-) affecting vascular tone (-tonin) [1]. While evidence suggesting a skeletal effect of 5-HT has been available for numerous years [2, 3], recent data has spurred interest into this compound. In particular, 5-HT derived from the gut has been observed to indirectly mediate the entire skeletal effects of low-density lipoprotein receptor-related protein 5 (LRP5) [4]. This represents a significant paradigm shift from the prevailing view that LRP5 (and the homologous LRP6) located on the cell surface membrane of osteoblasts exerts direct skeletal effects via Wnt/β-catenin signaling.
Central and peripheral effects of 5-HT
5-HT is a fascinating molecule as it exhibits separate central and peripheral functional identities. This duality results from the differential regulation of central and peripheral 5-HT synthesis. 5-HT is synthesized in two steps from the essential amino acid tryptophan, with the rate-limiting step being catalyzed by tryptophan hydroxylase (TPH). Given the critical role of TPH, expression for this enzyme has become a marker for 5-HT synthesis. There was classically one gene encoding for TPH (Tph1); however, a second isoform (Tph2) was recently identified [5]. The existence of a second Tph isoform has confirmed a duality in 5-HT signaling as Tph1 and Tph2 are preferentially expressed in the periphery and centrally, respectively. As 5-HT does not freely cross the blood-brain barrier due to its positive charge at physiological pH, centrally and peripherally synthesized 5-HT can function in relative isolation. This has enabled the investigation and therapeutic targeting of the independent central and peripheral effects of 5-HT.
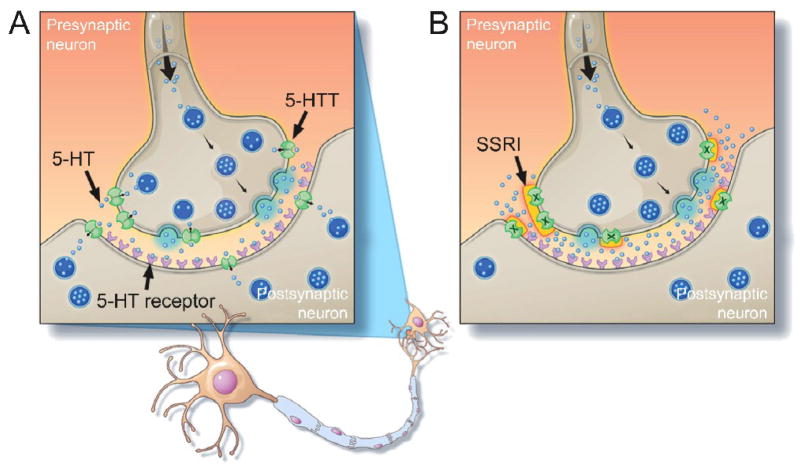
5-HT functions centrally as a neurotransmitter within the central nervous system (CNS) (Fig. 1A). It is synthesized utilizing TPH2 and released by neurons of the raphe nuclei to influence a range of behavioral, physiological and cognitive functions. These effects are mediated by seven families of membrane-bound 5-HT receptors (5-HT1 to 5-HT7) and are tightly regulated by a plasma membrane 5-HT transporter (5-HTT [also known as SERT]). The 5-HTT regulates the duration of 5-HT activity by actively transporting 5-HT using transmembrane ion gradients of Na+, Cl− and K+, and an internal negative membrane potential. Central 5-HT signaling is a frequent therapeutic target as it is hypothesized to play a key role in major depressive disorder and other affective conditions [6]. Pharmaceutical agents that antagonize the 5-HTT, such as selective serotonin reuptake inhibitors (SSRIs), are clinically popular as they potentiate 5-HT activity (Fig. 1B) and have been shown to effectively relieve depressive symptoms [7].
Figure 1.
A) 5-hydroxytryptamine (5-HT) signaling within the central nervous system. 5-HT is synthesized by presynaptic neurons and stored in vesicles. Vesicles bind with the cell membrane following a stimulus to release 5-HT into the synaptic cleft via exocytosis. Released 5-HT activates post-synaptic receptors to stimulate the post-synaptic neuron. Membrane-bound 5-HT transporters (5-HTT) uptake released 5-HT to control the duration of 5-HT effects and recycle or degrade 5-HT. B) The effects of 5-HTT inhibition using a selective serotonin reuptake inhibitor (SSRI) on 5-HT signaling. Inhibition of the 5-HTT prevents uptake of 5-HT from resulting in its accumulation within the synaptic cleft and the prolonging receptor activation.
5-HT has major functions outside of the CNS, as suggested by the fact that 95% of 5-HT is located in the periphery. Peripheral 5-HT is predominantly produced utilizing TPH1 by enterochromaffin (EC) cells in the gastrointestinal (GI) tract where it functions as a paracrine factor. EC cells secrete 5-HT in response to mucosal stimulation after which it diffuses to act on enterocytes and enteric nerve endings to stimulate peristalsis and mucus secretion [8]. As in the CNS, the duration of 5-HT signaling within the GI tract is influenced by its removal from the extracellular space by the 5-HTT which is located on epithelial cells of the intestinal mucosa [9]. Interference with this uptake enables persistent binding of 5-HT to its receptors and has been associated with GI tract symptoms, including temporary nausea and diarrhea in individuals taking SSRIs [10]. Similarly, altered 5-HT levels within the GI tract have been associated with irritable bowel syndrome (IBS), with impaired 5-HT release and its reduced uptake being linked to diarrhea-and constipation-predominant IBS, respectively [11].
A proportion of 5-HT synthesized within the GI tract reaches the cardiovascular (CV) system where most (>95%) is rapidly taken up by platelets using the 5-HTT. Platelets possess the 5-HTT but cannot synthesize 5-HT as they do not express Tph. Platelets store 5-HT in dense granules and release it upon activation to stimulate platelet aggregation and blood clotting following tissue injury, as well as an array of other physiological effects including blood vessel constriction or dilation (depending on the 5-HT receptors activated), and smooth muscle cell hypertrophy and hyperplasia [12]. These hemostatic effects of 5-HT have been implicated in the etiology of pathological CV conditions including valvular heart disease and pulmonary hypertension. In addition, platelet-derived 5-HT has non-hemostatic functions including acting as a hepatocyte proliferative agent important for liver regeneration [13]. The remaining <5% of circulating 5-HT stays free in the plasma. The function of this extracellular 5-HT remains unknown, but it may conceivably act as a hormone influencing target cells at distant sites.
Emerging role of 5-HT signaling in the skeleton
Growing evidence suggests 5-HT has peripheral effects beyond the GI tract and CV system, including effects within the skeleton [14]. 5-HT receptors have been identified in all the major bone cell types (osteoblasts, osteocytes and osteoclasts), and stimulation of these receptors influences bone cell activities [2, 15–19]. Similarly, each major bone cell type possesses a 5-HTT which is highly specific for 5-HT uptake into these cells [2, 15–18]. These findings indicate that bone cells possess functional pathways for both responding to and regulating the uptake of 5-HT.
A number of in vitro studies have confirmed the functionality of 5-HT signaling in bone cells, but offer contrasting evidence as to its effects [20]. Some suggest a direct stimulatory effect on bone formation pathways with 5-HT increasing prostaglandin E2 release from osteocyte-like (MLO-Y4) cells and enhancing proliferation of MC3T3-E1 cells and primary human osteoblasts [17, 19, 21]. The latter effects were mediated by 5-HT binding to 5-HT2A, 2B, and 2C receptors [17, 19], with 5-HT binding to 5-HT2B receptors also contributing to matrix mineralization by differentiated osteoblasts from the mesoblastic (C1) cell line [22]. In contrast, other investigations suggest an inhibitory effect of 5-HT on bone formation with 5-HT reducing proliferation of primary murine osteoblasts [4] and inhibiting nitric oxide release from mouse-derived osteoblasts [19].
