Abstract
Recent progress in understanding visceral afferents, some of it reviewed in the present issue, serves to underscore how little is known about the aging of the visceral afferents in the gastrointestinal (GI) tract. In spite of the clinical importance of the issue--with age, GI function often becomes severely compromised--only a few initial observations on age-related structural changes of visceral afferents are available. Primary afferent cell bodies in both the nodose ganglia and dorsal root ganglia lose Nissl material and accumulate lipofucsin, inclusions, aggregates, and tangles. Additionally, in changes that we focus on in the present review, vagal visceral afferent terminals in both the muscle wall and the mucosa of the GI tract exhibit age-related structural changes. In aged animals, both of the vagal terminal types examined, namely intraganglionic laminar endings and villus afferents, exhibit dystrophic or regressive morphological changes. These neuropathies are associated with age-related changes in the structural integrity of the target organs of the affected afferents, suggesting that local changes in trophic environment may give rise to the aging of GI innervation. Given the clinical relevance of GI tract aging, a more complete understanding both of how aging alters the innervation of the gut and of how such changes might be mitigated should be made research priorities.
Keywords: aging, intestines, intraganglionic laminar endings, intramuscular arrays, myenteric, senescence, stomach, trophic factors, vagus, villus afferents
Introduction
Little is known about how the visceral afferent innervation of the gastrointestinal (GI) tract ages. A survey of the history of the question illustrates the point: Thirty years ago, a full fifty years after the early descriptions of visceral afferents by Langley and others, Brizzee and Ordy (1979) provided a cogent summary of what was then known about the effects of age on such afferents. They noted that (1) almost nothing was known about structural changes associated with aging of visceral afferents, and (2) motor networks in the ANS had received by that point in time “much more attention” than the afferents.
Presently, another thirty years on, the field’s knowledge of how aging affects visceral afferents is just as unsatisfactory as it was in 1979, and the two assertions of Brizzee and Ordy are still an apt summary of the state of visceral sensory neuroscience generally and certainly, more particularly, of our understanding of the afferents innervating the gut.
Given the prominent and often debilitating effects of aging on GI function and health, the dearth of information is especially unsatisfactory. The complications and disorders of GI function that afflict the elderly and compromise their quality of life, and that often further complicate a variety of other age-related diseases, have been extensively reviewed in recent years (e.g., O’Mahony et al., 2002; Newton, 2004; Hays and Roberts, 2006; Norton, 2006; Crane and Talley, 2007; Morley, 2007; Trinh and Prabhakar, 2007; Roach and Christie, 2008). The recognition of these significant health problems has led, recently, to examinations of the age-related changes in the intrinsic motor networks innervating the GI tract (for reviews see: Hall, 2002; Wade, 2002; Wiley, 2002; Saffrey, 2004; Wade and Cowen, 2004; Phillips and Powley, 2007; Camilleri et al., 2008; Cersosimo and Benarroch, 2008). Somewhat surprisingly, though, the awareness of clinical problems has not produced a comparable amount of attention to the afferent limb of the innervation.
Such an oversight cannot be rationalized with a claim that many of the functional disturbances (e.g., acid reflux, dyspepsia, constipation, diarrhea, and irritable bowel syndrome) are manifested on the motor side. Motor symptoms are as easily produced by upstream distortions or losses of the afferent limb of a reflex loop as by direct compromise of motor neurons. Furthermore, motor disturbances--whatever their root causes--are more likely to remain uncompensated or uncorrected if the afferents monitoring the motor responses are impaired and not generating appropriate feedback.
Why there has been little to no attention paid to aging visceral afferents is not entirely clear, but at least one factor contributing to this inertia would seem to be the technical difficulties in isolating and analyzing the specific pool of afferents that innervate a particular viscus or organ system. The cell bodies of the visceral afferents to the gut are intermingled with other visceral afferents (in the case of both nodose ganglia and dorsal root ganglia [DRG] afferents) as well as with other primary afferents (in the case of DRG afferents), and the different pools of afferent neuronal somata cannot be identified unambiguously on morphological grounds. Similarly, the neurites of visceral afferents run--both to their central relay nuclei and their peripheral targets--in mixed nerve bundles within which they cannot be distinguished from afferent fibers to other organs, or even efferents, on strictly morphological grounds. Even within their target organs, visceral afferents are complexly admixed with elements of the local neural networks, making identification difficult.
Below, we briefly address these issues as we review the limited information that is available on the aging of visceral afferents to the GI tract. We also illustrate--with observations from our recent experimental work--some advantages of identifying subpopulations of visceral afferents with neural tracers and immunohistochemistry. Such strategies now make it practical to distinguish the visceral afferents from other neuronal elements within the gut and to characterize fully their responses to aging.
In reviewing the available information on the visceral afferent innervation of the GI tract and illustrating some dystrophic features in identified afferent populations, we also discuss a few of the issues and questions that pertain to the aging processes in the visceral afferent innervations. For lack of more specific information, some of the issues and mechanistic hypotheses are of necessity drawn from investigations of other organs (e.g. the heart; Ai et al., 2007), other afferents (e.g., somatic primary afferents; Ulfhake et al., 2000, 2002), or even other classes of neurons (e.g., central nervous system neurons; Mattson and Magnus, 2006). Though it is unclear how closely extrapolations from other neuronal systems will apply to visceral afferents innervating the GI tract, the more detailed information available on the aging of some of these other neuronal systems may offer particularly useful, if provisional, frameworks for designing and evaluating more thorough analyses of the visceral afferents of the gut.
Visceral Afferent Neuron Cell Bodies and Aging
Most of the limited work on age-related changes in visceral afferents to the GI tract has been focused on the neuronal somata, not their neurites (Devor, 1991; Soltanpour et al., 1996; Soltanpour and Santer, 1996; Bergman and Ulfhake, 1998; Bergman et al., 1999; Ulfhake et al., 2000).
The visceral afferents innervating the gut originate from cells located, in the nodose ganglia of the vagus nerve or from cell bodies in the DRG situated immediately peripheral to the spinal cord. The neurons of the nodose ganglia and the caudal DRGs constitute the craniosacral division and supply most low threshold mechanoreceptors and chemoreceptors to, respectively, the proximal GI tract or the large intestine. The gut-innervating visceral afferents found in the thoracicolumbar DRGs supply primarily high threshold afferent and nocioceptor fibers to the GI tract (Janig, 1996; Grundy, 2002; Robinson and Gebhart, 2008).
The effects of age on neuronal somata in the nodose ganglia and the DRGs have been evaluated in a number of experiments, as reviewed by Brizzee and Ordy (1979) and more recently by Vega et al. (1993). In total, the work has failed to bring into focus any particular pattern of change in neuronal number or size. Most experiments, indeed, have not documented an unequivocal decrease in afferent neuronal number or a change in soma size in aging animals of different species. While a number of investigations have not observed a change in number or size (e.g., Soltanpour et al., 1996; Bergman and Ulfhake, 1998), certainly some experiments have described decreases in size or number of neurons, at least in advanced senescence (cf. Vega et al., 1993).
