Abstract
OBJECTIVE
In this study, we investigate the effects of a soft bone hemostatic wax comprised of water-soluble alkylene oxide copolymers (Ostene; Ceremed, Inc., Los Angeles, CA) on bone healing in a rat calvaria defect model. We compared the effects with a control (no hemostatic agent) and bone wax, an insoluble and nonresorbable material commonly used for bone hemostasis.
METHODS
Two bilateral 3-mm circular noncritical-sized defects were made in the calvariae of 30 rats. Alkylene oxide copolymer or bone wax was applied or no hemostatic material was used (control). After 3, 6, and 12 weeks, rats were sacrificed and the calvariae excised. Bone healing, expressed as fractional bone volume (± standard error of the mean), was measured by microcomputed tomography.
RESULTS
Immediate hemostasis was achieved equally with bone wax and alkylene oxide copolymer. Bone wax-filled defects remained unchanged at all time points with negligible healing observed. At 3 weeks, no evidence of alkylene oxide copolymer was observed at the application site, with fractional bone volume significantly greater than bone wax-treated defects (0.20 ± 0.03 versus 0.02 ± 0.01; P = 0.0003). At 6 and 12-weeks, alkylene oxide copolymer-treated defects continued to show significantly greater healing versus bone wax (0.18 ± 0.04 versus 0.05 ± 0.01 and 0.31 ± 0.04 versus 0.06 ± 0.02, respectively). At all time points, alkylene oxide copolymer-treated and control defects showed good healing with no significant difference.
CONCLUSION
Alkylene oxide copolymer is an effective hemostatic agent that does not inhibit osteogenesis or bone healing.
Keywords: Alkylene oxide copolymer, Bone, Bone wax, Calvaria, Hemostasis, Osteogenesis
For more than a century, softened beeswax (bone wax) has been used to achieve bone hemostasis; today, because of its ease of application and effectiveness, it remains one of the more common methods used. Its application in a clinical setting was first described in 1892 by Parker (27) and Horsley (16). Today, commercial formulations of bone wax are primarily comprised of beeswax, softened with 12% isopropyl palmitate and/or up to 30% soft paraffin wax (11, 13, 31, 36).
Numerous complications have been attributed to bone wax primarily because beeswax is an insoluble material, is not metabolized or resorbed, and, hence, remains indefinitely at the site of application (31, 40). The most common complications recognized as being associated with bone wax are inhibition of osteogenesis (2, 5, 8, 29, 30, 31, 40), infection (3, 12, 13, 19, 26, 31), foreign body response causing a giant cell reaction (“wax granuloma”), local inflammation, and pain (4, 11, 23, 29, 34, 36, 42). Other complications attributed to the use of bone wax include quadriplegia after spine surgery (10), lower extremity paralysis after thoracotomy (9), sigmoid sinus obstruction after mastoid surgery (15, 23), and cerebrospinal fluid leakage after cranial base surgery (7). The use of bone wax is one of few identified modifiable risk factors associated with mediastinitis after median sternotomy (6). Based on histological studies of autopsy cases, bone wax has been shown to interfere with sternal healing, causing chronic inflammation and giant cells up to 10 years after application (36).
Thus, good surgical practice minimizes the amount of bone wax used interoperatively. The use of bone wax is often avoided altogether in procedures in which fusion is critical for stability. Alternative topical hemostatic agents such as microfibrillar collagen, thrombin, gelatin, and oxidized cellulose have not supplanted the common use of bone wax, which may be due, in part, to bone wax’s immediacy and effectiveness of hemostasis, ease of use, low cost, and the fact that the aforementioned alternative bone hemostasic agents also interfere with bone healing and are not without complications (31).
The use of a water soluble wax, comprised solely of alkylene oxide block copolymers (poloxamers or Pluronics; BASF Corp., Florham Park, NJ), was first described by Wang et al. (39). Using a rat femur defect model, this water soluble wax achieved immediate and effective bone hemostasis, was absorbed within 48 hours, and did not interfere with bone healing. Alkylene oxide copolymers are non-metabolizable and are excreted, unchanged, primarily via renal excretion (14, 18).
Similarly, Ostene (Ceremed, Inc., Los Angeles, CA) is comprised solely of water soluble alkylene oxide copolymers and is a Food and Drug Administration-approved water soluble implant material indicated for use in the control of bleeding from bone surfaces (40). Hence, it is approved for use in cranial and spinal procedures as a bone hemostatic material. Because Ostene is water soluble, it does not remain at the site of application and should address all of the known reactions associated with bone wax.