Similar contrasting evidence has been provided in terms of the effect of 5-HT signaling on bone resorption pathways. Cells (RAW264.7 and human peripheral blood mononuclear cells stimulated with RANKL) treated with 5-HT (0.01–50 μM) or a SSRI (fluoxetine; 0.001–10 μM) increased their differentiation into osteoclast-like cells and increased their bone-resorption activity [17]. These observations suggest 5-HT may potentiate bone resorption. However, osteoclast differentiation and activity were decreased at the highest concentration of the SSRI (fluoxetine; 10 μM) [17]. Similarly, an alternative study found a SSRI (fluoxetine; 1–3 μM) to inhibit osteoclast formation from RAW264. 7 and bone marrow derived cells [23]. These later observations suggest that 5-HT may have anti-resorptive effects.
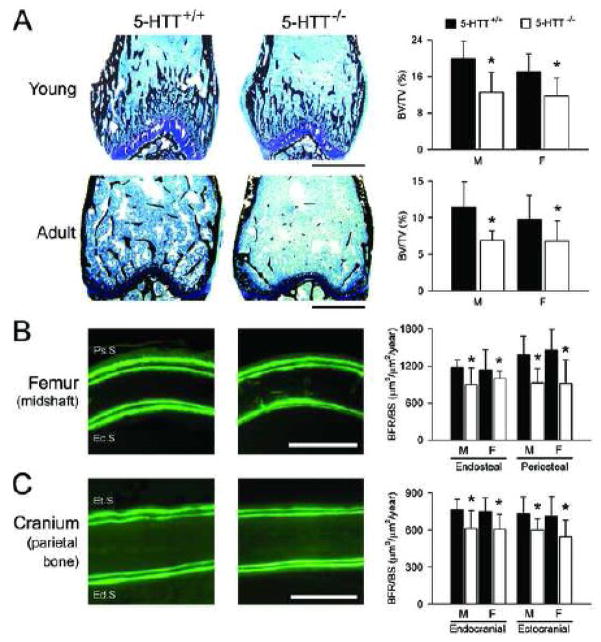
In vitro studies have confirmed the functionality of 5-HT signaling in bone cells, but their contrasting findings have yet to provide a clear picture as to its potential in vivo skeletal effects. The preponderance of evidence from in vivo preclinical studies indicates that increased 5-HT signaling has negative effects on bone [3, 24–27], though there are a number of studies suggesting the opposite [28–32]. These latter studies are disputable and outweighed by the larger and growing body of evidence suggesting detrimental skeletal effects of 5-HT [20]. In vivo studies into the skeletal effect of 5-HT signaling were initiated by the observation that mice with a null mutation in the 5-htt gene possessed a consistent skeletal phenotype of reduced mass, altered architecture and inferior mechanical properties (Fig. 2A) [3]. This potential detrimental effect of enhanced 5-HT signaling has been supported by numerous preclinical studies investigating the skeletal effects of SSRIs [3, 24–27]. These studies found young and adult rodents treated for four weeks with daily doses of a SSRI (fluoxetine; 5–20 mg/kg) to also exhibit reduced bone mass, altered skeletal architecture and reduced bone mechanical properties. These changes resulted from a reduction in bone formation in growing animals treated with the SSRI or possessing a null mutation in the 5-htt gene, and reduced bone formation and increased resorption in adult animals treated with the SSRI (Fig. 2B,C) [3, 26].
Figure 2.
Gene-mediated inhibition of the 5-hydroxytryptamine transporter (5-HTT) reduces: A) trabecular bone volume fraction (bone volume [BV]/total volume [TV]) within the distal femur of young (four-week-old) and adult (19-week-old) mice; B) femoral midshaft periosteal (Ps.S) and endosteal (Ec.S) surface bone formation (bone formation rate normalized for bone surface [BFR/BS]) in young (four-week-old) mice, and; C) cranial ectocranial (Et.S) and endocranial (Ed.S) surface BFR/BS in young (four-week-old) mice. 5-HTT+/+ = wild-type control mice; 5-HTT−/− = mice with null mutation in the 5-htt gene; M = male; F = female. Scale bars = 1 mm, 100 μm and 200 μm in A), B) and C) respectively. Error bars show mean ± SD. *Indicates P < 0.05 for genotype main effect, as determined by 2-way factorial ANOVA (genotype × sex). There were no significant genotype × sex interactions (all P > 0.05). Reproduced with permission of The Endocrine Society (©2005) from Warden et al. [3].
There are a number of putative explanations beyond direct effects at the bone cell level for the observed skeletal phenotype associated with 5-HTT inhibition in animal models. Primarily, the impact of central inhibition of the 5-HTT on the skeleton has not been explored in detail. Pharmacological agents that antagonize the 5-HTT are currently non-selective such that studies investigating the skeletal effects of these agents inhibited the transporter both centrally and peripherally. Similarly, the study investigating the skeletal effects of gene-mediated inhibition of the 5-HTT utilized an animal model possessing a global (central and peripheral) null mutation in the 5-htt gene rather than a tissue-specific null mutant. This non-specific pharmacological- and gene-mediated inhibition of the 5-HTT enables upstream central and peripheral effects of 5-HT to potentially contribute to the observed skeletal changes.
An established central effect of gene- and pharmacological-mediated inhibition of the 5-HTT in mice is heightened anxiety-like behavior, which manifests in a hypoactive locomotor behavioral phenotype [33, 34]. Reduced cage activity may contribute to the bone changes observed with 5-HTT inhibition. However, this does not fully explain the reduced bone formation and mineral content in the cranial bones of 5-htt mutant mice, a site unlikely influenced by genotype differences in physical activity levels (Fig. 2C) [3]. Similarly, physical inactivity does not explain why mice treated with a SSRI (fluoxetine; 10–20 mg/kg) exhibit a skeletal phenotype, as animals treated with a tricyclic antidepressant [TCA] (desipramine; 20 mg/kg) show equivalent physical inactivity but do not show a consistent skeletal phenotype [24, 25]. Further, a SSRI (fluoxetine; 20 mg/kg) reduced bone formation in tail-suspended mice which effectively removes the physical inactivity phenotype induced by these agents and normalizes skeletal loading between treated and control animals [25]. Collectively, these data suggest that centrally-mediated altered loading does not appear responsible for the negative skeletal effects of 5-HTT inhibition.
Another potential central mechanism for the observed skeletal phenotype with 5-HTT inhibition is its effect on circulating levels of skeletally relevant hormones and biochemical mediators. 5-HT pathways directly modulate numerous hormonal and biochemical pathways, including those in the hypothalamic-pituitary-adrenal axis [6]. Modulation of these pathways may indirectly contribute to the skeletal phenotype observed with 5-HTT inhibition, with a previous clinical pilot study linking altered growth hormone to the skeletal response to SSRIs [35]. However, 5-htt null mutant mice did not display significant differences in serum-measured, skeletally-relevant hormones or biochemical markers, including insulin-like growth factor 1 [3]. This is consistent with previous reports demonstrating no genotype effects of gene-mediated inhibition of the 5-HTT on plasma corticosterone or adrenocorticotropic hormone levels unless the animals were stressed [36].