Though the extent--or even the occurrence--of afferent cell loss with age is controversial, evidence of metabolic changes of the somata is routinely observed. Afferent somata in both the nodose ganglia and DRG appear to express less basophilic Nissl material (i.e., they stain less well), and exhibit accumulations of lipofuscin and translucent vacuoles. In addition, particularly in late senescence, afferent somata often accumulate a variety of inclusions, granules, filamentous bodies, masses and tangles. Changes in glia also occur in the ganglia housing the visceral afferents. Descriptions of the changes in visceral afferent neuronal somata and glia with age can be found in Brizzee and Ordy (1979), Koistinaho (1986), Koistinaho et al. (1990), Vega et al., (1993), Soltanpour et al. (1996).
An assessment of the aging of visceral afferents innervating the GI tract based on these changes in cell bodies in the afferent ganglia is greatly complicated, however, by the fact that none of the descriptions were based specifically on an afferent population projecting to the gut. The nodose ganglia are comprised entirely of visceral afferents, but these afferents project to a variety of organs. Thus an observation of a neuronal change in the nodose ganglia with age cannot be unequivocally associated with the subpopulation innervating the GI tract. The ambiguities are even greater for the DRGs since primary somatic afferents and visceral afferents (projecting to multiple sites or multiple organs) coexist in the same ganglia.
Tentatively, it would seem likely that the visceral afferents to the GI tract exhibit the same changes in Nissl substance expression and the same aggregations of lipofuscin and inclusions that have been described globally for the heterogeneous populations of afferents with cell bodies in nodose ganglia and the DRGs. The conclusion, though, needs to be tempered by the observation that investigators have observed in other neural systems that neurons in different sites and different subpopulations display different aging patterns (Andrew, 1956; Finch, 1993; Anderton, 1997, 2002; Phillips et al., 2003b; Mattson and Magnus, 2006; Phillips and Powley, 2007; Braak and Del Tredici, 2008).
Aging of Vagal Afferent Terminals in the Gut
As discussed elsewhere (Powley and Phillips, 2005) injections of neural tracers into pools of visceral afferents make it possible to assign arbitrary chemical signatures or “phenotypes” to the afferents and then to identify their terminals in their target sites. Similarly, afferents expressing distinctive neuropeptides can be processed immunohistochemically to identify their neurites throughout different tissues. And, unlike the traditional confusion as to which organ is innervated by a given neuron in a ganglion, examinations of labeled visceral afferent terminals at their target sites are unambiguous as to the type of afferent and the site of innervation. These strategies have now been used extensively to characterize the normal or young-adult structural organization of the afferents in gut (e.g., Neuhuber, 1987; Berthoud and Powley, 1992; Berthoud et al., 1997; Fox et al., 2000; Wang and Powley, 2000), but they have been only used in a limited way to look at aging of the same terminals (Phillips and Powley, 2001, 2007). For present purposes though, it is instructive to add even these few limited observations to a survey of visceral afferent aging. The observations focus on a mechanoreceptor in the muscle wall, the intraganglionic laminar ending, and a putative chemoreceptor in the mucosa, the villus afferent.
The Intraganglionic Laminar Ending (IGLE)
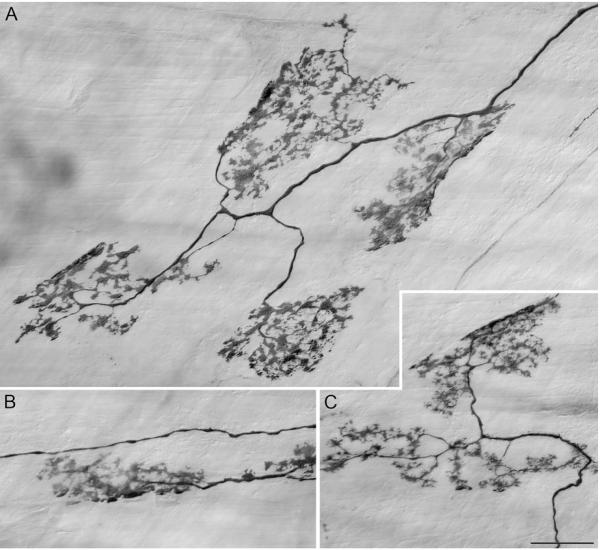
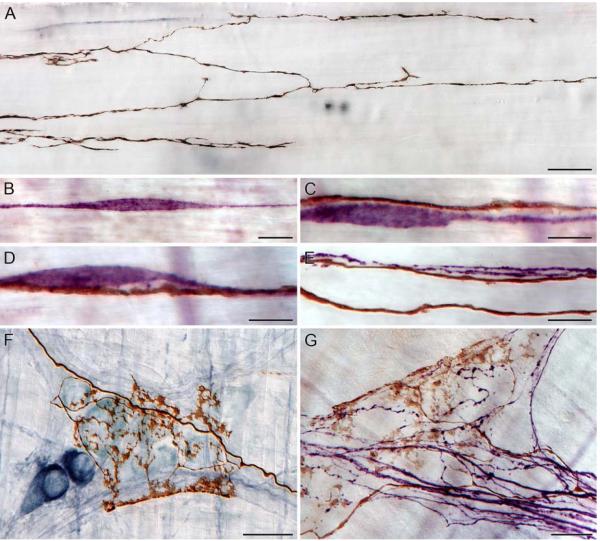
The first vagal visceral afferent to the GI tract recognized and described was the intraganglionic laminar ending (IGLE; Nonidez, 1946; Rodrigo et al., 1975). This ending is well characterized as to its structure (Neuhuber and Cleric, 1990; Berthoud and Neuhuber, 2000; Phillips and Powley, 2000; Powley and Phillips, 2002; Neuhuber et al., 2006), distribution within the GI tract (Berthoud et al., 1997; Wang and Powley, 2000), and functional operation (Zagorodnyuk and Brookes, 2000; Zagorodnyuk et al., 2001, 2003). In brief, IGLEs are plates or leaflets of puncta formed at the surface of myenteric ganglia throughout the GI tract (see Fig. 1). The puncta in the main are located at the surface of a ganglion where they effectively are sandwiched between the ganglionic neuronal somata and one of the smooth muscle layers, with some of the puncta insinuated or interdigitated between the neuronal cell bodies. An individual vagal IGLE afferent will typically branch and divide so as to produce several separate IGLE plates (ranging from one to perhaps 18 or 20; Fig. 1 illustrates 7 such IGLEs issued by a single afferent) innervating multiple neighboring myenteric ganglia, with the receptive field of the afferent corresponding to the arbor of plates the fiber issues.
Figure 1.
Vagal afferents that terminate in the myenteric plexus are considered putative tension receptors; they produce endings known as intraganglionic laminar endings (IGLEs). In an adult rat (3 months of age), a single well labeled axon terminating in the corpus adjacent to the greater curvature had a receptive field consisting of approximately 15 separate IGLEs. Seven of the 15 IGLEs are pictured in panels A (4 IGLEs), B (1 IGLE), and C (2 IGLEs); the receptive field of an afferent that terminates in IGLEs is thought to encompass the area occupied by the cluster of IGLEs. IGLEs were visualized by injecting dextran-tetramethylrhodamine-biotin (D-TMR-B; D-3312; 15%, MW 10k, lysine fixable, Molecular Probes, OR) into the nodose ganglia followed 14d later with a permanent DAB immunohistochemical protocol (Powley and Phillips, 2005). Scale Bar in C = 25 μm for A-C.