To test the hypothesis that Ostene alkylene oxide copolymer does not impair bone healing, this study was designed to compare the effect of alkylene oxide copolymer versus no hemostasis on bone healing and to compare the effects of 2 effective bone hemostatic agents (alkylene oxide copolymer and bone wax) on bone healing in a noncritical-sized calvaria defect model.
MATERIALS AND METHODS
Materials
Alkylene oxide copolymer (Ostene) was provided by Ceremed Inc., and bone wax was purchased from Ethicon, Inc. (Summerfield, NJ).
Rat Calvaria Defect Model
The animal protocol (No. 2005–119–01) was approved by the University of California, Los Angeles, Chancellor’s Research Committee in conformity with the National Institutes of Health guidelines for the care and use of laboratory animals (Department of Health and Human Services publication No. [NIH] 85–23, revised 1985).
Noncritical-sized bilateral bone defects and microcomputed tomography (microCT) analyses were performed based on previous studies (1, 28, 37). Thirty 3-month-old male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were used for this study. Rats were anesthetized, and anesthesia was maintained with isoflurane. Surgery was performed using standard aseptic techniques. Two 3-mm bilateral, circular defects were made in the calvariae with a trephine drill under constant irrigation. Care was taken to avoid injury to the underlying dura mater. Sufficient alkylene oxide copolymer or bone wax was applied to the defect to achieve immediate hemostasis (7 defects per time point). Control defects had no hemostatic material added (6 defects per time point). For each group of 10 animals, bone wax, alkylene oxide copolymer, or control treatment of the bilateral defects was performed as follows, with the number of animals per pairing shown in brackets: bone wax/bone wax [2], alkylene oxide copolymer/alkylene oxide copolymer [2], control/control [1], bone wax/alkylene oxide copolymer [1], control/alkylene oxide copolymer [2], and control/bone wax [2]. The wound was closed with monofilament sutures. All animals were given buprenorphine (0.05 mg/kg) for 48 hours postsurgery.
At 3, 6 and 12 weeks after surgery, 10 rats were sacrificed using an intravenous overdose of pentobarbital, and the calvariae were excised. Calvariae were placed in neutral buffered formalin for 48 hours, washed in water for 8 hours, and stored in 70% ethanol. Photographs of the excised calvariae were taken and observed for remaining hemostatic agent.
Evaluation of Bleeding or Hemostasis
The surgeon (TLA) was blinded to whether alkylene oxide copolymer or bone wax was used to fill the bony defects. After application of alkylene oxide copolymer or bone wax, the surgeon was asked to comment on the handling and effectiveness of hemostasis. Subjective criteria included ease of application of the hemostatic material, the time from application of the material to cessation of bleeding, and whether hemostasis was maintained for the duration of the surgical procedure.
MicroCT Analysis
Calvariae were scanned in 70% ethanol using a Scanco40 scanner (μCT-40; Scanco Medical, Bassersdorf, Switzerland) with a voxel isotropic resolution of 15 μm and an x-ray energy of 55 KVp and 72 μA.
Five to six calvariae were accommodated in the scanning tube for each scan. Each scan time was approximately 3 hours and accounted for 1000 projections/180 degrees, 200 milliseconds integration time, and 2048 charge coupled device detector array. A calibration phantom of hydroxyapatite was used. Segmented images were obtained using a Gaussian filter (sigma, 1.2; support, 1). Threshold for the image binarization was set as 25% of the maximal grayscale for all the scans. Volumetric analysis was performed by obtaining volumes of interest, using the Scanco software to include only the mineralized tissue formed inward during healing and separate it from the rest of the calvaria. The architectural parameters obtained from the binarized volumes of interest were as follows: total volume (TV) in mm3, bone volume (BV) in mm3, and bone volume fraction (BV/TV). TV is the volume of the whole defect (diameter, 3 mm), BV represents the volume of the mineralized tissue formed during healing, and BV/TV is the relative bone volume which normalizes the volume of the mineralized tissue formed during healing by taking into account differences in thickness of the specimen.
Histology
After microCT analysis, calvariae were trimmed to size and then dehydrated in increasing concentrations of ethanol, cleared with xylene, and then embedded, undecalcified, in methyl methacrylate. Coronal sections were cut at a thickness of 5 μm from the middle of the defect using a microtome (Jung Supercut; Reichert-Jung, Nuβloch, Germany) and stained with toluidine blue (pH, 6.4). Photomicrographs of sections were obtained using a ScanScope XT System (Aperio Technologies, Inc., Vista, CA) at a magnification of ×20. Sections were examined by 2 independent experts who were blinded to the 3 treatment groups for mineralized bone quality; cellular activity, including osteoblasts and osteoid; and any evidence of fibrotic tissue formation within the defect site.