Inhibition of the 5-HTT within the GI tract may be a peripheral upstream mechanism for the skeletal phenotype observed with gene- and pharmacological-mediated 5-HTT inhibition. Mice with a null mutation in the 5-htt gene have altered colorectal motility consistent with the function of the 5-HTT in regulating peristalsis [37]; however, this does not appear sufficient to significantly alter their nutritional status. The bowel continues to function in 5-htt null mice [37], and these mice gain equivalent weight and have comparable survival to wild-type littermates [38]. Similarly, SSRI-treated mice exhibit a skeletal phenotype independent of any drug effects on food intake or body weight [3, 25, 26]. Thus, upstream peripheral effects within the GI tract do not appear to contribute to the skeletal phenotype observed with 5-HTT inhibition.
Clinical studies confirm the preclinical evidence demonstrating a negative effect of altered 5-HT signaling on bone health. In particular, clinical use of SSRIs has been shown to be associated with increased postmenopausal bone loss [39] and fracture risk [40]. These studies are described in detail in an accompanying paper [41].
Gut-derived 5-HT mediates the skeletal effects of LRP5
For 5-HT pathways within the skeleton to be biologically relevant bone cells need access to 5-HT. Bone cells may produce 5-HT themselves as osteoblasts, osteocytes and osteoclasts all express Tph1 [4, 16, 17]. However, autocrine/paracrine 5-HT signaling has yet to be confirmed in bone with bone cells not secreting measurable levels of 5-HT [4]. Instead, recent evidence provided by Yadav et al. [4] suggests that 5-HT derived from the GI tract and transported by the CV system is the major source for skeletal 5-HT. This is an important observation as it suggests 5-HT functions as an endocrine signaling molecule and may be a potential target for novel therapeutics. The finding was made all-the-more striking by the concomitant observation that gut-derived 5-HT acted as a downstream mediator for the entire skeletal effects of LRP5.
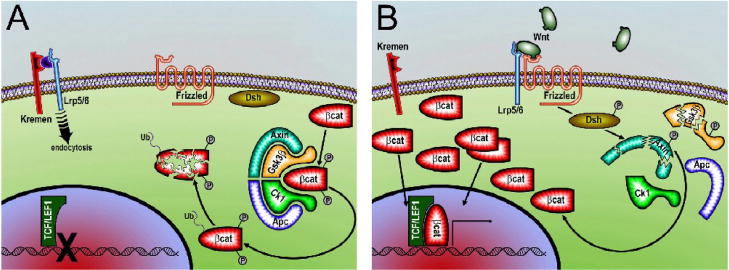
Interest into the skeletal effects of LRP5 was initially prompted by the discoveries that loss- and gain-of-function mutations in its gene cause osteoporosis pseudoglioma (OPPG) syndrome and a high bone mass (HBM) phenotype, respectively [42–44]. These effects are commonly believed to result from LRP5 modulation of Wnt/β-catenin signaling in osteoblasts [45]. LRP5 (and the homologous LRP6) acts as a co-receptor together with frizzled for Wnt proteins. Binding of Wnt to its LRP5 and frizzled co-receptors inactivates glycogen synthase kinase-3β (GSK-3β) allowing for cytoplasmic accumulation of β-catenin which subsequently translocates into the nucleus to influence gene transcription (Fig. 3). These observations have directed recent research and drug discovery efforts towards the Wnt/β-catenin signaling pathway, which has resulted in the discovery of additional mediators in the pathway and the development of potentially anabolic compounds such as neutralizing antibodies for sclerostin and Dickkopf-1 (Dkk-1) [46, 47]. Although there is little doubt that the Wnt/β-catenin signaling pathway plays an important role in the skeleton, the observation that the entire skeletal effects of LRP5 are indirect and mediated by gut-derived 5-HT opposes the existing paradigm of its role in directly activating osteoblastic Wnt/β-catenin signaling.
Figure 3.
Elements of Wnt/β-catenin signaling. A) Lack of Wnt signaling (i.e. via soluble Frizzled related protein [sFrp] antagonism of Wnt, Dickkopf [Dkk] antagonism of low-density lipoprotein receptor-related protein 5/6 [Lrp5/6], or other means) permits stabilization of the Axin-adenomatosis polyposis coli (Apc) complex, which facilitates phosphorylation of β-catenin by glycogen synthase kinase 3 (Gsk3) and casein kinase 1 (Ck1). Phosphorylated β-catenin is subsequently ubiquitin-tagged (Ub) by β-Trcp for proteosome degradation, and consequently does not accumulate in the cytoplasm in sufficient quantities to allow translocation to the nucleus. The Tcf/Lef 1 transcription factor requires β-catenin binding to initiate gene transcription. B) Activated Wnt signaling occurs through Wnt binding and complexing of Lrp5/6 with Frizzled. Formation of the trimeric receptor–ligand complex induces degradation of the Axin complex through phosphorylated Dishevelled (Dsh) signaling. Loss of the β-catenin phosphorylation machinery allows β-catenin to remain stable (unphosphorylated) and accumulate in the cytoplasm, to the point where translocation to the nucleus occurs and gene transcription is initiated.
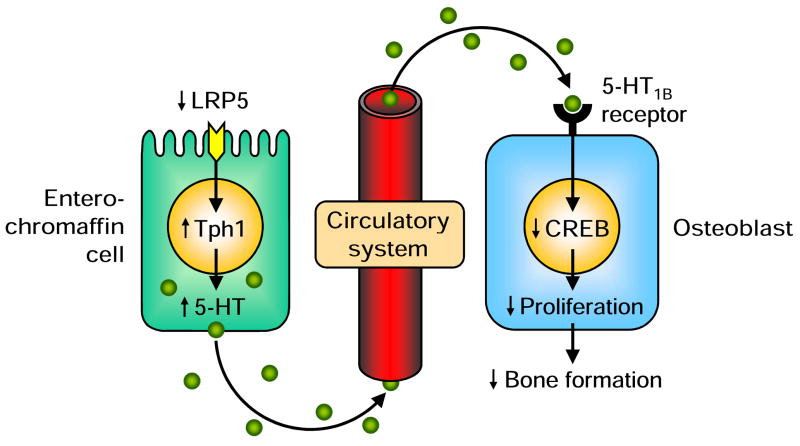
The initial motivation leading to the identification of the LRP5–5-HT–osteoblast pathway was to better understand the skeletal role of LRP5. Bones from mice with a loss-of-function mutation in Lrp5 (Lrp5−/−) exhibited a specific molecular signature of decreased expression of genes associated with cell proliferation, but not osteoblast differentiation, bone matrix deposition, or osteoclast differentiation [4]. As Lrp5−/− bones reportedly had a paucity of osteoblasts while ex vivo osteoblasts from Lrp5−/− animals proliferated normally, it was hypothesized that an extracellular signal not originating from osteoblasts contributed to the reduced cell proliferation in Lrp5−/− bones (i.e. that LRP5 was influencing the skeleton indirectly). Subsequent analyses found Tph1 to be the most differentially expressed gene in Lrp5−/− bones, and that Lrp5−/− mice also had overexpression of Tph1 in the duodenum as well as elevated circulating levels of 5-HT [4]. Establishing a link between the elevated 5-HT and bone, 5-HT inhibited ex vivo proliferation of osteoblasts from both Lrp5−/− and wild-type mice, and Lrp5−/− mice fed a low-tryptophan diet or treated with an inhibitor of 5-HT synthesis exhibited reduced circulating 5-HT levels and normalization of their skeletal phenotype [4]. These combined findings provided preliminary evidence that elevated circulating 5-HT contributed to the decreased bone formation and mass observed in Lrp5−/− mice.