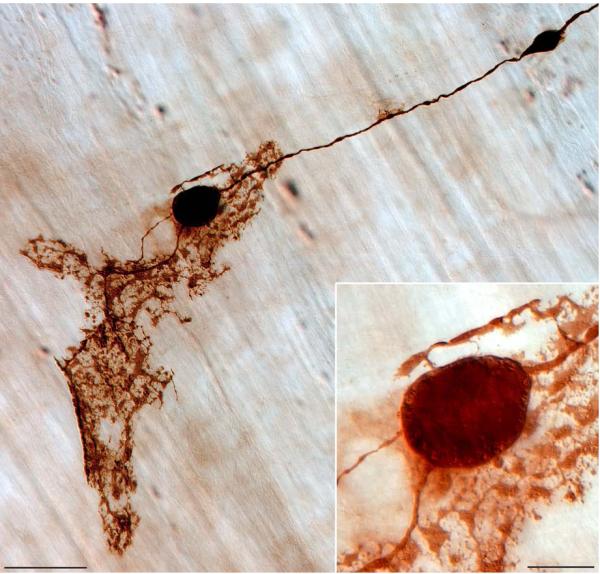
As the gut and its innervations age, IGLEs develop two conspicuous types of dystrophic or heteroplastic changes. One of these age-related changes consists of dilations and bulbous swellings, some of them quite dramatic, of the neurite forming the IGLEs or even the IGLE plates themselves (Fig. 2). These swellings can be dense masses as large as a myenteric neuron (Inset in Fig. 2), and they commonly contain organelles or vacuoles (mitochondria?) that appear clear because they do not incorporate the dextran tracer used to visualize the morphology of the afferent’s axon and terminal.
Figure 2.
Degenerative changes are illustrated in a vagal afferent neurite prior to terminating in an IGLE (located towards the greater curvature of the forestomach of an aged rat; 22 months of age); adapted from a panel which originally appeared in Phillips and Powley, 2007. Two heteroplastic varicosities stand out in marked contrast to the considerably smaller diameter of the axon from which they originate, and the presence of small lucent vesicles (possible mitochondria?) around the circumference of the larger of the two varicosities--shown at higher power in the Inset--further validates the assumption of a degenerative fate for this particular afferent. Scale Bar = 50 μm; 15 μm in inset.
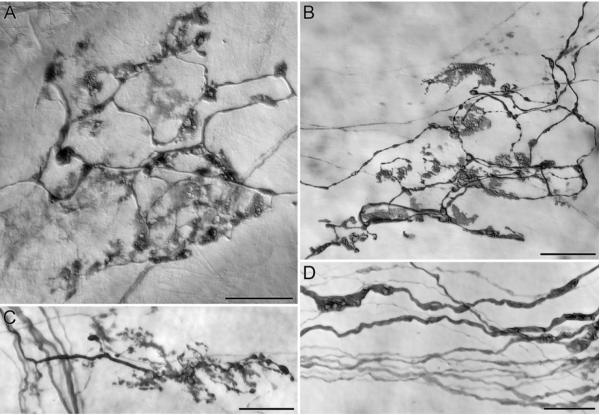
The second type of dystrophic change in aged IGLEs consists of a contraction of the IGLE from its typical extensive plate of fine puncta to a simpler and smaller involuted structure or plate (Fig. 3A-C). Many of the apparently involuted and shrunken puncta fill densely with tracer and exhibit vacuoles much like parent neurites described above (Fig. 2). In contrast to these dark, densely contracted puncta, some aged IGLEs also seem to contain other more lightly labeled “ghost” puncta that may represent a different stage of a developing involution. Dilations and swellings containing translucent vacuoles are also seen in the afferent neurites as they course in myenteric connectives and before they ramify into IGLE plates (Fig. 3D).
Figure 3.
IGLEs in the stomachs of two different aged rats (22 months of age) injected in the nodose ganglia with D-TMR-B showed a range of morphological changes consistent with age-induced deterioration. These IGLEs, which were “aged” in appearance, had numerous inclusions with unstained central cores that occurred along the length of the secondary and tertiary branches (A; ending located directly below the lower esophageal sphincter in the corpus), and laminar plates that were diffuse and 2-dimensional (B; ending located on the greater curvature side of the pyloric sphincter in the transition zone between the antrum and the corpus) in comparison to their young adult counterparts (see Fig. 1). In addition, in contrast to the highly arborizing adult IGLEs, some aged IGLEs appeared to be pruned back to the parent axon and less leafy by comparison (C; ending located in the antrum). Finally, within axonal bundles of D-TMR-B labeled aged vagal afferents (D; bundle was located in the corpus in close proximity to the IGLE shown in panel A), axons of normal diameter ran parallel to axons with engorged regions that were three to four times as thick. Scale Bars = 25 μm.
The dystrophic patterns in aging IGLEs clearly document that the visceral afferents supplied by the vagus to the myenteric plexus undergo age-related morphological changes at the target site. These profiles suggest the possibility that, as the afferents age, they lose trophic support from the target tissues, and this issue is discussed below. Additional questions about these dystrophic IGLE patterns should, however, be mentioned here: To date, we have made only limited observations on the visceral afferents in the NIH Fischer 344 rat model of aging in the course of surveys focused on the aging of the enteric innervation of the gut. We have not yet performed quantitative assessments of the time course of the regressive changes noted in afferents, the regional distributions of the changes, or the relative frequencies of the changes. That such changes do occur in aging, though, underscores the need for more complete analyses and for investigations of the underlying mechanisms.
Vagal Villus Afferents
A second vagal visceral afferent, the villus afferent, also undergoes age-related dystrophic or regressive changes. Mucosal afferents found in intestinal villi have been examined with electrophysiological protocols for decades, and (at least partial) morphological descriptions of them have been reported (Hill, 1927; Berthoud et al, 1995; Powley et al., 2005; Phillips and Powley, 2007). We recently completed a more exhaustive analysis of these endings in villi (Powley et al., unpublished observation) of adult rats. Here, though, we note how such endings age.
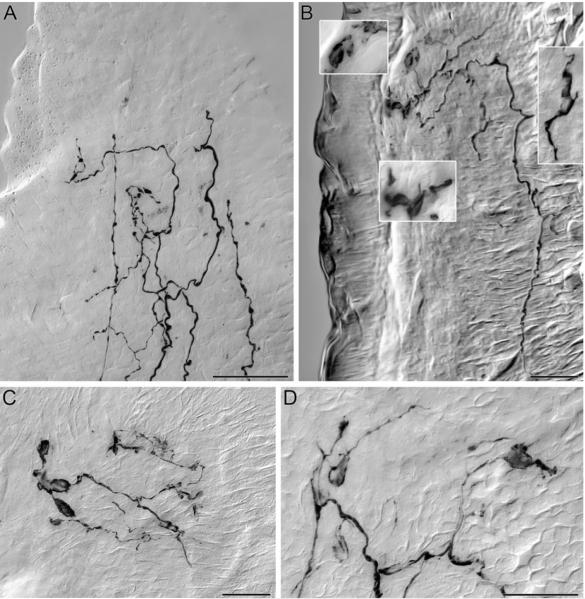
The vagal villus afferents enter one or more villi and travel to the apical or luminal tip of the structure(s), repeatedly bifurcating into simple open arbors of terminals as they travel (Fig. 4A). In an adult rat, these villus arbors consist of simple, relatively smooth terminal neurites with only modest and inconspicuous varicosities.