Statistical Analysis
MicroCT values (BV, TV, BV/TV) were reported as means and standard errors. Results were compared by 2-way analysis of variance with post-test repeated measures analyzed by the Bonferroni procedure (Prism; GraphPad Software Inc., San Diego, CA). A P value of less than 0.05 was considered statistically significant.
RESULTS
Immediate bone hemostasis was achieved in 100% of the defects treated with alkylene oxide copolymer or bone wax, and hemostasis was equally effective with either material. No differences in the handling characteristics or application of either material to the defect were noted.
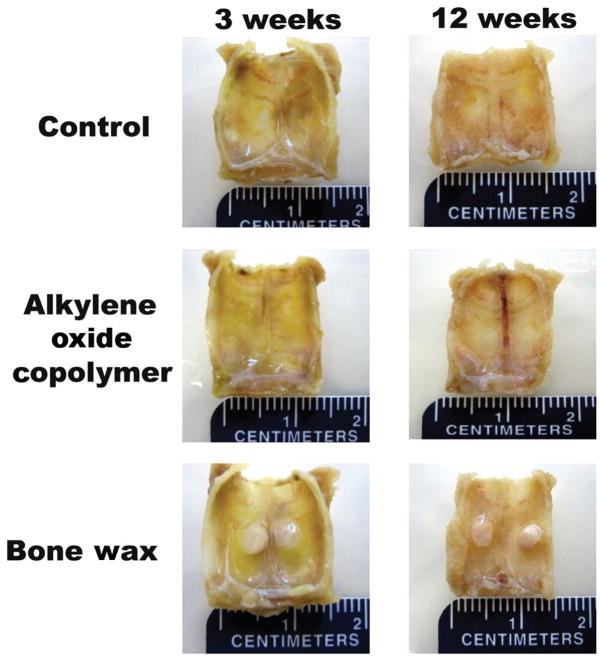
Figure 1 shows representative images of excised calvariae after 3 and 12 weeks. For 100% of the defects, bone wax remained unchanged at 12 weeks postsurgery, whereas no evidence of alkylene oxide copolymer was observed at 3 weeks postsurgery and was comparable in appearance to untreated (control) defects.
FIGURE 1.
Representative photographs of excised calvariae at 3 and 12 weeks after surgery.
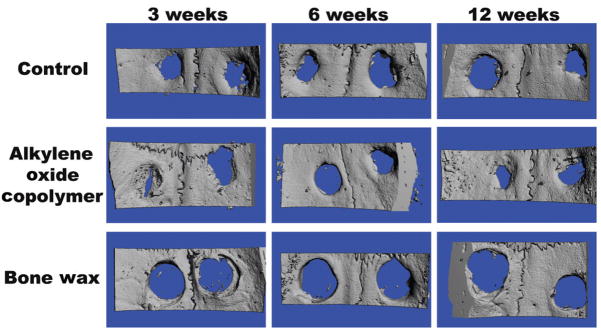
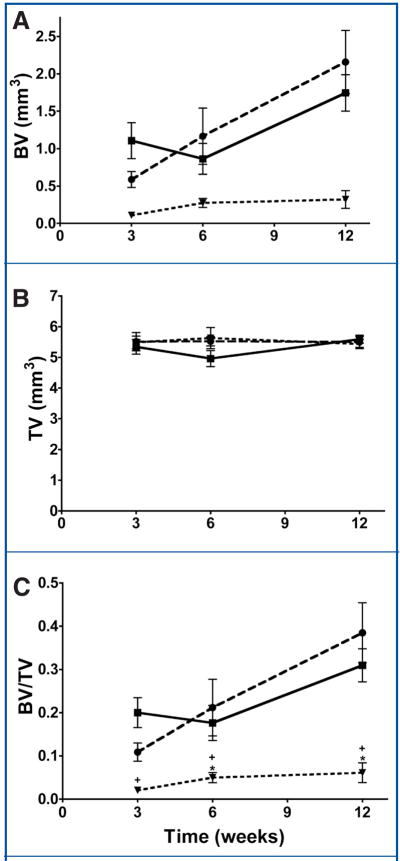
Representative microCT-derived three-dimensional renderings are shown in Figure 2, with mean BV, TV, and BV/TV data shown in Figure 3. The TV, representing the analyzed defect volume, was similar among groups and time-points (Fig. 3B).
