Abstract
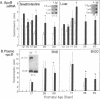
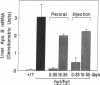
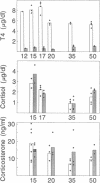
Thyroid hormone has been implicated as an important factor in rodent development. We have used a strain of mice with a recessive mutation producing congenital primary hypothyroidism (C.RF/J-hyt/+) to study the effects of thyroid hormone on developmental changes in the expression of genes encoding a number of proteins involved in lipid metabolism and transport. Total cellular RNA was prepared from the small intestine and liver of hyt/hyt mice and their unaffected littermates (+/?) at various times during postnatal development. RNA blots were probed with apolipoprotein A-I, A-II, A-IV, B, and E cDNAs plus cDNAs encoding the low density lipoprotein receptor, 3-hydroxy-3-methylglutaryl coenzyme A reductase, and three cytoplasmic hydrophobic ligand-binding proteins (two fatty acid-binding proteins and a protein that binds all-trans-retinol). Hypothyroidism results in small changes (1.5- to 5-fold) in the concentration of many of these mRNAs in liver and small intestine between postnatal days 15 and 50. A much greater tissue-specific effect was noted on apolipoprotein B (apoB) gene expression. In euthyroid +/? animals, apoB mRNA levels fall by a factor of 30 in liver between days 20 and 35 without a comparable decrease in the small intestine. This liver-specific decrease does not occur in hyt/hyt animals. The normal decrease in hepatic apoB mRNA levels is accompanied by a decrease in plasma apoB-100 but not apoB-48. No reduction in either form of plasma apoB was noted in hyt/hyt animals. Mutant hyt/hyt mice given thyroxine from birth to 35 days had liver apoB mRNA levels comparable to those in +/? littermates. In contrast, hepatic apoB mRNA concentrations did not fall to normal levels in hyt/hyt mice given thyroxine from postnatal days 15 to 35. All treatment groups have comparable levels of plasma corticosteroids. These data suggest that (i) there is a critical period or a required response time during postnatal development for thyroid hormone action on apoB gene expression, (ii) thyroid hormone's effect on apoB is tissue specific, and (iii) the hyt/hyt mouse represents a useful system to evaluate the developmental effects of thyroid hormone on specific gene expression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers D. H., Strauss A. W., Ockner R. K., Bass N. M., Gordon J. I. Cloning of a cDNA encoding rat intestinal fatty acid binding protein. Proc Natl Acad Sci U S A. 1984 Jan;81(2):313–317. doi: 10.1073/pnas.81.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamer W. G., Cresswell L. A. Defective thyroid ontogenesis in fetal hypothyroid (hyt/hyt) mice. Anat Rec. 1982 Mar;202(3):387–393. doi: 10.1002/ar.1092020311. [DOI] [PubMed] [Google Scholar]
- Beamer W. J., Eicher E. M., Maltais L. J., Southard J. L. Inherited primary hypothyroidism in mice. Science. 1981 Apr 3;212(4490):61–63. doi: 10.1126/science.7209519. [DOI] [PubMed] [Google Scholar]
- Birkenmeier E. H., Gordon J. I. Developmental regulation of a gene that encodes a cysteine-rich intestinal protein and maps near the murine immunoglobulin heavy chain locus. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2516–2520. doi: 10.1073/pnas.83.8.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguski M. S., Elshourbagy N., Taylor J. M., Gordon J. I. Comparative analysis of repeated sequences in rat apolipoproteins A-I, A-IV, and E. Proc Natl Acad Sci U S A. 1985 Feb;82(4):992–996. doi: 10.1073/pnas.82.4.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguski M. S., Elshourbagy N., Taylor J. M., Gordon J. I. Rat apolipoprotein A-IV contains 13 tandem repetitions of a 22-amino acid segment with amphipathic helical potential. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5021–5025. doi: 10.1073/pnas.81.16.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- Davidson N. O., Magun A. M., Brasitus T. A., Glickman R. M. Intestinal apolipoprotein A-I and B-48 metabolism: effects of sustained alterations in dietary triglyceride and mucosal cholesterol flux. J Lipid Res. 1987 Apr;28(4):388–402. [PubMed] [Google Scholar]
- De Nayer P. Thyroid hormone action at the cellular level. Horm Res. 1987;26(1-4):48–57. doi: 10.1159/000180685. [DOI] [PubMed] [Google Scholar]
- Demmer L. A., Levin M. S., Elovson J., Reuben M. A., Lusis A. J., Gordon J. I. Tissue-specific expression and developmental regulation of the rat apolipoprotein B gene. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8102–8106. doi: 10.1073/pnas.83.21.8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshourbagy N. A., Boguski M. S., Liao W. S., Jefferson L. S., Gordon J. I., Taylor J. M. Expression of rat apolipoprotein A-IV and A-I genes: mRNA induction during development and in response to glucocorticoids and insulin. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8242–8246. doi: 10.1073/pnas.82.23.8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Folz R. J., Gordon J. I. Deletion of the propeptide from human preproapolipoprotein A-II redirects cotranslational processing by signal peptidase. J Biol Chem. 1986 Nov 5;261(31):14752–14759. [PubMed] [Google Scholar]