Figure 4.
In young adult rats, vagal afferents supply extensive networks of free nerve endings terminating at the apical tip of the intestinal villi immediately subjacent to the epithelium (A; 3 months of age), and function as possible chemoreceptors detecting the chemical composition of the contents of the lumen. A single vagal fiber enters the villus (B; bottom right) in an aged rat (22 months of age) and climbs to the apical tip where it gives off terminal processes immediately below the epithelial wall. The Insets in panel B are enlargements of regions of the free nerve endings that have contracted into flattened plates of dystrophic neurites. Panels C and D are similar examples of the flatted ovid, fusiform morphology of the dystrophic vagal afferent intravillous arbors in villi of aged rats (22 months of age). Nickel enhanced DAB was used to permanently visualize the D-TMR-B labeled vagal afferent innervation of the villus in 100 μm thick transverse sections of mucosa sampled from the proximal duodenum. Scale Bars = 25 μm; D applies to insets in B.
The vagal villus afferents in aged animals, however, exhibit signs of regression or dystrophy. The arbors within the villi tend to contract and simplify, retaining fewer terminal elements (Fig. 4B). In addition, the terminal processes develop flattened and enlarged features reminiscent of lamellipodia. These features often appear thin and flattened (e.g. Fig. 4B-D), extending from one side of the affected neurite, and they often contain darker regions or masses aggregated within the structure.
In sum, villus afferents, the vagal visceral afferents innervating the intestinal mucosa (and constituting presumptive chemoreceptors), undergo dystrophic changes with aging. Their terminal arbors regress or involute, and they develop lamellar extensions or swellings. This pattern, much like that described above for IGLEs, may well represent some loss of trophic support and stimulation for the visceral afferents (see additional discussion below). In addition-- or alternatively--the pattern could reflect some cumulative consequences of the chronic wear and tear and damage that the epithelium and mucosal tissues experience. Much like the case of the IGLE observations discussed above, our observations on aging patterns of vagal villus afferents are as yet limited. Our observations do, however, establish that the afferents undergo dystrophic remodeling with aging, but should be tempered by the fact that more systematic and quantitative assessments of the location(s), extent, and progression of the changes need to be provided in the near future. Observations on other visceral afferents, including other vagal afferents as well as DRG afferents, are similarly needed.
Considering a Trophic Explanation of Gastrointestinal Visceral Afferent Aging
As suggested in the introduction, surprisingly little else is known about the effects of aging on the structure of the visceral afferents innervating the GI tract. Additional conclusions at the present time have to rely on comparisons and extrapolations from other systems (e.g., visceral afferents to other organs, other types of afferents, etc.) and models or general principles developed from other processes (e.g., degenerative changes; pathological effects).
Perhaps the most promising model for further exploration of the age-related changes in the visceral afferent innervations of the gut may be a trophic explanation. Such an explanation would be consistent with the structural effects of aging that have been observed, and are briefly reviewed above, upon neuronal somata in the nodose ganglia and the DRGs. In addition, the patterns of age-related changes we have observed in IGLEs and villus afferent terminals are consistent with a deterioration or a waning of trophic influences.
A trophic explanation also finds support in observations drawn from other neural systems. There is evidence that trophic factors are involved in the age-related neuronal changes documented for enteric neurons (Wade and Cowen, 2004; Thrasivoulou et al., 2006). Furthermore, trophic factors are strongly implicated in the age-related neuronal changes seen in other classes of primary sensory neurons (e.g., Cowen, 1993; Ulfhake et al., 2000, 2002).
Additional observations also implicate trophic factors in the organization of vagal visceral afferents, and, hence, link visceral afferents in the GI tract to a trophic factor explanation of age-related dystrophies. The observations are of two kinds. First, for some of the vagal visceral afferents, evidence exists indicating that the afferents are affected by trophic factor manipulations. Second, for some of the vagal visceral afferents, evidence also exists that the tissues that they target undergo age-related changes, thus suggesting that the trophic stimulation of the afferents does change with aging. These indirect observations can be considered briefly for the two most thoroughly studied vagal visceral afferents in the gut, namely IGLEs and intramuscular arrays (IMAs).
The Organization of IGLEs Suggests Their Structure is Controlled by Trophic Influences
Three converging lines of evidence are consistent with a role for trophic factors in maintaining IGLE morphology and, by implication, with roles for diminished trophic agents in the aging of the visceral afferents.
One set of observations comes from examinations of the relationships between IGLEs and their target tissues. These putative mechanoreceptor endings are tightly insinuated on and in the ganglia of the myenteric plexus, where their puncta articulate in characteristic and specialized ways with different enteric neurons (Fig. 5F) and neurites (Fig. 5G). Ultrastructural evidence indicates that the IGLEs form appositions and vesicle-containing synapse-like contacts with myenteric neurons (Neuhuber, 1987; Neuhuber et al., 2006; c.f., Fig. 9 in Powley et al. 2008). Also, critically, these IGLE puncta can be found in apposition with cholinergic myenteric neurons (Fig. 5F). As we (Phillips et al., 2003b) and others (Abalo et al., 2005; Thrasivoulou et al., 2006) have shown, age-related neuronal losses in the myenteric ganglia are specific to the cholinergic--and include the calretinin subpopulation of cholinergic neurons (cf. Fig. 6B,C)-- phenotype. Thus, since IGLEs contact myenteric elements that are lost with age and since the afferent terminals undergo the involution and dystrophic changes described above, changes in trophic support for the IGLEs with age seem particularly likely.
Figure 5.
Intramuscular arrays (IMAs) are vagal afferent terminals located in the smooth muscle layers of the GI tract, and are thought to be mechanoreceptors involved in the detect of changes in length of the end-organs that they innervate. The morphology of IMAs consists of a parent neurite that upon entering the smooth muscle layer branches several times into fine individual processes running for several millimeters in the same orientation as the muscle layer in which the neurites terminate, creating a distinct pattern of parallel elements with occasional bridging elements (brown fibers; A). Within the smooth muscle layers, interstitial cells of Cajal of the intramuscular type (purple cells; B-D) are in close contact with IMAs (reddish-brown fibers; C-E), indicating possible communication between the two (cf. Powley et al., 2008). In the same study, IMAs running in nerve bundles within the muscle layers made contact with individual neurites (purple fibers; E). The same dynamic exists for the vagal afferent innervations of the myenteric plexus, with IGLEs (brown terminals) in close contact with both neurons (dark blue/nitrergic; light blue/cholinergic; F), and axons (purple fibers; G). Scale Bars = 25 μm for A,F,G; 12 μm for B; 10 μm for C-E.