To establish a more compelling link between LRP5, 5-HT and bone, transgenic mice with either gut- or osteoblast-specific loss- or gain-of-function of Lrp5 were generated. This was achieved by crossing mice harboring either a floxed loss- or gain-of-function allele of Lrp5 with either Villin (gut-specific) or collagen type 1 (osteoblast-specific) promoter-driven Cre transgenic mice. Gut-specific deletion of Lrp5 recapitulated the high circulating 5-HT and skeletal phenotype of Lrp5−/− mice, whereas osteoblast-specific deletion did not [4]. Conversely, overexpression of Lrp5 in the gut via the insertion of cDNA encoding the HBM mutation of Lrp5 (G171V) decreased circulating 5-HT levels and generated a high bone mass phenotype, whereas osteoblast-specific overexpression of Lrp5 did not [4]. These findings support Lrp5 regulating bone formation indirectly via effects in the gut as opposed to via direct effects on osteoblasts. Confirming 5-HT as the intermediary between gut LRP5 and the skeleton, gut-specific deletion of Tph1 lowered circulating 5-HT and produced a high bone mass phenotype, whereas osteoblast-specific deletion of Tph1 did not [4].
For elevated circulating 5-HT to function as a hormone that directly inhibits bone formation it must act on 5-HT receptors located on the cell surface membrane of osteoblasts. Three 5-HT receptors were found to be expressed in osteoblasts—5-HT1B, 2A, and 2B [4]. Mice with either global deletion of Htr2a or osteoblast-specific deletion of Htr2b did not display skeletal phenotypes; however, the same was not true for mice with global or osteoblast-specific deletion of at least one allele of the Htr1b gene [4]. These latter animals displayed a similar HBM phenotype to mice with the HBM mutation of Lrp5 (G171V) in the gut, suggesting that the 5-HT1B receptor mediates 5-HT effects in osteoblasts. This was confirmed in vitro with primary osteoblasts from Htr2a and Htr2b null mutant mice responding similarly to 5-HT as osteoblasts from wild-type animals, whereas osteoblasts from Htr1b null mutant mice were unresponsive to 5-HT conditioned media [4]. Additional studies subsequently showed the transcriptional mediator of 5-HT signaling in osteoblasts to be cAMP response element binding (CREB) protein, a transcription factor that can function as both a positive and negative regulator of gene transcription. 5-HT binding to the 5-HT1B receptor was suggested to inhibit Creb expression which in turn inhibited CycD1 expression and osteoblast proliferation [4].
Does gut-derived 5-HT completely explain the skeletal effects of LRP5?
The preceding body of work eloquently demonstrated the presence of a novel skeletal regulatory pathway whereby LRP5 effects were mediated indirectly by gut-derived 5-HT (Fig. 4). As with all paradigm changing findings, this observation poses as many questions as it does answers which will no doubt become the subject of substantial scientific scrutiny over the coming years. In particular, the complete absence of a direct skeletal effect of LRP5 is likely to be an area of intense debate and subsequent investigation, particularly considering that this finding appears at odds with existing evidence.
Figure 4.
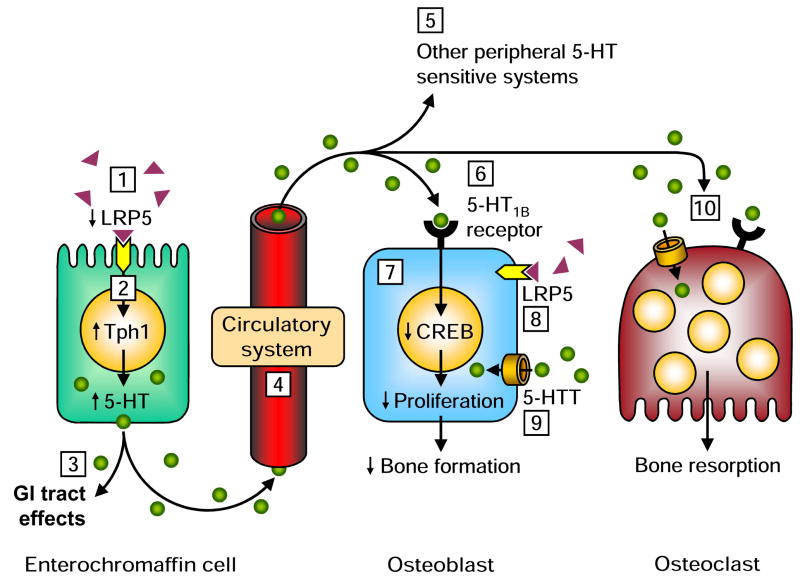
Schematic of the low-density lipoprotein receptor-related protein 5 (LRP5)–5-hydroxytryptamine (5-HT)–osteoblast pathway observed by Yadav et al. [4]. LRP5 was inhibitory of tryptophan hydroxylase 1 (Tph1) expression and resultant 5-HT synthesis in enterochromaffin cells in the gut, but had no direct effect on osteoblasts. A reduction in gut LRP5 increased Tph1 expression and 5-HT synthesis resulting in an increase in circulating 5-HT levels. Binding of 5-HT to osteoblastic 5-HT1B receptors resulted in a reduction in cAMP response element binding (CREB) protein expression contributing to a reduction in osteoblast proliferation and bone formation.
Two independent groups have observed direct skeletal effects of gain-of-function mutations in Lrp5 [48, 49]. Babij et al. [48] overexpressed the human Lrp5 (G171V) gain-of-function mutation in mouse osteoblasts using the 3.6-kb rat type-I collagen promoter. Transgenic mouse lines overexpressing this mutation in osteoblasts were found to have a HBM phenotype consistent with that observed in humans. These investigators also report that osteoblastic overexpression of the wild-type receptor also conferred an increase in bone mass, though not to the same degree as that induced by osteoblastic G171V overexpression. These observations suggest LRP5 can have direct skeletal effects, though there are plausible explanations for this disparate finding to Yadav et al. [4]. In particular, the genetic engineering strategies utilized in the two sets of studies differed greatly. Babij et al. [48] created a transgenic that exhibited significantly elevated expression levels of the G171V cDNA. Conversely, Yadav et al. [4] knocked in cDNA for the G171V mutation into the Lrp5 coding locus, so the endogenous promoter specified proper physiologic levels and proper spatiotemporal distribution of the receptor. The Yadav et al. [4] model was engineered with the benefit of having a floxed stop sequence 5′ of the cDNA, so that expression of the G171V allele could be activated in specific tissues via Cre-mediated recombination. In light of these differences in mouse models, it is possible that Lrp5 and its gain-of-function mutation only have direct skeletal effects when present in overabundance, and not when expressed at physiological levels. Alternatively, the discrepancy in the observations of Babij et al. [48] and Yadav et al. [4] may result from the use of promoters targeting osteoblasts at different stages in their lineage. The 3.6-kb rat type-I collagen promoter used by Babij et al. [48] is active in early mesenchymal progenitors, including preosteoblasts and osteoblasts, while the 2.3-kb rat type-I collagen promoter used by Yadav et al. [4] to activate the G171V allele is more restricted, showing expression in mature osteoblasts and osteocytes. It is possible that Lrp5 signaling within the skeleton is essential at early stages of the osteoblast lineage and that Yadav et al. [4] drove the Lrp5 gain-of-function in osteoblasts only after it had exerted its early direct skeletal effects.