FIGURE 2.
Representative microcomputed tomography (microCT)-derived, 3-dimensional renderings of rat calvariae at 3, 6 and 12 weeks after surgery.
FIGURE 3.
Graphs showing microCT data for control (untreated) (●), alkylene oxide copolymer-treated (■), and bone wax-treated (◀) defects at 3, 6, and 12 weeks after surgery. A, mean mineralized bone volume (BV, mm3). B, total volume (TV, mm3). C, bone volume fraction (BV/TV). Asterisks indicate a P value of less than 0.05.
For bone wax-treated defects, negligible healing of the defects was observed at 3, 6, and 12 weeks postsurgery, as represented by BV/TV values of 0.02 ± 0.01, 0.05 ± 0.01, and 0.06 ± 0.02, respectively (Fig. 3C). This is shown in the representative microCT images at 3 and 12 weeks (Fig. 2).
At 3 weeks postsurgery, the BV/TV values for alkylene oxide copolymer-treated and untreated (control) defects were 0.20 ± 0.01 and 0.11 ± 0.02, respectively, which were significantly greater than bone wax-treated defects (P = 0.0003 and 0.0014, respectively) (Fig. 3C). Although alkylene oxide copolymer-treated defects showed a higher mean BV/TV versus untreated (control) defects at 3 weeks, these data did not reach significance (P = 0.0550) (Fig. 3C).
At 6 and 12 weeks post-surgery, bone healing (BV/TV) in alkylene oxide copolymer-treated and untreated (control) defects was comparable (alkylene oxide copolymer, 0.18 ± 0.04; control, 0.21 ± 0.06 at 6 weeks; alkylene oxide copolymer, 0.31 ± 0.04; control, 0.38 ± 0.07 at 12 weeks), with rapid mineralization observed at all time points, indicative that alkylene oxide copolymer did not interfere with bone healing (Figs. 2 and 3). Alkylene oxide copolymer-treated and untreated (control) defects showed significant bone healing (BV/TV) compared with bone wax treated defects at 6 and 12 weeks (alkylene oxide copolymer/control; P = 0.0112/0.0233 and P = 0.0012/0.0062, respectively).
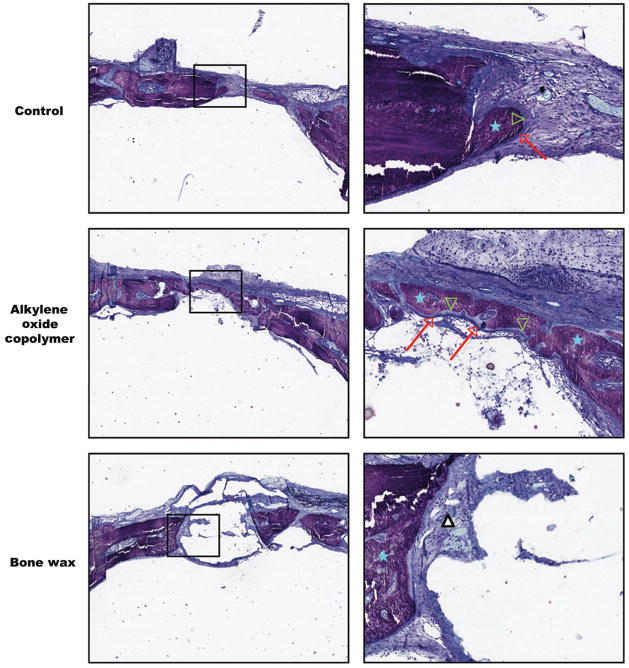
To verify the quality of bone healing observed with microCT, histology was performed on undecalcified sections. Representative histological images of coronal sections from the center of the defect at 3 weeks post-surgery are shown in Figure 4. Untreated (control) and alkylene oxide copolymer-treated defects demonstrated newly formed bone rich in cellular activity, as can be seen by the mineralization front of osteoblasts and osteoid. Bone wax treated defects at 3 weeks post-surgery showed minimal bone healing at the defect margins and mainly fibrotic tissue observed at the edges of the defect and around the site of the applied bone wax (Fig. 4). It is worthy to note that bone wax is not visible in the histology images because the aromatic solvent (xylene) used during tissue processing for the histological analyses dissolved the bone wax from the calvaria section. After 6 and 12 weeks post-surgery, continued bone healing was clearly evident for the untreated (control) and alkylene oxide copolymer-treated defects, with mineralized tissue extending toward the center of the defect. Conversely, negligible mineralization was observed at 6 and 12 weeks for bone wax-treated defects with fibrous tissue formation within the site of the defect and around the applied bone wax comparable to the observations at 3 weeks (images not shown).