Figure 6.
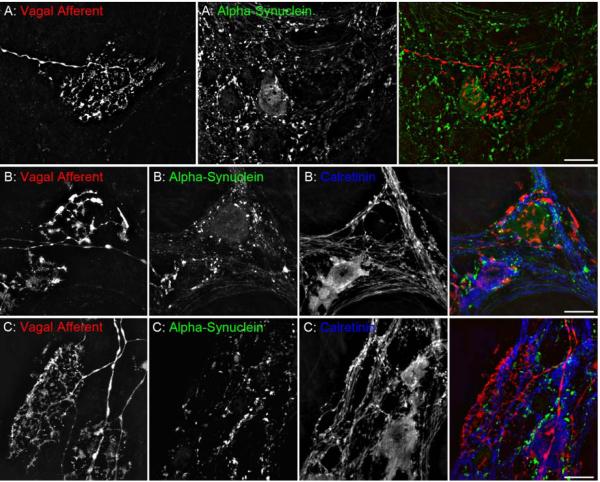
Disturbances in autonomic function are common in the elderly and those afflicted with age-related diseases. Excessive accumulation of alpha-synuclein, a protein implicated in the development of Parkinson’s disease, results in compromised function in terminals and reduced viability of neurons. Therefore, selective expression of alpha-syuclein in the extrinsic and intrinsic nerves that coordinate different aspects of GI function could provide the basis for the degeneration and subsequent loss of function described in the present review. Virtually all vagal preganglionic projections to the stomach express alpha-synuclein, both in axons and terminal varicosities in apposition with myenteric neurons (Phillips et al., 2008); whereas, IGLEs do not (A). Furthermore, alpha-synuclein is expressed in a subpopulation of myenteric neurons (B) with approximately 90% of those in the forestomach being nitrergic and 92% in the corpus-antrum co-localized with markers for cholinergic neurons (e.g., calbindin and calretinin; C). Dextran-Texas Red (D-TR; D-1863; 5%, MW 10k, lysine fixable, Molecular Probes, OR) was injected into the nodose ganglia to label IGLEs in the stomach, followed by immunofluorescent labeling of alpha-synuclein (1:2500; mouse; 610787; BD Biosciences, CA) with ALEXA Fluor 488 (1:500; A11029; Molecular Probes, OR), and calretinin (1:16,000; 7699/4; Swant, Switzerland) with ALEXA Fluor 350 (1:500; S11249; Molecular Probes, OR). Scale bars = 20 μm.
A second set of observations, based on a trophic factor knockout model, indicates that the morphology and distribution of intestinal IGLEs depends specifically on the expression of trophic factor NT-4: In a comparison between IGLEs in mice which the gene for NT-4 is knocked out and IGLEs in littermate controls that express NT-4, Fox et al. (2001a) found that the absence of the trophic factor led to extensive losses (~80-90%) of IGLEs in the duodenum and ileum.
The third set of observations is that IGLEs evidence plasticity and reorganization when they are challenged. When IGLEs are compromised by trauma to the stomach wall (cf. Phillips and Powley, 2005) or, even more drastically, when the visceral afferents are axotomized (Phillips et al., 2000, 2003a), the afferents exhibit regeneration, growth cones, and sprouting, and the neurites eventually reform IGLE plates. After insults, though, the endings often remain deformed or dystrophic, in some ways similar to the profiles of aging endings, suggesting that the tissue trauma may have reduced or distorted the local trophic environment.
The Organization of Vagal Intramuscular Arrays (IMAs) Suggests that Their Structure is Controlled by Trophic Influences
Another subpopulation of vagal visceral afferents, the IMA, has yet to be systematically examined for possible age-related dystrophic changes. On the other hand, though, far more is known about these endings and the way they articulate with their targets than is known about the villus afferents discussed above. [Too little is yet known about the articulations of villus afferents with other villous tissues to evaluate a trophic hypothesis of the aging of the villus afferents, though it is worth noting that, consistent with a trophic explanation of the patterns of aging of vagal villus afferents, mucosal villi are known to change with age (Ecknauer et al., 1982; Penzes and Regius, 1985; Holt et al., 1988; Atillasoy and Holt, 1993).] IMAs, much like IGLEs, appear to be controlled by trophic relationships with their GI targets.
IMAs, which have only recently been characterized structurally (e.g., Berthoud and Powley, 1992; Fox et al., 2000; Phillips and Powley, 2000; Powley and Phillips, 2002; Powley et al., 2008), are parallel arrays of terminals in smooth muscle issued by individual vagal afferents (Fig. 5A). The parallel telodendria of the IMAs run in conjunction with networks of interstitial cells of Cajal (ICCs; Fig. 5B), and this afferent-ICC complex is oriented parallel to the smooth muscle fibers (Fig. 5C,D). As illustrated in Fig. 5E, similar to ICCs, the telodendria of IMAs intertwine tightly with the axons of visceral afferents (presumably, based on their CGRP phenotype, originating from DRGs; Phillips et al., 2006, 2007, Phillips and Powley, 2007).
In ways analogous to the observations on IGLE plasticity, three types of observations indirectly suggest that the structural features of IMAs are responsive to trophic factors. One set of observations is structural. Intramuscular array telodendria intertwine closely with the processes of ICCs, appearing to make contact (Fig. 5C,D). A recent ultrastructural study (Powley et al., 2008) confirms that IMAs form pre-junctional thickenings with varicosities and appositions with ICCs. Such structural interactions suggest, that the IMAs might derive trophic as well as structural support from the ICCs and potentially other elements such as CGRP-positive DRG fibers (Fig. 5E). And, since ICCs have recently been shown to decline with age (Bernard et al., 2007; Camilleri et al., 2008), it would seem likely that IMAs might well undergo complementary losses or remodeling similar to that described by Fox et al. (2001b) in the trophic factor NT-4 knockout mouse.
A second set of observations, based on an analysis of IMA structure in animals with mutation affecting ICCs, indicates that IMAs do draw trophic support from ICCs. Fox et al. (2001b) have observed that c-Kit mutant mice (that lack c-Kit the receptor kinase and hence lack ICCs in the forestomach) have a selective loss (~70%) of IMAs in the forestomach. In a parallel experimental analysis, Fox et al. (2002) have also observed that mice mutant for steel factor, the c-Kit receptor ligand, display comparable losses and disruptions of IMAs.
Finally, a third set of observations also points to the conclusion that the structure of visceral afferent IMAs is dependent on trophic factors, thus making the prediction that IMAs may well undergo regressive or dystrophic changes with age. Our experiments looking at vagal afferent remodeling after injury to the stomach wall (Phillips and Powley, 2005) or axotomy (Phillips et al., 2000, 2003a) indicated that IMAs can regenerate and re-establish arrays of parallel telodendria, though the endings that differentiate tend to organize with significant distortion both in terms of the structure of the telodendria and in terms of the tissue targets they innervate. The patterns of plasticity again would appear to be particularly consonant with explanations in terms of altered trophic environments. Such a point also, in a sense, suggests a way of evaluating a trophic model of gut visceral afferent aging: We have yet to fully survey IMAs for age-related reorganizations, but the tight articulations with target tissues and the other indirect suggestions of trophic support for plasticity predict that alterations may be found.