Another approach was taken by Warman and colleagues [49]. They used a similar strategy as Yadav et al. [4] to knock-in the mouse Lrp5 G171V mutation (and also another HBM-causing mutation, A214V), though some notable exceptions exist. Rather than knocking in the G171V cDNA, Warman and colleagues [49] knocked in exons 3 and 4 (exon 3 houses the G171V and A214V mutation), leaving those intronic regions and the remainder of the gene intact (and untagged), with the exception of a floxed Neo cassette 5′ of exon 3. In stark contrast to the findings of Yadav et al. [4], Warman and colleagues [49] demonstrated that mice carrying single copies of the osteoblast-specific (using the Col2.3-Cre transgene) Lrp5 gain-of-function mutations exhibited significantly increased femoral bone mass, compared to knock-in mice not harboring the Col2.3-Cre transgene. Since Lrp5 expression was regulated by the endogenous promoter sequence, the gain in bone mass observed by Warman’s group [49] was unlikely to be the result of increased expression, but rather a direct result of expressing physiologic levels of G171V or A214V mutations in osteoblasts. It is possible that Yadav et al. [4] overlooked a skeletal phenotype in their mice carrying the HBM mutation of Lrp5 (G171V) as only one skeletal site (lumbar vertebrae) and one type of bone (trabecular bone) was apparently assessed in their studies. Alternatively, the knock-in generated by Warman’s group [49] was reported to have higher femoral trabecular bone mass, but no data on vertebral bone mass have been reported.
The data of Yadav et al. [4] are also difficult to reconcile with the observed integral role of LRP5 in skeletal mechanotransduction. Sawakami et al. [50] found the skeletal anabolic response to mechanical loading to be completely blocked in Lrp5−/− mice. For this observation to be mediated indirectly by gut-derived 5-HT it would require the loaded site to somehow alter LRP5 signaling in the gut to modify circulating 5-HT levels. The altered level of circulating 5-HT would then need to be transported back precisely to the loaded skeletal site to inhibit the site-specific bone formation response that occurs with loading. This does not seem feasible. Though, it is possible that Lrp5−/− mice have deficient mechanosensitivity simply because of their elevated circulating 5-HT levels, with LRP5 not actually being involved in mechanotransduction. This would require 5-HT to influence bone cell mechanosensitivity, a hypothesis not supported by the observation that mice possessing a null mutation in the 5-htt gene have no change in their ability to respond to mechanical stimuli [3]. Further, primary osteoblasts isolated from Lrp5−/− mice exhibit altered responses to fluid shear stress (mechanical loading) indicating a direct skeletal role of LRP5 in mechanotransduction [50]. It is possible that mechanical loading and other stimuli produce a local environment that promotes direct rather than indirect skeletal effects of LRP5. This may explain why Sawakami et al. [50] showed Lrp5−/− mice to have deficient osteoblast differentiation but not proliferation in response to mechanical loading, whereas the indirect skeletal effects of LRP5 in Yadav et al.’s [4] animals resulted exclusively from changes in osteoblast proliferation.
The preceding evidence suggests LRP5 does have direct skeletal effects, in addition to the indirect effects clearly demonstrated by Yadav et al. [4]. This is supported by clinical genome-wide studies which consistently identify LRP5 as contributing to the heritable component of bone mass whereas no 5-HT related genes have yet to be found [51–53]. Much further research will be required before the direct and indirect effects of LRP5 are completely understood. In addition, it is not yet possible to state that LRP5 is not a genuine co-receptor for Wnts in bone, as suggested by Yadav et al. [4]. It has been shown that bone cells unresponsive to Wnt acquire canonical Wnt responsiveness when transfected with LRP5 [43, 54]. Similarly, these cells lose their ability to respond to Wnt if transfected with an LRP5 mutant lacking the cytoplasmic C-terminal tail, known to be required for intracellular transduction via Axin recruitment to the plasma membrane. These studies demonstrate that LRP5 is a genuine Wnt receptor [55].
Is there more to know about skeletal 5-HT signaling?
In addition to clarifying the direct and indirect skeletal effects of LRP5, and whether LRP5 is a genuine co-receptor for Wnts, the skeletal role of 5-HT requires much elaboration. 5-HT clearly influences the skeleton; however, many questions remain. It remains unknown as to how LRP5 affects Tph1 expression in EC cells of the gut, with there possibly being a yet to be identified ligand for gut LRP5 mediating a currently unknown molecular pathway leading to altered Tph1 expression. It also remains to be shown how 5-HT synthesized in the gut reaches bone cells to activate 5-HT receptors. Yadav et al. [4] demonstrated 5-HT transport to be via the CV system, as evident by elevated serum 5-HT levels. However, assessment of serum 5-HT levels provides only a picture of whole-blood (intra- and extra-cellular) 5-HT levels. As indicated earlier, most circulating 5-HT (>95%) is stored intracellularly in platelets in dense granules. This sequestered 5-HT is unlikely to be the source for the skeletal effects of 5-HT as it is typically released only following platelet activation which occurs during clotting. Instead, the more likely source of skeletal 5-HT is the small amount (<5% of circulating 5-HT) in the plasma. This 5-HT is extracellular enabling it to function as a hormone that binds with osteoblastic 5-HT receptors. The influence of LRP5 on circulating platelet-free plasma levels of 5-HT is currently unknown, and the consequences of LRP5-mediated elevation of circulating 5-HT on other peripheral 5-HT sensitive systems (including the GI tract and CV system) also requires consideration. Similarly, the initial motivating observation that Tph1 is the most differentially expressed gene in Lrp5−/− mice requires further exploration as this does not fit with gut-derived 5-HT being the sole source of skeletal 5-HT, albeit the data obtained from genetically manipulated animal models are convincing.
The skeletal effects of 5-HT were found to involve the osteoblastic 5-HT1B receptor, with activation of this receptor leading to reduced bone formation via a decrease in osteoblast proliferation [4]. This may be one mechanism by which 5-HT influences the skeleton, yet the possibility for other mechanisms and effects of 5-HT exist. Others have shown the presence and functionality of 5-HT signaling in osteoclastic- and osteocytic-like cells [15–17], and the presence of 5-HT receptors other than 5-HT1B in osteoblasts [2, 15–19, 22, 29]. In particular, Collet et al. [29] found global deletion of Htr2b in mice to cause a skeletal phenotype of reduced bone mass and formation, with ex vivo cell cultures from these animals showing decreased osteoblast proliferation, differentiation and osteogenic capacity. Yadav et al. [4] did not observe a skeletal phenotype following osteoblast-specific deletion of Htr2b in their mice; however, this contrasting finding may have resulted from the driving of Htr2b effects only in mature osteoblasts and osteocytes, and not at earlier time points in the osteoblastic lineage. The reduced proliferation of osteoblasts from animals lacking functional 5-HT2B receptors observed by Collet et al. [29] suggests 5-HT activation of these receptors may have beneficial bone effects. Thus, it is possible that 5-HT has both anabolic and catabolic skeletal effects, with the prevailing effect being determined by the extracellular concentration of 5-HT and the respective 5-HT receptor/s activated. Yadav et al. [4] did not find a change in proliferation of primary osteoblasts from Htr2b null mutant mice when treated with 5-HT; however, they investigated the effect of a single 5-HT concentration (50 μM), a concentration up to 50,000 times greater than estimated in vivo platelet-free plasma 5-HT concentrations (1 nM) [56]. Investigation of a more physiological 5-HT concentration may have yielded a different result as bone cell responses to 5-HT have been observed to be concentration dependent [2, 17, 19].