FIGURE 4.
Histological analysis of bone healing in calvarial defects. Representative images of 5-μm thick coronal sections from the center of the defect at 3 weeks after surgery. Toluidine blue staining demonstrates mineralized bone (blue stars), osteoblasts (red arrows), and osteoid (green arrowheads) in the control and alkylene oxide copolymer-treated defects, with mostly fibrous tissue observed at the site of the bone wax treated defects (white triangle). Both low (× 2, left) and high magnification (× 10, right) are shown.
DISCUSSION
This investigation was designed to evaluate bone healing after application of a water soluble bone hemostasis material (alkylene oxide copolymer) using a rat calvaria noncritical-sized defect model. The purpose of using a noncritical-sized defect was to determine whether alkylene oxide copolymer affected bone healing compared with the absence of any hemostatic agent (control defects) or to bone wax, a widely used bone hemostatic material that has been reported to inhibit bone healing (30, 31, 39, 40).
Bone healing, as measured by high resolution microCT, progressed with time similarly to that in alkylene oxide copolymer and untreated defects, indicating that alkylene oxide copolymer does not interfere with bone healing. Similarly, using a rat femur defect model, the application of water soluble wax comprised of Pluronic copolymers as a bone hemostasis material showed good healing as early as 10 days after surgery, and healing up to 42 days was comparable to untreated defects, as determined from x-ray and histological analyses (39). In the clinical setting, minimal use and removal of excess bone wax is recommended due to its insoluble and nonmetabolizable nature; our findings suggest that the conservative use and removal of excess alkylene oxide copolymer after application to bone may not be necessary.
To our knowledge, the data in this report is the first to quantifiably show that a bone hemostasis material (alkylene oxide copolymer) does not interfere with bone healing when compared with no hemostasis material. It is worth noting that at 3 weeks, the mean BV/TV for alkylene oxide copolymer-treated defects was nearly 2-fold greater than untreated defects indicative of an early onset of bone healing compared with control, although these data did not reach significance(P = 0.0550). As alkylene oxide copolymer does not act biochemically but rather by mechanical occlusion, allowing clotting to occur upstream from transected vessels in cancellous bone, and because alkylene oxide copolymers are rapidly cleared and not metabolized (14, 18), one may postulate that this apparent early onset of healing is due to the absence of a clot within the defect combined with rapid clearance of the hemostatic material. These data clearly warrant further studies to fully elucidate whether improved early bone healing can be achieved with alkylene oxide copolymer compared with untreated defects.
Effective bone hemostasis is critical for many surgical procedures, and bone wax is one of the oldest agents in use today. The use of cautery and styptics (22) that are immediately damaging to tissues are either obsolete or have very limited application in modern bone hemostasis. As early as 1924, in Carson’s Modern Operative Surgery (38), the use of bone wax was recommended to prevent bone healing and to create a pseudo-arthroses as part of an arthroplasty procedure. The author expressed caution to avoid excessive use of bone wax and highly recommended research to find an alternative to this material (38). Recently, the application of bone wax was recommended to prevent heterotopic ossification of the elbow (20).
The adverse effects of bone wax have been well documented (3, 4, 8, 13, 19, 23, 29, 34, 36, 42) and are reviewed in detail elsewhere (31, 40). Several experimental bone hemostasis agents have been described in the literature, including oxidized cellulose/poly(ethylene glycol) (12), fibrin/collagen paste (33), gelatin paste (41), patient-derived fibrin sealant (21), polyorthoester (33, 35), and hydroxyapatite putty (25), but none of these are in widespread use. Alternative commercially available bone hemostatic agents, such as microfibrillar collagen, thrombin, gelatin, and oxidized cellulose, are reported to interfere with bone healing (11, 24, 31, 41). Similarly to bone wax, the use of gelatin is contraindicated for use in contaminated fields (31), and additional complications, primarily due to excessive swelling of the hemostatic material, are noted for gelatin, microfibrillar collagen, and oxidized cellulose (17, 31, 32). The continued use of bone wax may be due, in part, to the lack of effective alternative agents that are simple to use and achieve immediate and sustained bone hemostasis. Undesirable handling properties and the immediate cost of the hemostatic agent may also be a factor. As a recently introduced bone hemostatic material, the cost of a 2.5-g unit of Ostene is less than $75, comparable in price to porcine gelatin (Gelfoam; Pharmacia & Upjohn, Kalamazoo, MI). The use of Ostene may bring about a marked overall cost reduction when compared with the potential subsequent cost of treating adverse reactions to bone wax (e.g., reoperation, management of infection and pain).