Do Vagal Afferents and Efferents Age Differently?
As reported and reviewed elsewhere, efferent innervations of the gut also develop heteroplastic swellings and dystrophic features with age (Phillips et al., 2006, 2007; Phillips and Powley, 2007; cf. Fig. 2G in Walter et al., 2009 for an example of a dystrophic vagal extrinsic efferent), with such plastic changes considered to be a result of changes in the trophic control of efferents (Cowen, 1993; Andrews, 1996; Cowen and Gavazzi, 1998). Thus, it may be the case that afferents and efferents develop comparable dystrophic and regressive morphological patterns because of similar age-related changes in the trophic support of the neural projections.
One set of observations, however, suggests that there are some differences in the aging processes of the vagal afferents and efferents. The protein alpha-synuclein is expressed in some enteric neurons and in all vagal preganglionic efferents to the gut (Phillips et al., 2008), and aggregations or misfolded masses of alpha-synuclein occur in many of the age-related neuropathies in the intrinsic and extrinsic gut efferents (Phillips et al., unpublished), but the protein is not (or very seldom) expressed in vagal afferents to the gut (e.g., Fig. 6A). Conceivably, of course, the afferents may express other proteins that are susceptible to misfolding and aggregations, but regardless, it would appear that aging of vagal afferents must involve at least somewhat different mechanisms than does aging of efferents innervating the GI tract. This is particularly relevant to the evolving hypothesis that for certain age-related diseases, such as Parkinson’s, the sensory components of the nervous system remain intact while the motor areas--particularly the visceromotor systems--progressively degenerate (Braak et al., 2006; 2007; Cersosimo and Benarrock, 2008; Braak and Del Tredici, 2009).
Summary
Visceral afferents, which are responsible for initiating GI reflexes and providing feedback about functional adjustments of the gut, exhibit structural remodeling with aging. Vagal IGLEs and villus afferents, the two types of visceral afferent terminals that have been examined in the gut to date, both display dystrophic and regressive changes with aging. Such deficits, one found in the muscle wall and one found in the mucosa, are consistent with diminished trophic support. But whatever the mechanism, such dystrophic features presumably compromise both afferent feedback from the aged gut and reflexes key to gastrointestinal health. Given the strategic functions of gut visceral afferents and given the evidence that they deteriorate with age, as does GI function, more systematic evaluations of visceral afferent aging are certainly needed.
Acknowledgment
This work was supported by National Institute of Health Grants NIH DK61317, DK27627, and HD52112.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abalo R, Jose Rivera A, Vera G, Isabel Martin M. Ileal myenteric plexus in aged guinea-pigs: loss of structure and calretinin-immunoreactive neurones. Neurogastroenterol Motil. 2005;17:123–132. doi: 10.1111/j.1365-2982.2004.00612.x. [DOI] [PubMed] [Google Scholar]
- Ai J, Gozal D, Li L, Wead WB, Chapleau MW, Wurster R, Yang B, Li H, Liu R, Cheng Z. Degeneration of vagal efferent axons and terminals in cardiac ganglia of aged rats. J Comp Neurol. 2007;504:74–88. doi: 10.1002/cne.21431. [DOI] [PubMed] [Google Scholar]
- Anderton BH. Changes in the ageing brain in health and disease. Philos Trans R Soc Lond B Biol Sci. 1997;352:1781–1792. doi: 10.1098/rstb.1997.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderton BH. Ageing of the brain. Mech Ageing Dev. 2002;123:811–817. doi: 10.1016/s0047-6374(01)00426-2. [DOI] [PubMed] [Google Scholar]
- Andrew W. Structural alterations with aging in the nervous system. J Chron Dis. 1956;3:575–596. doi: 10.1016/0021-9681(56)90155-2. [DOI] [PubMed] [Google Scholar]
- Andrews TJ. Autonomic nervous system as a model of neuronal aging: the role of target tissues and neurotrophic factors. Microsc Res Tech. 1996;35:2–19. doi: 10.1002/(SICI)1097-0029(19960901)35:1<2::AID-JEMT2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Atillasoy E, Holt PR. Gastrointestinal proliferation and aging. J Gerontol. 1993;48:B43–49. doi: 10.1093/geronj/48.2.b43. [DOI] [PubMed] [Google Scholar]
- Bergman E, Fundin BT, Ulfhake B. Effects of aging and axotomy on the expression of neurotrophin receptors in primary sensory neurons. J Comp Neurol. 1999;410:368–386. doi: 10.1002/(sici)1096-9861(19990802)410:3<368::aid-cne2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Bergman E, Ulfhake B. Loss of primary sensory neurons in the very old rat: neuron number estimates using the disector method and confocal optical sectioning. J Comp Neurol. 1998;396:211–222. [PubMed] [Google Scholar]
- Bernard C, Gibbons S, Lurken M, Schmalz P, Roeder J, Linden D, Cima R, Dozois E, Larson D, Camilleri M, Hicks G, Farrugia G. Effect of ag on the enteric nervous system of the human colon. Neurogastroenterol Motil. 2007;19(Suppl 3):39. doi: 10.1111/j.1365-2982.2008.01245.x. abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Kressel M, Raybould HE, Neuhuber WL. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat Embryol (Berl) 1995;191:203–212. doi: 10.1007/BF00187819. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Patterson LM, Neumann F, Neuhuber WL. Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat Embryol (Berl) 1997;195:183–191. doi: 10.1007/s004290050037. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Powley TL. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J Comp Neurol. 1992;319:261–276. doi: 10.1002/cne.903190206. [DOI] [PubMed] [Google Scholar]
- Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Invited Article: Nervous system pathology in sporadic Parkinson disease. Neurology. 2008;70:1916–1925. doi: 10.1212/01.wnl.0000312279.49272.9f. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Neuroanatomy and pathology of sporadic Parkinson’s disease. Adv Anat Embryol Cell Biol. 2009;201:1–119. [PubMed] [Google Scholar]
- Braak H, Sastre M, Bohl JR, de Vos RA, Del Tredici K. Parkinson’s disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol (Berl) 2007;113:421–429. doi: 10.1007/s00401-007-0193-x. [DOI] [PubMed] [Google Scholar]
- Brizzee KR, Ordy JM. Effects of age on the visceral afferent components of the autonomic nervous system. In: Ordy JM, Brizzee KR, editors. Sensory systems and communication in the elderly. Raven Press; New York: 1979. pp. 283–296. [Google Scholar]