The negative skeletal effects of elevated circulating 5-HT also do not explain the negative skeletal effects of SSRIs. Administration of a single-dose of an SSRI (fluoxetine) transiently increases plasma 5-HT levels [56]; however, chronic administration of the same SSRI over 1–2 weeks results in substantial reductions in both whole-blood and plasma 5-HT levels [56, 57]. Applying the findings of Yadav et al. [4], the reduction in circulating 5-HT with chronic SSRI administration suggests these agents would lead to increased osteoblast proliferation and bone formation, and a HBM phenotype. Preclinical and clinical studies indicate the opposite and, thus, other explanations for the negative skeletal effects of SSRIs need to be explored. One possible scenario is that SSRIs impact the skeleton by directly inhibiting the 5-HTT located on bone cell membranes. This may increase local 5-HT levels, in spite of decreased circulating 5-HT, by reducing its removal from the bone cell microenvironment. Another possible scenario is that inhibition of the 5-HTT induces skeletal effects via a yet to be identified central or upstream peripheral effect. These hypotheses obviously require further exploration.
The skeletal influence of altered 5-HT levels in clinical populations also needs to be investigated. Yadav et al. [4] provided preliminary data to suggest that patients with OPPG and a HBM phenotype have elevated and decreased circulating 5-HT levels, respectively [4]. These data are consistent with their preclinical data; however, require confirmation due to the investigation of very small sample sizes (OPPG, n=3; HBM, n=2) and the fact that both patients and controls had circulating 5-HT levels within the normal expected clinical range. In addition, there is a need to explore skeletal status in clinical conditions wherein circulating 5-HT levels are altered. One such condition is carcinoid syndrome which results from GI tract tumors (‘carcinoids’) producing extremely high circulating levels of 5-HT [58].
Summary
Mounting evidence supports 5-HT as an important regulatory compound in bone, with the most conclusive data being recently provided by the eloquent work of Yadav et al. [4]. They clearly demonstrated the existence of a novel regulatory pathway whereby gut-derived 5-HT mediated the complete skeletal effects of LRP5. The overwhelming evidence leaves little doubt as to whether LRP5 has indirect skeletal effects; however, many questions remain in relation to the LRP5–5-HT–osteoblast pathway (Fig. 5). In particular, important questions remain regarding whether LRP5 also has direct skeletal effects and whether these effects are mediated by the Wnt/β-catenin pathway. Irrespective of the outcome of future studies investigating the direct skeletal effects of LRP5, targeting of components of the Wnt/β-catenin pathway appears promising for bone anabolism. Whether targeting of 5-HT signaling can also be developed as an intervention for bone, without compromise to other 5-HT sensitive systems, will be determined with further work. One starting point may be the investigation of orally-active, peripheral-specific inhibitors of Tph1 [59, 60]. These agents selectively inhibit 5-HT synthesis in the periphery as they are unable to cross the blood-brain barrier and, based on the work of Yadav et al. [4], should be potently anabolic within the skeleton. Similarly, further work is required to establish the full skeletal effects of agents currently being used to modulate 5-HT signaling for the management of conditions in other systems. This includes the clinically popular SSRIs which inhibit the 5-HTT for the management of major depressive disorder and other affective conditions.
Figure 5.
Unanswered questions regarding the low-density lipoprotein receptor-related protein 5 (LRP5)—5-hydroxytryptamine (5-HT)—osteoblast pathway.
1) What is the ligand for LRP5 within enterochromaffin cells?
2) What is the molecular pathway between LRP5 and tryptophan hydroxylase 1 (Tph1) within enterochromaffin cells?
3) Does LRP5-mediated 5-HT synthesis by enterochromaffin cells have any local effects on the gastrointestinal (GI) tract?
4) How are LRP5-mediated changes in circulating 5-HT levels transported to bone cells (i.e. is it sequestered in platelets or free within the plasma)?
5) What effect does LRP5-mediated changes in circulating 5-HT levels have on other peripheral 5-HT sensitive systems, including heart valves and the pulmonary circulation?
6) Does activation of the 5-HT1B receptor completely explain the skeletal effects of 5-HT or are other 5-HT receptors involved depending on the prevailing conditions?
7) Does 5-HT solely influence osteoblast proliferation or does it also influence differentiation depending on the prevailing conditions?
8) Are there direct skeletal effects of LRP5 under alternative conditions (i.e. at different stages within the osteoblastic lineage or during mechanical loading)?
9) What is the role of the osteoblastic 5-HT transporter (5-HTT) in regulating the skeletal effects of gut-derived 5-HT?
10) Does the LRP5-mediated change in circulating 5-HT levels influence the functional 5-HT pathways observed in osteoclasts?
Acknowledgments
Completion of this review was enabled by support from National Institutes of Health grants AR-052018 (to M.M.B.), AR-051926 (to E.M.H.) and AR-53237 (to A.G.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rapport MM, Green AA, Page IH. Serum vasoconstrictor (serotonin). IV. Isolation and characterization. J Biol Chem. 1948;176:1243–51. [PubMed] [Google Scholar]
- 2.Bliziotes MM, Eshleman AJ, Zhang X-W, Wiren KM. Neurotransmitter action in osteoblasts: expression of a functional system for serotonin receptor activation and reuptake. Bone. 2001;29:477–86. doi: 10.1016/s8756-3282(01)00593-2. [DOI] [PubMed] [Google Scholar]
- 3.Warden SJ, Robling AG, Sanders MS, Bliziotes MM, Turner CH. Inhibition of the serotonin (5-hydroxytryptamine) transporter reduces bone accrual during growth. Endocrinology. 2005;146:685–93. doi: 10.1210/en.2004-1259. [DOI] [PubMed] [Google Scholar]
- 4.Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schutz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, Mann JJ, Hen R, Ducy P, Karsenty G. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135:825–37. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 6.aan het Rot M, Mathew SJ, Charney DS. Neurobiological mechanisms in major depressive disorder. CMAJ. 2009;180:305–13. doi: 10.1503/cmaj.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arroll B, Macgillivray S, Ogston S, Reid I, Sullivan F, Williams B, Crombie I. Efficacy and tolerability of tricyclic antidepressants and SSRIs compared with placebo for treatment of depression in primary care: a meta-analysis. Ann Fam Med. 2005;3:449–56. doi: 10.1370/afm.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Wade PR, Chen J, Jaffe B, Kassem IS, Blakely RD, Gershon MD. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci. 1996;16:2352–64. doi: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trindade E, Menon D, Topfer LA, Coloma C. Adverse effects associated with selective serotonin reuptake inhibitors and tricyclic antidepressants: a meta-analysis. CMAJ. 1998;159:1245–52. [PMC free article] [PubMed] [Google Scholar]