CONCLUSION
Alkylene oxide copolymer achieved immediate and effective bone hemostasis equivalent to bone wax. Compared with untreated (control) defects, alkylene oxide copolymer did not affect osteogenesis or bone healing.
Acknowledgments
We thank Renata C. Pereira, Ph.D., (Department of Pediatric Nephrology, UCLA School of Medicine, Los Angeles, CA) for preparation and staining of the histology sections and Sarah Dry, M.D., and Dorina Gui, M.D., Ph.D., (Tissue Procurement Core Laboratory, Department of Pathology and Laboratory Medicine, UCLA School of Medicine, Los Angeles, CA) for providing the microscopic images of toluidine blue-stained sections.
ABREVIATIONS
- BV
bone volume
- microCT
microcomputed tomography
- TV
total volume
Footnotes
Disclosure
This study was conducted using Ostene, manufactured by Ceremed, Inc. The husband of Clara E. Magyar, Ph.D., (Jonathan K. Armstrong, Ph.D.) is an employee and shareholder of Ceremed, Inc. Financial support for this work was provided by Ceremed, Inc.; 3643 Lenawee Avenue; Los Angeles, CA, 90016.
Contributor Information
Clara E. Magyar, Department of Diagnostic and Surgical Sciences, School of Dentistry, University of California, Los Angeles, Los Angeles, California
Tara L. Aghaloo, Department of Diagnostic and Surgical Sciences, School of Dentistry, University of California, Los Angeles, Los Angeles, California
Elisa Atti, Department of Diagnostic and Surgical Sciences, School of Dentistry, University of California, Los Angeles, Los Angeles, California
Sotirios Tetradis, Department of Diagnostic and Surgical Sciences, School of Dentistry, University of California, Los Angeles, Los Angeles, California
References
- 1.Aghaloo TL, Amantea CM, Cowan CM, Richardson JA, Wu BM, Parhami F, Tetradis S. Oxysterols enhance osteoblast differentiation in vitro and bone healing in vivo. J Orthop Res. 2007;25:1488–1497. doi: 10.1002/jor.20437. [DOI] [PubMed] [Google Scholar]
- 2.Alberius P, Klinge B, Sjögren S. Effects of bone wax on rabbit cranial bone lesions. J Craniomaxillofac Surg. 1987;15:63–67. doi: 10.1016/s1010-5182(87)80020-3. [DOI] [PubMed] [Google Scholar]
- 3.Allison RT. Foreign body reactions and an associated histological artefact due to bone wax. Br J Biomed Sci. 1994;51:14–17. [PubMed] [Google Scholar]
- 4.Anfinsen OG, Sudmann B, Rait M, Bang G, Sudmann E. Complications secondary to the use of standard bone wax in seven patients. J Foot Ankle Surg. 1993;32:505–508. [PubMed] [Google Scholar]
- 5.Angelini GD, el-Ghamari FA, Butchart EG. Poststernotomy pseudo-arthrosis due to foreign body reaction to bone wax. Eur J Cardiothorac Surg. 1987;1:129–130. doi: 10.1016/1010-7940(87)90025-x. [DOI] [PubMed] [Google Scholar]
- 6.Baskett RJ, MacDougall CE, Ross DB. Is mediastinitis a preventable complication? A 10-year review. Ann Thorac Surg. 1999;67:462–465. doi: 10.1016/s0003-4975(98)01195-3. [DOI] [PubMed] [Google Scholar]
- 7.Bolger WE, Tadros M, Ellenbogen RG, Judy K, Grady MS. Endoscopic management of cerebrospinal fluid leak associated with the use of bone wax in skull-base surgery. Otolaryngol Head Neck Surg. 2005;132:418–420. doi: 10.1016/j.otohns.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Brightmore TG, Hayes P, Humble J, Morgan AD. Hemostasis and healing following median sternotomy. Langenbecks Arch Chir. 1975;(Suppl):39–41. doi: 10.1007/978-3-662-05557-1_8. [DOI] [PubMed] [Google Scholar]