- Camilleri M, Cowen T, Koch TR. Enteric neurodegeneration in ageing. Neurogastroenterol Motil. 2008;20:418–429. doi: 10.1111/j.1365-2982.2008.01134.x. [DOI] [PubMed] [Google Scholar]
- Cersosimo MG, Benarroch EE. Neural control of the gastrointestinal tract: implications for Parkinson disease. Mov Disord. 2008;23:1065–1075. doi: 10.1002/mds.22051. [DOI] [PubMed] [Google Scholar]
- Cowen T. Ageing in the autonomic nervous system: a result of nerve-target interactions? A review. Mech Ageing Dev. 1993;68:163–173. doi: 10.1016/0047-6374(93)90148-k. [DOI] [PubMed] [Google Scholar]
- Cowen T, Gavazzi I. Plasticity in adult and ageing sympathetic neurons. Prog Neurobiol. 1998;54:249–288. doi: 10.1016/s0301-0082(97)00071-3. [DOI] [PubMed] [Google Scholar]
- Crane SJ, Talley NJ. Chronic gastrointestinal symptoms in the elderly. Clin Geriatr Med. 2007;23:721–734. v. doi: 10.1016/j.cger.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Devor M. Chronic pain in the aged: possible relation between neurogenesis, involution and pathophysiology in adult sensory ganglia. J Basic Clin Physiol Pharmacol. 1991;2:1–15. doi: 10.1515/jbcpp.1991.2.1-2.1. [DOI] [PubMed] [Google Scholar]
- Ecknauer R, Vadakel T, Wepler R. Intestinal morphology and cell production rate in aging rats. J Gerontol. 1982;37:151–155. doi: 10.1093/geronj/37.2.151. [DOI] [PubMed] [Google Scholar]
- Finch CE. Neuron atrophy during aging: programmed or sporadic? Trends Neurosci. 1993;16:104–110. doi: 10.1016/0166-2236(93)90134-8. [DOI] [PubMed] [Google Scholar]
- Fox EA, Phillips RJ, Baronowsky EA, Byerly MS, Jones S, Powley TL. Neurotrophin-4 deficient mice have a loss of vagal intraganglionic mechanoreceptors from the small intestine and a disruption of short-term satiety. J Neurosci. 2001a;21:8602–8615. doi: 10.1523/JNEUROSCI.21-21-08602.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox EA, Phillips RJ, Byerly MS, Baronowsky EA, Chi MM, Powley TL. Selective loss of vagal intramuscular mechanoreceptors in mice mutant for steel factor, the c-Kit receptor ligand. Anat Embryol (Berl) 2002;205:325–342. doi: 10.1007/s00429-002-0261-x. [DOI] [PubMed] [Google Scholar]
- Fox EA, Phillips RJ, Martinson FA, Baronowsky EA, Powley TL. Vagal afferent innervation of smooth muscle in the stomach and duodenum of the mouse: morphology and topography. J Comp Neurol. 2000;428:558–576. doi: 10.1002/1096-9861(20001218)428:3<558::aid-cne11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Fox EA, Phillips RJ, Martinson FA, Baronowsky EA, Powley TL. C-Kit mutant mice have a selective loss of vagal intramuscular mechanoreceptors in the forestomach. Anat Embryol (Berl) 2001b;204:11–26. doi: 10.1007/s004290100184. [DOI] [PubMed] [Google Scholar]
- Grundy D. Neuroanatomy of visceral nociception: vagal and splanchnic afferent. Gut. 2002;51(Suppl 1):i2–5. doi: 10.1136/gut.51.suppl_1.i2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall KE. Aging and neural control of the GI tract. II. Neural control of the aging gut: can an old dog learn new tricks? Am J Physiol Gastrointest Liver Physiol. 2002;283:G827–832. doi: 10.1152/ajpgi.00162.2002. [DOI] [PubMed] [Google Scholar]
- Hays NP, Roberts SB. The anorexia of aging in humans. Physiol Behav. 2006;88:257–266. doi: 10.1016/j.physbeh.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Hill CJ. A contribution to our knowledge of the enteric plexuses. Phil Trans B. 1927;215:355–387. [Google Scholar]
- Holt PR, Yeh KY, Kotler DP. Altered controls of proliferation in proximal small intestine of the senescent rat. Proc Natl Acad Sci U S A. 1988;85:2771–2775. doi: 10.1073/pnas.85.8.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janig W. Neurobiology of visceral afferent neurons: neuroanatomy, functions, organ regulations and sensations. Biol Psychol. 1996;42:29–51. doi: 10.1016/0301-0511(95)05145-7. [DOI] [PubMed] [Google Scholar]
- Koistinaho J. Difference in the age-related accumulation of lipopigments in the adrenergic and nonadrenergic peripheral neurons in the male rat. Gerontology. 1986;32:300–307. doi: 10.1159/000212808. [DOI] [PubMed] [Google Scholar]
- Koistinaho J, Hartikainen K, Hatanp K, Hervonen A. Age pitments in different populations of peripheral neurons in vivo and in vitro. In: Porta EA, editor. Lipofuscian and ceroid pigments. Plenum Press; New York: 1990. pp. 49–58. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE. Constipation and irritable bowel syndrome in the elderly. Clin Geriatr Med. 2007;23:823–832. vi–vii. doi: 10.1016/j.cger.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Neuhuber WL. Sensory vagal innervation of the rat esophagus and cardia: a light and electron microscopic anterograde tracing study. J Auton Nerv Syst. 1987;20:243–255. doi: 10.1016/0165-1838(87)90153-6. [DOI] [PubMed] [Google Scholar]
- Neuhuber WL, Clerc N. Afferent innervation of the esophagus in cat and rat. In: Zenker W, Neuhuber WL, editors. The Primary afferent neuron : a survey of recent morpho-functional aspects. Plenum Press; New York: 1990. pp. 93–107. [Google Scholar]
- Neuhuber WL, Raab M, Berthoud HR, Worl J. Innervation of the mammalian esophagus. Adv Anat Embryol Cell Biol. 2006;185:1–73. back cover. [PubMed] [Google Scholar]
- Newton JL. Changes in upper gastrointestinal physiology with age. Mech Ageing Dev. 2004;125:867–870. doi: 10.1016/j.mad.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Nonidez JF. Afferent nerves in the intermuscular plexus of the dog’s oesophagus. J Comp Neurol. 1946;85:177–189. doi: 10.1002/cne.900850204. [DOI] [PubMed] [Google Scholar]
- Norton C. Constipation in older patients: effects on quality of life. Br J Nurs. 2006;15:188–192. doi: 10.12968/bjon.2006.15.4.20542. [DOI] [PubMed] [Google Scholar]
- O’Mahony D, O’Leary P, Quigley EM. Aging and intestinal motility: a review of factors that affect intestinal motility in the aged. Drugs Aging. 2002;19:515–527. doi: 10.2165/00002512-200219070-00005. [DOI] [PubMed] [Google Scholar]
- Penzes L, Regius O. Changes in the intestinal microvillous surface area during reproduction and ageing in the female rat. J Anat. 1985;140(Pt 3):389–396. [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Baronowsky EA, Powley TL. Regenerating vagal afferents reinnervate gastrointestinal tract smooth muscle of the rat. J Comp Neurol. 2000;421:325–346. [PubMed] [Google Scholar]