- 11.Spiller R. Serotonin and GI clinical disorders. Neuropharmacology. 2008;55:1072–80. doi: 10.1016/j.neuropharm.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Ni W, Watts SW. 5-hydroxytryptamine in the cardiovascular system: focus on the serotonin transporter (SERT) Clin Exp Pharmacol Physiol. 2006;33:575–83. doi: 10.1111/j.1440-1681.2006.04410.x. [DOI] [PubMed] [Google Scholar]
- 13.Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104–7. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- 14.Warden SJ, Bliziotes MM, Wiren KM, Eshleman AJ, Turner CH. Neural regulation of bone and the skeletal effects of serotonin (5-hydroxytryptamine) Mol Cell Endocrinol. 2005;242:1–9. doi: 10.1016/j.mce.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Battaglino R, Fu J, Spate U, Ersoy U, Joe M, Sedaghat L, Stashenko P. Serotonin regulates osteoclast differentiation via its transporter. J Bone Miner Res. 2004;19:1420–31. doi: 10.1359/JBMR.040606. [DOI] [PubMed] [Google Scholar]
- 16.Bliziotes M, Eshleman A, Burt-Pichat B, Zhang XW, Hashimoto J, Wiren K, Chenu C. Serotonin transporter and receptor expression in osteocytic MLO-Y4 cells. Bone. 2006;39:1313–21. doi: 10.1016/j.bone.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gustafsson BI, Thommesen L, Stunes AK, Tommeras K, Westbroek I, Waldum HL, Slordahl K, Tamburstuen MV, Reseland JE, Syversen U. Serotonin and fluoxetine modulate bone cell function in vitro. J Cell Biochem. 2006;98:139–51. doi: 10.1002/jcb.20734. [DOI] [PubMed] [Google Scholar]
- 18.Hirai T, Tokumo K, Tsuchiya D, Nishio H. Expression of mRNA for 5-HT2 receptors and proteins related to inactivation of 5-HT in mouse osteoblasts. J Pharmacol Sci. 2009;109:319–23. doi: 10.1254/jphs.08243sc. [DOI] [PubMed] [Google Scholar]
- 19.Westbroek I, van der Plas A, de Rooij KE, Klein-Nulend J, Nijweide PJ. Expression of serotonin receptors in bone. J Biol Chem. 2001;276:28961–8. doi: 10.1074/jbc.M101824200. [DOI] [PubMed] [Google Scholar]
- 20.Warden SJ, Haney EM. Skeletal effects of serotonin (5-hydroxytryptamine) transporter inhibition: Evidence from in vitro and animal-based studies. J Musculoskelet Neuronal Interact. 2008;8:121–32. [PMC free article] [PubMed] [Google Scholar]
- 21.Bliziotes M, Eshleman A, Burt-Pichat B, Zhang XW, Hashimoto J, Wiren K, Chenu C. Serotonin transporter and receptor expression in osteocytic MLO-Y4 cells. Bone. 2006;39:1313–21. doi: 10.1016/j.bone.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Locker M, Bitard J, Collet C, Poliard A, Mutel V, Launay JM, Kellermann O. Stepwise control of osteogenic differentiation by 5-HT(2B) receptor signaling: nitric oxide production and phospholipase A2 activation. Cell Signal. 2006;18:628–39. doi: 10.1016/j.cellsig.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Battaglino R, Fu J, Spate U, Ersoy U, Joe M, Sedaghat L, Stashenko P. Serotonin regulates osteoclast differentiation via its transporter. J Bone Miner Res. 2004;19:1420–31. doi: 10.1359/JBMR.040606. [DOI] [PubMed] [Google Scholar]
- 24.Bonnet N, Bernard P, Beaupied H, Bizot JC, Trovero F, Courteix D, Benhamou CL. Various effects of antidepressant drugs on bone microarchitectecture, mechanical properties and bone remodeling. Toxicol Appl Pharmacol. 2007;221:111–8. doi: 10.1016/j.taap.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Warden SJ, Hassett SM, Bond JL, Rydberg J, Grogg J, Hilles E, Fuchs RK, Bliziotes MM, Turner CH. Psychotropic drugs have contrasting skeletal effects that are independent of their negative effects on activity levels. J Bone Miner Res. 2008;23:S481. doi: 10.1016/j.bone.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warden SJ, Nelson IR, Fuchs RK, Bliziotes MM, Turner CH. Serotonin (5-hydroxytryptamine) transporter inhibition causes bone loss in adult mice independently of estrogen deficiency. Menopause. 2008;15:1176–83. doi: 10.1097/gme.0b013e318173566b. [DOI] [PubMed] [Google Scholar]
- 27.Westbroek I, Waarsing JH, van Leeuwen JP, Waldum H, Reseland JE, Weinans H, Syversen U, Gustafsson BI. Long-term fluoxetine administration does not result in major changes in bone architecture and strength in growing rats. J Cell Biochem. 2007;101:360–8. doi: 10.1002/jcb.21177. [DOI] [PubMed] [Google Scholar]
- 28.Battaglino R, Vokes M, Schulze-Spate U, Sharma A, Graves D, Kohler T, Muller R, Yoganathan S, Stashenko P. Fluoxetine treatment increases trabecular bone formation in mice. J Cell Biochem. 2007;100:1387–94. doi: 10.1002/jcb.21131. [DOI] [PubMed] [Google Scholar]
- 29.Collet C, Schiltz C, Geoffroy V, Maroteaux L, Launay JM, de Vernejoul MC. The serotonin 5-HT2B receptor controls bone mass via osteoblast recruitment and proliferation. FASEB J. 2008;22:418–27. doi: 10.1096/fj.07-9209com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gustafsson BI, Westbroek I, Waarsing JH, Waldum H, Solligard E, Brunsvik A, Dimmen S, van Leeuwen JP, Weinans H, Syversen U. Long-term serotonin administration leads to higher bone mineral density, affects bone architecture, and leads to higher femoral bone stiffness in rats. J Cell Biochem. 2006;97:1283–91. doi: 10.1002/jcb.20733. [DOI] [PubMed] [Google Scholar]
- 31.Sibilia V, Pagani F, Lattuada N, Greco A, Guidobono F. Linking chronic tryptophan deficiency with impaired bone metabolism and reduced bone accrual in growing rats. J Cell Biochem. doi: 10.1002/jcb.22189. (in press) [DOI] [PubMed] [Google Scholar]
- 32.Yirmiya R, Goshen I, Bajayo A, Kreisel T, Feldman S, Tam J, Trembovler V, Csernus V, Shohami E, Bab I. Depression induces bone loss through stimulation of the sympathetic nervous system. Proc Natl Acad Sci U S A. 2006;103:16876–81. doi: 10.1073/pnas.0604234103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–30. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- 34.Holmes A, Yang RJ, Murphy DL, Crawley JN. Evaluation of antidepressant-related behavioral responses in mice lacking the serotonin transporter. Neuropsychopharmacology. 2002;27:914–23. doi: 10.1016/S0893-133X(02)00374-3. [DOI] [PubMed] [Google Scholar]
- 35.Weintrob N, Cohen D, Klipper-Aurbach Y, Zadik Z, Dickerman Z. Decreased growth during therapy with selective serotonin reuptake inhibitors. Arch Pediatr Adolesc Med. 2002;156:696–701. doi: 10.1001/archpedi.156.7.696. [DOI] [PubMed] [Google Scholar]
- 36.Tjurmina OA, Armando I, Saavedra JM, Li Q, Murphy DL. Life-long serotonin reuptake deficiency results in complex alterations in adrenomedullary responses to stress. Ann N Y Acad Sci. 2004;1018:99–104. doi: 10.1196/annals.1296.011. [DOI] [PubMed] [Google Scholar]