- 9.Butterworth J, Douglas-Akinwande A. Lower extremity paralysis after thoracotomy or thoracic epidural: Image first, ask questions later. Anesth Analg. 2007;104:201–203. doi: 10.1213/01.ane.0000250362.34569.20. [DOI] [PubMed] [Google Scholar]
- 10.Cirak B, Unal O. Iatrogenic quadriplegia and bone wax. Case illustration. J Neurosurg. 2000;92(Suppl):248. doi: 10.3171/spi.2000.92.2.0248. [DOI] [PubMed] [Google Scholar]
- 11.Finn MD, Schow SR, Schneiderman ED. Osseous regeneration in the presence of four common hemostatic agents. J Oral Maxillofac Surg. 1992;50:608–612. doi: 10.1016/0278-2391(92)90443-4. [DOI] [PubMed] [Google Scholar]
- 12.Geary JR, Franz VK. New absorbable wax. Ann Surg. 1950;132:1128–1137. doi: 10.1097/00000658-195012000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibbs L, Kakis A, Weinstein P, Conte JE., Jr Bone wax as a risk factor for surgical-site infection following neurospinal surgery. Infect Control Hosp Epidemiol. 2004;25:346–348. doi: 10.1086/502403. [DOI] [PubMed] [Google Scholar]
- 14.Grindel JM, Jaworski T, Emanuele RM, Culbreth P. Pharmacokinetics of a novel surface-active agent, purified poloxamer 188, in rat, rabbit, dog and man. Biopharm Drug Dispos. 2002;23:87–103. doi: 10.1002/bdd.297. [DOI] [PubMed] [Google Scholar]
- 15.Hadeishi H, Yasui N, Suzuki A. Mastoid canal and migrated bone wax in the sigmoid sinus: Technical report. Neurosurgery. 1995;36:1220–1224. doi: 10.1227/00006123-199506000-00028. [DOI] [PubMed] [Google Scholar]
- 16.Horsley V. Antiseptic wax [Letter] Br Med J. 1892;1:1165. [Google Scholar]
- 17.Ibarrola JL, Bjorenson JE, Austin BP, Gerstein H. Osseous reactions to three hemostatic agents. J Endod. 1985;11:75–83. doi: 10.1016/s0099-2399(85)80123-0. [DOI] [PubMed] [Google Scholar]
- 18.Jewell RC, Khor SP, Kisor DF, LaCroix KA, Wargin WA. Pharmacokinetics of RheothRx injection in healthy male volunteers. J Pharm Sci. 1997;86:808–812. doi: 10.1021/js960491e. [DOI] [PubMed] [Google Scholar]
- 19.Johnson P, Fromm D. Effects of bone wax on bacterial clearance. Surgery. 1981;89:206–209. [PubMed] [Google Scholar]
- 20.Kamineni S, Maritz NG, Morrey BF. Proximal radial resection for posttraumatic radioulnar synostosis: A new technique to improve forearm rotation. J Bone Joint Surg. 2002;84:745–751. doi: 10.2106/00004623-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Kjaergard HK, Trumbull HR. Bleeding from the sternal marrow can be stopped using vivostat patient-derived fibrin sealant. Ann Thorac Surg. 2000;69:1173–1175. doi: 10.1016/s0003-4975(99)01560-x. [DOI] [PubMed] [Google Scholar]
- 22.Light RU. Hemostasis in neurosurgery. J Neurosurg. 1945;2:414–434. [Google Scholar]
- 23.Low WK, Sim CS. Bone wax foreign body granuloma in the mastoid. J Otorhinolaryngol Relat Spec. 2002;64:38–40. doi: 10.1159/000049267. [DOI] [PubMed] [Google Scholar]
- 24.Mattsson T, Anneroth G, Köndell PA, Nordenram A. ACP and Surgicel in bone hemostasis. A comparative experimental and histologic study. Swed Dent J. 1990;14:57–62. [PubMed] [Google Scholar]
- 25.Momota Y, Miyamoto Y, Ishikawa K, Takechi M, Yuasa T, Tatehara S, Nagayama M, Suzuki K. Evaluation of feasibility of hydroxyapatite putty as a local hemostatic agent for bone. J Biomed Mater Res. 2002;63:542–547. doi: 10.1002/jbm.10332. [DOI] [PubMed] [Google Scholar]
- 26.Nelson DR, Buxton TB, Luu QN, Rissing JP. The promotional effect of bone wax on experimental Staphylococcus aureus osteomyelitis. J Thorac Cardiovasc Surg. 1990;99:977–980. [PubMed] [Google Scholar]