- Phillips RJ, Baronowsky EA, Powley TL. Long-term regeneration of abdominal vagus: Efferents fail while afferents succeed. J Comp Neurol. 2003a;455:222–237. doi: 10.1002/cne.10470. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Kieffer EJ, Powley TL. Aging of the myenteric plexus: neuronal loss is specific to cholinergic neurons. Auton Neurosci. 2003b;106:69–83. doi: 10.1016/S1566-0702(03)00072-9. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Pairitz JC, Powley TL. Age-related neuronal loss in the submucosal plexus of the colon of Fischer 344 rats. Neurobiol Aging. 2007;28:1124–1137. doi: 10.1016/j.neurobiolaging.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev. 2000;34:1–26. doi: 10.1016/s0165-0173(00)00036-9. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. As the gut ages: timetables for aging of innervation vary by organ in the Fischer 344 rat. J Comp Neurol. 2001;434:358–377. doi: 10.1002/cne.1182. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Plasticity of vagal afferents at the site of an incision in the wall of the stomach. Auton Neurosci. 2005;123:44–53. doi: 10.1016/j.autneu.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Innervation of the gastrointestinal tract: patterns of aging. Auton Neurosci. 2007;136:1–19. doi: 10.1016/j.autneu.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Rhodes BS, Powley TL. Effects of age on sympathetic innervation of the myenteric plexus and gastrointestinal smooth muscle of Fischer 344 rats. Anat Embryol (Berl) 2006;211:673–683. doi: 10.1007/s00429-006-0123-z. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Walter GC, Wilder SL, Baronowsky EA, Powley TL. Alpha-synuclein-immunopositive myenteric neurons and vagal preganglionic terminals: Autonomic pathway implicated in Parkinson’s disease? Neuroscience. 2008;153:733–750. doi: 10.1016/j.neuroscience.2008.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powley TL, Chi MM, Schier LA, Phillips RJ. Obesity: should treatments target visceral afferents? Physiol Behav. 2005;86:698–708. doi: 10.1016/j.physbeh.2005.08.059. [DOI] [PubMed] [Google Scholar]
- Powley TL, Phillips RJ. Musings on the wanderer: what’s new in our understanding of vago-vagal reflexes? I. Morphology and topography of vagal afferents innervating the GI tract. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1217–1225. doi: 10.1152/ajpgi.00249.2002. [DOI] [PubMed] [Google Scholar]
- Powley TL, Phillips RJ. Advances in neural tracing of vagal afferent nerves and terminals. In: Undem BJ, Weinreich D, editors. Advances in vagal afferent neurobiology. Taylor & Francis; Boca Raton: 2005. pp. 123–145. [Google Scholar]
- Powley TL, Wang XY, Fox EA, Phillips RJ, Liu LW, Huizinga JD. Ultrastructural evidence for communication between intramuscular vagal mechanoreceptors and interstitial cells of Cajal in the rat fundus. Neurogastroenterol Motil. 2008;20:69–79. doi: 10.1111/j.1365-2982.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- Roach M, Christie JA. Fecal incontinence in the elderly. Geriatrics. 2008;63:13–22. [PubMed] [Google Scholar]
- Robinson DR, Gebhart GF. Inside information: the unique features of visceral sensation. Mol Interv. 2008;8:242–253. doi: 10.1124/mi.8.5.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo J, Hernandez J, Vidal MA, Pedrosa JA. Vegetative innervation of the esophagus. II. Intraganglionic laminar endings. Acta Anat (Basel) 1975;92:79–100. doi: 10.1159/000144431. [DOI] [PubMed] [Google Scholar]
- Saffrey MJ. Ageing of the enteric nervous system. Mech Ageing Dev. 2004;125:899–906. doi: 10.1016/j.mad.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Soltanpour N, Baker DM, Santer RM. Neurons and microvessels of the nodose (vagal sensory) ganglion in young adult and aged rats: morphometric and enzyme histochemical studies. Tissue Cell. 1996;28:593–602. doi: 10.1016/s0040-8166(96)80062-0. [DOI] [PubMed] [Google Scholar]
- Soltanpour N, Santer RM. Preservation of the cervical vagus nerve in aged rats: morphometric and enzyme histochemical evidence. J Auton Nerv Syst. 1996;60:93–101. doi: 10.1016/0165-1838(96)00038-0. [DOI] [PubMed] [Google Scholar]
- Thrasivoulou C, Soubeyre V, Ridha H, Giuliani D, Giaroni C, Michael GJ, Saffrey MJ, Cowen T. Reactive oxygen species, dietary restriction and neurotrophic factors in age-related loss of myenteric neurons. Aging Cell. 2006;5:247–257. doi: 10.1111/j.1474-9726.2006.00214.x. [DOI] [PubMed] [Google Scholar]
- Trinh C, Prabhakar K. Diarrheal diseases in the elderly. Clin Geriatr Med. 2007;23:833–856. vii. doi: 10.1016/j.cger.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Ulfhake B, Bergman E, Edstrom E, Fundin BT, Johnson H, Kullberg S, Ming Y. Regulation of neurotrophin signaling in aging sensory and motoneurons: dissipation of target support? Mol Neurobiol. 2000;21:109–135. doi: 10.1385/MN:21:3:109. [DOI] [PubMed] [Google Scholar]
- Ulfhake B, Bergman E, Fundin BT. Impairment of peripheral sensory innervation in senescence. Auton Neurosci. 2002;96:43–49. doi: 10.1016/s1566-0702(01)00368-x. [DOI] [PubMed] [Google Scholar]
- Vega JA, Calzada B, Del Valle ME. Age-induced changes in the mammalian autonomic and sensory ganglia. In: Amenta F, editor. Aging of the Autonomic Nervous System. CRC Press; Boca Raton, Florida: 1993. pp. 37–67. [Google Scholar]
- Wade PR. Aging and neural control of the GI tract. I. Age-related changes in the enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2002;283:G489–495. doi: 10.1152/ajpgi.00091.2002. [DOI] [PubMed] [Google Scholar]
- Wade PR, Cowen T. Neurodegeneration: a key factor in the ageing gut. Neurogastroenterol Motil. 2004;16(Suppl 1):19–23. doi: 10.1111/j.1743-3150.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- Walter GC, Phillips RJ, Baronowsky EA, Powley TL. Versatile, high-resolution anterograde labeling of vagal efferent projections with dextran amines. J Neurosci Methods. 2009;178:1–9. doi: 10.1016/j.jneumeth.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FB, Powley TL. Topographic inventories of vagal afferents in gastrointestinal muscle. J Comp Neurol. 2000;421:302–324. [PubMed] [Google Scholar]
- Wiley JW. Aging and neural control of the GI tract: III. Senescent enteric nervous system: lessons from extraintestinal sites and nonmammalian species. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1020–1026. doi: 10.1152/ajpgi.00224.2002. [DOI] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Brookes SJ. Transduction sites of vagal mechanoreceptors in the guinea pig esophagus. J Neurosci. 2000;20:6249–6255. doi: 10.1523/JNEUROSCI.20-16-06249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, Brookes SJ. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol. 2001;534:255–268. doi: 10.1111/j.1469-7793.2001.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, Costa M, Brookes SJ. Mechanotransduction by intraganglionic laminar endings of vagal tension receptors in the guinea-pig oesophagus. J Physiol. 2003;553:575–587. doi: 10.1113/jphysiol.2003.051862. [DOI] [PMC free article] [PubMed] [Google Scholar]