- 37.Chen JJ, Li Z, Pan H, Murphy DL, Tamir H, Koepsell H, Gershon MD. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001;21:6348–61. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–55. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- 39.Diem SJ, Blackwell TL, Stone KL, Yaffe K, Haney EM, Bliziotes MM, Ensrud KE. Use of antidepressants and rates of hip bone loss in older women: the study of osteoporotic fractures. Arch Intern Med. 2007;167:1240–5. doi: 10.1001/archinte.167.12.1240. [DOI] [PubMed] [Google Scholar]
- 40.Richards JB, Papaioannou A, Adachi JD, Joseph L, Whitson HE, Prior JC, Goltzman D. Effect of selective serotonin reuptake inhibitors on the risk of fracture. Arch Intern Med. 2007;167:188–94. doi: 10.1001/archinte.167.2.188. [DOI] [PubMed] [Google Scholar]
- 41.Haney EM, Warden SJ, Bliziotes MM. The effects of medications that block the serotonin transporter on bone health in older adults: implications for screening. Bone. (submitted) [Google Scholar]
- 42.Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–21. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 43.Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–23. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 44.Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, Lappe MM, Spitzer L, Zweier S, Braunschweiger K, Benchekroun Y, Hu X, Adair R, Chee L, FitzGerald MG, Tulig C, Caruso A, Tzellas N, Bawa A, Franklin B, McGuire S, Nogues X, Gong G, Allen KM, Anisowicz A, Morales AJ, Lomedico PT, Recker SM, Van Eerdewegh P, Recker RR, Johnson ML. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70:11–9. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baron R, Rawadi G. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology. 2007;148:2635–43. doi: 10.1210/en.2007-0270. [DOI] [PubMed] [Google Scholar]
- 46.Glantschnig H, Hampton R, Wei N, Scott K, Nantermet P, Zhao J, Chen F, Fisher J, Su Q, Pennypacker B, Cusick T, Sandhu P, Reszka A, Strohl W, Flores O, Wang F, Kimmel D, An Z. Fully human anti-DKK1 antibodies increase bone formation and resolve osteopenia in mouse models of estrogen-deficiency induced bone loss. J Bone Miner Res. 2008;23:S60. [Google Scholar]
- 47.Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, Gao Y, Shalhoub V, Tipton B, Haldankar R, Chen Q, Winters A, Boone T, Geng Z, Niu QT, Ke HZ, Kostenuik PJ, Simonet WS, Lacey DL, Paszty C. Sclerostin antibody treatment increases bone formation, bone mass and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009;24:578–88. doi: 10.1359/jbmr.081206. [DOI] [PubMed] [Google Scholar]
- 48.Babij P, Zhao W, Small C, Kharode Y, Yaworsky PJ, Bouxsein ML, Reddy PS, Bodine PV, Robinson JA, Bhat B, Marzolf J, Moran RA, Bex F. High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res. 2003;18:960–74. doi: 10.1359/jbmr.2003.18.6.960. [DOI] [PubMed] [Google Scholar]
- 49.Cui Y, Niziolek PJ, Robling AG, Warman ML. G171V and A214V Lrp5 knock-in mice have increased bone mass and strength, and can help precisely define the in vivo functions of Lrp5 during bone growth and homeostasis. J Bone Miner Res. 2008;23:S2. [Google Scholar]
- 50.Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, Li J, Maye P, Rowe DW, Duncan RL, Warman ML, Turner CH. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem. 2006;281:23698–711. doi: 10.1074/jbc.M601000200. [DOI] [PubMed] [Google Scholar]
- 51.Kaufman JM, Ostertag A, Saint-Pierre A, Cohen-Solal M, Boland A, Van Pottelbergh I, Toye K, de Vernejoul MC, Martinez M. Genome-wide linkage screen of bone mineral density (BMD) in European pedigrees ascertained through a male relative with low BMD values: evidence for quantitative trait loci on 17q21–23, 11q12–13, 13q12–14, and 22q11. J Clin Endocrinol Metab. 2008;93:3755–62. doi: 10.1210/jc.2008-0678. [DOI] [PubMed] [Google Scholar]
- 52.Richards JB, Rivadeneira F, Inouye M, Pastinen TM, Soranzo N, Wilson SG, Andrew T, Falchi M, Gwilliam R, Ahmadi KR, Valdes AM, Arp P, Whittaker P, Verlaan DJ, Jhamai M, Kumanduri V, Moorhouse M, van Meurs JB, Hofman A, Pols HA, Hart D, Zhai G, Kato BS, Mullin BH, Zhang F, Deloukas P, Uitterlinden AG, Spector TD. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet. 2008;371:1505–12. doi: 10.1016/S0140-6736(08)60599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Meurs JB, Trikalinos TA, Ralston SH, Balcells S, Brandi ML, Brixen K, Kiel DP, Langdahl BL, Lips P, Ljunggren O, Lorenc R, Obermayer-Pietsch B, Ohlsson C, Pettersson U, Reid DM, Rousseau F, Scollen S, Van Hul W, Agueda L, Akesson K, Benevolenskaya LI, Ferrari SL, Hallmans G, Hofman A, Husted LB, Kruk M, Kaptoge S, Karasik D, Karlsson MK, Lorentzon M, Masi L, McGuigan FE, Mellstrom D, Mosekilde L, Nogues X, Pols HA, Reeve J, Renner W, Rivadeneira F, van Schoor NM, Weber K, Ioannidis JP, Uitterlinden AG. Large-scale analysis of association between LRP5 and LRP6 variants and osteoporosis. JAMA. 2008;299:1277–90. doi: 10.1001/jama.299.11.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, 2nd, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157:303–14. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baron R. Wnt signaling, LRP5 and gut sertonin: have we been targeting the right pathway for the wrong reasons? IBMS BoneKEy. 2009;6:86–93. [Google Scholar]
- 56.Rothman RB, Zolkowska D, Baumann MH. Serotonin (5-HT) transporter ligands affect plasma 5-HT in rats. Ann N Y Acad Sci. 2008;1139:268–84. doi: 10.1196/annals.1432.042. [DOI] [PubMed] [Google Scholar]
- 57.Zolkowska D, Baumann MH, Rothman RB. Chronic fenfluramine administration increases plasma serotonin (5-hydroxytryptamine) to nontoxic levels. J Pharmacol Exp Ther. 2008;324:791–7. doi: 10.1124/jpet.107.132654. [DOI] [PubMed] [Google Scholar]
- 58.Kulke MH, Mayer RJ. Carcinoid tumors. N Engl J Med. 1999;340:858–68. doi: 10.1056/NEJM199903183401107. [DOI] [PubMed] [Google Scholar]
- 59.Liu Q, Yang Q, Sun W, Vogel P, Heydorn W, Yu XQ, Hu Z, Yu W, Jonas B, Pineda R, Calderon-Gay V, Germann M, O’Neill E, Brommage R, Cullinan E, Platt K, Wilson A, Powell D, Sands A, Zambrowicz B, Shi ZC. Discovery and characterization of novel tryptophan hydroxylase inhibitors that selectively inhibit serotonin synthesis in the gastrointestinal tract. J Pharmacol Exp Ther. 2008;325:47–55. doi: 10.1124/jpet.107.132670. [DOI] [PubMed] [Google Scholar]
- 60.Shi ZC, Devasagayaraj A, Gu K, Jin H, Marinelli B, Samala L, Scott S, Stouch T, Tunoori A, Wang Y, Zang Y, Zhang C, Kimball SD, Main AJ, Sun W, Yang Q, Nouraldeen A, Yu XQ, Buxton E, Patel S, Nguyen N, Swaffield J, Powell DR, Wilson A, Liu Q. Modulation of peripheral serotonin levels by novel tryptophan hydroxylase inhibitors for the potential treatment of functional gastrointestinal disorders. J Med Chem. 2008;51:3684–7. doi: 10.1021/jm800338j. [DOI] [PubMed] [Google Scholar]