- 27.Parker R. Aural pyaemia successfully treated by removing putrid thrombus of jugular vein and lateral sinus. Br Med J. 1892;1:1076–1077. [Google Scholar]
- 28.Plachokova AS, van den Dolder J, Stoelinga PJ, Jansen JA. Early effect of platelet-rich plasma on bone healing in combination with an osteoconductive material in rat cranial defects. Clin Oral Implants Res. 2007;18:244–251. doi: 10.1111/j.1600-0501.2006.01327.x. [DOI] [PubMed] [Google Scholar]
- 29.Robicsek F, Masters TN, Littman L, Born GV. The embolization of bone wax from sternotomy incisions. Ann Thorac Surg. 1981;31:357–359. doi: 10.1016/s0003-4975(10)60967-8. [DOI] [PubMed] [Google Scholar]
- 30.dos Santos Neto FL, Volpon JB. Experimental nonunion in dogs. Clin Orthop Relat Res. 1984;187:260–271. [PubMed] [Google Scholar]
- 31.Schonauer C, Tessitore E, Barbagallo G, Albanese V, Moraci A. The use of local agents: Bone wax, gelatin, collagen, oxidized cellulose. Eur Spine J. 2004;13(Suppl 1):S89–S96. doi: 10.1007/s00586-004-0727-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Short HD. Paraplegia associated with the use of oxidized cellulose in postero-lateral thoracotomy incisions. Ann Thorac Surg. 1990;50:288–290. doi: 10.1016/0003-4975(90)90751-q. [DOI] [PubMed] [Google Scholar]
- 33.Solheim E, Pinholt EM, Bang G, Sudmann E. Effect of local hemostatics on bone induction in rats: A comparative study of bone wax, fibrin-collagen paste, and bioerodible polyorthoester and demineralized bone with and without gentamicin. J Biomed Mater Res. 1992;26:791–800. doi: 10.1002/jbm.820260608. [DOI] [PubMed] [Google Scholar]
- 34.Sorrenti SJ, Cumming WJ, Miller D. Reaction of the human tibia to bone wax. Clin Orthop Relat Res. 1984;182:293–296. [PubMed] [Google Scholar]
- 35.Sudmann B, Anfinsen OG, Bang G, Koppang R, Stolen SO, Koppang HS, Sudmann E. Assessment in rats of a new bioerodible bone-wax-like polymer. Acta Orthop Scand. 1993;64:336–339. doi: 10.3109/17453679308993639. [DOI] [PubMed] [Google Scholar]
- 36.Sudmann B, Bang G, Sudmann E. Histologically verified bone wax (beeswax) granuloma after median sternotomy in 17 of 18 autopsy cases. Pathology. 2006;38:138–141. doi: 10.1080/00313020600561732. [DOI] [PubMed] [Google Scholar]
- 37.Verna C, Bosch C, Dalstra M, Wikesjö UM, Trombelli L. Healing patterns in calvarial defects following guided bone regeneration in rats. A micro-CT analysis. J Clin Periodontol. 2002;29:865–870. doi: 10.1034/j.1600-051x.2002.290912.x. [DOI] [PubMed] [Google Scholar]
- 38.Verrall PJ. Operation on Joints. In: Carson HW, editor. Modern Operative Surgery. London: Cassell & Co; 1924. p. 69. [Google Scholar]
- 39.Wang MY, Armstrong JK, Fisher TC, Meiselman HJ, McComb GJ, Levy ML. A new, pluronic-based, bone hemostatic agent that does not impair osteogenesis. Neurosurgery. 2001;49:962–968. doi: 10.1097/00006123-200110000-00031. [DOI] [PubMed] [Google Scholar]
- 40.Wellisz T, Armstrong JK, Cambridge J, Fisher TC. Ostene, a new water-soluble bone hemostasis agent. J Craniofac Surg. 2006;17:420–425. doi: 10.1097/00001665-200605000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Wilkinson HA, Baker S, Rosenfeld S. Gelfoam paste in experimental laminectomy and cranial trephination: Hemostasis and bone healing. J Neurosurg. 1981;54:664–667. doi: 10.3171/jns.1981.54.5.0664. [DOI] [PubMed] [Google Scholar]
- 42.Wolvius EB, van der Wal KG. Bone wax as a cause of a foreign body granuloma in a cranial defect: A case report. Int J Oral Maxillofac Surg. 2003;32:656–658. doi: 10.1054/ijom.2002.0394. [DOI] [PubMed] [Google Scholar]