- Gordon J. I., Alpers D. H., Ockner R. K., Strauss A. W. The nucleotide sequence of rat liver fatty acid binding protein mRNA. J Biol Chem. 1983 Mar 10;258(5):3356–3363. [PubMed] [Google Scholar]
- Henning S. J. Postnatal development: coordination of feeding, digestion, and metabolism. Am J Physiol. 1981 Sep;241(3):G199–G214. doi: 10.1152/ajpgi.1981.241.3.G199. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levin M. S., Li E., Ong D. E., Gordon J. I. Comparison of the tissue-specific expression and developmental regulation of two closely linked rodent genes encoding cytosolic retinol-binding proteins. J Biol Chem. 1987 May 25;262(15):7118–7124. [PubMed] [Google Scholar]
- Li E., Demmer L. A., Sweetser D. A., Ong D. E., Gordon J. I. Rat cellular retinol-binding protein II: use of a cloned cDNA to define its primary structure, tissue-specific expression, and developmental regulation. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5779–5783. doi: 10.1073/pnas.83.16.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusis A. J., Taylor B. A., Quon D., Zollman S., LeBoeuf R. C. Genetic factors controlling structure and expression of apolipoproteins B and E in mice. J Biol Chem. 1987 Jun 5;262(16):7594–7604. [PubMed] [Google Scholar]
- Lusis A. J., West R., Mehrabian M., Reuben M. A., LeBoeuf R. C., Kaptein J. S., Johnson D. F., Schumaker V. N., Yuhasz M. P., Schotz M. C. Cloning and expression of apolipoprotein B, the major protein of low and very low density lipoproteins. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4597–4601. doi: 10.1073/pnas.82.14.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean J. W., Fukazawa C., Taylor J. M. Rat apolipoprotein E mRNA. Cloning and sequencing of double-stranded cDNA. J Biol Chem. 1983 Jul 25;258(14):8993–9000. [PubMed] [Google Scholar]
- McNamara D. J., Quackenbush F. W., Rodwell V. W. Regulation of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase. Developmental pattern. J Biol Chem. 1972 Sep 25;247(18):5805–5810. [PubMed] [Google Scholar]
- Mehrabian M., Callaway K. A., Clarke C. F., Tanaka R. D., Greenspan M., Lusis A. J., Sparkes R. S., Mohandas T., Edmond J., Fogelman A. M. Regulation of rat liver 3-hydroxy-3-methylglutaryl coenzyme A synthase and the chromosomal localization of the human gene. J Biol Chem. 1986 Dec 5;261(34):16249–16255. [PubMed] [Google Scholar]
- Moog F., Denes A. E., Powell P. M. Disaccharidases in the small intestine of the mouse: normal development and influence of cortisone, actinomycin D, and cycloheximide. Dev Biol. 1973 Nov;35(1):143–159. doi: 10.1016/0012-1606(73)90012-2. [DOI] [PubMed] [Google Scholar]
- Noguchi T., Sugisaki T., Satoh I., Kudo M. Partial restoration of cerebral myelination of the congenitally hypothyroid mouse by parenteral or breast milk administration of thyroxine. J Neurochem. 1985 Nov;45(5):1419–1426. doi: 10.1111/j.1471-4159.1985.tb07208.x. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Mariash C. N., Kinlaw W. B., Wong N. C., Freake H. C. Advances in our understanding of thyroid hormone action at the cellular level. Endocr Rev. 1987 Aug;8(3):288–308. doi: 10.1210/edrv-8-3-288. [DOI] [PubMed] [Google Scholar]
- Powell L. M., Wallis S. C., Pease R. J., Edwards Y. H., Knott T. J., Scott J. A novel form of tissue-specific RNA processing produces apolipoprotein-B48 in intestine. Cell. 1987 Sep 11;50(6):831–840. doi: 10.1016/0092-8674(87)90510-1. [DOI] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Shire J. G., Beamer W. G. Adrenal changes in genetically hypothyroid mice. J Endocrinol. 1984 Sep;102(3):277–280. doi: 10.1677/joe.0.1020277. [DOI] [PubMed] [Google Scholar]
- Sweetser D. A., Heuckeroth R. O., Gordon J. I. The metabolic significance of mammalian fatty-acid-binding proteins: abundant proteins in search of a function. Annu Rev Nutr. 1987;7:337–359. doi: 10.1146/annurev.nu.07.070187.002005. [DOI] [PubMed] [Google Scholar]
- Weinberger C., Thompson C. C., Ong E. S., Lebo R., Gruol D. J., Evans R. M. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986 Dec 18;324(6098):641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Bishop R. W., Brown M. S., Goldstein J. L., Russell D. W. Deletion in cysteine-rich region of LDL receptor impedes transport to cell surface in WHHL rabbit. Science. 1986 Jun 6;232(4755):1230–1237. doi: 10.1126/science.3010466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh K., Moog F. Intestinal lactase activity in the suckling rat: influence of hypophysectomy and thyroidectomy. Science. 1974 Jan 11;183(4120):77–79. doi: 10.1126/science.183.4120.77. [DOI] [PubMed] [Google Scholar]